Abstract
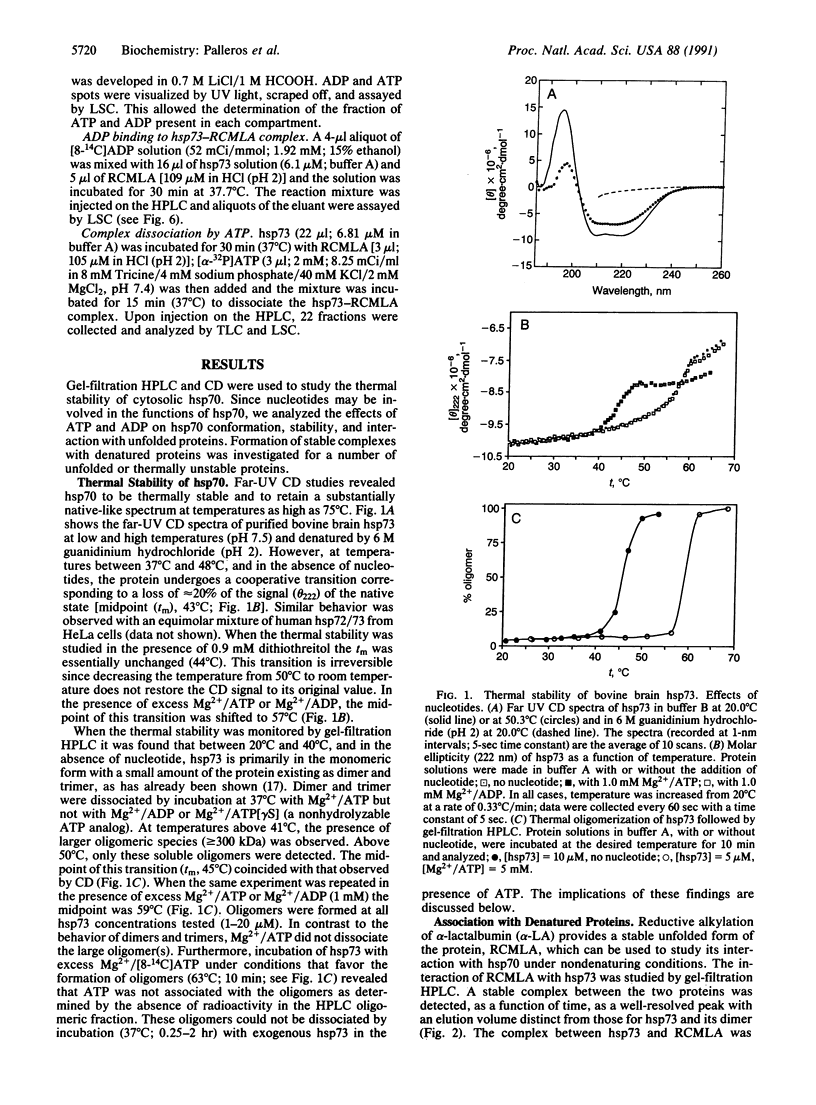
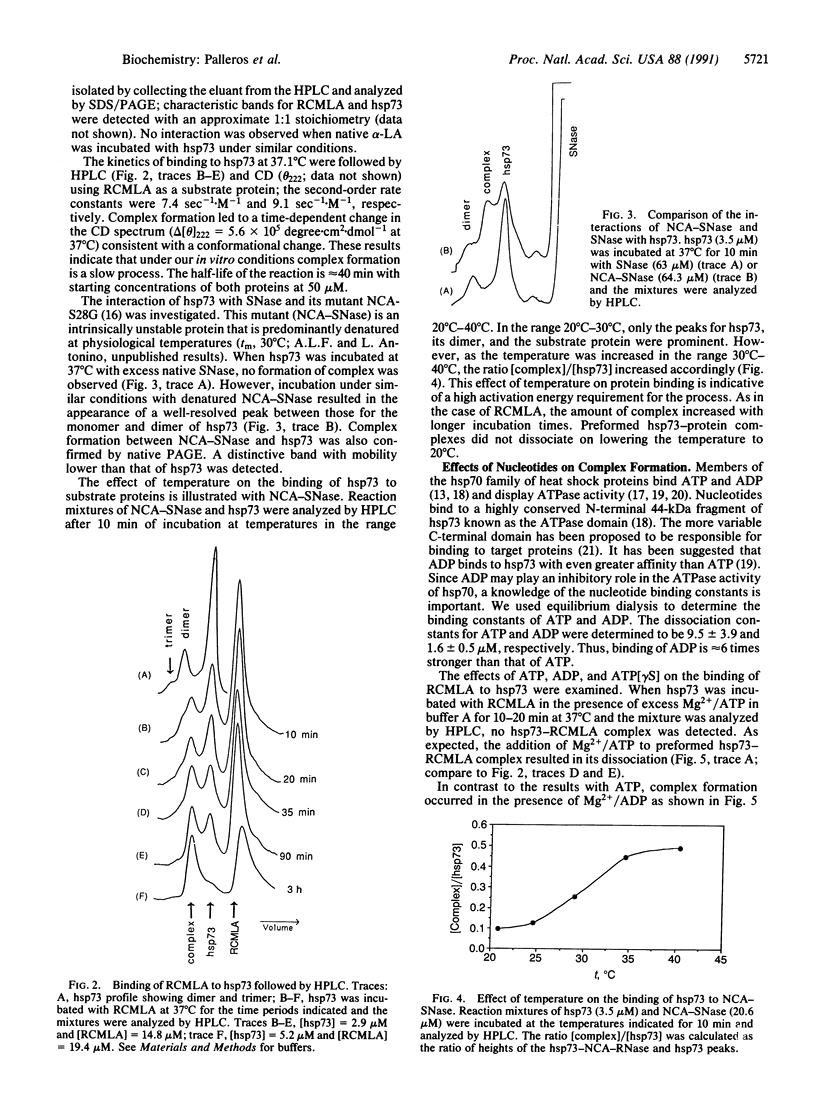
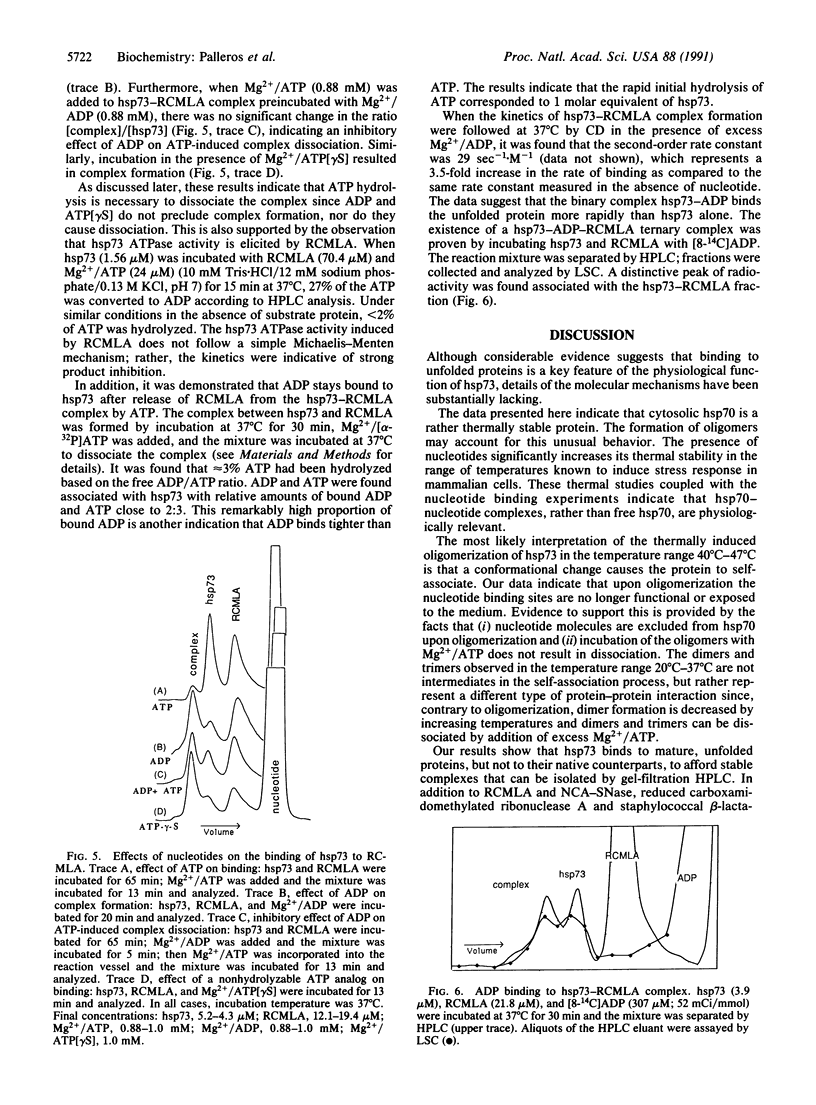
Circular dichroism and HPLC gel filtration were used to show that cytosolic hsp70 is thermally stable but undergoes a conformational transition (midpoint, 43 degrees C; 57 degrees C in the presence of ATP or ADP) leading to oligomerization. hsp70 binds to unfolded, but not to folded, proteins in a temperature-dependent manner; complex formation is significant only at physiologically relevant temperatures. hsp70 binds ADP more tightly than ATP to form a binary complex, which binds to the unfolded protein more rapidly than free hsp70. ADP also inhibits the ATP-induced dissociation of the hsp70-protein complex. A regulatory role for the hsp70-nucleotide binary complexes is proposed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckmann R. P., Mizzen L. E., Welch W. J. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990 May 18;248(4957):850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Bell O. E., Jr, Cain C. E., Sulya L. L., White H. B., Jr The occurrence of polyunsaturated aldehydes in choline-containing phosphoglycerides of a human brain meningioma. Biochim Biophys Acta. 1967 Oct 2;144(2):481–484. doi: 10.1016/0005-2760(67)90183-x. [DOI] [PubMed] [Google Scholar]
