Abstract
Objectives
One of the most common head and neck cancers is nasopharynx cancer. Knowledge about the incidence and mortality of this disease and its distribution in terms of geographical areas is necessary for further study and better planning. Therefore, this study was conducted with the aim of determining the incidence and mortality rates of nasopharynx cancer and its relationship with the Human Development Index (HDI) in Asia in 2012.
Methods
The aim of this ecologic study was to assess the correlation between age-specific incidence rate (ASIR) and age-specific mortality rate (ASMR) with HDI and its components, which include the following: life expectancy at birth, mean years of schooling, and gross national income per capita. Data about SIR and SMR for every Asian country for 2012 were obtained from the global cancer project. We used the correlation bivariate method for the assessment. Statistical significance was assumed if p < 0.05. All reported p values are two-sided. Statistical analyses were performed using SPSS (Version 15.0, SPSS Inc.).
Results
A total of 68,272 cases (males, 71.02%; females, 28.97%; sex ratio, 2.45) and 40,530 mortalities (males, 71.63%; females, 28.36%; sex ratio, 2.52) were recorded in Asian countries in 2012. The five countries with the highest ASIR of nasopharynx cancer were Malaysia, Singapore, Indonesia, Vietnam, and Brunei, and the five countries with the highest ASMR were Indonesia, Vietnam, Singapore, Malaysia, and Brunei. The correlation between HDI and ASIR was 0.097 (p = 0.520) [0.105 in men (p = 0.488) and 0.119 in women (p = 0.901)]. The correlation between HDI and ASMR was –0.102 (p = 0.502) [–0.072 in men (p = 0.633) and –0.224 in women (p = 0.134)].
Conclusion
Nasopharynx cancer is native to Southeast Asia. The highest incidence and mortality rates are found in Malaysia, Singapore, Indonesia, Vietnam, and Brunei. No significant relation was found between the standardized incidence and mortality rates of nasopharynx cancer and the HDI components. Further studies are recommended in Southeast Asian countries in order to find the etiology of cancer, as well as its diagnosis and treatment.
Keywords: Asia, epidemiology, HDI, incidence, mortality, nasopharynx cancer
1. Introduction
Cancer is the leading cause of mortality, with a high financial burden, and one of the major public health concerns at the international level 1, 2. Head and neck malignancies are among the relatively common cancers in humans that affect several anatomic sites of the head and the neck [3]. One of the most common head and neck cancers is nasopharynx cancer 4, 5, which has a very unique distribution pattern. Worldwide, about 86,500 cases of nasopharynx cancer and 50,000 deaths arising from it [6] are reported annually. According to the International Agency for Research on Cancer report in 2008, more than 80% of patients with nasopharynx cancer are in Asia, and only 5% of these cancers are reported in Europe. Specifically, 71% of new nasopharynx cancer cases are recorded in East and Southeast Asia, and 29% are diagnosed in South and Central Asia and North and East Africa [7].
Nasopharynx cancer is native to Southeast Asia, where the prevalence rate is 15–50 cases per 100,000 people [8]. For the United States and the rest of the world, the incidence of less than 1 case per 100,000 people per year is reported [9]. In addition to geographic diversity, it seems that some ethnic groups are prone to nasopharynx cancer. These groups include the Bidayuh in Borneo, the Nagas in northern India, and the Inuits in the North pole, which have an age-standardized incidence rate of more than 16 per 100,000 people among men each year [10].
In terms of heterogeneous epidemiological patterns, in addition to nonenvironmental risk factors such as sex, ethnicity, and family history [11], other factors—such as smoking [12], salted fish consumption, especially in childhood 12, 13, 14, 15, 16, 17, 18, nitrosamine in some food items traditionally used in southern China 19, 20, and use of traditional herbal medicines in the Asian population 12, 21, 22, 23, as well as nonfood risk factors such as occupational exposures to formaldehyde, wood dust, smoke, and chemicals 7, 12, 22, 24—may be involved in the pathogenesis of nasopharynx carcinoma [25]. Nasopharynx cancer, in comparison to other head and neck tumors, has different epidemiological, staging, and treatment characteristics. Most patients are diagnosed when they are in advanced stages 26, 27.
The Human Development Index (HDI) is a useful tool to compare the incidence and mortality rates of cancer at the global level 28, 29, 30 and is one of the indicators to check the status of illnesses and deaths between countries. In fact, this index has been observed to be related with the incidence and mortality rates of many diseases; it is considered a good index to obtain information regarding the status of a specific disease in different countries. The HDI is composed of three basic dimensions: life expectancy at birth, adult literacy rate, and gross domestic product (GDP) per capita. The relationship between HDI and some cancers is studied, and investigating this relationship can lead to a more accurate understanding of cancer and its risk factors distribution [31], and it is also suggested to be used for other cancers. Although nutritional and communicable diseases are common causes of death in countries with a low HDI, it is anticipated that by 2030, one common cause of death in these countries will be noncommunicable diseases such as cancer [32]. Because awareness about nasopharynx cancer incidence and mortality can be useful for health programs and research activities, and considering the possible role of the HDI, this study was conducted with the aim of evaluating the incidence and mortality of nasopharynx carcinoma and its relationship with the development index and its components in Asia in 2012.
2. Materials and methods
We conducted an ecologic study in Asia to assess the correlation between age-specific incidence and mortality rate (ASR) with HDI and its components, which include the following: life expectancy at birth, mean years of schooling, and gross national income (GNI) per capita. The ASR data of all Asian countries for the year 2012 were obtained from the global cancer project (available at http://globocan.iarc.fr/Default.aspx) [33], and the HDI data was based on the Human Development Report 2013 [34], which contains information about HDI and its components for every country in the world in 2012.
2.1. Method of estimating ASRs in global cancer project by the International Agency for Research on Cancer
2.1.1. Age-specific incidence rate estimate
The methods of estimation are country-specific, and the quality of the estimation depends on the quality and amount of information available for each country. In theory, there are as many methods as countries, and because of the variety and the complexity of these methods, an overall quality score for the incidence and mortality estimates combined is almost impossible to establish. However, an alphanumeric scoring system that independently describes the availability of incidence and mortality data has been established at the country level. The combined score is presented together with the estimates for each country with the aim of providing a broad indication of the robustness of the estimation.
The methods used to estimate the sex- and age-specific incidence rates of cancer for a specific country fall into one of the following broad categories (by order of priority):
-
1.
Rates projected to 2012 (38 countries).
-
2.
Most recent rates applied to 2012 population (20 countries).
-
3.
Estimated from national mortality by modeling, using incidence mortality ratios derived from recorded data in country-specific cancer registries (13 countries).
-
4.
Estimated from national mortality estimates by modeling, using incidence mortality ratios derived from recorded data in local cancer registries in neighboring countries (9 European countries).
-
5.
Estimated from national mortality estimates using modeled survival (32 countries)
-
6.
Estimated as the weighted average of the local rates (16 countries)
-
7.
One cancer registry covering part of a country is used as representative of the country profile (11 countries).
-
8.
Age/sex-specific rates for “all cancers” were partitioned using data on relative frequency of different cancers (by age and sex) (12 countries).
-
9.
The rates are those of neighboring countries or registries in the same area (33 countries) 33, 35.
2.1.2. Age-specific mortality rate estimate
Depending on the degree of detail and accuracy of the national mortality data, six methods were used in the following order of priority:
-
1.
Rates projected to 2012 (69 countries).
-
2.
Most recent rates applied to 2012 population (26 countries).
-
3.
Estimated as the weighted average of regional rates (1 country).
-
4.
Estimated from national incidence estimates by modeling, using country-specific survival (2 countries).
-
5.
Estimated from national incidence estimates using modeled survival (83 countries).
