Introduction
Enthesitis is a central feature of spondyloarthritis (SpA). Although enthesitis has traditionally been considered to be a focal insertional disorder, advanced imaging and pathologic findings suggest that enthesitis is a diffuse process with effects on adjacent bone and soft tissue. As a result of repeated biomechanical stress, it appears that microdamage at the enthesis triggers an inflammatory response in the synovium, leading to synovitis. Along with mechanical stress, exogenous bacteria may play a role in activating the immune response, especially in genetically predisposed individuals whose major histocompatibility locus encodes the class I molecule HLA–B27. Recent studies in animal models suggest that autoimmunity against versican and fibrocartilage proteins, and bone morphogenetic protein (BMP) signaling play roles in enthesitis development. Finally, interleukin-23 (IL-23) has been implicated in enthesitis with inflammatory effects mediated through IL-17 and tumor necrosis factor (TNF), and new bone formation driven by IL-22.
Although prior therapeutic choices were limited to nonsteroidal antiinflammatory drugs (NSAIDs) and activity modification, in recent years TNF inhibitors have proven to be useful. Further research on the effects of IL-22 and IL-23 blockade is needed to understand the effects on the treated patient. While enthesitis is underdiagnosed by physical examination alone, the use of ultrasound has proven to be highly sensitive for the detection of enthesitis, with utility in monitoring response to therapy, and will be an invaluable tool for assessing the efficacy of newer treatments. This review summarizes the substantial progress that has been made in addressing the pathophysiology, molecular mechanisms, genetic associations, clinical features, diagnostic modalities, and treatment of enthesitis.
Definitions and evolution of the enthesis concept
Historic definition
Although the adjective “enthetic” derives from the ancient Greek word “enthetikos,” meaning “introduced into the body from without,” in the nineteenth century the adjective was increasingly used to refer to diseases that were “implanted into the body from external sources” (1). It was not until the twentieth century that the term “enthesis” was used as it is today, referring to focal insertional abnormalities at sites of bony attachments to tendons, ligaments, fascia, muscles, or joint capsules (2,3). The first suggestion that the enthesis is centrally affected in SpA was made by Ball in 1971 and was substantiated after a review of pathologic tissues from both patients with rheumatoid arthritis (RA) and patients with ankylosing spondylitis (AS), where he noted the presence of a unique inflammatory enthesopathy that could help to distinguish SpA from RA (2).
Broadening the definition of enthesis with the concept of the “enthesis organ”
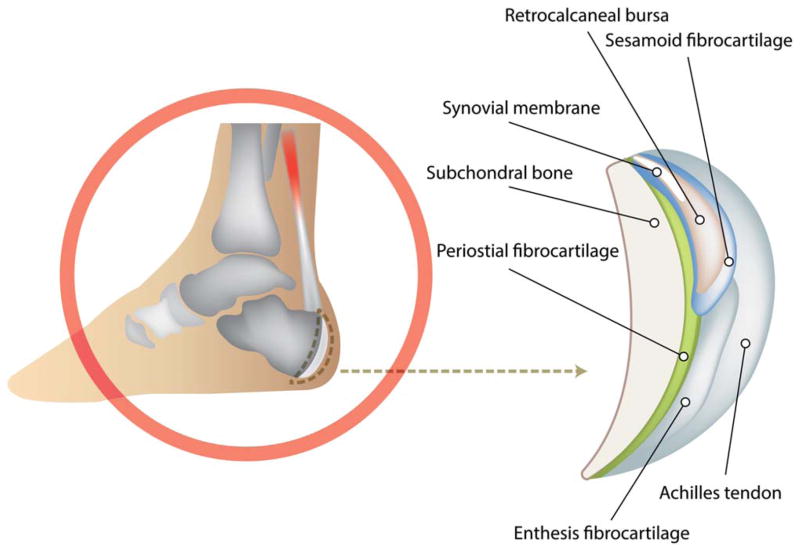
Magnetic resonance imaging (MRI) and ultrasound findings have suggested that enthesopathy encompasses pathologic changes extending to the adjacent bone and soft tissues (4). Likewise, it has been argued that this entity should be considered an “enthesis organ” encompassing not only the enthesis itself, but also the fibrocartilage, bursa, fat pad, adjacent trabecular bone networks, and deeper fascia (5) (Figure 1). Representing areas where hard and soft tissues meet, entheses are sites of concentrated stress with effects not only on the bony attachment interface and the enthesis itself, but also on these neighboring tissues (4–7).
Figure 1.
Illustration of the Achilles enthesis organ.
Entheses and mechanical stress
The concept of an enthesis organ was extended to that of a synovioentheseal complex (8,9), which refers to the relationship between the proinflammatory synovium and the avascular enthesis. In contrast to other skeletal locations, the enthesis is a site of repetitive biomechanical forces. High biomechanical stress at the enthesis triggers an inflammatory cascade with cytokine production by infiltrating monocytes and lymphocytes in the adjacent synovial tissue, resulting in an articular inflammatory response, and clinically leading to synovitis adjacent to attachment sites (8,9). Support for this theory of a dynamic response to biomechanical stress at the enthesis originates from animal models. In one experiment, botulinum toxin A injection delayed fibrocartilage development, suggesting that enthesis development is sensitive to mechanical environmental factors (10). In a mouse model that overexpresses TNF, enthesitis was reduced when the hind legs of the mice were made non–weight-bearing through tail suspension (11). Those authors proposed that triggering of mechanoreceptors via the MAPK pathway stimulates the production of inflammatory mediators.
As a result of biomechanical stress, adjacent bone reacts with formation of surface spurs or enthesophytes, observed both radiographically and on histologic examination (12). In early disease, there is destruction of superficial fibrocartilage, with vascular invasion and inflammatory cell infiltration, predominantly with macrophages (13). This leads to another important microanatomical feature, which is the presence of blood vessels at sites where synovium, subchondral bone, and bone marrow are close to each other. In early experiments using labeled phosphorus, Ball identified capillary-like vessels that pass through the enthesis to the marrow (2). Later studies described the presence of vascular channels penetrating cortical bone in the knees of mice adjacent to the cruciate ligaments with associated subclinical changes, including subchondral bone damage and microcyst formation. In the rat adjuvant-induced arthritis model, vascular channels provided a site for inflammatory tissue entry and osteoclast activation (14).
Whether enthesitis is a primary central lesion or a secondary process remains a matter of debate. Studies that have implicated enthesitis as the primary process include studies of TNF-transgenic mice, in which the earliest lesion appears to be in the enthesis (11). However, this may be model specific, and a number of reports have challenged the idea of enthesitis as the primary inflammatory lesion (15,16). In one study examining different stages of spontaneous tail spondylitis and peripheral arthritis in HLA–B27/hβ2m–transgenic mice, histologic samples displayed destructive synovitis with neutrophils and multinucleated giant cells rather than by enthesitis or osteitis (16). Among human studies examining biopsy specimens and MRIs of sacroiliac joints, synovitis and subchondral bone marrow changes were more prominent features while enthesitis was not (17,18). In a subsequent study, in patients with early untreated knee or ankle arthritis, analyses revealed a higher synovitis score by MRI in SpA than in RA, whereas there were no differences in the prevalence of enthesitis as assessed by perientheseal focal tissue, entheseal enhancement, and bone marrow edema (15). However, in light of substantial data in animal models highlighting 3 stages of tendon response to injury that have been defined by distinct pathologic changes, determining the initiating event in the enthesis may be confounded by the timing of the analysis (19–21).
