Abstract
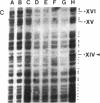
The genomic sequencing technique has been applied to assess the state of methylation in the DNA from human leukocyte subpopulations from healthy individuals and in the DNA from several individuals with myeloid or lymphatic leukemias or non-Hodgkin lymphomas. Leukocyte populations were purified by the high-gradient magnetic cell sorting technique. In the human tumor necrosis factor alpha (TNF-alpha) gene segment between nucleotides 300 and 1150, the specific methylation profile in the DNA from human granulocytes and monocytes is maintained in three cases of myeloid leukemia. In one such case, all 5-methyl-2'-deoxycytidine residues have been replaced by cytidine. In a chronic lymphatic T-cell leukemia, all 5-methyl-2'-deoxycytidine residues have been substituted by cytidine. In normal B lymphocytes, in two cases of chronic lymphatic B-cell leukemias and two cases of non-Hodgkin lymphomas, all 5'-CG-3' sequences in this gene segment are devoid of methylation. In the TNF-beta gene, DNA methylation is decreased in several examples of acute or chronic myeloid leukemias in comparison to normal human granulocytes or monocytes, whose DNA is almost completely methylated between nucleotides 700 and 900. In human T and B lymphocytes, the main producers of TNF-beta, in three instances of chronic lymphatic leukemias and two cases of non-Hodgkin lymphomas, all 5'-CG-3' sequences are unmethylated in this region. The DNA from the human HeLa cell line is highly methylated at all 5'-CG-3' sequences in the TNF-alpha and -beta genes. The TNF-alpha gene is transcribed in the cells of one case of acute myeloid leukemia in which the analyzed region of the TNF-alpha gene is completely unmethylated. The TNF-beta gene is not transcribed in any of the malignant cells tested.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandler L. A., DeClerck Y. A., Bogenmann E., Jones P. A. Patterns of DNA methylation and gene expression in human tumor cell lines. Cancer Res. 1986 Jun;46(6):2944–2949. [PubMed] [Google Scholar]
- Chandler L. A., Ghazi H., Jones P. A., Boukamp P., Fusenig N. E. Allele-specific methylation of the human c-Ha-ras-1 gene. Cell. 1987 Aug 28;50(5):711–717. doi: 10.1016/0092-8674(87)90329-1. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuturi M. C., Murphy M., Costa-Giomi M. P., Weinmann R., Perussia B., Trinchieri G. Independent regulation of tumor necrosis factor and lymphotoxin production by human peripheral blood lymphocytes. J Exp Med. 1987 Jun 1;165(6):1581–1594. doi: 10.1084/jem.165.6.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Gehrke C. W., Kuo K. C., Ehrlich M. Reduced genomic 5-methylcytosine content in human colonic neoplasia. Cancer Res. 1988 Mar 1;48(5):1159–1161. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983 Jan 6;301(5895):89–92. doi: 10.1038/301089a0. [DOI] [PubMed] [Google Scholar]
- Flatau E., Bogenmann E., Jones P. A. Variable 5-methylcytosine levels in human tumor cell lines and fresh pediatric tumor explants. Cancer Res. 1983 Oct;43(10):4901–4905. [PubMed] [Google Scholar]
- Gama-Sosa M. A., Slagel V. A., Trewyn R. W., Oxenhandler R., Kuo K. C., Gehrke C. W., Ehrlich M. The 5-methylcytosine content of DNA from human tumors. Nucleic Acids Res. 1983 Oct 11;11(19):6883–6894. doi: 10.1093/nar/11.19.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelz S. E., Vogelstein B., Hamilton S. R., Feinberg A. P. Hypomethylation of DNA from benign and malignant human colon neoplasms. Science. 1985 Apr 12;228(4696):187–190. doi: 10.1126/science.2579435. [DOI] [PubMed] [Google Scholar]
- Goldman J. M. Leucocyte separation and transfusion. Br J Haematol. 1974 Nov;28(3):271–275. doi: 10.1111/j.1365-2141.1974.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Kochanek S., Toth M., Dehmel A., Renz D., Doerfler W. Interindividual concordance of methylation profiles in human genes for tumor necrosis factors alpha and beta. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8830–8834. doi: 10.1073/pnas.87.22.8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltenyi S., Müller W., Weichel W., Radbruch A. High gradient magnetic cell separation with MACS. Cytometry. 1990;11(2):231–238. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- Nedospasov S. A., Shakhov A. N., Turetskaya R. L., Mett V. A., Azizov M. M., Georgiev G. P., Korobko V. G., Dobrynin V. N., Filippov S. A., Bystrov N. S. Tandem arrangement of genes coding for tumor necrosis factor (TNF-alpha) and lymphotoxin (TNF-beta) in the human genome. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):611–624. doi: 10.1101/sqb.1986.051.01.073. [DOI] [PubMed] [Google Scholar]
- Nedwin G. E., Naylor S. L., Sakaguchi A. Y., Smith D., Jarrett-Nedwin J., Pennica D., Goeddel D. V., Gray P. W. Human lymphotoxin and tumor necrosis factor genes: structure, homology and chromosomal localization. Nucleic Acids Res. 1985 Sep 11;13(17):6361–6373. doi: 10.1093/nar/13.17.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A. J., White R. Inheritance of allelic blueprints for methylation patterns. Cell. 1988 Jul 15;54(2):145–152. doi: 10.1016/0092-8674(88)90546-6. [DOI] [PubMed] [Google Scholar]
- Steffen M., Ottmann O. G., Moore M. A. Simultaneous production of tumor necrosis factor-alpha and lymphotoxin by normal T cells after induction with IL-2 and anti-T3. J Immunol. 1988 Apr 15;140(8):2621–2624. [PubMed] [Google Scholar]
- Sung S. S., Jung L. K., Walters J. A., Chen W., Wang C. Y., Fu S. M. Production of tumor necrosis factor/cachectin by human B cell lines and tonsillar B cells. J Exp Med. 1988 Nov 1;168(5):1539–1551. doi: 10.1084/jem.168.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter D., Westphal M., Doerfler W. Patterns of integration of viral DNA sequences in the genomes of adenovirus type 12-transformed hamster cells. Cell. 1978 Jul;14(3):569–585. doi: 10.1016/0092-8674(78)90243-x. [DOI] [PubMed] [Google Scholar]
- Toth M., Lichtenberg U., Doerfler W. Genomic sequencing reveals a 5-methylcytosine-free domain in active promoters and the spreading of preimposed methylation patterns. Proc Natl Acad Sci U S A. 1989 May;86(10):3728–3732. doi: 10.1073/pnas.86.10.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth M., Müller U., Doerfler W. Establishment of de novo DNA methylation patterns. Transcription factor binding and deoxycytidine methylation at CpG and non-CpG sequences in an integrated adenovirus promoter. J Mol Biol. 1990 Aug 5;214(3):673–683. doi: 10.1016/0022-2836(90)90285-T. [DOI] [PubMed] [Google Scholar]
- de Bustros A., Nelkin B. D., Silverman A., Ehrlich G., Poiesz B., Baylin S. B. The short arm of chromosome 11 is a "hot spot" for hypermethylation in human neoplasia. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5693–5697. doi: 10.1073/pnas.85.15.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]