Abstract
Chronic stress induces changes in neuronal functions in specific brain regions regulating sociability and mood-related behaviors. Recently we reported that stress-induced persistent upregulation of the neuropeptides orexin and melanin-concentrating hormone (MCH) in the basolateral amygdala (BLA) and the resulting activation of orexin receptors or MCH receptors within the BLA produced deficits in sociability and mood-related behaviors. In the present study, we investigated the neural targets that were innervated by BLA neurons containing orexin receptors or MCH receptors. The viral vector system AAV2-CaMKII-ChR2-eYFP was injected into the BLA to trace the axonal tracts of BLA neurons. This axon labeling analysis led us to identify the prelimbic and infralimbic cortices, nucleus accumbens (NAc), dorsal striatum, paraventricular nucleus (PVN), interstitial nucleus of the posterior limb of the anterior commissure, habenula, CA3 pyramidal neurons, central amygdala, and ventral hippocampus as the neuroanatomical sites receiving synaptic inputs of BLA neurons. Focusing on these regions, we then carried out stimulus-dependent c-Fos induction analysis after activating orexin receptors or MCH receptors of BLA neurons. Stereotaxic injection of an orexin receptor agonist or an MCH receptor agonist in the BLA induced c-Fos expression in the NAc, PVN, central amygdala, ventral hippocampus, lateral habenula and lateral hypothalamus, which are all potentially important for depression-related behaviors. Among these neural correlates, the NAc, PVN and central amygdala were strongly activated by stimulation of orexin receptors or MCH receptors in the BLA, whereas other BLA targets were differentially and weakly activated. These results identify a functional connectivity of BLA neurons regulated by orexin and MCH receptor systems in sociability and mood-related behaviors.
Keywords: BLA, Depression, Orexin, MCH, c-Fos
INTRODUCTION
Chronic stress alters various functional connectivities in the brain leading to impairments in sociability and mood-related behaviors [1,2]. An important neuroanatomical site closely linked to stress-induced changes in sociability and mood-related behaviors is the amygdala. The amygdala is hyperactivated in response to fear or anxiety-inducing stressors [3]. The amygdala needs to form short- or long-range functional connections with other brain regions in order to regulate behaviors. The amygdala establishes descending connectivity with various brain regions, including the prefrontal cortex, nucleus accumbens, hippocampus, dorsal raphe nucleus, and ventral tegmental area [4,5]. Delineation of functional connectivities of the amygdala in chronic stress responses is necessary to understand the neural mechanism(s) underlying stress-induced impairments in sociability and moodrelated behaviors.
Orexins A and B (also known as hypocretins 1 and 2) are neuropeptides that consist of 33 and 28 amino acids, respectively, both of which are derived from preproorexin [6,7]. Orexin neurons are mainly located in the lateral hypothalamus, from which they send axons to various regions of the brain [6,8]. The hypothalamic orexins play a key role in arousal, and the loss of the orexin gene function or orexin-producing neurons causes narcolepsy in humans and rodents [9]. Orexins produces these effects through the Gq protein-coupled receptors, orexin-1 and orexin-2 receptors, which are widely expressed in the brain [9,10]. Melanin-concentrating hormone (MCH) is a cyclic neuropeptide that consists of 19 amino acids, derived from a 165-amino acid preprohormone encoded by the Pmch gene. MCH exerts its effects through MCHR1 and MCHR2 receptors, which are also G protein-coupled receptors [11,12]. MCH neurons are distributed adjacent to orexin neurons in the lateral hypothalamus, and MCH neurons also project throughout the brain [13,14]. MCH is known to increase feeding [15, 16] and regulate motivation behavior [17]. Orexin and MCH transcripts are detected at low expression levels in many other brain regions including the hippocampus and amygdala [18,19,20], although the physiological significance of this expression is not clearly understood.
Recently, it was reported that chronic stress in mice produced depressive behaviors through the increased expression of orexin and MCH in the BLA. Moreover, stereotaxic injection of either orexin or MCH peptides within the BLA induced depression-like behaviors. In contrast, siRNA-mediated suppression of either orexin, MCH, or their receptors in the BLA blocked stress-induced depression-like behaviors [21]. Stress-induced upregulation of MCH in the BLA and hippocampus was shown to be involved in stress-induced anxiety-like behaviors [22]. These results suggest that BLA neurons bearing orexin and/or MCH receptor systems are important for regulating anxiety and depressive behaviors. These results also raise a question regarding which neural targets are regulated by the BLA neurons carrying orexin or MCH receptor systems. In the present study, we demonstrate that BLA neurons carrying orexin or MCH receptors in the BLA are functionally connected to the NAc, PVN and central amygdala in the brain.
MATERIALS AND METHODS
Animals
Male 7-week-old C57BL6 mice were purchased from Daehan BioLink (Eumsung, Chungbuk, Republic of Korea). All mice were housed in pairs in a standard clear plastic cage in a temperature(23~24℃)- and humidity (50~60%)-controlled environment under a 12 h light/dark cycle. All animals were handled in accordance with the animal care guidelines of Ewha Womans University (IACUC 2013-01-007).
Chronic restraint stress
Mice were treated with restraints as described previously [21,23]. In brief, mice were individually put in a well-ventilated, 50-ml polypropylene conical tube, were placed toward the conical side by pressure with a cut piece of a 15-ml conical tube, and were restrained for 2 h daily starting from 10 a.m. After each session of restraint, they were returned to their home cages with free access to food and water. This procedure was repeated each day for 14 days or the indicated time.