Contributing cellular and molecular mechanisms
Genetic susceptibility
It has long been known that AS susceptibility is largely genetically determined. The strongest genetic association is with the major histocompatibility complex (MHC)–encoded class I molecule, HLA–B27, and it is postulated that HLA–B27 contributes to ~40% of the overall risk for SpA (22). Protein misfolding of nascent HLA–B27 in the endoplasmic reticulum has been hypothesized to trigger an unfolded protein response with aberrant recognition by natural killer cell receptors (23). The HLA–B27–induced unfolded protein response in macrophages has been demonstrated in HLA–B27–transgenic rats and is associated with an increase in IL-23 production by these cells (24). Although HLA–B27 remains the dominant risk factor for susceptibility to the AS phenotype, other important influences of the MHC have been observed (25). More recently, Haroon et al (26) found a positive association of B*27:05:02 with enthesitis, dactylitis, and symmetric sacroiliitis in a cohort of psoriatic arthritis (PsA) patients, whereas B*44 haplotypes were associated with a decreased frequency of enthesitis, dactylitis, and joint fusion. Finally, investigators have recently focused on genes outside of the MHC region, such as ERAP1 and ERAP2, which code for aminopeptidases that are involved in MHC class I presentation (25,27). Although additional HLA class I and class II alleles have also been implicated, the scale and scope of gene identification to date have not yet matched the putative total genetic risk for SpA.
Microbial factors
Microbial infection with virulent organisms remote from affected joints, as well as gastrointestinal dysbiosis without a directly invading pathogen, are known features of certain phenotypes of SpA, and it has long been appreciated that microbial factors can lead to immune activation (28). Clinically, reactive arthritis (ReA) is known to follow infections with Chlamydia, Campylobacter, Shigella, or Yersinia. AS patients consistently have been found to have subclinical gut inflammation and increased gastrointestinal permeability (29,30). In animal models, HLA–B27–transgenic rats raised in germ-free environments do not develop intestinal inflammatory or peripheral joint disease, yet the disease recurs if rats are reconstituted with Bacteroides, supporting the role of gut flora in the development of joint inflammation (31). In a more recent study, colonoscopic biopsies of the terminal ileum of AS patients showed a discrete microbial signature as revealed by sequencing and quantitative polymerase chain reaction analysis of the 16S ribosomal RNA (16S rRNA) gene, exhibiting higher levels of 5 families of bacteria as compared to healthy controls (32). In that study there was no significant difference in the 16S rRNA copy number between patients with AS and controls, indicating that the observed differences were not due to bacterial overgrowth. It has been postulated that the combination of bacterial adjuvants and mechanical factors act synergistically to activate the immune response, particularly in genetically predisposed individuals (5).
Fibrocartilage and versican autoimmunity
A number of studies have indicated that autoimmunity against fibrocartilage proteins, including aggrecan, may underlie enthesitis and spondylitis (33). A model of SpA induced by immunizing BALB/c mice with the G1 globular domain of versican, leading to spondylitis and enthesitis, suggests that versican autoimmunity may also play a role in enthesitis (34). The inflammatory lesions are characterized by mononuclear cell infiltration at the entheseal insertions to the vertebrae, as is seen with AS, and are associated with angiogenesis which then progresses to cause destructive discitis (35).
Role of bone morphogens
In the DBA/1 mouse model, where mice develop spontaneously occurring arthritis that culminates in bone formation and joint ankylosis, male mice in crowded conditions developed arthritis in the hind paws that was entheseal, but not synovially based, with new bone formation driven by BMP-7 signaling (36). In that experiment, the incidence of arthritis was increased in mice that were caged together in crowded conditions, yet decreased when the mice were placed in larger cages (37). Thus, in addition to a genetic predisposition for enthesitis, this observation points to the role of environmental factors in the development of arthritis. Finally, immunohistochemical studies in SpA show increased synovial expression of BMP-2 and BMP-6, which is up-regulated by proinflammatory cytokines such as IL-1 and TNF, suggesting that synovial molecules contribute to chronic arthritis and joint ankylosis (36,38).
Role of proinflammatory cytokines
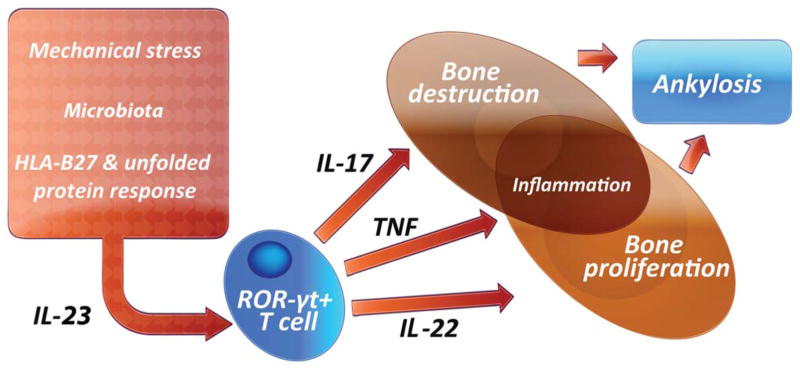
The role of IL-23 has been addressed as a major driver of cascades that lead to inflammation and bone remodeling in SpA. Alterations in AS susceptibility are related to the existence of single-nucleotide polymorphisms in the IL-23 receptor as demonstrated in genome-wide association studies, and serum levels of the IL-12/23 p40 subunit have been shown to be significantly higher in patients with PsA compared with controls (39,40). IL-23 is produced in the gut, suggesting that the intestinal mucosa is a key site of IL-23 production in SpA. Additionally, Chlamydia trachomatis also leads to induction of IL-23 via CHOP10. Taken together, these findings indicate that IL-23 is a pivotal cytokine and potentially central to the pathogenesis of SpA (41). Increased IL-17 expression by innate immune cells such as mast cells and neutrophils in SpA has been shown to target the facet joints and synovial tissue (42,43). In a subsequent set of investigations, Sherlock et al found that IL-23 could induce SpA by acting on an isolated population of CD3+CD4−CD8− entheseal resident lymphocytes, leading to increased expression of TNF and IL-6 in the enthesis. When IL-23 was overexpressed, mice developed enthesitis with inflammation, which spread into the adjacent synovium (41). Enthesitis was associated with new bone erosion. IL-23 promoted inflammation through IL-17 and TNF, whereas new bone formation was associated with overproduction of IL-22 (41,44) (Figure 2).
Figure 2.
Interleukin-23 (IL-23) is activated via a variety of pathways including the HLA–B27 unfolded protein response. IL-23 then activates resident T cells within the enthesis, which then promotes inflammation and bone remodeling, with inflammation mediated by IL-17 and osteoproliferation mediated by IL-22. The net result is bone ankylosis in the spine. RORγt = retinoic acid receptor–related orphan nuclear receptor γt; TNF = tumor necrosis factor. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/journal/doi/10.1002/art.39458/abstract
Additional support for the role of IL-23 comes from the SKG mouse model, in which curdlan (β-1,3-glucan) injections induce enthesitis and dactylitis. Arthritis and spondylitis were IL-23 dependent and were transferable to SCID mouse recipients with CD4+ T cells (45). In this model, disease severity was dependent on the external microbial environment and the host immunogenetic background. More recent work illustrates the differential impact of microbiota on specific pathologic features of SpA; ileitis development, ileal IL-23 expression, and lymph node IL-17A production were microbiota dependent, but arthritis was not (46). In curdlan-treated SKG mice, enthesitis was specifically dependent on IL-17A and IL-22 (47). The role of up-regulation of the IL-23/Th17 pathway in promoting joint inflammation and bone turnover is further supported by recent murine studies, with inhibition of the PsA phenotype after neutralization of IL-17A (48,49).
Clinical enthesitis in SpA
SpA is by definition a heterogeneous group of clinical entities long recognized as having unique phenotypes that include AS, ReA, PsA, enteropathic arthritis, and what has traditionally been referred to as undifferentiated arthritis. However, with advances in imaging and careful long-term followup observations, it appears that these diseases share common features, including subclinical spinal and peripheral joint inflammation, along with associations with microbes and gene identifications. In attempting to develop a model for an underlying unifying anatomical basis for SpA, an “enthesitis-based model” has been proposed as the basis for the osteitis, periostitis, and new bone formation that are seen in SpA (5). The association between enthesitis and adjacent osteitis has been further supported by imaging and cadaver studies, primarily in patients with PsA (50–52).
Regional sites
Patients with SpA have a remarkable propensity for inflammation at certain enthesis sites that are ubiquitous and numerous. Clinically, peripheral enthesitis is observed not only in all forms of SpA, but particularly frequently in juvenile-onset SpA. A number of patients with juvenile SpA are classified as having enthesitis-related arthritis (ERA), a heterogeneous subtype that includes some patients who predominantly have enthesitis, enthesitis and arthritis, or juvenile AS. Compared to other subtypes of juvenile idiopathic arthritis, ERA is associated with worse function, worse quality of life, and increased pain (53,54).