-
6.
The rates are those of neighboring countries or registries in the same area (3 countries) 33, 35.
2.2. Human Development Index
HDI is a composite measure of indicators along three dimensions: life expectancy, educational attainment, and command over the resources needed for a decent living. All groups and regions have seen notable improvement in all HDI components, with faster progress in low and medium HDI countries. On this basis, the world is becoming less unequal. Nevertheless, national averages hide large variations in human experience. Wide disparities remain within countries of both the North and the South, and income inequality within and between many countries has been rising [34].
2.3. Statistical analysis
In this study, we used the correlation bivariate method to assess the correlation between ASR and HDI and its components (life expectancy at birth, mean years of schooling, and GNI per capita). Statistical significance was assumed if p < 0.05. All reported p values are two-sided. Statistical analyses were performed using SPSS (Version 15.0: SPSS Inc., Chicago).
3. Results
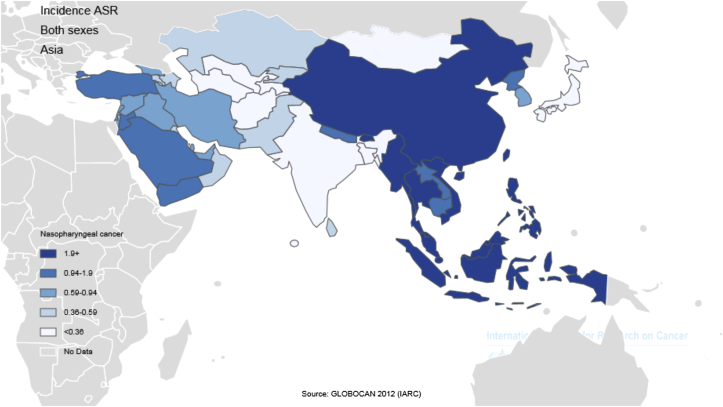
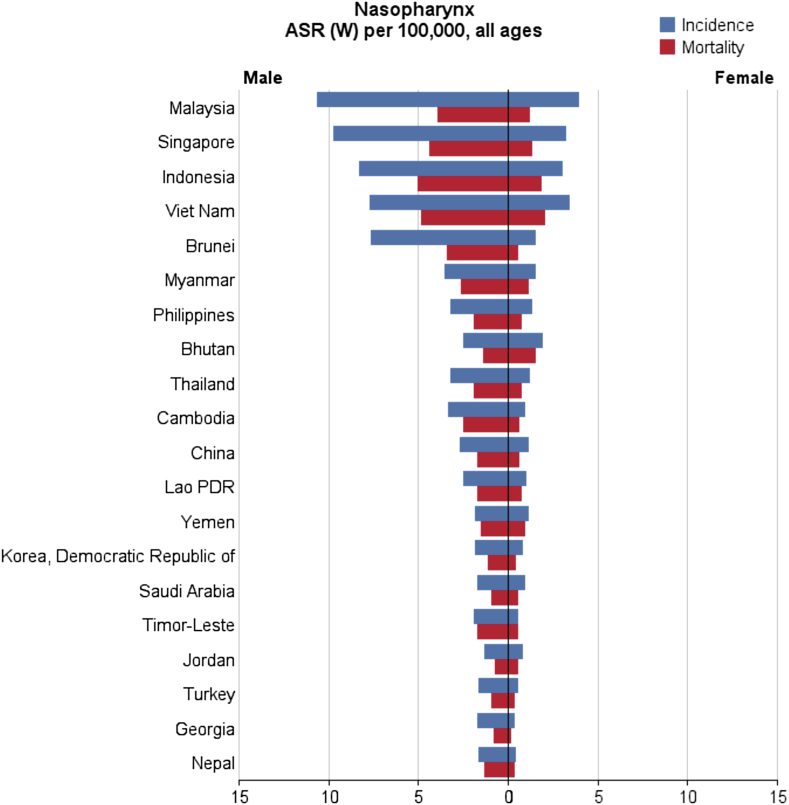
In general, a total of 68,272 nasopharynx cancer cases were recorded in Asian countries in 2012, of which 48,492 cases (2/71%) were diagnosed in men and 19,780 cases (97/28%) were diagnosed in women. These figures indicate that the sex (male/female) ratio is 45:2. Five countries with the highest number of new cases of nasopharynx cancer are as follows: (1) China with 33,198 cases; (2) Indonesia, 13,084 cases; (3) Vietnam, 4,931 cases; (4) India, 3,947 cases; (5) Malaysia, 2,030 cases; these five countries alone accounted for a total of 57,190 cases (76/83%) of all cases in Asia. In Asia, the five countries with the highest standardized incidence of nasopharynx cancer are as follows: (1) Malaysia, with a standardized rate of 7.2 per 100,000 people; (2) Singapore, 6.4 per 100,000 people; (3) Indonesia, 5.6 per 100,000 people; (4) Vietnam, 5.4 per 100,000 people; (5) Brunei, 5 per 100,000 people. Conversely, the five countries with the lowest standardized incidence of nasopharynx cancer are as follows: (1) Maldives, with a rate of 0 per 100,000 people; (2) Tajikistan, 0.1 per 100,000 people; (3) Japan, 0.2 per 100,000 people; (4) Bahrain, 0.2 per 100,000 people; (5) Uzbekistan, 0.3 per 100,000 people. The number and rate of standardized and crude incidence of this cancer in Asian countries according to sex are shown in Table 1. Countries are arranged based on standardized rates, from highest to lowest, in Table 1, so that countries with the highest and lowest standardized incidence rates in each sex are shown (Table 1, Figure 1, Figure 2).
Table 1.
Number and amount of standardized and crude incidence of nasopharynx cancer in Asian countries in 2012 (sorted by age-standardized incidence rate, from the highest to the lowest value).