- Chappell T. G., Konforti B. B., Schmid S. L., Rothman J. E. The ATPase core of a clathrin uncoating protein. J Biol Chem. 1987 Jan 15;262(2):746–751. [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Flaherty K. M., DeLuca-Flaherty C., McKay D. B. Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature. 1990 Aug 16;346(6285):623–628. doi: 10.1038/346623a0. [DOI] [PubMed] [Google Scholar]
- Flynn G. C., Chappell T. G., Rothman J. E. Peptide binding and release by proteins implicated as catalysts of protein assembly. Science. 1989 Jul 28;245(4916):385–390. doi: 10.1126/science.2756425. [DOI] [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Dissociation of clathrin from coated vesicles by the uncoating ATPase. J Biol Chem. 1990 Apr 25;265(12):6682–6687. [PubMed] [Google Scholar]
- Hemmingsen S. M., Woolford C., van der Vies S. M., Tilly K., Dennis D. T., Georgopoulos C. P., Hendrix R. W., Ellis R. J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988 May 26;333(6171):330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Hurtley S. M., Bole D. G., Hoover-Litty H., Helenius A., Copeland C. S. Interactions of misfolded influenza virus hemagglutinin with binding protein (BiP). J Cell Biol. 1989 Jun;108(6):2117–2126. doi: 10.1083/jcb.108.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes T. R., Kautz R. A., Goodman M. A., Gill J. F., Fox R. O. Transfer of a beta-turn structure to a new protein context. Nature. 1989 May 4;339(6219):73–76. doi: 10.1038/339073a0. [DOI] [PubMed] [Google Scholar]
- Kang P. J., Ostermann J., Shilling J., Neupert W., Craig E. A., Pfanner N. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature. 1990 Nov 8;348(6297):137–143. doi: 10.1038/348137a0. [DOI] [PubMed] [Google Scholar]