Enthesitis can be seen in 33–58% of patients with ReA and may be the only clinical manifestation in some whose disease has been triggered by an enteric infection (55). In SpA, the entheses of the lower extremities are more frequently involved than those of the upper limbs, and the heel is the most frequent site (55). In addition to the Achilles and plantar fascia insertions, identified sites of enthesitis include muscle attachments to the greater and lesser trochanters, the insertion of the quadriceps tendon at the upper patellar pole, the insertions of the patellar ligament at the lower patellar pole and the tibial tubercle, acromial and clavicular insertions of the deltoid muscle, and the insertions of the flexor and extensor tendons at the phalanges (55–57). It is unknown why there is a predilection for the entheses at the lower parts of the lower limbs, although it has been hypothesized that this may be due to the length, anatomy, and higher mechanical load at these sites.
Given the presumed role of repetitive biomechanical forces discussed above, it is not surprising that in patients with longstanding AS, those with occupational activities that required more bending, twisting, and stretching had more functional limitations and radiographic damage than those whose jobs required little or no dynamic flexibility (58). A recently published computer-based method that fully quantified syndesmophyte heights and volumes on computed tomography scans has revealed that syndesmophytes grow at different rates over time in AS patients, suggesting that mechanical factors local to the disc space may influence syndesmophyte formation (59). Clearly, there are sites that are not associated with SpA despite being sites of significant biomechanical stress, and perhaps it is the compressive and shear force nature of the stress as well as the putative role of antigen expression adjacent to the enthesis that may underlie this apparent discrepancy (5). Additionally, it cannot be discounted that the increased detection of enthesitis at the lower limbs is explained by the accessibility of these sites to ultrasound.
Diagnostic criteria and outcome measures
Enthesitis is often underdiagnosed in the clinic; clinical assessment and quantification of peripheral enthesitis in daily practice lacks sensitivity and specificity (56,60,61). Although both the Amor criteria (62) and the European Spondylarthropathy Study Group criteria (63) for SpA include peripheral enthesitis, there are limitations to these criteria with regard to the exact quantification of enthesitis. Two clinical methods have been designed and often implemented for evaluating enthesopathy in AS: Mander’s Entheseal Index and the Maastricht Ankylosing Spondylitis Enthesitis Score (MASES) (64,65). Both rely on pain elicited by local pressure of entheseal points. The intraarticular and deep location of entheseal insertions, however, makes quantification of enthesitis by physical examination alone difficult, and not surprisingly, these scoring systems have only moderate sensitivity and specificity for predicting positive sonogram results, depending on the entheseal site (66).
Imaging of the enthesis
Because of the clinical limitations described above and the poor sensitivity of markers of inflammation, it is necessary to rely on typical abnormalities seen on various imaging techniques to diagnose SpA. Plain radiographs are limited by their inability to show inflammation or soft tissue changes, although late chronic bony changes such as enthesophyte formation or occasional erosions can be seen at the attachment of the Achilles tendon or plantar aponeurosis. More sensitive methods such as ultrasound and MRI, which are useful in their ability to detect both inflammatory and chronic changes in enthesitis at both early and late stages, can be used.
MRI
MRI has changed the way we approach both the diagnosis and classification of SpA; it is particularly useful in detecting spinal disease in early AS when conventional radiographs are still normal (67). The use of fat-suppressed, fat-saturated, and water-sensitive MRI sequences has demonstrated that the extracapsular inflammation of joints quite often represents enthesitis with variable degrees of soft tissue and bone marrow edema (68,69) (Figures 3–5). The typical appearance of enthesitis on MRI includes soft tissue inflammatory changes outside the joint capsule and perientheseal bone marrow edema (70). Recent studies have examined the utility of whole-body MRI, which has shown promise in the detection of subclinical axial and peripheral enthesitis (71). Of course, MRI has limitations; structures that make up entheses have a low signal on conventional MRI, with low water accumulation in the areas where fibroblasts are tightly cross-linked. MRI is further limited by its cost and availability, and therefore ultrasound remains the preferred modality for the detection of enthesitis both in the clinical setting as well as in research.
Figure 3.
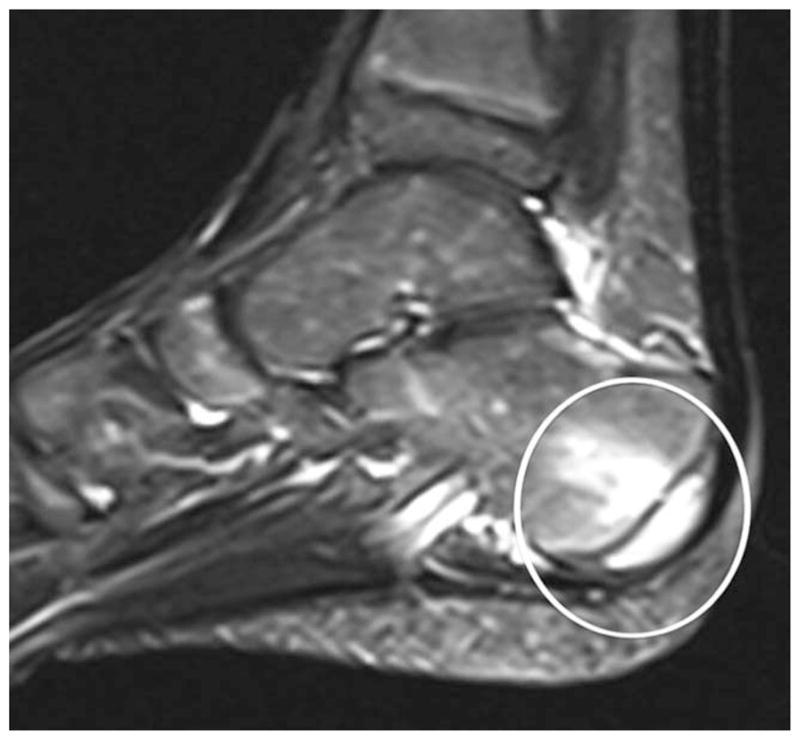
Achilles tendon insertion into the calcaneus. An abnormal signal is seen at the posterior calcaneus at the site of the Achilles entheseal insertion (encircled area) on magnetic resonance imaging using STIR sequences. Reproduced from ref. 68.
Figure 5.
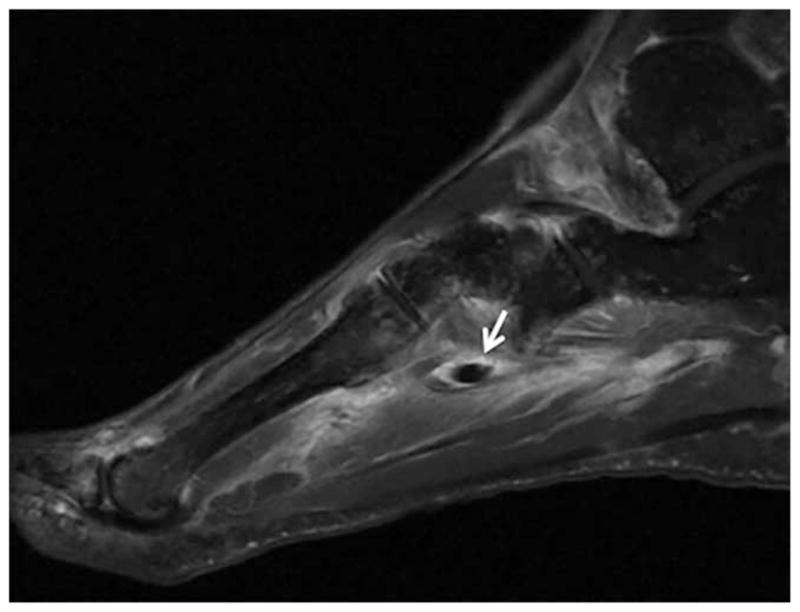
Fat-suppressed T1-weighted sequence magnetic resonance image (MRI) of the right midfoot of a 58-year-old woman with HLA–B27–positive peripheral spondyloarthropathy. The MRI demonstrates extensive enthesitis, synovitis, and tenosynovitis of the peroneus longus, peroneus brevis, tibialis posterior, flexor digitorum longus, and extensor digitorum tendons. This is characterized by excess fluid and enhancement in and around the tendon sheaths. Arrow indicates extensive edema and enhancement at the plantar aspect of the midfoot, involving insertions of intrinsic musculature and capsular ligaments consistent with enthesitis. Image courtesy of Dr. Joseph Robinson (Cedars-Sinai Medical Center).