Stereotaxic injection of peptides
Stereotaxic injection of orexin or MCH peptides was performed as described previously [21]. For stereotaxic injection, mice were anesthetized with 1.2% isoflurane, and orexin A (0.53 ng, 300 nM) or MCH peptide (0.12 ng, 100 nM) at a volume of 0.5 µl was injected, using a Hamilton syringe (10 µl syringe; 1701 model; #80030) with a 26s gauge needle and a 30 gauge tubing (OD, 0.3 mm; ID, 0.15 mm; volume, 0.18 µl/cm), into the BLA on both sides (stereotaxic coordinates: AP, -1.4; ML, ±3.0; DV, -4.8 mm). After 50 min of injection, mice were perfused with 4% paraformaldehyde via a transcardiac method, and brains were prepared for histological analysis. Orexin peptide (orexin A, an endogenous peptide agonist at orexin receptors; #1455) and MCH peptide (an endogenous peptide agonist at MCH receptors; #3806) were purchased from Tocris Bioscience (Bristol, U.K.). The sequence of orexin peptide was XPLPDCCRQKTCSCRLYELLHGAGN HAAGILTL (modifications: X=Glp, disulfide bridge between 6 and 12, and 7 and 14 residues, Leu-33=C-terminal amide), and that of MCH peptide was DFDMLRCMLGRVYRPCWQV (modification: disulfide bridge between 7~16 residues). Orexin and MCH peptides were resolved in 0.9% saline.
Stereotaxic injection of AAV2-CaMKII-ChR2-eYFP
Stereotaxic injection of AAV2-CaMKII-ChR2-eYFP was performed as described previously [24]. Adeno-associated virus (AAV) carrying the channel rhodopsin-2-eYFP fusion gene driven by the CaMKII promoter was purchased from the University of Pennsylvania Vector Core (AV-1-26969P, 1.15×1013 viral particles/ml; UPenn, Philadelphia, PA, USA). For stereotaxic injection, mice were anesthetized with ketamine hydrochloride and xylazine hydrochloride. AAV2-CaMKII-ChR2-eYFP (1.15×1012 viral particles/ml) was stereotaxically injected into the right BLA (AP, -1.4; ML, +3.0; DV, -4.8 mm) at a volume of 0.5 µl and a speed of 0.2 µl/min using a 30-G needle. After 18 days of injection, mice were sacrificed, followed by perfusion with 4% paraformaldehyde via a trans-cardiac method, and brains were prepared. The brains were coronally cut into 40-µm-thick sections with a vibratome (Leica VT 1000S; Leica Instruments, Nussloch, Germany). Fluorescent images were analyzed using an Olympus BX 51 microscope equipped with the X-cite 120 fluorescence illuminator (EXFO Life Science & Industrial Division, Ontario, Canada), a DP71 camera (Olympus), and MetaMorph Microscopy Automation & Image Analysis software (Molecular Devices, Sunnyvale, CA, USA).
Immunohistochemistry
Mice were perfused with 4% paraformaldehyde via a transcardiac method, and isolated brains were post-fixed in the same solution at 4℃ overnight. Brains were coronally cut into 40-µm-thick sections using a vibratome (Leica VT 1000S; Leica Instruments, Nussloch, Germany). Floating sections were blocked with 4% bovine serum albumin in PBS containing 0.1% Triton X-100 (PBST) for 1 h, and then reacted with anti-c-Fos antibody (sc-52, Santa Cruz Biotechnology, Santa Cruz, CA, USA), antiorexin receptor1/2 antibody (Santa Cruz Biotechnology), or anti- MCH receptor-1 antibody (Thermo Fisher Scientific, Pittsburgh, PA, USA) at 4℃ overnight. All sections were washed with PBST 3 times for 20 min each and reacted with anti-rabbit IgG (BA-1000, Vector Laboratories, Burlingame, CA, USA) diluted 1:200 in PBST for 1 h. Signals were visualized using an ABC Elite kit (PK-6200, Vector Laboratories). c-Fos DAB images were analyzed using an Olympus BX 51 microscope equipped with a DP71 camera and MetaMorph Microscopy Automation & Image Analysis software (Molecular Devices, Sunnyvale, CA, USA). Brain sections were prepared from 6~7 animals 50 min after orexin or MCH injection.
The levels of c-Fos expression in specific brain regions were quantified using a 5-point rating scale, as described previously [22,25]. c-Fos levels were assigned a numerical grade of 0~5, where 0 was lowest and +5 was highest. Specifically, the numerical grade of 0 was assigned for 0~50 c-Fos-positive cells/mm2 in a given area; +1 was for 51~100 c-Fos-positive cells/mm2; +2 was for 101~200 c-Fos-positive cells/mm2; +3 was for 201~350 c-Fos-positive cells/mm2; +4 was for 351~600 c-Fos-positive cells/mm2; and +5 was for >600 c-Fos-positive cells/mm2. For the hippocampus (the pyramidal cell layer and granule cell layer) and PVN, which showed high cell densities, c-Fos levels were assigned a numerical grade of 0~5 in regard to the relative fraction of c-Fos-positive cells among the total cells, where +1 was lowest, and +5 was highest. Average scores of c-Fos level in each region were rounded to the nearest tenth decimal.