| Nasopharynx—estimated incidence, all ages: both sexes |
Nasopharynx—estimated incidence, all ages: male |
Nasopharynx—estimated incidence, all ages: female |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Population | No. | Crude rate | Age-world-standardized incidence rate (ASR(W)) | Population | No. | Crude rate | Age-world-standardized incidence rate (ASR(W)) | Population | No. | Crude rate | ASR (W) |
| Malaysia | 2,030 | 6.9 | 7.2 | Malaysia | 1,487 | 10.0 | 10.6 | Malaysia | 543 | 3.8 | 3.9 |
| Singapore | 473 | 9.0 | 6.4 | Singapore | 353 | 13.3 | 9.7 | Vietnam | 1,630 | 3.6 | 3.4 |
| Indonesia | 13,084 | 5.3 | 5.6 | Indonesia | 9,355 | 7.7 | 8.3 | Singapore | 120 | 4.6 | 3.2 |
| Vietnam | 4,931 | 5.5 | 5.4 | Vietnam | 3,301 | 7.4 | 7.7 | Indonesia | 3,729 | 3.0 | 3.0 |
| Brunei | 15 | 3.6 | 5.0 | Brunei | 12 | 5.8 | 7.6 | Bhutan | 6 | 1.7 | 1.9 |
| Myanmar | 1,148 | 2.4 | 2.4 | Myanmar | 789 | 3.3 | 3.5 | Brunei | 3 | 1.5 | 1.5 |
| Philippines | 1,738 | 1.8 | 2.2 | Cambodia | 159 | 2.2 | 3.3 | Myanmar | 359 | 1.5 | 1.5 |
| Bhutan | 14 | 1.9 | 2.2 | Philippines | 1,199 | 2.5 | 3.2 | Philippines | 539 | 1.1 | 1.3 |
| Thailand | 1,867 | 2.7 | 2.1 | Thailand | 1,328 | 3.9 | 3.2 | Thailand | 539 | 1.5 | 1.2 |
| China | 33,198 | 2.4 | 1.9 | China | 23,581 | 3.3 | 2.7 | China | 9,617 | 1.5 | 1.1 |
| Cambodia | 220 | 1.5 | 1.9 | Bhutan | 8 | 2.0 | 2.5 | Yemen | 96 | 0.8 | 1.1 |
| Lao PDR | 81 | 1.3 | 1.7 | Lao PDR | 55 | 1.7 | 2.5 | Lao PDR | 26 | 0.8 | 1.0 |
| Yemen | 217 | 0.8 | 1.5 | Timor-Leste | 7 | 1.2 | 1.9 | Cambodia | 61 | 0.8 | 0.9 |
| Saudi Arabia | 295 | 1.0 | 1.3 | Yemen | 121 | 0.9 | 1.8 | Saudi Arabia | 83 | 0.6 | 0.9 |
| Korea, Democratic Republic of | 383 | 1.6 | 1.3 | Korea, Democratic Republic of | 244 | 2.0 | 1.8 | Korea, Democratic Republic of | 139 | 1.1 | 0.8 |
| Timor-Leste | 9 | 0.8 | 1.2 | Saudi Arabia | 212 | 1.3 | 1.7 | Jordan | 19 | 0.6 | 0.8 |
| Jordan | 51 | 0.8 | 1.1 | Georgia | 46 | 2.3 | 1.7 | Kuwait | 4 | 0.3 | 0.6 |
| Turkey | 779 | 1.0 | 1.0 | Nepal | 178 | 1.2 | 1.6 | Syrian Arab Republic | 51 | 0.5 | 0.6 |
| Nepal | 233 | 0.8 | 1.0 | Turkey | 588 | 1.6 | 1.6 | Turkey | 191 | 0.5 | 0.5 |
| Georgia | 57 | 1.3 | 0.9 | Jordan | 32 | 1.0 | 1.3 | Iraq | 56 | 0.3 | 0.5 |
| Syrian Arab Republic | 138 | 0.7 | 0.8 | Armenia | 19 | 1.3 | 1.2 | Timor-Leste | 2 | 0.3 | 0.5 |
| United Arab Emirates | 36 | 0.4 | 0.8 | Kyrgyzstan | 25 | 0.9 | 1.1 | Sri Lanka | 50 | 0.5 | 0.4 |
| Iraq | 139 | 0.4 | 0.7 | Syrian Arab Republic | 87 | 0.8 | 1.1 | Nepal | 55 | 0.4 | 0.4 |
| Qatar | 10 | 0.5 | 0.7 | Lebanon | 23 | 1.1 | 1.1 | Iran, Islamic Republic of | 140 | 0.4 | 0.4 |
| Lebanon | 31 | 0.7 | 0.7 | Korea, Republic of | 320 | 1.3 | 1.0 | United Arab Emirates | 10 | 0.4 | 0.4 |
| Korea, Republic of | 442 | 0.9 | 0.7 | Israel | 38 | 1.0 | 0.9 | Korea, Republic of | 122 | 0.5 | 0.4 |
| Iran, Islamic Republic of | 418 | 0.6 | 0.6 | Iraq | 83 | 0.5 | 0.9 | Lebanon | 8 | 0.4 | 0.3 |
| Israel | 53 | 0.7 | 0.6 | United Arab Emirates | 26 | 0.5 | 0.9 | Pakistan | 228 | 0.3 | 0.3 |
| Kyrgyzstan | 28 | 0.5 | 0.6 | Qatar | 9 | 0.6 | 0.8 | Israel | 15 | 0.4 | 0.3 |
| Armenia | 22 | 0.7 | 0.6 | Iran, Islamic Republic of | 278 | 0.7 | 0.8 | Georgia | 11 | 0.5 | 0.3 |
| Pakistan | 795 | 0.4 | 0.6 | State of Palestine | 10 | 0.5 | 0.8 | Mongolia | 3 | 0.2 | 0.3 |
| Kuwait | 11 | 0.4 | 0.5 | Pakistan | 567 | 0.6 | 0.8 | Kazakhstan | 25 | 0.3 | 0.3 |
| State of Palestine | 14 | 0.3 | 0.5 | Azerbaijan | 34 | 0.7 | 0.7 | Qatar | 1 | 0.2 | 0.3 |
| Kazakhstan | 75 | 0.5 | 0.4 | Kazakhstan | 50 | 0.6 | 0.7 | Uzbekistan | 26 | 0.2 | 0.2 |
| Azerbaijan | 42 | 0.4 | 0.4 | India | 2,956 | 0.5 | 0.5 | Oman | 2 | 0.2 | 0.2 |
| Sri Lanka | 95 | 0.4 | 0.4 | Bangladesh | 322 | 0.4 | 0.5 | Bahrain | 1 | 0.2 | 0.2 |
| Oman | 9 | 0.3 | 0.4 | Oman | 7 | 0.4 | 0.5 | Bangladesh | 112 | 0.1 | 0.2 |
| Mongolia | 8 | 0.3 | 0.4 | Afghanistan | 51 | 0.3 | 0.5 | India | 991 | 0.2 | 0.2 |
| India | 3,947 | 0.3 | 0.4 | Turkmenistan | 9 | 0.4 | 0.4 | State of Palestine | 4 | 0.2 | 0.2 |
| Bangladesh | 434 | 0.3 | 0.3 | Mongolia | 5 | 0.4 | 0.4 | Azerbaijan | 8 | 0.2 | 0.2 |
| Afghanistan | 66 | 0.2 | 0.3 | Japan | 421 | 0.7 | 0.4 | Afghanistan | 15 | 0.1 | 0.2 |
| Turkmenistan | 11 | 0.2 | 0.3 | Sri Lanka | 45 | 0.4 | 0.4 | Armenia | 3 | 0.2 | 0.1 |
| Uzbekistan | 64 | 0.2 | 0.3 | Kuwait | 7 | 0.4 | 0.4 | Kyrgyzstan | 3 | 0.1 | 0.1 |
| Bahrain | 4 | 0.3 | 0.2 | Uzbekistan | 38 | 0.3 | 0.3 | Turkmenistan | 2 | 0.1 | 0.1 |
| Japan | 553 | 0.4 | 0.2 | Bahrain | 3 | 0.4 | 0.2 | Japan | 132 | 0.2 | 0.1 |
| Tajikistan | 4 | 0.1 | 0.1 | Tajikistan | 4 | 0.1 | 0.1 | Maldives | 0 | 0.0 | 0.0 |
| Maldives | 0 | 0.0 | 0.0 | Maldives | 0 | 0.0 | 0.0 | Tajikistan | 0 | 0.0 | 0.0 |
ASR = age-specific incidence and mortality rate.
Figure 1.
Distribution standardized incidence rate of nasopharynx cancer in Asia in 2012 (extracted from GLOBALCAN). ASR = age-specific incidence and mortality rate.
Figure 2.
Standardized incidence and mortality rate of nasopharynx cancer in Asia in 2012 (extracted from GLOBALCAN). HDI = Human Development Index; LAO PDR = Laos People’s Democratic Republic.
Of the 40,530 people who died of nasopharynx cancer in Asia in 2012, 29,032 (63/71%) were men and 11,498 (36/28%) were women, which translates to a male/female mortality ratio of 52:2. The highest number of deaths occurred in China with 20,404 cases, followed by Indonesia with 7,391 cases, Vietnam with 2,885 cases, India with 2,836 cases, and Thailand with 1,114 cases. This brings the total of deaths to 34,630 (44/85%) in just these five countries.