Ultrasound
Ultrasound has indeed proven to be a highly useful and sensitive tool in the evaluation of enthesitis and improves the ability of the clinical examination to detect enthesopathy. In one study of 92 patients with PsA, ultrasound was useful in detecting subclinical entheseal involvement, independent of clinical examination and symptoms (72). In another study of 600 lower limb entheses, at least 1 ultrasound sign of enthesopathy was detected in 60% of clinically asymptomatic cases of enthesitis, thus demonstrating a higher sensitivity than physical examination (73).
Ultrasound may be most useful in the early diagnosis of SpA, and likewise, entheseal abnormalities can be detected prior to overt clinical disease. Nevertheless, in an older cross-sectional single-center study of 51 SpA patients and 24 controls, neither MRI nor power Doppler ultrasound (PDUS) discriminated between SpA and controls (74). In a prospective single-center cohort study of 118 patients with symptoms suggestive of SpA conducted by D’Agostino and colleagues (57), vascularization at cortical bone detected by PDUS of at least one enthesis provided good predictive value for diagnosing SpA with a sensitivity of 76.5% and a specificity of 81.3%. Indeed, PDUS is a sensitive and reliable technique used to detect increased blood flow in the enthesis revealing neovascularity and subclinical active inflammation (56,75) (Figure 6).
Figure 6.
A, Ultrasound image of a patient with psoriatic arthritis with enthesitis (long-axis view of the lateral epicondyle [high-frequency, 18-MHz probe]). Precise delineation of blood flow seen at the cortical interface on B-flow imaging is shown. Calcification is seen adjacent to the epicondyle. B and C, 3T magnetic resonance image of the same patient, in the same orientation as the ultrasound in A, showing proton density (B) and fat presaturation (C). Boxed areas show the region of the lateral epicondyle. Images courtesy of Dr. Ralph Thiele (University of Rochester, Rochester, NY). Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/journal/doi/10.1002/art.39458/abstract
Recent studies have indicated that ultrasound may accurately predict which patients will go on to develop SpA (51,57,75). In one investigation, ultrasound examination of Achilles erosions correlated with objective activity-based measurements of SpA outcomes, and was sensitive to change (76). In the study by D’Agostino and colleagues described above, vascularized enthesis as detected by PDUS combined with Amor’s criteria proved to be the only independent contributors to a diagnosis of SpA (57).
Finally, ultrasound may be used to monitor response to therapeutic interventions. A few studies have illustrated improvement in enthesitis shown on ultrasound after the use of TNF antagonists (77,78). In one investigation of 327 patients with active SpA who were treated with anti-TNF therapy for 6 months, cumulative entheseal morphologic abnormalities, intraenthesis and perienthesis, and bursitis were all significantly decreased on PDUS after 6 months of treatment (77). In another study, D’Agostino et al monitored regression of enthesitis using PDUS after treatment with infliximab (78), providing confirmatory evidence for the utility of ultrasound in a clinical research setting.
Treatment of enthesitis
Historically, treatment of clinical enthesitis had been limited to NSAIDs. Continuous use of NSAIDs not only controls symptoms of disease, but may also slow progression of bony changes in AS (79,80). Therefore, Assessment of SpondyloArthritis international Society/European League Against Rheumatism guidelines place optimal NSAID therapy as a cornerstone of the management plan for AS (81).
Treatment with TNF inhibitors is indicated in patients that do not respond to NSAID therapy. TNF inhibition with adalimumab, etanercept, infliximab, and golimumab has been shown to be efficacious in the treatment of enthesitis (82–87). Olivieri et al (88) have reported that adalimumab and etanercept are effective treatments of MRI-documented refractory heel enthesitis, with progressive improvement of bone edema in a 6-month period (88).
Agents that block IL-23 have the potential to inhibit both inflammation and altered bone remodeling, although further analysis of the effect of IL-22 and IL-23 blockade on bone pathologies in animal models and patients with PsA are needed to address this important therapeutic issue (89). Entheseal inflammation in a passive-transfer model of collagen antibody-induced arthritis was reduced by an antibody to the p19 subunit of IL-23, which was also associated with the down-regulation of several inflammatory mediators, such as IL-6 and IL-1β, and genes such as Rankl, Ctsk, and matrix metalloproteinases known to be involved in bone erosion (41). Both ustekinumab, a monoclonal antibody directed against the common p40 subunit of IL-12 and IL-23, and secukinumab, a human anti–IL-17A monoclonal antibody, have already demonstrated promise in PsA, with significant improvements in enthesitis (90,91).
Apremilast, an oral inhibitor of phosphodiesterase 4, which increases cAMP and thus modulates multiple proinflammatory mediators, has demonstrated efficacy in PsA, with significant improvements in the severity of both enthesitis and dactylitis evidenced by reductions in MASES over a 52-week period (92). Finally, bisphosphonates may also have a role in peripheral enthesitis felt to be refractory to NSAID therapy. In a 6-month randomized controlled comparison of intravenous pamidronate treatment of NSAID-refractory AS, patients treated with pamidronate showed symptomatic improvement with significant reductions in Bath Ankylosing Spondylitis Functional Index and Bath Ankylosing Spondylitis Disease Activity Index measurements together with regression of periarticular osteitis documented by MRI with gadolinium (93).
Treatment of patients with SpA enthesitis with currently available agents has not had universal success. In placebo-controlled trials of methotrexate and leflunomide in PsA, enthesitis measures were not assessed (94,95). In a randomized controlled trial, sulfasalazine was not effective for enthesitis (96). Other agents that have not demonstrated clinical efficacy in AS include tocilizumab, and lymphocyte-targeted therapies such as abatacept (97,98). Rituximab only showed modest therapeutic efficacy in SpA (99,100).
Conclusions
In summary, investigations and clinical observations uniformly point out with increasing clarity that the enthesis is much more than a simple attachment site. A number of studies have shown that it functions as a unit comprising adjacent tissues, including bone and fibro-cartilage linked to synovium, and serves as a way of dissipating stress over a wide area. Inflammation at the enthesis manifests in the adjacent synovium presumably via immunity to common antigens or via release of proinflammatory cytokines at the enthesis. Although work by Benjamin and McGonagle (9) suggests that the enthesis is the primary SpA lesion, the precise role of the enthesis in early stages of disease, especially regarding issues of cause or effect, remains an area of continued debate and discovery. Improved imaging modalities may in the future be able to detect enthesitis at different stages of disease. However, this will require a clinically diverse and large sample size to help address this question. Inflammation at the enthesis is likely modulated by multiple factors. A more complete role for genetic predisposition will require additional advances in gene sequencing and discovery. Repeated biomechanical stress with the resultant inflammatory response regulated by IL-17, IL-22, and IL-23 now provide clues as to why certain areas of the body are affected, and perhaps why others are not. The spine itself (the clinical hallmark of the disease) remains inaccessible to traditional enthesitis-focused research methodologies thus far. However, newer imaging techniques are on the horizon. Further examination into the role of the inflammatory mediators, including IL-17, IL-22, and IL-23 as well as potentially others, in driving enthesitis and bone formation will be important to direct our attention toward future therapeutic targeted pathways in patients with SpA.