- Kassenbrock C. K., Garcia P. D., Walter P., Kelly R. B. Heavy-chain binding protein recognizes aberrant polypeptides translocated in vitro. Nature. 1988 May 5;333(6168):90–93. doi: 10.1038/333090a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Mizzen L. A., Kabiling A. N., Welch W. J. The two mammalian mitochondrial stress proteins, grp 75 and hsp 58, transiently interact with newly synthesized mitochondrial proteins. Cell Regul. 1991 Feb;2(2):165–179. doi: 10.1091/mbc.2.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Rothman J. E. Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell. 1989 Nov 17;59(4):591–601. doi: 10.1016/0092-8674(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Schmid S. L., Braell W. A., Rothman J. E. ATP catalyzes the sequestration of clathrin during enzymatic uncoating. J Biol Chem. 1985 Aug 25;260(18):10057–10062. [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Rapid purification of mammalian 70,000-dalton stress proteins: affinity of the proteins for nucleotides. Mol Cell Biol. 1985 Jun;5(6):1229–1237. doi: 10.1128/mcb.5.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylicz M., LeBowitz J. H., McMacken R., Georgopoulos C. The dnaK protein of Escherichia coli possesses an ATPase and autophosphorylating activity and is essential in an in vitro DNA replication system. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6431–6435. doi: 10.1073/pnas.80.21.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]



