Abstract
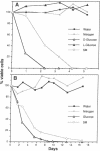
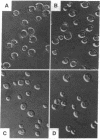
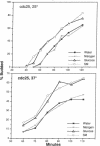
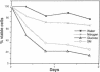
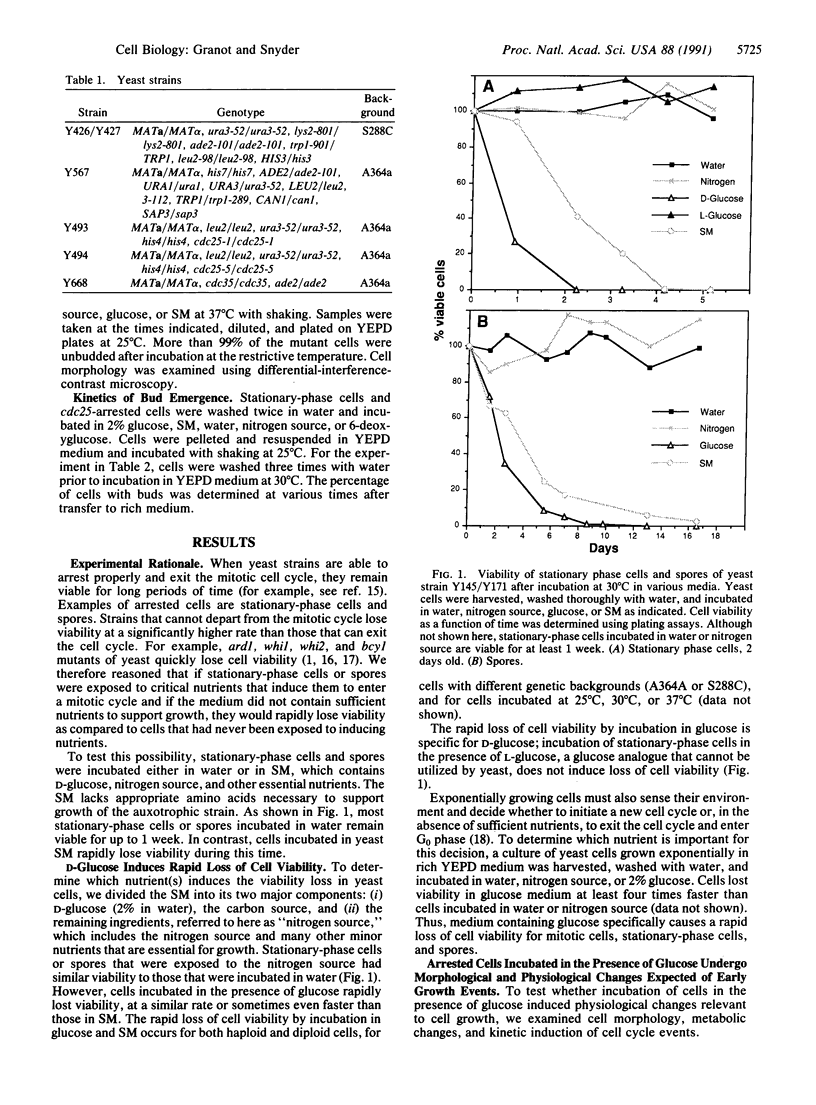
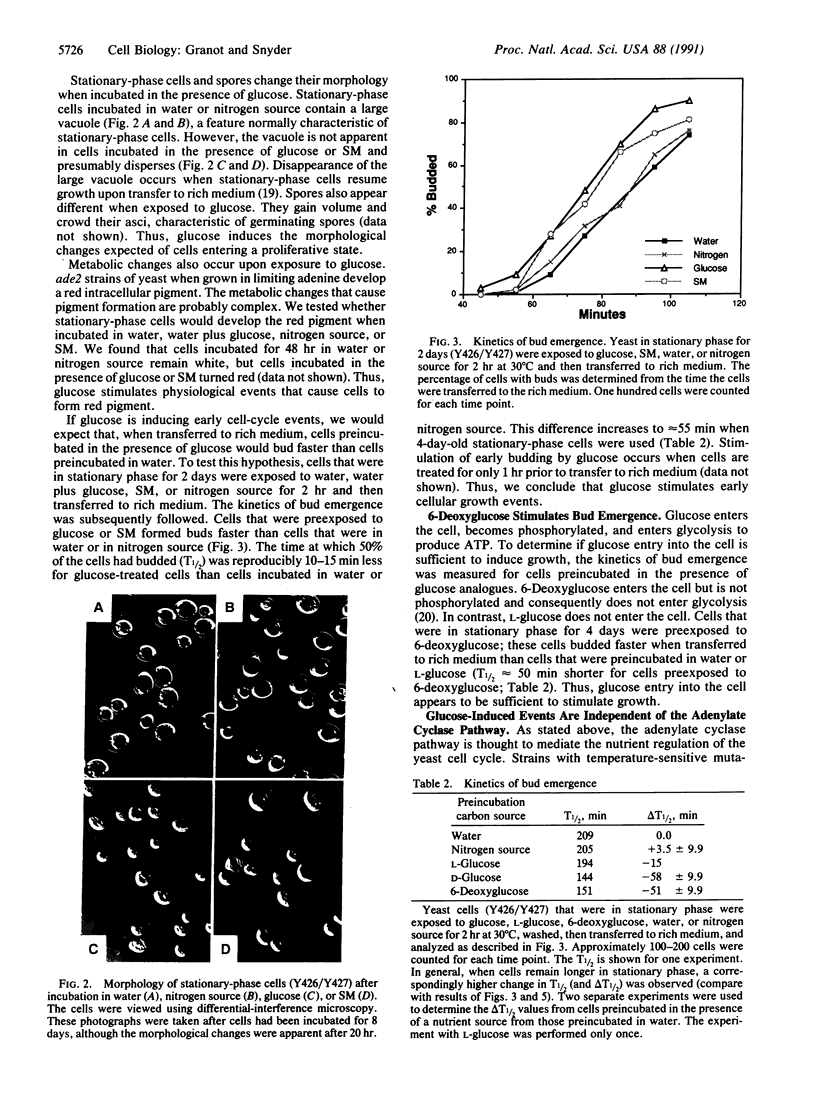
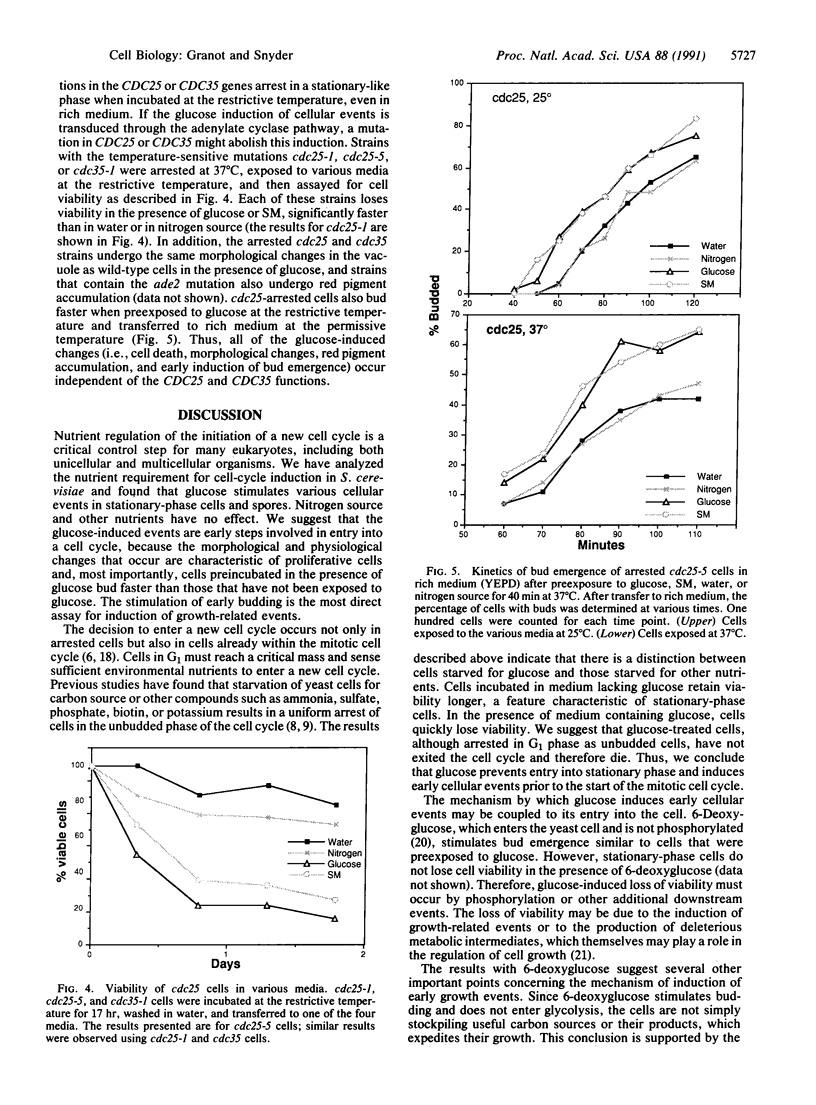
Nutrients play a critical role in the decision to initiate a new cell cycle. Addition of nutrients to arrested cells such as stationary-phase cells and spores induces them to begin growth. We have analyzed the nutrients required to induce early cellular events in yeast. When stationary-phase cells or spores are incubated in the presence of only glucose, morphological and physiological changes characteristic of mitotically growing cells are induced and, in the absence of additional nutrients to support growth, the cells rapidly lose viability. Preincubation of stationary-phase cells in the presence of glucose decreases the time required to reach bud emergence upon the subsequent addition of rich medium. These processes are specifically induced by D-glucose and not by other components such as nitrogen source or L-glucose. The glucose-induced events are independent of the adenylate cyclase pathway, since strains with a temperature-sensitive mutation in either the adenylate cyclase gene (CDC35) or its regulator (CDC25) undergo glucose-induced cellular changes when incubated at the restrictive temperature. We suggest that glucose triggers events in the induction of a new mitotic cell cycle and that these events are either prior to the adenylate cyclase pathway or are in an alternative pathway.