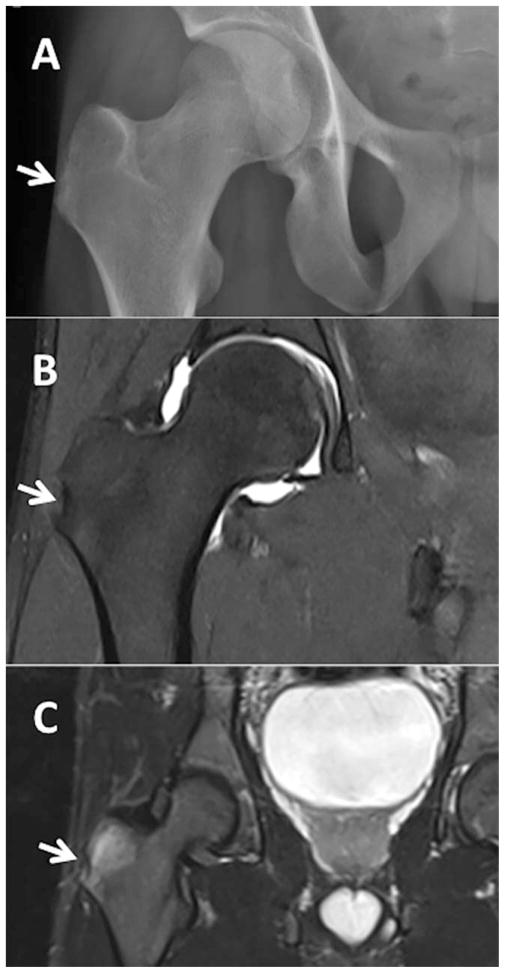
Figure 4.
Radiographic findings of enthesitis in a 21-year-old man with ankylosing spondylitis. A, Reference plain radiograph. B, Magnetic resonance image with T1 sequence showing a small erosion at the right greater trochanter. C, Axial T2 fat-saturated sequence of the right hip showing edema of the right gluteus minimus tendon at its insertion, consistent with enthesitis. Arrows indicate the region of the right greater trochanter. Images courtesy of Dr. Joseph Robinson (Cedars-Sinai Medical Center).
Acknowledgments
Supported in part by the NIH (National Institute of Arthritis and Musculoskeletal and Skin Diseases grant P01-AR-052915 and National Center for Advancing Translational Sciences grant UL1-TR-000124).
The authors wish to thank Joseph Robinson, MD (Cedars-Sinai Medical Center Department of Radiology) for assistance with MRI acquisition and interpretation.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published.
References
- 1.Lampman JH. Origin of enthesopathy. J Rheumatol. 1985;12:1030–1. [PubMed] [Google Scholar]
- 2.Ball J. Enthesopathy of rheumatoid and ankylosing spondylitis. Ann Rheum Dis. 1971;30:213–23. doi: 10.1136/ard.30.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francois RJ, Braun J, Khan MA. Entheses and enthesitis: a histopathologic review and relevance to spondyloarthritides. Curr Opin Rheumatol. 2001;13:255–64. doi: 10.1097/00002281-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin M, Moriggl B, Brenner E, Emery P, McGonagle D, Redman S. The “enthesis organ” concept: why enthesopathies may not present as focal insertional disorders. Arthritis Rheum. 2004;50:3306–13. doi: 10.1002/art.20566. [DOI] [PubMed] [Google Scholar]
- 5.McGonagle D, Stockwin L, Isaacs J, Emery P. An enthesitis based model for the pathogenesis of spondyloarthropathy: additive effects of microbial adjuvant and biomechanical factors at disease sites. J Rheumatol. 2001;28:2155–9. [PubMed] [Google Scholar]
- 6.Benjamin M, McGonagle D. The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. J Anat. 2001;199:503–26. doi: 10.1046/j.1469-7580.2001.19950503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGonagle D. Enthesitis: an autoinflammatory lesion linking nail and joint involvement in psoriatic disease. J Eur Acad Dermatol Venereol. 2009;23(Suppl 1):9–13. doi: 10.1111/j.1468-3083.2009.03363.x. [DOI] [PubMed] [Google Scholar]
- 8.McGonagle D, Lories RJ, Tan AL, Benjamin M. The concept of a “synovio-entheseal complex” and its implications for understanding joint inflammation and damage in psoriatic arthritis and beyond. Arthritis Rheum. 2007;56:2482–91. doi: 10.1002/art.22758. [DOI] [PubMed] [Google Scholar]
- 9.Benjamin M, McGonagle D. The enthesis organ concept and its relevance to the spondyloarthropathies. Adv Exp Med Biol. 2009;649:57–70. doi: 10.1007/978-1-4419-0298-6_4. [DOI] [PubMed] [Google Scholar]
- 10.Thomopoulos S, Kim HM, Rothermich SY, Biederstadt C, Das R, Galatz LM. Decreased muscle loading delays maturation of the tendon enthesis during postnatal development [published erratum appears in J Orthop Res 2009;27:141] J Orthop Res. 2007;25:1154–63. doi: 10.1002/jor.20418. [DOI] [PubMed] [Google Scholar]
- 11.Jacques P, Lambrecht S, Verheugen E, Pauwels E, Kollias G, Armaka M, et al. Proof of concept: enthesitis and new bone formation in spondyloarthritis are driven by mechanical strain and stromal cells. Ann Rheum Dis. 2014;73:437–45. doi: 10.1136/annrheumdis-2013-203643. [DOI] [PubMed] [Google Scholar]
- 12.Benjamin M, Rufai A, Ralphs JR. The mechanism of formation of bony spurs (enthesophytes) in the Achilles tendon. Arthritis Rheum. 2000;43:576–83. doi: 10.1002/1529-0131(200003)43:3<576::AID-ANR14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 13.McGonagle D, Marzo-Ortega H, O’Connor P, Gibbon W, Hawkey P, Henshaw K, et al. Histological assessment of the early enthesitis lesion in spondyloarthropathy. Ann Rheum Dis. 2002;61:534–7. doi: 10.1136/ard.61.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Binks D, Matzelle M, Bergin D, Hodgson RJ, Tan AL, Gravallese EM, et al. The frequency of bone marrow oedema adjacent to the cruciate ligament peri-entheseal vascular channels in inflammatory and degenerative arthritis [abstract] Arthritis Rheum. 2013;65(Suppl):S25–6. [Google Scholar]
- 15.Paramarta JE, van der Leij C, Gofita I, Yeremenko N, van de Sande MG, de Hair MJ, et al. Peripheral joint inflammation in early onset spondyloarthritis is not specifically related to enthesitis. Ann Rheum Dis. 2014;73:735–40. doi: 10.1136/annrheumdis-2012-203155. [DOI] [PubMed] [Google Scholar]
- 16.Van Duivenvoorde LM, Dorris ML, Satumtira N, van Tok MN, Redlich K, Tak PP, et al. Relationship between inflammation, bone destruction, and osteoproliferation in the HLA–B27/human β2-microglobulin–transgenic rat model of spondylarthritis. Arthritis Rheum. 2012;64:3210–9. doi: 10.1002/art.34600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francois RJ, Gardner DL, Degrave EJ, Bywaters EG. Histopathologic evidence that sacroiliitis in ankylosing spondylitis is not merely enthesitis: systematic study of specimens from patients and control subjects. Arthritis Rheum. 2000;43:2011–24. doi: 10.1002/1529-0131(200009)43:9<2011::AID-ANR12>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 18.Muche B, Bollow M, Francois RJ, Sieper J, Hamm B, Braun J. Anatomic structures involved in early- and late-stage sacroiliitis in spondylarthritis: a detailed analysis by contrast-enhanced magnetic resonance imaging. Arthritis Rheum. 2003;48:1374–84. doi: 10.1002/art.10934. [DOI] [PubMed] [Google Scholar]
- 19.Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med. 2009;43:409–16. doi: 10.1136/bjsm.2008.051193. [DOI] [PubMed] [Google Scholar]
- 20.Aspenberg P. Stimulation of tendon repair: mechanical loading, GDFs and platelets: a mini-review. Int Orthop. 2007;31:783–9. doi: 10.1007/s00264-007-0398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apostolakos J, Durant TJ, Dwyer CR, Russell RP, Weinreb JH, Alaee F, et al. The enthesis: a review of the tendon-to-bone insertion. Muscles Ligaments Tendons J. 2014;4:333–42. [PMC free article] [PubMed] [Google Scholar]
- 22.Reveille JD. The genetic basis of spondyloarthritis. Ann Rheum Dis. 2011;70(Suppl 1):i44–50. doi: 10.1136/ard.2010.140574. [DOI] [PubMed] [Google Scholar]