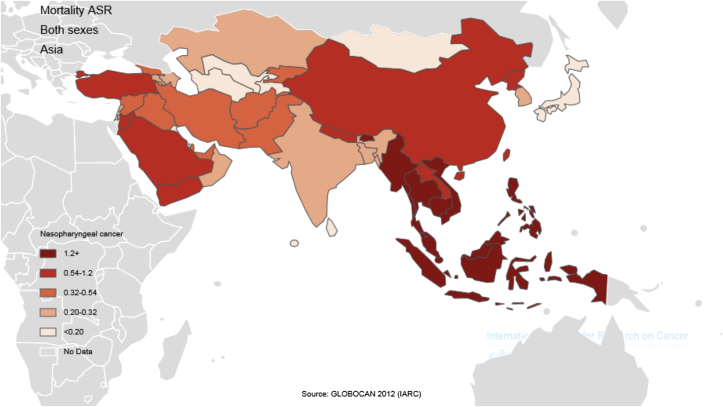
The five countries with the highest standardized mortality rate of nasopharynx cancer are as follows: (1) Indonesia, with a standardized rate of 3.3 per 100,000 people; (2) Vietnam, 3.3 per 1000,000 people; (3) Singapore, 2.8 per 100,000 people; (4) Malaysia, 2.5 per 100,000 people; (5) Brunei, 2.1 per 100,000 people. Conversely, the five countries with the lowest standardized mortality rate of nasopharynx cancer are as follows: Maldives and Tajikistan, with a rate of 0 per 100,000 people; followed by Bahrain, Kuwait, and Israel with a rate of 0.1 per 100,000 people. The number and rate of standardized and crude mortality of this cancer in Asian countries by sex are shown in Table 2. Countries are arranged based on standardized rates, from highest to lowest, in Table 2, so that countries with the highest and lowest standardized rates in each sex are shown (Table 2 and Figure 2, Figure 3).
Table 2.
Number and amount of standardized and crude mortality of nasopharynx cancer in Asian countries in 2012 (sorted by age-standardized incidence rate, from the highest to the lowest value).
| Nasopharynx—estimated mortality, all ages: both sexes |
Nasopharynx—estimated mortality, all ages: female |
Nasopharynx—estimated mortality, all ages: male |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Population | No. | Crude rate | Age-world-standardized incidence rate (ASR(W)) | Population | Number | Crude rate | Age-world-standardized incidence rate (ASR(W)) | Population | No. | Crude rate | ASR (W) |
| Indonesia | 7,391 | 3.0 | 3.3 | Indonesia | 5,283 | 4.3 | 5.0 | Vietnam | 954 | 2.1 | 2.0 |
| Vietnam | 2,885 | 3.2 | 3.3 | Vietnam | 1,931 | 4.4 | 4.8 | Indonesia | 2,108 | 1.7 | 1.8 |
| Singapore | 218 | 4.1 | 2.8 | Singapore | 168 | 6.3 | 4.4 | Bhutan | 4 | 1.1 | 1.5 |
| Malaysia | 698 | 2.4 | 2.5 | Malaysia | 533 | 3.6 | 3.9 | Singapore | 50 | 1.9 | 1.3 |
| Brunei | 6 | 1.5 | 2.1 | Brunei | 5 | 2.4 | 3.4 | Malaysia | 165 | 1.1 | 1.2 |
| Myanmar | 785 | 1.6 | 1.8 | Myanmar | 538 | 2.2 | 2.6 | Myanmar | 247 | 1.0 | 1.1 |
| Bhutan | 8 | 1.1 | 1.4 | Cambodia | 105 | 1.5 | 2.5 | Yemen | 65 | 0.5 | 0.9 |
| Cambodia | 144 | 1.0 | 1.4 | Thailand | 793 | 2.3 | 1.9 | Philippines | 272 | 0.6 | 0.7 |
| Philippines | 873 | 0.9 | 1.3 | Philippines | 601 | 1.2 | 1.9 | Lao PDR | 16 | 0.5 | 0.7 |
| Thailand | 1,114 | 1.6 | 1.3 | Lao PDR | 35 | 1.1 | 1.7 | Thailand | 321 | 0.9 | 0.7 |
| Lao PDR | 51 | 0.8 | 1.2 | Timor-Leste | 6 | 1.0 | 1.7 | China | 5,686 | 0.9 | 0.6 |
| China | 20,404 | 1.5 | 1.2 | China | 14,718 | 2.1 | 1.7 | Cambodia | 39 | 0.5 | 0.6 |
| Yemen | 152 | 0.6 | 1.2 | Yemen | 87 | 0.7 | 1.5 | Jordan | 9 | 0.3 | 0.5 |
| Timor-Leste | 8 | 0.7 | 1.1 | Bhutan | 4 | 1.0 | 1.4 | Timor-Leste | 2 | 0.3 | 0.5 |
| Nepal | 164 | 0.5 | 0.8 | Nepal | 125 | 0.8 | 1.3 | Saudi Arabia | 38 | 0.3 | 0.5 |
| Saudi Arabia | 136 | 0.5 | 0.7 | Korea, Democratic Republic of | 148 | 1.2 | 1.1 | Brunei | 1 | 0.5 | 0.5 |
| Korea, Democratic Republic of | 215 | 0.9 | 0.7 | Saudi Arabia | 98 | 0.6 | 0.9 | Syrian Arab Republic | 29 | 0.3 | 0.4 |
| Jordan | 22 | 0.3 | 0.6 | Turkey | 300 | 0.8 | 0.9 | Korea, Democratic Republic of | 67 | 0.5 | 0.4 |
| Turkey | 397 | 0.5 | 0.5 | Georgia | 22 | 1.1 | 0.8 | Nepal | 39 | 0.2 | 0.3 |
| Syrian Arab Republic | 77 | 0.4 | 0.5 | Kyrgyzstan | 14 | 0.5 | 0.8 | Iraq | 32 | 0.2 | 0.3 |
| Iraq | 82 | 0.2 | 0.5 | Syrian Arab Republic | 48 | 0.4 | 0.7 | Turkey | 97 | 0.3 | 0.3 |
| Qatar | 4 | 0.2 | 0.4 | Jordan | 13 | 0.4 | 0.7 | Pakistan | 150 | 0.2 | 0.2 |
| Georgia | 27 | 0.6 | 0.4 | Iraq | 50 | 0.3 | 0.6 | Iran, Islamic Republic of | 72 | 0.2 | 0.2 |
| Pakistan | 532 | 0.3 | 0.4 | Armenia | 10 | 0.7 | 0.6 | Sri Lanka | 26 | 0.2 | 0.2 |
| Kyrgyzstan | 16 | 0.3 | 0.4 | Qatar | 4 | 0.3 | 0.6 | Afghanistan | 12 | 0.1 | 0.2 |
| United Arab Emirates | 13 | 0.2 | 0.4 | Pakistan | 382 | 0.4 | 0.6 | United Arab Emirates | 3 | 0.1 | 0.2 |
| Iran, Islamic Republic of | 214 | 0.3 | 0.3 | Lebanon | 12 | 0.6 | 0.5 | Oman | 1 | 0.1 | 0.1 |
| Afghanistan | 54 | 0.2 | 0.3 | State of Palestine | 6 | 0.3 | 0.5 | Uzbekistan | 14 | 0.1 | 0.1 |
| State of Palestine | 8 | 0.2 | 0.3 | Afghanistan | 42 | 0.2 | 0.5 | Bangladesh | 74 | 0.1 | 0.1 |
| Lebanon | 15 | 0.3 | 0.3 | Iran, Islamic Republic of | 142 | 0.4 | 0.4 | Kazakhstan | 12 | 0.1 | 0.1 |
| Armenia | 12 | 0.4 | 0.3 | Korea, Republic of | 145 | 0.6 | 0.4 | India | 742 | 0.1 | 0.1 |