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cameron S., Levin L., Zoller M., Wigler M. cAMP-independent control of sporulation, glycogen metabolism, and heat shock resistance in S. cerevisiae. Cell. 1988 May 20;53(4):555–566. doi: 10.1016/0092-8674(88)90572-7. [DOI] [PubMed] [Google Scholar]
- Eilam Y., Othman M. Activation of Ca2+ influx by metabolic substrates in Saccharomyces cerevisiae: role of membrane potential and cellular ATP levels. J Gen Microbiol. 1990 May;136(5):861–866. doi: 10.1099/00221287-136-5-861. [DOI] [PubMed] [Google Scholar]
- Gillies R. J., Ugurbil K., den Hollander J. A., Shulman R. G. 31P NMR studies of intracellular pH and phosphate metabolism during cell division cycle of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2125–2129. doi: 10.1073/pnas.78.4.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Saccharomyces cerevisiae cell cycle. Bacteriol Rev. 1974 Jun;38(2):164–198. doi: 10.1128/br.38.2.164-198.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. C., Pringle J. R., Hartwell L. H. Coordination of growth with cell division in the yeast Saccharomyces cerevisiae. Exp Cell Res. 1977 Mar 1;105(1):79–98. doi: 10.1016/0014-4827(77)90154-9. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K., Miyajima A., Arai K., Matsumoto K. Possible involvement of RAS-encoded proteins in glucose-induced inositolphospholipid turnover in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8172–8176. doi: 10.1073/pnas.83.21.8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka T., Powers S., McGill C., Fasano O., Strathern J., Broach J., Wigler M. Genetic analysis of yeast RAS1 and RAS2 genes. Cell. 1984 Jun;37(2):437–445. doi: 10.1016/0092-8674(84)90374-x. [DOI] [PubMed] [Google Scholar]