- 23.Colbert RA, Tran TM, Layh-Schmitt G. HLA-B27 misfolding and ankylosing spondylitis. Mol Immunol. 2014;57:44–51. doi: 10.1016/j.molimm.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeLay ML, Turner MJ, Klenk EI, Smith JA, Sowders DP, Colbert RA. HLA–B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 2009;60:2633–43. doi: 10.1002/art.24763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortes A, Pulit SL, Leo PJ, Pointon JJ, Robinson PC, Weisman MH, et al. Major histocompatibility complex associations of ankylosing spondylitis are complex and involve further epistasis with ERAP1. Nat Commun. 2015;6:7146. doi: 10.1038/ncomms8146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haroon M, Winchester R, Giles JT, Heffernan E, FitzGerald O. Certain class I HLA alleles and haplotypes implicated in susceptibility play a role in determining specific features of the psoriatic arthritis phenotype. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2014-205461. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Breban M, Costantino F, Andre C, Chiocchia G, Garchon HJ. Revisiting MHC genes in spondyloarthritis. Current Rheumatology Reports. 2015;17:516. doi: 10.1007/s11926-015-0516-1. [DOI] [PubMed] [Google Scholar]
- 28.Hacker G, Redecke V, Hacker H. Activation of the immune system by bacterial CpG-DNA. Immunology. 2002;105:245–51. doi: 10.1046/j.0019-2805.2001.01350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacques P, Van Praet L, Carron P, Van den Bosch F, Elewaut D. Pathophysiology and role of the gastrointestinal system in spondyloarthritides. Rheum Dis Clin North Am. 2012;38:569–82. doi: 10.1016/j.rdc.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Matzkies FG, Targan SR, Berel D, Landers CJ, Reveille JD, McGovern DP, et al. Markers of intestinal inflammation in patients with ankylosing spondylitis: a pilot study. Arthritis Res Ther. 2012;14:R261. doi: 10.1186/ar4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taurog JD, Richardson JA, Croft JT, Simmons WA, Zhou M, Fernandez-Sueiro JL, et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–64. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costello ME, Ciccia F, Willner D, Warrington N, Robinson PC, Gardiner B, et al. Intestinal dysbiosis in ankylosing spondylitis. Arthritis Rheumatol. 2015;67:686–91. doi: 10.1002/art.38967. [DOI] [PubMed] [Google Scholar]
- 33.Guerassimov A, Zhang Y, Banerjee S, Cartman A, Webber C, Esdaile J, et al. Autoimmunity to cartilage link protein in patients with rheumatoid arthritis and ankylosing spondylitis. J Rheumatol. 1998;25:1480–4. [PubMed] [Google Scholar]
- 34.Shi SL, Ciurli C, Cartman A, Pidoux I, Poole AR, Zhang Y. Experimental immunity to the G1 domain of the proteoglycan versican induces spondylitis and sacroiliitis, of a kind seen in human spondylarthropathies. Arthritis Rheum. 2003;48:2903–15. doi: 10.1002/art.11270. [DOI] [PubMed] [Google Scholar]
- 35.Zhang YP, Guerassimov A, Leroux JY, Cartman A, Webber C, Lalic R, et al. Arthritis induced by proteoglycan aggrecan G1 domain in BALB/c mice. Evidence for T cell involvement and the immunosuppressive influence of keratan sulfate on recognition of T and B cell epitopes. J Clin Invest. 1998;101:1678–86. doi: 10.1172/JCI1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lories RJ, Derese I, Luyten FP. Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J Clin Invest. 2005;115:1571–9. doi: 10.1172/JCI23738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braem K, Carter S, Lories RJ. Spontaneous arthritis and ankylosis in male DBA/1 mice: further evidence for a role of behavioral factors in “stress-induced arthritis”. Biol Proced Online. 2012;14:10. doi: 10.1186/1480-9222-14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lories RJ, Derese I, Ceuppens JL, Luyten FP. Bone morphogenetic proteins 2 and 6, expressed in arthritic synovium, are regulated by proinflammatory cytokines and differentially modulate fibroblast-like synoviocyte apoptosis. Arthritis Rheum. 2003;48:2807–18. doi: 10.1002/art.11389. [DOI] [PubMed] [Google Scholar]
- 39.Wellcome Trust Case Control Consortium, Australo-Anglo-American Spondylitis Consortium (TASC) Burton PR, Clayton DG, Cardon LR, Craddock N, et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet. 2007;39:1329–37. doi: 10.1038/ng.2007.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reveille JD. Genetics of spondyloarthritis-beyond the MHC. Nat Rev Rheumatol. 2012;8:296–304. doi: 10.1038/nrrheum.2012.41. [DOI] [PubMed] [Google Scholar]
- 41.Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4−CD8− entheseal resident T cells. Nat Med. 2012;18:1069–76. doi: 10.1038/nm.2817. [DOI] [PubMed] [Google Scholar]
- 42.Noordenbos T, Yeremenko N, Gofita I, van de Sande M, Tak PP, Canete JD, et al. Interleukin-17–positive mast cells contribute to synovial inflammation in spondylarthritis. Arthritis Rheum. 2012;64:99–109. doi: 10.1002/art.33396. [DOI] [PubMed] [Google Scholar]
- 43.Appel H, Maier R, Wu P, Scheer R, Hempfing A, Kayser R, et al. Analysis of IL-17+ cells in facet joints of patients with spondyloarthritis suggests that the innate immune pathway might be of greater relevance than the Th17-mediated adaptive immune response. Arthritis Res Ther. 2011;13:R95. doi: 10.1186/ar3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lories RJ, McInnes I. Primed for inflammation: enthesis resident cells. Nat Med. 2012;18:1018–9. doi: 10.1038/nm.2854. [DOI] [PubMed] [Google Scholar]
- 45.Ruutu M, Thomas G, Steck R, Degli-Esposti MA, Zinkernagel MS, Alexander K, et al. β-glucan triggers spondylarthritis and Crohn’s disease–like ileitis in SKG mice. Arthritis Rheum. 2012;64:2211–22. doi: 10.1002/art.34423. [DOI] [PubMed] [Google Scholar]
- 46.Rehaume LM, Mondot S, Aguirre de Carcer D, Velasco J, Benham H, Hasnain SZ, et al. ZAP-70 genotype disrupts the relationship between microbiota and host, leading to spondyloarthritis and ileitis in SKG mice. Arthritis Rheumatol. 2014;66:2780–92. doi: 10.1002/art.38773. [DOI] [PubMed] [Google Scholar]
- 47.Benham H, Rehaume LM, Hasnain SZ, Velasco J, Baillet AC, Ruutu M, et al. Interleukin-23 mediates the intestinal response to microbial β-1,3-glucan and the development of spondyloarthritis pathology in SKG mice. Arthritis Rheumatol. 2014;66:1755–67. doi: 10.1002/art.38638. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto M, Nakajima K, Takaishi M, Kitaba S, Magata Y, Kataoka S, et al. Psoriatic inflammation facilitates the onset of arthritis in a mouse model. J Invest Dermatol. 2015;135:445–53. doi: 10.1038/jid.2014.426. [DOI] [PubMed] [Google Scholar]
- 49.Khmaladze I, Kelkka T, Guerard S, Wing K, Pizzolla A, Saxena A, et al. Mannan induces ROS-regulated, IL-17A-dependent psoriasis arthritis-like disease in mice. Proc Natl Acad Sci U S A. 2014;111:E3669–78. doi: 10.1073/pnas.1405798111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan AL, Benjamin M, Toumi H, Grainger AJ, Tanner SF, Emery P, et al. The relationship between the extensor tendon enthesis and the nail in distal interphalangeal joint disease in psoriatic arthritis—a high-resolution MRI and histological study. Rheumatology (Oxford) 2007;46:253–6. doi: 10.1093/rheumatology/kel214. [DOI] [PubMed] [Google Scholar]
- 51.Naredo E, Moller I, de Miguel E, Batlle-Gualda E, Acebes C, Brito E, et al. High prevalence of ultrasonographic synovitis and enthesopathy in patients with psoriasis without psoriatic arthritis: a prospective case-control study. Rheumatology (Oxford) 2011;50:1838–48. doi: 10.1093/rheumatology/ker078. [DOI] [PubMed] [Google Scholar]