| India | 2,836 | 0.2 | 0.3 | United Arab Emirates | 10 | 0.2 | 0.4 | Georgia | 5 | 0.2 | 0.1 |
| Bangladesh | 288 | 0.2 | 0.3 | India | 2,094 | 0.3 | 0.4 | Lebanon | 3 | 0.1 | 0.1 |
| Korea, Republic of | 185 | 0.4 | 0.3 | Bangladesh | 214 | 0.3 | 0.4 | Korea, Republic of | 40 | 0.2 | 0.1 |
| Kazakhstan | 36 | 0.2 | 0.2 | Azerbaijan | 18 | 0.4 | 0.4 | Azerbaijan | 4 | 0.1 | 0.1 |
| Azerbaijan | 22 | 0.2 | 0.2 | Kazakhstan | 24 | 0.3 | 0.4 | State of Palestine | 2 | 0.1 | 0.1 |
| Oman | 4 | 0.1 | 0.2 | Turkmenistan | 5 | 0.2 | 0.3 | Kyrgyzstan | 2 | 0.1 | 0.1 |
| Sri Lanka | 49 | 0.2 | 0.2 | Mongolia | 4 | 0.3 | 0.3 | Armenia | 2 | 0.1 | 0.1 |
| Turkmenistan | 6 | 0.1 | 0.2 | Oman | 3 | 0.2 | 0.3 | Turkmenistan | 1 | 0.0 | 0.1 |
| Mongolia | 5 | 0.2 | 0.2 | Israel | 9 | 0.2 | 0.2 | Mongolia | 1 | 0.1 | 0.1 |
| Uzbekistan | 35 | 0.1 | 0.2 | Uzbekistan | 21 | 0.2 | 0.2 | Japan | 90 | 0.1 | 0.1 |
| Japan | 324 | 0.3 | 0.1 | Japan | 234 | 0.4 | 0.2 | Israel | 1 | 0.0 | 0.0 |
| Israel | 10 | 0.1 | 0.1 | Sri Lanka | 23 | 0.2 | 0.2 | Tajikistan | 0 | 0.0 | 0.0 |
| Kuwait | 2 | 0.1 | 0.1 | Kuwait | 2 | 0.1 | 0.1 | Maldives | 0 | 0.0 | 0.0 |
| Bahrain | 1 | 0.1 | 0.1 | Bahrain | 1 | 0.1 | 0.1 | Bahrain | 0 | 0.0 | 0.0 |
| Tajikistan | 2 | 0.0 | 0.0 | Tajikistan | 2 | 0.1 | 0.1 | Qatar | 0 | 0.0 | 0.0 |
| Maldives | 0 | 0.0 | 0.0 | Maldives | 0 | 0.0 | 0.0 | Kuwait | 0 | 0.0 | 0.0 |
ASR = age-specific incidence and mortality rate.
Figure 3.
Distribution standardized mortality rate of nasopharynx cancer in Asia in 2012 (extracted from GLOBALCAN). HDI = Human Development Index.
In Table 3, values of HDI and its components for all Asian countries, arranged according to HDI, are shown. Thus, Asian countries were classified in terms of HDI as follows: three countries in very high category, four countries in top category, 35 countries in the medium category, three countries in low category, and a country in uncertain category.
Table 3.
Human Development Index (HDI) and its components in Asian countries in 2012.
| Population | HDI | Life expectancy at birth | Mean years of schooling | GNI/capita | |
|---|---|---|---|---|---|
| Very high human development | Japan | 0.912 | 83.6 | 11.6 | 32,545 |
| Korea, Republic of | 0.909 | 80.7 | 11.6 | 28,231 | |
| Israel | 0.900 | 81.9 | 11.9 | 26,224 | |
| Singapore | 0.895 | 81.2 | 10.1 | 52,613 | |
| Brunei | 0.855 | 78.1 | 8.6 | 45,690 | |
| Qatar | 0.834 | 78.5 | 7.3 | 87,478 | |
| United Arab Emirates | 0.818 | 76.7 | 8.9 | 42,716 | |
| High human development | Bahrain | 0.796 | 75.2 | 9.4 | 19,154 |
| Kuwait | 0.790 | 74.7 | 6.1 | 52,793 | |
| Saudi Arabia | 0.782 | 74.1 | 7.8 | 22,616 | |
| Malaysia | 0.769 | 74.5 | 9.5 | 13,676 | |
| Kazakhstan | 0.754 | 67.4 | 10.4 | 10,451 | |
| Georgia | 0.745 | 73.9 | 12.1 | 5,005 | |
| Lebanon | 0.745 | 72.8 | 7.9 | 12,364 | |
| Iran, Islamic Republic of | 0.742 | 73.2 | 7.8 | 10,695 | |
| Azerbaijan | 0.734 | 70.9 | 11.2 | 8,153 | |
| Oman | 0.731 | 73.2 | 5.5 | 24,092 | |
| Armenia | 0.729 | 74.4 | 10.8 | 5,540 | |
| Turkey | 0.722 | 74.2 | 6.5 | 13,710 | |
| Sri Lanka | 0.715 | 75.1 | 9.3 | 5,170 | |
| Medium human development | Jordan | 0.700 | 73.5 | 8.6 | 5,272 |
| China | 0.699 | 73.7 | 7.5 | 7,945 | |
| Turkmenistan | 0.698 | 65.2 | 9.9 | 7,782 | |
| Thailand | 0.690 | 74.3 | 6.6 | 7,722 | |
| Maldives | 0.688 | 77.1 | 5.8 | 7,478 | |
| Mongolia | 0.675 | 68.8 | 8.3 | 4,245 | |
| State of Palestine | 0.670 | 73.0 | 8.0 | 3,359 | |
| Philippines | 0.654 | 69.0 | 8.9 | 3,752 | |
| Uzbekistan | 0.654 | 68.6 | 10 | 3,201 | |
| Syrian Arab Republic | 0.648 | 76.0 | 5.7 | 4,674 | |
| Indonesia | 0.629 | 69.8 | 5.8 | 4,154 | |
| Kyrgyzstan | 0.622 | 68.0 | 9.3 | 2,009 | |
| Tajikistan | 0.622 | 67.8 | 9.8 | 2,119 | |
| Vietnam | 0.617 | 75.4 | 5.5 | 2,970 | |
| Iraq | 0.590 | 69.6 | 5.6 | 3,557 | |
| Timor-Leste | 0.576 | 62.9 | 4.4 | 5,446 | |
| India | 0.554 | 65.8 | 4.4 | 3,285 | |
| Cambodia | 0.543 | 63.6 | 5.8 | 2,095 | |
| Lao PDR | 0.543 | 67.8 | 4.6 | 2,435 | |
| Bhutan | 0.538 | 67.6 | 2.3 | 5,246 | |
| Low human development | Bangladesh | 0.515 | 69.2 | 4.8 | 1,785 |
| Pakistan | 0.515 | 65.7 | 4.9 | 2,566 | |
| Myanmar | 0.498 | 65.7 | 3.9 | 1,817 | |
| Nepal | 0.463 | 69.1 | 3.2 | 1,137 | |
| Yemen | 0.458 | 65.9 | 5.3 | 928 | |
| Afghanistan | 0.374 | 49.1 | 3.1 | 1,000 | |
| Other countries or territories | Korea, Democratic People’s Republic of | — | — | — | — |
GNI = gross national income.