Real-time PCR analysis
Real-time PCR was carried out as described previously [21]. Total RNA was purified from the basolateral amygdala using TRIzol reagent (Life Technologies, Carlsbad, CA, USA) and treated with DNase I to avoid genomic DNA contamination. Reverse transcription of total RNA (2 µg) was carried out using a reverse transcription system (Promega, MO, USA) in a volume of 20 µl. Real-time PCR was performed with a mix of 10 µl of 2X iQTM SYBR Green Supermix (Bio-Rad Laboratories, Foster City, CA, USA), 1 µl each of 5 pmol/µl forward and reverse primers, and 4 µl of cDNA (1/8 dilution of the convert) in a total volume of 20 µl using the CFX 96 Real-Time PCR System Detector (Bio-Rad Laboratories; Foster City, CA, USA). The primer sets used were 5'-TTGGACCACTGCACTGAAGA-3' and 5'-CCCAGGGAACCTTTGTAGAAG-3' for orexin, 5'-TGAACG ATGATGACAATAAGAA-3' and 5'- TCAGAGCGAGGTAAGGTT-3' for MCH, 5'-AGAAGGTGGTGAAGCAGGCATC-3' and 5'-CGA AGGTGGAAGAGTGGGAGTTG-3' for GAPDH and 5'-GCTGCC ATCTGTTTTACGG-3' and 5'-TGACTGGTGCCTGATGAACT-3' for L32.
Sociability test
The sociability test was performed as described previously [23]. At all times, the testing room was masked with 65 dB white noise and was lit by 20 lux through indirect illumination. Mice were brought to the testing room 30 min prior to the start of the behavioral test. In brief, the sociability test field consisted of a U-shaped open field (45×45×40 cm) with a portioning wall at the central point, so that each open field had closed and open squares on both sides. The open field was made of a cream-colored FOAMAX (Expanded PVC; LG Ltd., Korea) chamber. On the test day, the subject mouse was allowed to explore the U-shaped field for 5 min, and the locomotive track and the time spent in each field were recorded. This procedure was regarded as habituation to the test field. While the subject mouse was returned to its home cage for 2 min, a social target (a 10~12-week-old male B6 mouse) was put in the grid enclosure (12 cm in diameter×33 cm in height) and positioned in a closed square, and an empty grid enclosure was placed on the opposite side of the U-shaped field. Then, the subject mouse was allowed to freely explore the U-shaped field for 5 min, and the time spent in each field and locomotive track were recorded. The closed square containing a social target in the grid enclosure was defined as the target-zone, whereas the closed square containing an unanimated grid enclosure was defined as the non-target zone. The locomotive track and the time spent in each field were recorded using a video tracking system (SMART; Panlab S.I., Barcelona, Spain).
Tail suspension test
The tail suspension test was performed as previously described [23]. The test room was illuminated with indirect illumination of 250 lx. In brief, mice were individually suspended 50 cm above the surface of a table for 6 min by fixing their tails using adhesive tape onto the ceiling of a white box. During the 6-min period, the summed immobility time was recorded. Immobility was defined as the total time during which all limbs and body were motionless.
Forced swim test
The forced swim test was performed as previously described [23]. In brief, mice were placed in a transparent Plexiglass cylinder (height: 27 cm, diameter: 15 cm) containing water (a depth of 15 cm) at 24℃ for 6 min. The cumulative immobility time for the last 5 min was counted. Immobility was defined as floating with all limbs motionless. The test room was illuminated with 250 lx.
Marble burying test
The marble burying test was carried out as described previously [26]. Empty home cages were filled with smooth bedding (JRS 3-4; J. Rettenmaier & Söhne, Rosenberg, Germany) to a height of 5 cm from the cage floor. Identical glass marbles (diameter: 1.5 cm) were evenly placed on top of the bedding throughout the cage. Mice were individually allowed to freely explore the cage for 30 min, and afterward, the number of successfully buried marbles was counted. Marble "burying" was defined as less than 25% of a marble being visible.
Statistical analysis
Two-sample comparisons were carried out using the Student's t-test, and multiple comparisons were performed using one-way ANOVA and the Newman-Keuls post hoc test. GraphPad PRISM 6 software (GraphPad Software. Inc., San Diego, CA, USA) was used to perform statistical analyses. All data are presented as mean±S.E.M. and statistical difference was accepted at the 5% level unless otherwise indicated.
RESULTS
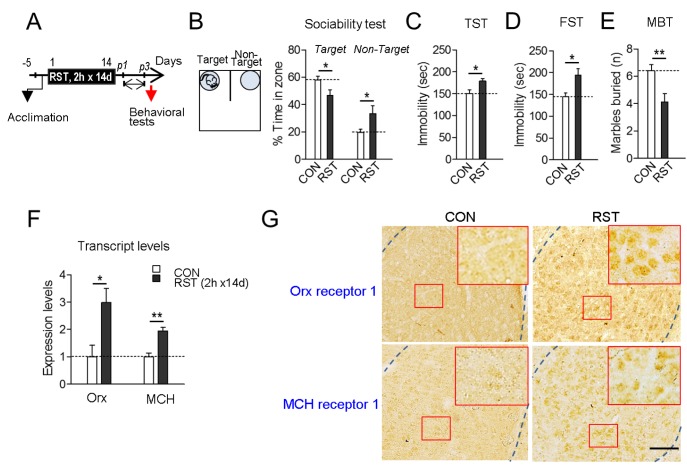
Mice subjected to restraint for 2 h daily for 14 days (2 h×14 d RST) exhibited reduced sociability in the sociability test, increased immobility in the FST and TST, and reduced numbers of buried marbles in the marble-burying test (Fig. 1A~E). These stressevoked mice had increased expression of orexin and MCH in the BLA (Fig. 1F), which was consistent with previous reports [21,27]. We also confirmed that orexin receptors and MCH receptors were also expressed in the BLA (Fig. 1G).