- 52.Yasser R, Yasser E, Hanan D, Rasker JJ. Enthesitis in seronegative spondyloarthropathies with special attention to the knee joint by MRI: a step forward toward understanding disease pathogenesis. Clin Rheumatol. 2011;30:313–22. doi: 10.1007/s10067-010-1655-4. [DOI] [PubMed] [Google Scholar]
- 53.Weiss P, Beukelman T, Schanberg LE, Kimura Y, Colbert RA CARRAnet Investigators. Enthesitis is a significant predictor of decreased quality of life, function, and arthritis-specific pain across juvenile idiopathic arthritis (JIA) categories: preliminary analyses from the CARRAnet registry. Arthritis Rheum. 2011;63(Suppl):S105. [Google Scholar]
- 54.Weiss PF. Evaluation and treatment of enthesitis-related arthritis. Curr Med Lit Rheumatol. 2013;32:33–41. [PMC free article] [PubMed] [Google Scholar]
- 55.D’Agostino MA, Olivieri I. Enthesitis. Best Pract Res Clin Rheumatol. 2006;20:473–86. doi: 10.1016/j.berh.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 56.D’Agostino MA, Said-Nahal R, Hacquard-Bouder C, Brasseur JL, Dougados M, Breban M. Assessment of peripheral enthesitis in the spondylarthropathies by ultrasonography combined with power Doppler: a cross-sectional study. Arthritis Rheum. 2003;48:523–33. doi: 10.1002/art.10812. [DOI] [PubMed] [Google Scholar]
- 57.D’Agostino MA, Aegerter P, Bechara K, Salliot C, Judet O, Chimenti MS, et al. How to diagnose spondyloarthritis early? Accuracy of peripheral enthesitis detection by power Doppler ultrasonography. Ann Rheum Dis. 2011;70:1433–40. doi: 10.1136/ard.2010.138701. [DOI] [PubMed] [Google Scholar]
- 58.Ward MM, Reveille JD, Learch TJ, Davis JC, Jr, Weisman MH. Occupational physical activities and long-term functional and radiographic outcomes in patients with ankylosing spondylitis. Arthritis Rheum. 2008;59:822–32. doi: 10.1002/art.23704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tan S, Yao J, Flynn JA, Yao L, Ward MM. Quantitative syndesmophyte measurement in ankylosing spondylitis using CT: longitudinal validity and sensitivity to change over 2 years. Ann Rheum Dis. 2015;74:437–43. doi: 10.1136/annrheumdis-2013-203946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wiell C, Norregaard J, Szkudlarek M, Hasselquist M, Moller JM, Terslev L, et al. Ultrasonography of finger joints, tendons and entheses in patients with spondyloarthropathy: a comparison with clinical examination and MRI. Ann Rheum Dis. 2005;64(Suppl III):327. [Google Scholar]
- 61.Wiell C, Norregaard J, Szkudlarek M, Hasselquist M, Moller JM, Terslev L, et al. Ultrasonography of lower extremity tendons and entheses in patients with spondyloarthropathy: a comparison with clinical examination and MRI. Ann Rheum Dis. 2005;64(Suppl III):378. [Google Scholar]
- 62.Amor B, Dougados M, Mijiyawa M. Criteria for the classification of spondylarthropathies. Rev Rhum Mal Osteoartic. 1990;57:85–9. In French. [PubMed] [Google Scholar]
- 63.Dougados M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A, et al. the European Spondylarthropathy Study Group. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991;34:1218–27. doi: 10.1002/art.1780341003. [DOI] [PubMed] [Google Scholar]
- 64.Mander M, Simpson JM, McLellan A, Walker D, Goodacre JA, Dick WC. Studies with an enthesis index as a method of clinical-assessment in ankylosing spondylitis. Ann Rheum Dis. 1987;46:197–202. doi: 10.1136/ard.46.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heuft-Dorenbosch L, Spoorenberg A, van Tubergen A, Landewe R, van ver Tempel H, Mielants H, et al. Assessment of enthesitis in ankylosing spondylitis. Ann Rheum Dis. 2003;62:127–32. doi: 10.1136/ard.62.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klauser AS, Wipfler E, Dejaco C, Moriggl B, Duftner C, Schirmer M. Diagnostic values of history and clinical examination to predict ultrasound signs of chronic and acute enthesitis. Clin Exp Rheumatol. 2008;26:548–53. [PubMed] [Google Scholar]
- 67.Braun J, Bollow M, Eggens U, Konig H, Distler A, Sieper J. Use of dynamic magnetic resonance imaging with fast imaging in the detection of early and advanced sacroiliitis in spondylarthropathy patients. Arthritis Rheum. 1994;37:1039–45. doi: 10.1002/art.1780370709. [DOI] [PubMed] [Google Scholar]
- 68.McGonagle D, Marzo-Ortega H, O’Connor P, Gibbon W, Pease C, Reece R, et al. The role of biomechanical factors and HLA–B27 in magnetic resonance imaging–determined bone changes in plantar fascia enthesopathy. Arthritis Rheum. 2002;46:489–93. doi: 10.1002/art.10125. [DOI] [PubMed] [Google Scholar]
- 69.Marzo-Ortega H, McGonagle D, O’Connor P, Emery P. Efficacy of etanercept in the treatment of the entheseal pathology in resistant spondylarthropathy: a clinical and magnetic resonance imaging study. Arthritis Rheum. 2001;44:2112–7. doi: 10.1002/1529-0131(200109)44:9<2112::AID-ART363>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 70.Aquino MR, Tse SML, Gupta S, Rachlis AC, Stimec J. Whole-body MRI of juvenile spondyloarthritis: protocols and pictorial review of characteristic patterns. Pediatr Radiol. 2015;45:754–62. doi: 10.1007/s00247-015-3319-7. [DOI] [PubMed] [Google Scholar]
- 71.Poggenborg RP, Eshed I, Ostergaard M, Sorensen IJ, Moller JM, Madsen OR, et al. Enthesitis in patients with psoriatic arthritis, axial spondyloarthritis and healthy subjects assessed by ‘head-to-toe’ whole-body MRI and clinical examination. Ann Rheum Dis. 2014;74:823–9. doi: 10.1136/annrheumdis-2013-204239. [DOI] [PubMed] [Google Scholar]
- 72.Bandinelli F, Prignano F, Bonciani D, Bartoli F, Collaku L, Candelieri A, et al. Ultrasound detects occult entheseal involvement in early psoriatic arthritis independently of clinical features and psoriasis severity. Clin Exp Rheumatol. 2013;31:219–24. [PubMed] [Google Scholar]
- 73.Ruta S, Gutierrez M, Pena C, Garcia M, Arturi A, Filippucci E, et al. Prevalence of subclinical enthesopathy in patients with spondyloarthropathy: an ultrasound study. J Clin Rheumatol. 2011;17:18–22. doi: 10.1097/RHU.0b013e318204a6f8. [DOI] [PubMed] [Google Scholar]
- 74.Feydy A, Lavie-Brion MC, Gossec L, Lavie F, Guerini H, Nguyen C, et al. Comparative study of MRI and power Doppler ultrasonography of the heel in patients with spondyloarthritis with and without heel pain and in controls. Ann Rheum Dis. 2012;71:498–503. doi: 10.1136/annrheumdis-2011-200336. [DOI] [PubMed] [Google Scholar]
- 75.De Miguel E, Munoz-Fernandez S, Castillo C, Cobo-Ibanez T, Martin-Mola E. Diagnostic accuracy of enthesis ultrasound in the diagnosis of early spondyloarthritis. Ann Rheum Dis. 2011;70:434–9. doi: 10.1136/ard.2010.134965. [DOI] [PubMed] [Google Scholar]
- 76.De Miguel E, Falcao S, Castillo C, Plasencia C, Garcia M, Branco JC, et al. Enthesis erosion in spondyloarthritis is not a persistent structural lesion. Ann Rheum Dis. 2011;70:2008–10. doi: 10.1136/annrheumdis-2011-200352. [DOI] [PubMed] [Google Scholar]