3.1. Checking the relationship between standardized incidence rate and HDI
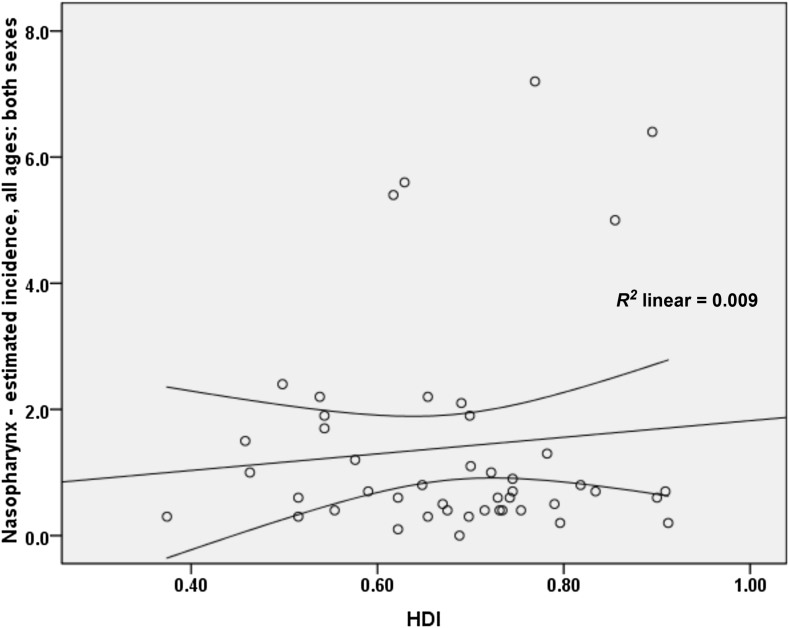
A correlation of 0.097 was obtained between the standardized incidence rate of nasopharynx cancer and HDI; however, this relation was not statistically significant (p = 0.520). As for the correlation between components of the HDI and the standardized rate, we obtained the following results: a correlation of 0.174 between the standardized incidence rate and life expectancy at birth (p = 0.247), a negative correlation of 0.057 with the average years of schooling (p = 0.705), and a correlation of 0.134 with the level of income per person of the population (p = 0.375; Figure 4).
Figure 4.
Correlation between HDI and standardized incidence rate of nasopharynx cancer in Asia in 2012. ASR = age-specific incidence and mortality rate.
In men, a correlation of 0.105 was observed between the standardized incidence rate of nasopharynx cancer and the HDI; however, this relation was not statistically significant (p = 0.488).
A correlation was also seen between the components of the HDI and the standardized incidence. Specifically, we obtained a correlation of 0.174 between the standardized incidence rate and life expectancy at birth (p = 0.253), a negative correlation of 0.031 with the average years of schooling (p = 0.836), and a correlation of 0.125 with the level of income per person of the population (p = 0.407).
In women, a correlation of 0.019 was found between the standardized incidence rate of nasopharynx cancer and the HDI, but this relation was not statistically significant (p = 0.901). A correlation between components of the HDI and the standardized incidence rate was also found. Specifically, we noted a correlation of 0.134 between the standardized incidence rate and life expectancy at birth (p = 0.376), a negative correlation of 0.142 with the average years of schooling (p = 0.346), and a correlation of 0.059 with the level of income per person of the population (p = 0.696).
3.2. Checking the relationship between standardized mortality rate and HDI
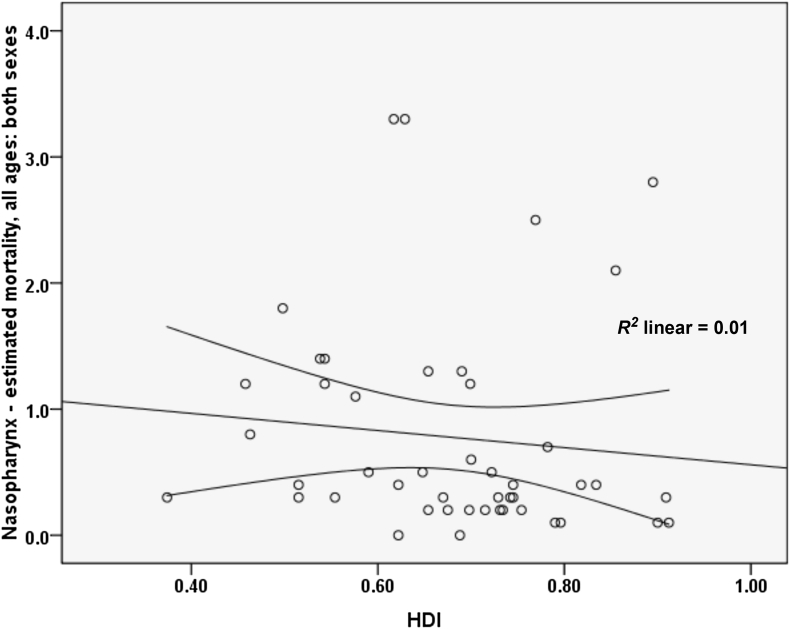
A negative correlation of 0.102 was found between the standardized mortality rate of nasopharynx cancer and HDI; however, this relation was not statistically significant (p = 0.502). A correlation was also found between components of the HDI and the standardized rate. Specifically, we noted a correlation of 0.034 between the standardized incidence rate and life expectancy at birth (p = 0.824), a negative correlation of 0.238 with the average years of schooling (p = 0.112), and a correlation of 0.001 with the level of income per person of the population (p = 0.994; Figure 5).
Figure 5.
Correlation between HDI and standardized mortality rate of nasopharynx cancer in Asia in 2012. ASR = age-specific incidence and mortality rate.
In men, we observed a correlation of 0.072 between the standardized mortality rate of nasopharynx cancer and the HDI, but this relation was not statistically significant (p = 0.633). Furthermore, a correlation between components of the HDI and standardized mortality rate was also found. Specifically, we noted a correlation of 0.040 between the standardized incidence rate and life expectancy at birth (p = 0.792), a negative correlation of 0.188 with the average years of schooling (p = 0.211), and a correlation of 0.014 with the level of income per person of the population (p = 0.924).
In women, a negative correlation of 0.224 was found between the standardized mortality rate of nasopharynx cancer and the HDI, but this relation was not statistically significant (p = 0.134). Also, a negative correlation was noted between components of the HDI and the standardized rate. Specifically, we observed a negative correlation of 0.044 between the standardized incidence rate and life expectancy at birth (p = 0.773), a negative correlation of 0.345 with the average years of schooling (p = 0.019), and a negative correlation of 0.014 with the level of income per person of the population (p = 0.350).
4. Discussion
Overall, 68,272 new cases and 40,530 deaths attributed to nasopharynx cancer were recorded in Asian countries in 2012; the sex (male/female) ratio of developing the disease is 2.45, and the sex ratio of nasopharynx cancer mortality is 2.52. The prevalence of nasopharynx cancer in developing countries is higher, and this is attributed to the higher exposure of inhabitants to a variety of risk factors and lower budget allocations for health. For these people, diagnosis is often made when the disease is already in advanced stages, and, with the lack of access to treatment, the metastasis of nasopharynx cancer can be observed more often in these areas 36, 37.
In this study, no significant relationship was found between the standardized incidence and mortality rate of nasopharynx cancer and the HDI. Moreover, no significant positive relationship was observed between standardized incidence and mortality rate of nasopharynx cancer with proper income level (GDP) and level of education or knowledge (mean years of schooling). In other studies, the increase in life expectancy leads to an increase in global cancer burden and future changes in population growth and aging, indicating that new cases of nasopharynx cancer and mortality in elderly people will increase and that this increase will be noticeable in countries with low HDI compared to countries with high HDI (76% vs. 25%) 26, 28, 38, 39. Furthermore, according to other studies, people who have higher education typically have more healthy habits and behaviors than those with a low education level, especially in terms of cancer incidence and mortality 40, 41. In studies focusing on nasopharynx cancer, the relationship between nasopharynx cancer with socioeconomic status, lifestyle, and geographical positions is known [12]. Thus, in Asian countries, there is an increased incidence of nasopharynx cancer in lower economic classes, especially among men [12]. Also, no difference was found between the deaths of people living in rural areas with low socioeconomic status and the people who live in urban areas 42, 43.