Fig. 1. Chronic restraint stress produced deficits in sociability and mood-related behaviors, and led to upregulation of orexin and MCH in the BLA. (A) Experimental design for treatment of mice with restraints for 2 h daily for 14 days (2 h×14 d RST) and subsequent behavioral tests on post-stress days 1~3 (p1~p3). Arrow: time point for tissue preparation. (B) Social interaction levels in the U-shaped field assay. The U-shaped field with a target mouse placed in a grid cage on one side and an unanimated cage on the other side is depicted (left panel). Social interaction levels were quantified by % time spent in the target and non-target zones for 5 min. The target and non-target zones correspond to the closed squares of the U-field. (C, D) Immobility time in the TST (C) and FST (D). (E) The number of marbles buried in a 30-min session of free exploration. (F) Real-time PCR data showing stressinduced up-regulation of orexin and MCH transcripts in the basolateral amygdala (BLA). n=6 animals, with 6 repeats of PCR. (G) Photomicrographs showing immunohistochemical staining of orexin receptor-1 (top panels) and MCH receptor-1 (bottom panels) in the BLA of mice treated with 2 h×14 d RST (RST) and their controls (CON). High magnifications (inset) of the squared regions (red box) in the BLA are presented. Scale bar, 200 µm. Data are presented as the mean±SEM. * and ** denote differences from the control at p<0.05 and p<0.01, respectively (for behaviors, n=7 animals for each group).
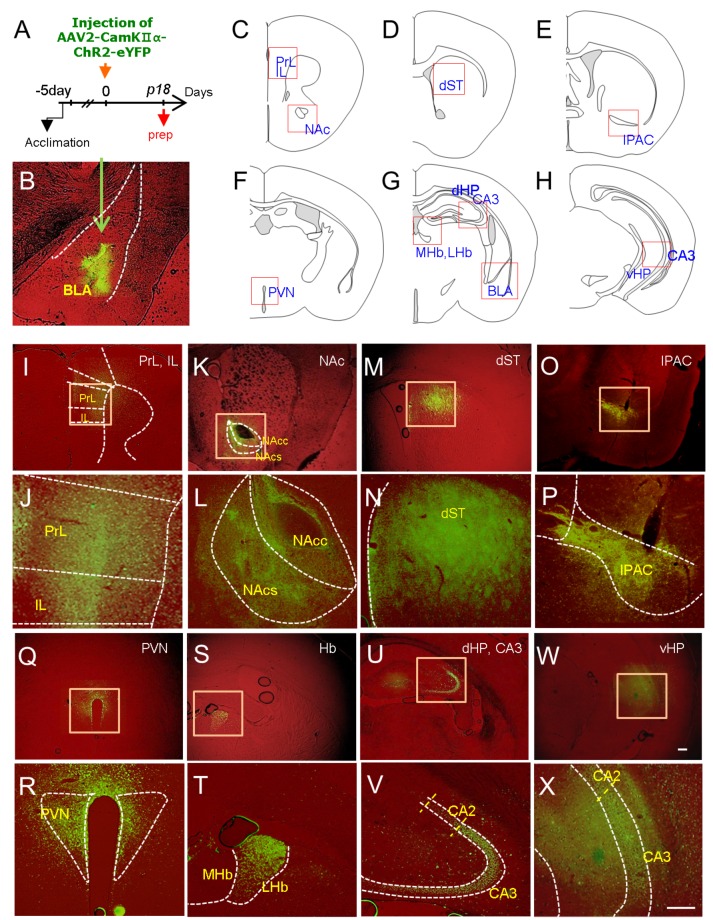
Next, we attempted to visualize the brain regions that were innervated by BLA neurons using the viral vector system AAV2-CaMKII-ChR2-eYFP, which allowed us to label axonal projections of infected neurons due to the expression of eYFP, as demonstrated previously [24]. Eighteen days after the stereotaxic injection of AAV2-CaMKII-ChR2-eYFP into the BLA, mice were sacrificed and axonal tracts of BLA neurons labeled by eYFP were analyzed in brain sections (Fig. 2A and B). This analysis identified the medial prefrontal cortex (prelimbic and infralimbic cortices), NAc (core and shell), dorsal striatum, PVN, medial and lateral habenula, interstitial nucleus of the posterior limb of the anterior commissure (IPAC), CA3 pyramidal neurons, ventral hippocampus, BLA, and central amygdala (CeA) as the neural targets that received synaptic inputs from BLA neurons (Fig. 2C~X). The BLA target regions identified by this analysis in part overlapped with the BLA neural targets inferred from Allen Lab brain image collections (http://connectivity.brain-map.org/projection/experiment/277710753; http://connectivity.brain-map. org/projection/experiment/113144533). The BLA neural targets read from Allen Lab brain mapping image collections were the mPFCX, dorsal striatum, CPu, NAc, VP, and CeA.