- 77.Naredo E, Batlle-Gualda E, Garcia-Vivar ML, Garcia-Aparicio AM, Fernandez-Sueiro JL, Fernandez-Prada M, et al. Power Doppler ultrasonography assessment of entheses in spondyloarthropathies: response to therapy of entheseal abnormalities. J Rheumatol. 2010;37:2110–7. doi: 10.3899/jrheum.100136. [DOI] [PubMed] [Google Scholar]
- 78.D’Agostino MA, Breban M, Said-Nahal R, Dougados M. Refractory inflammatory heel pain in spondylarthropathy: a significant response to infliximab documented by ultrasound [letter] Arthritis Rheum. 2002;46:840–1. doi: 10.1002/art.513. [DOI] [PubMed] [Google Scholar]
- 79.Poddubnyy D, Rudwaleit M, Haibel H, Listing J, Marker-Hermann E, Zeidler H, et al. Effect of non-steroidal anti-inflammatory drugs on radiographic spinal progression in patients with axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Ann Rheum Dis. 2012;71:1616–22. doi: 10.1136/annrheumdis-2011-201252. [DOI] [PubMed] [Google Scholar]
- 80.Kroon F, Landewe R, Dougados M, van der Heijde D. Continuous NSAID use reverts the effects of inflammation on radiographic progression in patients with ankylosing spondylitis. Ann Rheum Dis. 2012;71:1623–9. doi: 10.1136/annrheumdis-2012-201370. [DOI] [PubMed] [Google Scholar]
- 81.Braun J, van den Berg R, Baraliakos X, Boehm H, Burgos-Vargas R, Collantes-Estevez E, et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis. 2011;70:896–904. doi: 10.1136/ard.2011.151027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marzo-Ortega H, McGonagle D, Jarrett S, Haugeberg G, Hensor E, O’Connor P, et al. Infliximab in combination with methotrexate in active ankylosing spondylitis: a clinical and imaging study. Ann Rheum Dis. 2005;64:1568–75. doi: 10.1136/ard.2004.022582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baraliakos X, Davis J, Tsuji W, Braun J. Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis before and after therapy with the tumor necrosis factor α receptor fusion protein etanercept. Arthritis Rheum. 2005;52:1216–23. doi: 10.1002/art.20977. [DOI] [PubMed] [Google Scholar]
- 84.Antoni C, Krueger GG, de Vlam K, Birbara C, Beutler A, Guzzo C, et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis. 2005;64:1150–7. doi: 10.1136/ard.2004.032268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Genovese MC, Mease PJ, Thomson GT, Kivitz AJ, Perdok RJ, Weinberg MA M02-570 Study Group. Safety and efficacy of adalimumab in treatment of patients with psoriatic arthritis who had failed disease modifying antirheumatic drug therapy [published erratum appears in J Rheumatol 2007;34:1439] J Rheumatol. 2007;34:1040–50. [PubMed] [Google Scholar]
- 86.Kavanaugh A, McInnes I, Mease P, Krueger GG, Gladman D, Gomez-Reino J, et al. Golimumab, a new human tumor necrosis factor α antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four–week efficacy and safety results of a randomized, placebo-controlled study [published erratum appears in Arthritis Rheum 2010;62:2555] Arthritis Rheum. 2009;60:976–86. doi: 10.1002/art.24403. [DOI] [PubMed] [Google Scholar]
- 87.Van der Heijde D, Schiff MH, Sieper J, Kivitz AJ, Wong RL, Kupper H, et al. Adalimumab effectiveness for the treatment of ankylosing spondylitis is maintained for up to 2 years: long-term results from the ATLAS trial. Ann Rheum Dis. 2009;68:922–9. doi: 10.1136/ard.2007.087270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Olivieri I, Giasi V, Scarano E, Gigliotti P, D’Angelo S, Padula A. A brief course of anti-TNF-α therapy can cure recurrent episodes of HLA-B27-associated severe and refractory heel enthesitis [letter] Clin Exp Rheumatol. 2009;27:1057. [PubMed] [Google Scholar]
- 89.Hreggvidsdottir HS, Noordenbos T, Baeten DL. Inflammatory pathways in spondyloarthritis. Mol Immunol. 2014;57:28–37. doi: 10.1016/j.molimm.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 90.McInnes IB, Kavanaugh A, Gottlieb AB, Puig L, Rahman P, Ritchlin C, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382:780–9. doi: 10.1016/S0140-6736(13)60594-2. [DOI] [PubMed] [Google Scholar]
- 91.McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, improves active psoriatic arthritis: 24-week efficacy and safety data from a phase 3 randomized, multicenter, double-blind, placebo-controlled study using subcutaneous dosing [abstract] Arthritis Rheumatol. 2014;66:3529. [Google Scholar]
- 92.Kavanaugh A, Mease PJ, Gomez-Reino JJ, Adebajo AO, Wollenhaupt J, Gladman DD, et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol. 2015;42:479–88. doi: 10.3899/jrheum.140647. [DOI] [PubMed] [Google Scholar]
- 93.Maksymowych WP, Lambert R, Jhangri GS, Leclercq S, Chiu P, Wong B, et al. Clinical and radiological amelioration of refractory peripheral spondyloarthritis by pulse intravenous pamidronate therapy. J Rheumatol. 2001;28:144–55. [PubMed] [Google Scholar]
- 94.Kingsley GH, Kowalczyk A, Taylor H, Ibrahim F, Packham JC, McHugh NJ, et al. A randomized placebo-controlled trial of methotrexate in psoriatic arthritis. Rheumatology (Oxford) 2012;51:1368–77. doi: 10.1093/rheumatology/kes001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kaltwasser JP, Nash P, Gladman D, Rosen CF, Behrens F, Jones P, et al. for the Treatment of Psoriatic Arthritis Study Group. Efficacy and safety of leflunomide in the treatment of psoriatic arthritis and psoriasis: a multinational, double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheum. 2004;50:1939–50. doi: 10.1002/art.20253. [DOI] [PubMed] [Google Scholar]
- 96.Clegg DO, Reda DJ, Mejias E, Cannon GW, Weisman MH, Taylor T, et al. Comparison of sulfasalazine and placebo in the treatment of psoriatic arthritis: a Department of Veterans Affairs Cooperative Study. Arthritis Rheum. 1996;39:2013–20. doi: 10.1002/art.1780391210. [DOI] [PubMed] [Google Scholar]
- 97.Song IH, Heldmann F, Rudwaleit M, Haibel H, Weiss A, Braun J, et al. Treatment of active ankylosing spondylitis with abatacept: an open-label, 24-week pilot study. Ann Rheum Dis. 2011;70:1108–10. doi: 10.1136/ard.2010.145946. [DOI] [PubMed] [Google Scholar]
- 98.Lekpa FK, Poulain C, Wendling D, Soubrier M, De Bandt M, Berthelot JM, et al. Is IL-6 an appropriate target to treat spondyloarthritis patients refractory to anti-TNF therapy? A multicentre retrospective observational study. Arthritis Res Ther. 2012;14:R53. doi: 10.1186/ar3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Song IH, Heldmann F, Rudwaleit M, Listing J, Appel H, Haug-Rost I, et al. One-year follow-up of ankylosing spondylitis patients responding to rituximab treatment and re-treated in case of a flare. Ann Rheum Dis. 2013;72:305–6. doi: 10.1136/annrheumdis-2012-201926. [DOI] [PubMed] [Google Scholar]
- 100.Wendling D, Dougados M, Berenbaum F, Brocq O, Schaeverbeke T, Mazieres B, et al. on behalf of the French Society of Rheumatology and the Club Rhumatismes et Inflammation. Rituximab treatment for spondyloarthritis. A nationwide series: data from the AIR registry of the French Society of Rheumatology. J Rheumatol. 2012;39:2327–31. doi: 10.3899/jrheum.120201. [DOI] [PubMed] [Google Scholar]