In this study, Malaysia, Singapore, Indonesia, Vietnam, and Brunei are the five countries with the highest incidence rate. It is noteworthy that Singapore and Brunei are classified as very high HDI countries, Malaysia is considered a country with a high HDI, and Indonesia and Vietnam are countries with medium HDI. According to previous studies in Singapore, nasopharynx cancer is the eighth most common cancer in men, with age-standardized incidence of 9.5 per 100,000 per year [44]. In Indonesia, a relatively high incidence is reported—at least 5.7 among men and 1.9 among women per 100,000 people—compared with the global incidence average of 1.9 among men and 0.8 among women for every 100,000 individuals [45]. It should be noted, however, that the actual incidence of nasopharynx cancer in Indonesia is not known owing to incomplete registration [11].
Regardless of ethnicity, genetics plays an important part in the pathogenesis of nasopharynx cancer. The incidence of nasopharynx cancer is 20–50 times higher in South China compared with Western countries. It is notable that second and third generations of Chinese people who immigrated to the United States (a low prevalence area) are still at risk of high nasopharynx cancer despite cultural assimilation compared with the resident population [46]. In the present study, Indonesia, Vietnam, Singapore, Malaysia, and Brunei were the five countries with the highest death rates from this cancer. Other studies have shown that one of the main causes of death in Indonesia is nasopharynx cancer, and in early detection, 80% of the patients are already at an advanced stage of the disease. In Indonesia, primary healthcare is generally handled by health centers named Puskesmas. Lack of knowledge among the general practitioners working in these centers regarding the various aspects of nasopharynx cancer, may lead to a delay in diagnosis [11]. Thus, only about 14% of patients who have metastasis at the initial diagnosis and 29% of patients without metastasis show response to therapy, about 70–95% response to therapy is good, so in general response to the therapy is poor. [36]. In Singapore, older patients diagnosed with stage 2 or stage 3 are at higher risk of recurrence and lower overall survival [47]. Eighty percent of patients are now at stage 3 or 4 of the disease, which leads to lower survival and death [11]. In the past two decades, the treatment of nasopharynx cancer has been considerably enhanced by the use of chemotherapy and radiotherapy at the same time. However, the overall incidence of patients with metastasis remains at 25–34%, and survival of these patients is low 48, 49.
5. Conclusion
Nasopharynx cancer is the native cancer of Southeast Asia. So that the highest incidence and mortality are related to Southeast Asian countries including Malaysia, Singapore, Indonesia, Vietnam and Brunei. It was not seen a significant relation between the standardized incidence and mortality rate of nasopharynx cancer and the Human Development Index components. Further studies are recommended in Southeast Asian countries in order to find the etiology of cancer, diagnosis and treatment.
5.1. Limitations of the study
This was an ecological study. The ecological fallacy will occur if results are inferred and concluded at the individual level.
Conflicts of interest
The authors declare that they have no conflicts of interest for this work.
Acknowledgments
We appreciate the cooperation of all employees involved in data collection in the GLOBOCAN project and World Bank.
References
- 1.Keyghobadi N., Rafiemanesh H., Mohammadian-Hafshejani A. Epidemiology and trend of cancers in the province of Kerman: southeast of Iran. Asian Pac J Cancer Prev. 2015 Apr;16(4):1409–1413. doi: 10.7314/apjcp.2015.16.4.1409. [DOI] [PubMed] [Google Scholar]
- 2.Razi S., Rafiemanesh H., Ghoncheh M. Changing trends of types of skin cancer in Iran. Asian Pac J Cancer Prev. 2015 Dec;16(12):4955–4958. doi: 10.7314/apjcp.2015.16.12.4955. [DOI] [PubMed] [Google Scholar]
- 3.Devita V.T., Hellman S., Rosenberg S.A., editors. Principles and practice of oncology. Lippincott, Williams &Wilkims; Philadelphia (PA): 2001. pp. 1880–1904. [Google Scholar]
- 4.Tsao S.W.L.K., Huang D.P. Nasopharyngeal carcinoma. In: Tselis A.C., Jenson H., editors. Epstein–Barr virus. Taylor & Francis; New York (NY): 2006. pp. 273–295. [Google Scholar]
- 5.Raab-Traub N. Epstein–Barr virus in the pathogenesis of NPC. In: Robertson E.S., editor. Epstein–Barr virus. Caister Academic Press; Wymondham Norfolk (UK): 2005. pp. 71–92. [Google Scholar]
- 6.Parkin D.M., Bray F., Ferlay J. Global cancer statistics, 2002. CA Cancer J Clin. 2005 Mar-Apr;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 7.Chang E.T., Adami H.-O. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomark Prev. 2006 Oct;15(10):1765–1777. doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- 8.Rubin P., Bakemeier R., Kiackov S. 6th ed. National Cancer Institute; USA: 1983. Clinical oncology; pp. 2–10. [Google Scholar]
- 9.Wang Y., Zhang Y., Ma S. Racial differences in nasopharyngeal carcinoma in the United States. Cancer Epidemiol. 2013 Dec;37(6):793–802. doi: 10.1016/j.canep.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wee J., Ha T.C., Loong S., Qian C. Is nasopharyngeal cancer really a “Cantonese cancer”? Chin J Cancer. 2010 May;29(5):517–526. doi: 10.5732/cjc.009.10329. [DOI] [PubMed] [Google Scholar]
- 11.Fles R., Wildeman M.A., Sulistiono B. Knowledge of general practitioners about nasopharyngeal cancer at the Puskesmas in Yogyakarta, Indonesia. BMC Med Educ. 2010 Nov;10(1):1. doi: 10.1186/1472-6920-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mimi C.Y., Yuan J.-M. Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol. Dec 2002;12(6):421–426. doi: 10.1016/s1044579x02000858. [DOI] [PubMed] [Google Scholar]
- 13.Mimi C.Y., Ho J.H., Lai S.-H. Cantonese-style salted fish as a cause of nasopharyngeal carcinoma: report of a case-control study in Hong Kong. Cancer Res. 1986 Feb;46(2):956–961. [PubMed] [Google Scholar]
- 14.Yu M., Mo C., Chong W. Preserved foods and nasopharyngeal carcinoma: a case-control study in Guangxi, China. Cancer Res. 1988 Apr;48(7):1954–1959. [PubMed] [Google Scholar]
- 15.Yu M.C., Huang T.B., Henderson B.E. Diet and nasopharyngeal carcinoma: a case-control study in Guangzhou, China. Int J cancer. 1989 Jun;43(6):1077–1082. doi: 10.1002/ijc.2910430621. [DOI] [PubMed] [Google Scholar]
- 16.Ning J.-P., Mimi C.Y., Wang Q.-S. Consumption of salted fish and other risk factors for nasopharyngeal carcinoma (NPC) in Tianjin, a low-risk region for NPC in the People's Republic of China. J Natl Cancer Inst. 1990 Feb;82(4):291–296. doi: 10.1093/jnci/82.4.291. [DOI] [PubMed] [Google Scholar]