Fig. 2. Visualization of brain regions targeted by BLA neurons using the AAV2-CaMKII-ChR2-eYFP vector system. (A) Experimental design for stereotaxic injection of AAV2-CaMKIIα-ChR2-eYFP in the BLA and time point for tissue preparation (prep). AAV2-CaMKIIα-ChR2-eYFP (1.15×1012 viral particles/ml) was injected into the right BLA, and eYFP expression was examined 18 days after injection. Six animals were analyzed using this procedure, and brains with mislocalized injections in the BLA were excluded. (B-X) eYFP fluorescence images labeling the BLA (B, injection site), medial prefrontal cortex (PrL and IL; C, I, J), nucleus accumbens, core and shell (NAc-C and NAc-Sh; C , K, L), dorsal striatum (dST; D, M, N), interstitial nucleus of the posterior limb of the anterior commissure (IPAC; E, O, P), paraventricular nucleus of the hypothalamus (PVN; F, Q, R), medial and lateral habenula (MHb, LHb; G, S, T), CA3 of the dorsal hippocampus (dHP; G, U, V), and CA3 of the ventral hippocampus (vHP; H, W, X). The areas examined and presented with eYFP fluorescence images are presented on the diagrams (C-H). Higher magnifications of squared boxes in each region are presented (J, L, N, P, R, T, V and X). Scale bars, 200 µm.
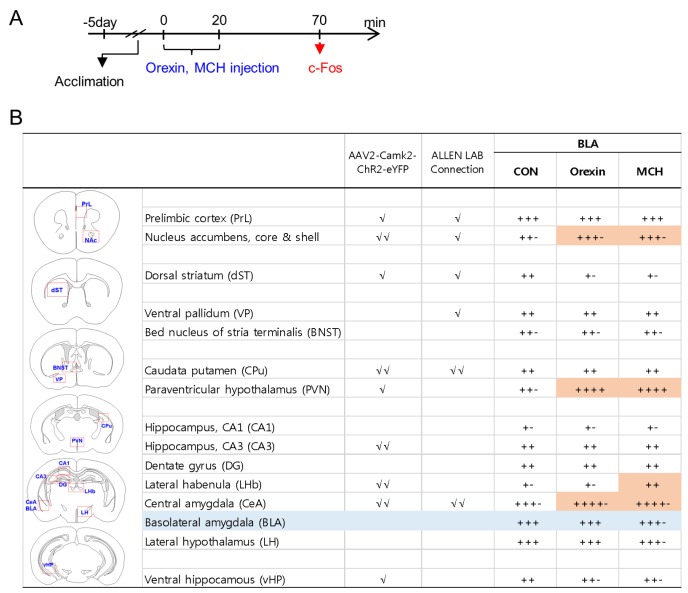
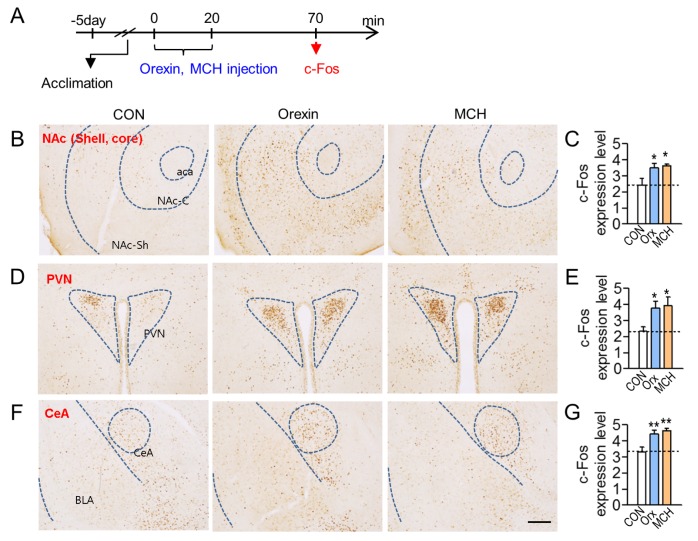
Recently, we reported that depressive-like behaviors were induced 50 min after injection of orexin or MCH peptides in the BLA [21]. To identify the neural targets that were stimulated by BLA neurons containing orexin receptors or MCH receptors, we injected orexin or MCH peptides stereotaxically into the BLA. Fifty minutes after the injection of each peptide, mice were sacrificed, and orexin- or MCH–induced c-Fos expression levels were analyzed with a focus on the mapped target regions of BLA neurons (Fig. 3A and 4A). The injection of orexin or MCH peptides induced c-Fos expression in the identified neural targets of BLA neurons relative to the vehicle-injected control. These regions were the NAc, PVN and central amygdala, ventral hippocampus, lateral habenula and lateral hypothalamus (Fig. 3B). Among these regions, the NAc, PVN and central amygdala were identified as the brain regions that were commonly and strongly activated after stimulating orexin receptors or MCH receptors in the BLA (Fig. 3 and 4).