- Lillie S. H., Pringle J. R. Reserve carbohydrate metabolism in Saccharomyces cerevisiae: responses to nutrient limitation. J Bacteriol. 1980 Sep;143(3):1384–1394. doi: 10.1128/jb.143.3.1384-1394.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra P. K., Lobo Z. Control of glycolytic enzyme synthesis in yeast by products of the hexokinase reaction. J Biol Chem. 1971 Jan 25;246(2):489–499. [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Ishikawa T. Control of cell division in Saccharomyces cerevisiae mutants defective in adenylate cyclase and cAMP-dependent protein kinase. Exp Cell Res. 1983 Jun;146(1):151–161. doi: 10.1016/0014-4827(83)90333-6. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Ishikawa T. Genetic analysis of the role of cAMP in yeast. Yeast. 1985 Sep;1(1):15–24. doi: 10.1002/yea.320010103. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Oshima Y., Ishikawa T. Isolation and characterization of yeast mutants deficient in adenylate cyclase and cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2355–2359. doi: 10.1073/pnas.79.7.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K., Inoue Y., Rhee H., Kimura A. 2-Oxoaldehyde metabolism in microorganisms. Can J Microbiol. 1989 Apr;35(4):423–431. doi: 10.1139/m89-065. [DOI] [PubMed] [Google Scholar]
- Piñon R. Folded chromosomes in non-cycling yeast cells: evidence for a characteristic g0 form. Chromosoma. 1978 Jul 31;67(3):263–274. doi: 10.1007/BF02569039. [DOI] [PubMed] [Google Scholar]