- 17.Aiyar A., Tyree C., Sugden B. The plasmid replicon of EBV consists of multiple cis-acting elements that facilitate DNA synthesis by the cell and a viral maintenance element. EMBO J. 1998 Nov;17(21):6394–6403. doi: 10.1093/emboj/17.21.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho J. University of Tokyo Press; Tokyo (Japan): 1971. Genetic and environmental factors in nasopharyngeal carcinoma. Recent advances in human tumor virology and immunology; pp. 275–295. [Google Scholar]
- 19.Zou X.N., Lu S.H., Liu B. Volatile N-nitrosamines and their precursors in chinese salted fish—a possible etological factor for NPC in China. Int J Cancer. 1994 Oct;59(2):155–158. doi: 10.1002/ijc.2910590202. [DOI] [PubMed] [Google Scholar]
- 20.Huang D., Ho J., Webb K. Volatile nitrosamines in salt-preserved fish before and after cooking. Food Cosmet Toxicol. 1981 Apr;19:167–171. doi: 10.1016/0015-6264(81)90353-9. [DOI] [PubMed] [Google Scholar]
- 21.Zheng Y., Tuppin P., Hubert A. Environmental and dietary risk factors for nasopharyngeal carcinoma: a case-control study in Zangwu County, Guangxi, China. Br J Cancer. 1994 Mar;69(3):508–514. doi: 10.1038/bjc.1994.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.West S., Hildesheim A., Dosemeci M. Non-viral risk factors for nasopharyngeal carcinoma in the philippines: results from a case-control study. Int J Cancer. 1993 Nov;55(5):722–727. doi: 10.1002/ijc.2910550504. [DOI] [PubMed] [Google Scholar]
- 23.Hildesheim A., West S., DeVeyra E. Herbal medicine use, Epstein–Barr virus, and risk of nasopharyngeal carcinoma. Cancer Res. 1992 Jun;52(11):3048–3051. [PubMed] [Google Scholar]
- 24.Hildesheim A., Dosemeci M., Chan C.-C. Occupational exposure to wood, formaldehyde, and solvents and risk of nasopharyngeal carcinoma. Cancer Epidemiol Biomark Prev. 2001 Nov;10(11):1145–1153. [PubMed] [Google Scholar]
- 25.Chua M.L., Wee J.T., Hui E.P. Nasopharyngeal carcinoma. Lancet. 2016 Mar;387(10022):1012–1024. doi: 10.1016/S0140-6736(15)00055-0. [DOI] [PubMed] [Google Scholar]
- 26.Chan A.T., TP, Johnson P.J. Nasopharyngeal carcinoma. Ann Oncol. 2002 Jul;13:1007–1015. doi: 10.1093/annonc/mdf179. [DOI] [PubMed] [Google Scholar]
- 27.Agulnik M., Siu L. State-of-the-art management of nasopharyngeal carcinoma: current and future directions. Br J Cancer. 2005 Mar;92(5):799–806. doi: 10.1038/sj.bjc.6602449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bray F., Jemal A., Grey N. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol. 2012 Aug;13(8):790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 29.Ghoncheh M., Mohammadian-Hafshejani A., Salehiniya H. Incidence and mortality of breast cancer and their relationship to development in Asia. Asian Pac J Cancer Prev. 2015 Dec;16(14):6081–6087. doi: 10.7314/apjcp.2015.16.14.6081. [DOI] [PubMed] [Google Scholar]
- 30.Ghoncheh M., Mirzaei M., Salehiniya H. Incidence and mortality of breast cancer and their relationship with the human development index (HDI) in the world in 2012. Asian Pac J Cancer Prev. 2015 Dec;16(18):8439–8443. doi: 10.7314/apjcp.2015.16.18.8439. [DOI] [PubMed] [Google Scholar]
- 31.Pakzad R., Mohammadian-Hafshejani A., Ghoncheh M., Pakzad I., Salehiniya H. The incidence and mortality of lung cancer and their relationship to development in Asia. Translational lung cancer research. 2015;4(6):763–774. doi: 10.3978/j.issn.2218-6751.2015.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner K.H., Brath H. A global view on the development of non communicable diseases. Prev Med. 2012 May;54:S38–S41. doi: 10.1016/j.ypmed.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Ferlay J., Soerjomataram I., Ervik M. International Agency for Research on Cancer; Lyon (France): 2013. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC Cancer Base No. 11 [Internet]http://globocan.iarc.fr [cited 2016 Feb 2]. Available from: [Google Scholar]
- 34.Malik K. 2013. Human development report 2013. The rise of the south: Human progress in a diverse world (March 15, 2013) UNDP–HDRO Human Development Reports. [Google Scholar]
- 35.Ferlay J., Soerjomataram I., Dikshit R. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015 Mar;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 36.Adham M., Stoker S.D., Wildeman M.A. Current status of cancer care for young patients with nasopharyngeal carcinoma in Jakarta, Indonesia. PloS One. 2014 July;9(7):e102353. doi: 10.1371/journal.pone.0102353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wildeman M.A., Fles R., Herdini C. Primary treatment results of nasopharyngeal carcinoma (NPC) in Yogyakarta, Indonesia. PloS One. 2013 May;8(5):e63706. doi: 10.1371/journal.pone.0063706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giovino G.A., Mirza S.A., Samet J.M. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet. 2012 Aug;380(9842):668–679. doi: 10.1016/S0140-6736(12)61085-X. [DOI] [PubMed] [Google Scholar]
- 39.Mirzaei M., Hosseini S.A., Ghoncheh M. Epidemiology and trend of head and neck cancers in Iran. Global J Health Sci. 2016 May;8(1):189–193. doi: 10.5539/gjhs.v8n1p189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stelmach W., Kaczmarczyk-Chalas K., Bielecki W. The impact of income, education and health on lifestyle in a large urban population of Poland (Cindi programme) Int J Occup Med Environ Health. 2004 Feb;17(3):393–401. [PubMed] [Google Scholar]
- 41.Shi L. Sociodemographic characteristics and individual health behaviors. Southern Med J. 1998 Oct;91(10):933–941. doi: 10.1097/00007611-199810000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Mimi C.Y., Ho J.H., Ross R.K. Nasopharyngeal carcinoma in Chinese—salted fish or inhaled smoke? Prev Med. 1981 Jan;10(1):15–24. doi: 10.1016/0091-7435(81)90002-5. [DOI] [PubMed] [Google Scholar]
- 43.Armstrong R., Kutty M.K., Dharmalingam S. Incidence of nasopharyngeal carcinoma in Malaysia, 1968–1977. Br J Cancer. 1979 Oct;40(4):557–567. doi: 10.1038/bjc.1979.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teo M.C., Soo K.C. Cancer trends and incidences in Singapore. Jpn J Clin Oncol. 2013 Mar;43(3):219–224. doi: 10.1093/jjco/hys230. [DOI] [PubMed] [Google Scholar]
- 45.Rafiemanesh H., Mehtarpoor M., Mohammadian-Hafshejani A. Cancer epidemiology and trends in Sistan and Baluchestan province. Iran. Med J Islamic Rep Iran. 2015 Aug;29:254. [PMC free article] [PubMed] [Google Scholar]
- 46.Buell P. The effect of migration on the risk of nasopharyngeal cancer among Chinese. Cancer Res. 1974;34(5):1189–1191. [PubMed] [Google Scholar]
- 47.Mak H.W., Lee S.H., Chee J. Clinical outcome among nasopharyngeal cancer patients in a multi-ethnic society in Singapore. PloS One. 2015;10(5):e0126108. doi: 10.1371/journal.pone.0126108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen M.-Y., Jiang R., Guo L. Locoregional radiotherapy in patients with distant metastases of nasopharyngeal carcinoma at diagnosis. Chin J Cancer. 2013;32(11):604–613. doi: 10.5732/cjc.013.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee A.W., Lin J.C., Ng W.T. Current management of nasopharyngeal cancer. Semin Radiat Oncol. 2012 July;22(3):233–244. doi: 10.1016/j.semradonc.2012.03.008. [DOI] [PubMed] [Google Scholar]