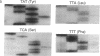
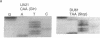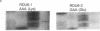Abstract
We previously showed that the preferred mutation induced by (+/-)-3 alpha,4 beta-dihydroxy-1 alpha,2 alpha-epoxy- 1,2,3,4-tetrahydrobenzo[c]phenanthrene (BcPHDE) in the dihydrofolate reductase gene in Chinese hamster ovary cells was a purine to thymine transversion on the nontranscribed strand at the sequence 5'-RRR-3' (R is a purine and the mutated base is underlined). To determine whether the observed mutational strand specificity was due to bias in the phenotypic selection, we designed a nonsense-codon reversion assay in which a triple purine target was present on both strands and all R----T transversion mutations yielded amino acid substitutions that were compatible with dihydrofolate reductase enzyme activity. From the size of the targets, a 2:1 ratio of mutations at the purines on the nontranscribed strand was expected if the DNA strands were mutationally equivalent. We isolated a total of 66 BcPHDE-induced revertants of two mutants that carry point mutations at either the 5' or the 3' end of the gene. All reversions at the 5' end arose by substitution on the nontranscribed strand; those at the 3' end showed a strand bias that favored this strand by 7:1. For both mutants, R----T transversions accounted for 88% of all the induced base changes. Thus, in this system, mutational strand bias is independent of the selection for phenotype. The results are consistent with the model of preferential repair of the transcribed strand as proposed by others. The involvement of RNA polymerase in the selective repair recruitment is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison L. A., Moyle M., Shales M., Ingles C. J. Extensive homology among the largest subunits of eukaryotic and prokaryotic RNA polymerases. Cell. 1985 Sep;42(2):599–610. doi: 10.1016/0092-8674(85)90117-5. [DOI] [PubMed] [Google Scholar]
- Armstrong J. D., Kunz B. A. Site and strand specificity of UVB mutagenesis in the SUP4-o gene of yeast. Proc Natl Acad Sci U S A. 1990 Nov;87(22):9005–9009. doi: 10.1073/pnas.87.22.9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigger C. A., Strandberg J., Yagi H., Jerina D. M., Dipple A. Mutagenic specificity of a potent carcinogen, benzo[c]phenanthrene (4R,3S)-dihydrodiol (2S,1R)-epoxide, which reacts with adenine and guanine in DNA. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2291–2295. doi: 10.1073/pnas.86.7.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A., Smith C. A., Okumoto D. S., Hanawalt P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985 Feb;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Carothers A. M., Grunberger D. DNA base changes in benzo[a]pyrene diol epoxide-induced dihydrofolate reductase mutants of Chinese hamster ovary cells. Carcinogenesis. 1990 Jan;11(1):189–192. doi: 10.1093/carcin/11.1.189. [DOI] [PubMed] [Google Scholar]
- Carothers A. M., Steigerwalt R. W., Urlaub G., Chasin L. A., Grunberger D. DNA base changes and RNA levels in N-acetoxy-2-acetylaminofluorene-induced dihydrofolate reductase mutants of Chinese hamster ovary cells. J Mol Biol. 1989 Aug 5;208(3):417–428. doi: 10.1016/0022-2836(89)90506-8. [DOI] [PubMed] [Google Scholar]
- Carothers A. M., Urlaub G., Mucha J., Grunberger D., Chasin L. A. Point mutation analysis in a mammalian gene: rapid preparation of total RNA, PCR amplification of cDNA, and Taq sequencing by a novel method. Biotechniques. 1989 May;7(5):494-6, 498-9. [PubMed] [Google Scholar]
- Carothers A. M., Urlaub G., Mucha J., Harvey R. G., Chasin L. A., Grunberger D. Splicing mutations in the CHO DHFR gene preferentially induced by (+/-)-3 alpha,4 beta-dihydroxy-1 alpha,2 alpha-epoxy-1,2,3,4- tetrahydrobenzo[c]phenanthrene. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5464–5468. doi: 10.1073/pnas.87.14.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. H., Maher V. M., McCormick J. J. Effect of excision repair by diploid human fibroblasts on the kinds and locations of mutations induced by (+/-)-7 beta,8 alpha-dihydroxy-9 alpha,10 alpha-epoxy-7,8,9,10- tetrahydrobenzo[a]pyrene in the coding region of the HPRT gene. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8680–8684. doi: 10.1073/pnas.87.21.8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiduschek E. P., Tocchini-Valentini G. P. Transcription by RNA polymerase III. Annu Rev Biochem. 1988;57:873–914. doi: 10.1146/annurev.bi.57.070188.004301. [DOI] [PubMed] [Google Scholar]
- Ho L., Bohr V. A., Hanawalt P. C. Demethylation enhances removal of pyrimidine dimers from the overall genome and from specific DNA sequences in Chinese hamster ovary cells. Mol Cell Biol. 1989 Apr;9(4):1594–1603. doi: 10.1128/mcb.9.4.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziej P. A., Woychik N., Liao S. M., Young R. A. RNA polymerase II subunit composition, stoichiometry, and phosphorylation. Mol Cell Biol. 1990 May;10(5):1915–1920. doi: 10.1128/mcb.10.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhani H. D., Bohr V. A., Hanawalt P. C. Differential DNA repair in transcriptionally active and inactive proto-oncogenes: c-abl and c-mos. Cell. 1986 May 9;45(3):417–423. doi: 10.1016/0092-8674(86)90327-2. [DOI] [PubMed] [Google Scholar]