Fig. 3. c-Fos induction profiles in various brain regions following the stimulation of orexin receptors or MCH receptors in the BLA. (A) Experimental design for stereotaxic injection with orexin peptide (0.53 ng/each side) or MCH peptide (0.12 ng/each side) in the BLA, and the time point for tissue preparation (arrow). (B) Orexin- or MCH-induced c-Fos expression levels were quantified in brain sections from 6~7 animals using a 5-point rating system, and the average value for each region is presented. BLA target sites were classified as the region(s) receiving moderate (√) or extensive (√√) projections from the BLA on the basis of eYFP expression levels (AAV2-CaMKIIα-ChR2-eYFP tracking data in as seen Fig. 2, and eGFP expression levels from the Allen Lab mouse brain mapping collections (experiments #277710753 and #113144533). The areas counted for c-Fos expression in each region are marked with a red square on the diagrams. Orange colors on the table represent differences greater than an 1-scale value in c-Fos induction levels between the control and injection groups.
Fig. 4. Representative photomicrographs showing c-Fos induction in the brain regions that were activated by orexin or MCH injection in the BLA. (A) Experimental design for stereotaxic injection with orexin or MCH peptides in the BLA, and time point for tissue preparation (arrow). Orexin or MCH peptides were injected into the BLA as described in Fig. 3A. (B-G) Photomicrographs showing c-Fos expression in the NAc (B, C), PVN (D, E), and CeA (F, G) following the injection of orexin (Orx) or MCH in the BLA. These three regions were commonly activated by the injection of orexin and MCH in the BLA. Quantification levels of c-Fos relative to the control are presented (C, E, G). Scale bar, 200 µm. Data are presented as the mean±SEM. * and ** denote differences from the control at p<0.05 and p<0.01, respectively (n=6-7 animals). aca, anterior commissure, anterior; NAc-C, nucleus accumbens, core; NAc-Sh, nucleus accumbens, shell; PVN paraventricular nucleus of the hypothalamus; CeA, central amygdala; BLA, basolateral amygdala.
DISCUSSION
Recently we reported that stress-induced upregulation of the neuropeptides orexin and MCH in the BLA and the resulting activation of orexin receptors or MCH receptors within the BLA produced deficits in sociability and mood-related behaviors [21]. In the present study, we demonstrate that BLA neurons carrying orexin and MCH receptors comprise a functional connection with the NAc, PVN, and CeA. Considering that upregulation of the orexin and MCH receptor systems in the BLA produced depression-like behaviors [21], our results support the possibility that the orexin and MCH receptor systems in the BLA mediate stress-induced deficits in sociability and emotion-related behaviors through the BLA-NAc, BLA-PVN, and/or BLA-CeA circuits. Recently, it was reported that the BLA-CeA circuit regulated anxiety [28], the BLA-NAc pathway regulated depression-related behavior [29], and the BLA-ventral hippocampus (vHP) circuit mediated sociability and anxiety-related behaviors [30,31]. Considering these, further studies are warranted to explore which neural circuits among these BLA circuits mediate neuropeptide-induced behavioral changes in a chronic stress state.
Previously, we demonstrated that restraint stress or physical exercise induced c-Fos expression in various brain regions [22,25]. Semiquantitative analyses indicated that the c-Fos levels induced by stress were +4 in the NAc, +4 in the PVN, and +3 in the CeA [22,25], whereas the c-Fos levels induced by either orexin- or MCH-injection in the BLA were +3.5 in the NAc, +4 in the PVN, and +4.5 in the CeA (Fig. 3B). In contrast, the c-Fos levels induced by stress were +5 in the PrL, +4 in the lateral habenula, and +3 in the dorsal striatum [22,25], whereas the c-Fos levels induced by orexin- or MCH-injection in the BLA were +3 in the PrL, +2 in the lateral habenula, and +1.5 in the dorsal striatum (Fig. 3B). Thus, the c-Fos levels in the NAc, PVN, and CeA induced by orexin or MCH injection were similar to those induced by stress, while the c-Fos levels in the PrL, lateral habenula, and dorsal striatum as well as in other regions induced by orexin or MCH injection were relatively low compared to those induced by stress. Because these experiments were carried out in separate sets of experiments, more careful examination will be required in the future. Nonetheless, these results suggest some useful and interrelated points regarding the role of the neuropeptide systems in the BLA, summarized as follows.
First, stimulation of orexin receptors or MCH receptors in the BLA induced similar, though not identical, levels and patterns of c-Fos expression in the brain, suggesting that the orexin and MCH receptor systems in the BLA might have overlapping functions, possibly con-expression of these receptors in a neuron [21]. The results of the present study are consistent with a previous report that orexin- or MCH-injection in the BLA commonly increased p-CaMKIIα, and produced social impairment and depressive behaviors. Moreover, both orexin KO mice and MCH KO mice had reduced p-CaMKIIα levels in the BLA, and were resilient to chronic stress [21].
Second, the c-Fos expression levels in most of the BLA target regions of Veh-injection controls were relatively higher than the base-line c-Fos levels examined in untreated, naive mice [22,25]. Recently, we reported that, in naive mice, the base-line c-Fos expression levels were 0~+1 (0~100 c-Fos positive cells/mm2) in most brain regions [22,25]. Whereas in the present study, the c-Fos expression levels in Veh-injection controls were +2~+3 (101~350 c-Fos positive cells/mm2) in many brain regions examined (Fig. 3B). Restraint stress increased the c-Fos expression levels to +2~+5 (101~>650 c-Fos positive cells/mm2) in various regions of the brain [22,25]. Considering these results together, we speculate that the weakly increased base-line c-Fos levels in Veh-injection controls might be attributed to the stimulation of BLA neurons by a needle injection and/or vehicle (0.9% saline) infusion. However, considering that Veh-injection in the BLA did not induce behavioral changes in various tests [21,32,33]. We speculate that weakly increased c-Fos levels in Veh-injection controls present non-specific activation of BLA neurons and therefore, they do not likely lead to behavioral changes.