- Snow R. An enrichment method for auxotrophic yeast mutants using the antibiotic 'nystatin'. Nature. 1966 Jul 9;211(5045):206–207. doi: 10.1038/211206a0. [DOI] [PubMed] [Google Scholar]
- Sudbery P. E., Goodey A. R., Carter B. L. Genes which control cell proliferation in the yeast Saccharomyces cerevisiae. Nature. 1980 Nov 27;288(5789):401–404. doi: 10.1038/288401a0. [DOI] [PubMed] [Google Scholar]
- Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., Broek D., Cameron S., Broach J., Matsumoto K., Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985 Jan;40(1):27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- Unger M. W., Hartwell L. H. Control of cell division in Saccharomyces cerevisiae by methionyl-tRNA. Proc Natl Acad Sci U S A. 1976 May;73(5):1664–1668. doi: 10.1073/pnas.73.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno I., Fukami K., Kato H., Takenawa T., Ishikawa T. Essential role for phosphatidylinositol 4,5-bisphosphate in yeast cell proliferation. Nature. 1988 May 12;333(6169):188–190. doi: 10.1038/333188a0. [DOI] [PubMed] [Google Scholar]
- Whiteway M., Szostak J. W. The ARD1 gene of yeast functions in the switch between the mitotic cell cycle and alternative developmental pathways. Cell. 1985 Dec;43(2 Pt 1):483–492. doi: 10.1016/0092-8674(85)90178-3. [DOI] [PubMed] [Google Scholar]
- Wiemken A., Matile P., Moor H. Vacuolar dynamics in synchronously budding yeast. Arch Mikrobiol. 1970;70(2):89–103. doi: 10.1007/BF00412200. [DOI] [PubMed] [Google Scholar]