- Melera P. W., Davide J. P., Hession C. A., Scotto K. W. Phenotypic expression in Escherichia coli and nucleotide sequence of two Chinese hamster lung cell cDNAs encoding different dihydrofolate reductases. Mol Cell Biol. 1984 Jan;4(1):38–48. doi: 10.1128/mcb.4.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon I., Hanawalt P. C. Induction of the Escherichia coli lactose operon selectively increases repair of its transcribed DNA strand. Nature. 1989 Nov 2;342(6245):95–98. doi: 10.1038/342095a0. [DOI] [PubMed] [Google Scholar]
- Mellon I., Spivak G., Hanawalt P. C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987 Oct 23;51(2):241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet M. L., Young R. A. Intragenic and extragenic suppressors of mutations in the heptapeptide repeat domain of Saccharomyces cerevisiae RNA polymerase II. Genetics. 1989 Dec;123(4):715–724. doi: 10.1093/genetics/123.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet M., Scafe C., Sexton J., Young R. Eucaryotic RNA polymerase conditional mutant that rapidly ceases mRNA synthesis. Mol Cell Biol. 1987 May;7(5):1602–1611. doi: 10.1128/mcb.7.5.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruess-Schwartz D., Baird W. M., Yagi H., Jerina D. M., Pigott M. A., Dipple A. Stereochemical specificity in the metabolic activation of benzo(c)phenanthrene to metabolites that covalently bind to DNA in rodent embryo cell cultures. Cancer Res. 1987 Aug 1;47(15):4032–4037. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sancar G. B., Smith F. W. Interactions between yeast photolyase and nucleotide excision repair proteins in Saccharomyces cerevisiae and Escherichia coli. Mol Cell Biol. 1989 Nov;9(11):4767–4776. doi: 10.1128/mcb.9.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scafe C., Martin C., Nonet M., Podos S., Okamura S., Young R. A. Conditional mutations occur predominantly in highly conserved residues of RNA polymerase II subunits. Mol Cell Biol. 1990 Mar;10(3):1270–1275. doi: 10.1128/mcb.10.3.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scafe C., Nonet M., Young R. A. RNA polymerase II mutants defective in transcription of a subset of genes. Mol Cell Biol. 1990 Mar;10(3):1010–1016. doi: 10.1128/mcb.10.3.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scicchitano D. A., Hanawalt P. C. Repair of N-methylpurines in specific DNA sequences in Chinese hamster ovary cells: absence of strand specificity in the dihydrofolate reductase gene. Proc Natl Acad Sci U S A. 1989 May;86(9):3050–3054. doi: 10.1073/pnas.86.9.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby C. P., Sancar A. Transcription preferentially inhibits nucleotide excision repair of the template DNA strand in vitro. J Biol Chem. 1990 Dec 5;265(34):21330–21336. [PubMed] [Google Scholar]
- Sentenac A. Eukaryotic RNA polymerases. CRC Crit Rev Biochem. 1985;18(1):31–90. doi: 10.3109/10409238509082539. [DOI] [PubMed] [Google Scholar]
- Smerdon M. J., Thoma F. Site-specific DNA repair at the nucleosome level in a yeast minichromosome. Cell. 1990 May 18;61(4):675–684. doi: 10.1016/0092-8674(90)90479-x. [DOI] [PubMed] [Google Scholar]
- Stillman D. J., Better M., Geiduschek E. P. Electron-microscopic examination of the binding of a large RNA polymerase III transcription factor to a tRNA gene. J Mol Biol. 1985 Sep 20;185(2):451–455. doi: 10.1016/0022-2836(85)90417-6. [DOI] [PubMed] [Google Scholar]
- Urlaub G., McDowell J., Chasin L. A. Use of fluorescence-activated cell sorter to isolate mutant mammalian cells deficient in an internal protein, dihydrofolate reductase. Somat Cell Mol Genet. 1985 Jan;11(1):71–77. doi: 10.1007/BF01534736. [DOI] [PubMed] [Google Scholar]
- Urlaub G., Mitchell P. J., Ciudad C. J., Chasin L. A. Nonsense mutations in the dihydrofolate reductase gene affect RNA processing. Mol Cell Biol. 1989 Jul;9(7):2868–2880. doi: 10.1128/mcb.9.7.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos J. M., Wauthier E. L. Differential introduction of DNA damage and repair in mammalian genes transcribed by RNA polymerases I and II. Mol Cell Biol. 1991 Apr;11(4):2245–2252. doi: 10.1128/mcb.11.4.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieling H., Van Rooijen M. L., Groen N. A., Zdzienicka M. Z., Simons J. W., Lohman P. H., van Zeeland A. A. DNA strand specificity for UV-induced mutations in mammalian cells. Mol Cell Biol. 1989 Mar;9(3):1277–1283. doi: 10.1128/mcb.9.3.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe A. P., Jordan E., Brown D. D. A bacteriophage RNA polymerase transcribes through a Xenopus 5S RNA gene transcription complex without disrupting it. Cell. 1986 Feb 14;44(3):381–389. doi: 10.1016/0092-8674(86)90459-9. [DOI] [PubMed] [Google Scholar]