Third, the c-Fos levels induced by orexin- or MCH-injection in the PrL and lateral habenula (LHb) were not significant relative to the vehicle-injection control (Fig. 3B). This reactivity contrasts with the previous findings that the PrL and LHb were among the areas responsive to stress or exercise at the highest levels [22,25]. These results suggest that the PrL and LHb are less important in mediating the behavioral effects of orexin- or MCH-injection in the BLA demonstrated in the previous study [21].
Fourth, given that orexin receptors and MCH receptors are expressed in the glutamatergic and GABAergic neurons in the BLA [21], and that GABAergic interneurons control the excitability of glutamatergic projection neurons [34,35,36], it is possible that the activation of orexin and MCH receptors in glutamatergic neurons directly modulates the excitability of glutamatergic projection neurons, whereas the activation of orexin and MCH receptors in local GABAergic interneurons might afford a modulatory role by changing the excitability of glutamatergic projection neurons.
Among several subregions of the amygdala, the BLA is critical for regulating stress and depressive behaviors. Stimulus-dependent c-Fos induction analyses in rodents have revealed that the BLA is activated by stress [25] and exercise [22,37]. Chronic stress in rodents produces sociability deficits, increased anxiety and depressive behaviors, whereas exercise produces opposing effects on these behavioral changes [21,22,38,39]. Recently, we demonstrated that these stress-induced sociability impairments and depression-related behaviors, and exercise-induced counteractive effects on these behavioral changes all could be experimentally shifted to an opposite direction when BLA activity was blocked [21,32,33]. These results support the notion that the BLA functions as a critical neuroanatomical node integrating various types of emotion-related information and regulating mood-related behaviors.
Overall, the present study demonstrates that the BLA forms functional connections with the NAc, PVN, and CeA to mediate the physiological effects of increased activation of orexin and MCH receptors in the BLA. These circuits might be responsible for the stress-induced impairments in sociability and moodrelated behaviors and their reversal by exercise, reported in a previous study [21].
ACKNOWLEDGEMENTS
This research was supported by a grant (HI15C1834) from the Ministry of Health and Welfare, Republic of Korea.
References
- 1.Riga D, Matos MR, Glas A, Smit AB, Spijker S, Van den Oever MC. Optogenetic dissection of medial prefrontal cortex circuitry. Front Syst Neurosci. 2014;8:230. doi: 10.3389/fnsys.2014.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knapska E, Radwanska K, Werka T, Kaczmarek L. Functional internal complexity of amygdala: focus on gene activity mapping after behavioral training and drugs of abuse. Physiol Rev. 2007;87:1113–1173. doi: 10.1152/physrev.00037.2006. [DOI] [PubMed] [Google Scholar]
- 5.Sah P, Faber ES, Lopez De, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- 6.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G proteincoupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 7.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scammell TE, Winrow CJ. Orexin receptors: pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2011;51:243–266. doi: 10.1146/annurev-pharmtox-010510-100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakurai T. The role of orexin in motivated behaviours. Nat Rev Neurosci. 2014;15:719–731. doi: 10.1038/nrn3837. [DOI] [PubMed] [Google Scholar]
- 11.Saito Y, Nagasaki H. The melanin-concentrating hormone system and its physiological functions. Results Probl Cell Differ. 2008;46:159–179. doi: 10.1007/400_2007_052. [DOI] [PubMed] [Google Scholar]
- 12.Macneil DJ. The role of melanin-concentrating hormone and its receptors in energy homeostasis. Front Endocrinol(Lausanne) 2013;4:49. doi: 10.3389/fendo.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hervieu G. Melanin-concentrating hormone functions in the nervous system: food intake and stress. Expert Opin Ther Targets. 2003;7:495–511. doi: 10.1517/14728222.7.4.495. [DOI] [PubMed] [Google Scholar]
- 14.Pissios P, Bradley RL, Maratos-Flier E. Expanding the scales: the multiple roles of MCH in regulating energy balance and other biological functions. Endocr Rev. 2006;27:606–620. doi: 10.1210/er.2006-0021. [DOI] [PubMed] [Google Scholar]
- 15.Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 16.Rossi M, Choi SJ, O'Shea D, Miyoshi T, Ghatei MA, Bloom SR. Melanin-concentrating hormone acutely stimulates feeding, but chronic administration has no effect on body weight. Endocrinology. 1997;138:351–355. doi: 10.1210/endo.138.1.4887. [DOI] [PubMed] [Google Scholar]
- 17.Duncan EA, Proulx K, Woods SC. Central administration of melanin-concentrating hormone increases alcohol and sucrose/quinine intake in rats. Alcohol Clin Exp Res. 2005;29:958–964. doi: 10.1097/01.alc.0000167741.42353.10. [DOI] [PubMed] [Google Scholar]
- 18.Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hervieu GJ, Cluderay JE, Harrison D, Meakin J, Maycox P, Nasir S, Leslie RA. The distribution of the mRNA and protein products of the melanin-concentrating hormone (MCH) receptor gene, slc-1, in the central nervous system of the rat. Eur J Neurosci. 2000;12:1194–1216. doi: 10.1046/j.1460-9568.2000.00008.x. [DOI] [PubMed] [Google Scholar]
- 20.García-Fuster MJ, Parks GS, Clinton SM, Watson SJ, Akil H, Civelli O. The melanin-concentrating hormone (MCH) system in an animal model of depression-like behavior. Eur Neuropsychopharmacol. 2012;22:607–613. doi: 10.1016/j.euroneuro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim TK, Kim JE, Park JY, Lee JE, Choi J, Kim H, Lee EH, Kim SW, Lee JK, Kang HS, Han PL. Antidepressant effects of exercise are produced via suppression of hypocretin/orexin and melanin-concentrating hormone in the basolateral amygdala. Neurobiol Dis. 2015;79:59–69. doi: 10.1016/j.nbd.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Kim TK, Han PL. Chronic stress and moderate physical exercise prompt widespread common activation and limited differential activation in specific brain regions. Neurochem Int. 2016;99:252–261. doi: 10.1016/j.neuint.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Park JY, Kim TK, Choi J, Lee JE, Kim H, Lee EH, Han PL. Implementation of a two-dimensional behavior matrix to distinguish individuals with differential depression states in a rodent model of depression. Exp Neurobiol. 2014;23:215–223. doi: 10.5607/en.2014.23.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim H, Kim TK, Kim JE, Park JY, Lee Y, Kang M, Kim KS, Han PL. Adenylyl cyclase-5 in the dorsal striatum function as a molecular switch for the generation of behavioral preferences for cue-directed food choices. Mol Brain. 2014;7:77. doi: 10.1186/s13041-014-0077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim KS, Han PL. Mice lacking adenylyl cyclase-5 cope badly with repeated restraint stress. J Neurosci Res. 2009;87:2983–2993. doi: 10.1002/jnr.22119. [DOI] [PubMed] [Google Scholar]
- 26.Baek IS, Park JY, Han PL. Chronic antidepressant treatment in normal mice induces anxiety and impairs stresscoping ability. Exp Neurobiol. 2015;24:156–168. doi: 10.5607/en.2015.24.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim TK, Han PL. Physical exercise counteracts stressinduced upregulation of melanin-concentrating hormone in the brain and stress-induced persisting anxiety-like behaviors. Exp Neurobiol. 2016;25:163–173. doi: 10.5607/en.2016.25.4.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16:317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- 29.Ramirez S, Liu X, MacDonald CJ, Moffa A, Zhou J, Redondo RL, Tonegawa S. Activating positive memory engrams suppresses depression-like behaviour. Nature. 2015;522:335–339. doi: 10.1038/nature14514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron. 2013;79:658–664. doi: 10.1016/j.neuron.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Felix-Ortiz AC, Tye KM. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J Neurosci. 2014;34:586–595. doi: 10.1523/JNEUROSCI.4257-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim TK, Lee JE, Kim JE, Park JY, Choi J, Kim H, Lee EH, Han PL. G9a-mediated regulation of OXT and AVP expression in the basolateral amygdala mediates stressinduced lasting behavioral depression and its reversal by exercise. Mol Neurobiol. 2016;53:2843–2856. doi: 10.1007/s12035-015-9160-z. [DOI] [PubMed] [Google Scholar]
- 33.Choi J, Kim JE, Kim TK, Park JY, Lee JE, Kim H, Lee EH, Han PL. TRH and TRH receptor system in the basolateral amygdala mediate stress-induced depression-like behaviors. Neuropharmacology. 2015;97:346–356. doi: 10.1016/j.neuropharm.2015.03.030. [DOI] [PubMed] [Google Scholar]
- 34.Kukkonen JP, Holmqvist T, Ammoun S, Akerman KE. Functions of the orexinergic/hypocretinergic system. Am J Physiol Cell Physiol. 2002;283:C1567–C1591. doi: 10.1152/ajpcell.00055.2002. [DOI] [PubMed] [Google Scholar]
- 35.Woodruff AR, Sah P. Networks of parvalbumin positive interneurons in the basolateral amygdala. J Neurosci. 2007;27:553–563. doi: 10.1523/JNEUROSCI.3686-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guyon A, Conductier G, Rovere C, Enfissi A, Nahon JL. Melanin-concentrating hormone producing neurons: Activities and modulations. Peptides. 2009;30:2031–2039. doi: 10.1016/j.peptides.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 37.Greenwood BN, Strong PV, Loughridge AB, Day HE, Clark PJ, Mika A, Hellwinkel JE, Spence KG, Fleshner M. 5-HT2C receptors in the basolateral amygdala and dorsal striatum are a novel target for the anxiolytic and antidepressant effects of exercise. PLoS One. 2012;7:e46118. doi: 10.1371/journal.pone.0046118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim KS, Han PL. Optimization of chronic stress paradigms using anxiety- and depression-like behavioral parameters. J Neurosci Res. 2006;83:497–507. doi: 10.1002/jnr.20754. [DOI] [PubMed] [Google Scholar]
- 39.Anderson E, Shivakumar G. Effects of exercise and physical activity on anxiety. Front Psychiatry. 2013;4:27. doi: 10.3389/fpsyt.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]