Abstract
Background
Oxidative stress in primary aldosteronism (PA) is thought to worsen aldosterone-induced damage by activating proinflammatory processes. Therefore, we investigated whether inflammatory markers associated with oxidative stress is increased with negative impacts on heart function as evaluated by echocardiography in patients with PA.
Methods
Thirty-two subjects (mean age, 50.3±11.0 years; 14 males, 18 females) whose aldosterone-renin ratio was more than 30 among patients who visited Severance Hospital since 2010 were enrolled. Interleukin-1β (IL-1β), IL-6, IL-8, monocyte chemoattractant protein 1, tumor necrosis factor α (TNF-α), and matrix metalloproteinase 2 (MMP-2), and MMP-9 were measured. All patients underwent adrenal venous sampling with complete access to both adrenal veins.
Results
Only MMP-2 level was significantly higher in the aldosterone-producing adenoma (APA) group than in the bilateral adrenal hyperplasia (BAH). Patients with APA had significantly higher left ventricular (LV) mass and A velocity, compared to those with BAH. IL-1β was positively correlated with left atrial volume index. Both TNF-α and MMP-2 also had positive linear correlation with A velocity. Furthermore, MMP-9 showed a positive correlation with LV mass, whereas it was negatively correlated with LV end-systolic diameter.
Conclusion
These results suggest the possibility that some of inflammatory markers related to oxidative stress may be involved in developing diastolic dysfunction accompanied by LV hypertrophy in PA. Further investigations are needed to clarify the role of oxidative stress in the course of cardiac remodeling.
Keywords: Cytokines, Hyperaldosteronism, Heart diseases
INTRODUCTION
Primary aldosteronism (PA) is the most common and curable form of secondary hypertension (HTN) [1]. According to previous reports, the prevalence of PA was thought to be less than 1%
of patients with mild to moderate essential HTN [2]. However, it has recently been estimated that PA is much more prevalent than previously believed, accounting for as many as 20% of patients with HTN [3]. It is mainly caused by autonomous aldosterone production from a unilateral aldosterone-producing adenoma (APA) or bilateral adrenal hyperplasia (BAH). Distinguishing APA from BAH is important because effective treatment differs according to subtypes [2].
Increasing evidence has shown that patients with PA experience more cardiovascular events related to blood pressure-independent effects due to the excess of aldosterone itself, compared to those with essential HTN [4,5]. Moreover, prolonged elevated aldosterone levels can contribute to the development and progression of stroke, myocardial infarction, atrial fibrillation [4], and chronic kidney disease by inducing renal damage [6]. Some researchers have performed studies using echocardiography [7] or cardiac magnetic resonance imaging [5] to evaluate clinical impact of PA. These deleterious effects on target organ damage appear to be involved with variable aldosterone-mediated mechanisms including vascular inflammation [8] and the immune system [9], but the exact mechanism has not been fully clarified.
Oxidative stress, which leads to endothelial dysfunction and organ damage [10], is thought to worsen aldosterone-induced damage by activating proinflammatory process. Zia et al. [10] stated that oxidative stress upregulates redox-sensitive nuclear transcription factor-κB and a proinflammatory gene cascade, such as tumor necrosis factor α (TNF-α) and monocyte chemoattractant protein 1 (MCP-1). Petramala et al. [11] demonstrated that patients with PA showed increased nicotinamide adenine dinucleotide phosphate (NADPH) oxidase plasma levels and urinary isoprostanes than those with essential HTN. In addition, Stehr et al. [12] found that malondialdehyde as an oxidative stress marker and amino terminal propeptides of type I known as a myocardial collagen deposition marker were significantly higher in PA, compared to the essential HTN. Meanwhile, according to one study, antioxidant N-acetylcysteine protects pancreatic β-cells against aldosterone-induced oxidative stress and apoptosis in vivo and in vitro [13].
Given this background, we investigated the relationships between variable inflammatory markers associated with oxidative stress and echocardiographic parameters in patients with PA. Moreover, as APA usually shows higher aldosterone levels than BAH, we hypothesized that cardiac structure or functional changes due to adverse aldosterone-induced oxidative stress in APA may become more aggravated than those in BAH.
METHODS
Study participants
Thirty-two patients with PA were enrolled among those who visited the HTN clinic at Severance Hospital from November 2010 to September 2012. The inclusion criteria were as follows: subjects whose aldosterone-renin ratio (ARR) was more than 30; who were confirmed with PA through saline loading test and adrenal venous sampling (AVS); for whom blood samples for the measurement of inflammatory markers were obtained. The patients included 14 males and 18 females, and their mean age was 50.3±11.0 years. A positive result was defined as post-saline loading plasma aldosterone concentration (PAC) over 8.5 ng/dL [14]. The protocol was approved by the Ethics Committee of the Yonsei University College of Medicine, and all participants provided written consent (IRB No. 4-2014-0237). All investigations were performed in accordance with the principles of the Declaration of Helsinki.
Subtype classification
To classify accurately subtypes of PA, adrenal computed tomography (CT) was performed in three phases before AVS in all patients, with a 64-channel multiple detector CT scanner (Discovery CT750HD, GE Healthcare, Milwaukee, WI, USA). The CT protocol was the same as previously described [15]. However, CT has the potential to be misleading by demonstrating unilateral nodules in some patients with bilateral disease [2], and therefore AVS was also conducted to determine whether the site of aldosterone hypersecretion was unilateral or bilateral.
According to a previous study [15] in which we found that C-arm CT can enhance confidence of right adrenal vein catheterization during AVS, we performed AVS using the same protocol. All patients underwent C-arm CT (Axiom Artis dTA, Siemens, Erlangen, Germany) assisted sequential bilateral AVS with 100% successful access to both adrenal veins during continuous intravenous infusion of tetracosactide (Synacthen, Dalim Biotech, Hwaseong, Korea). Blood sampling was conducted when C-arm CT images revealed proper catheter position. Venous samples were obtained at least 30 minutes after initiation of intravenous tetracosactide administration. Adequate catheterization in AVS was defined as a cortisol ratio in adrenal vein and inferior vena cava blood samples equal to or more than 3:1. In addition, when the adrenal venous blood cortisol-corrected aldosterone ratio was greater than 2.6 after tetracosactide stimulation, a unilateral lesion was considered to be present on the high-value side [14].
Analysis for inflammatory markers associated with oxidative stress
Blood samples for the measurement of inflammatory markers related to oxidative stress were collected from all patients. The concentrations of interleukin-1β (IL-1β), IL-6, IL-8, MCP-1, and TNF-α were measured using a Human Cytokine Magnetic kit (#HCYTOMAG-60K, Millipore, Billerica, MA, USA). Assays for matrix metalloproteinase 2 (MMP-2) and MMP-9 were performed using the Human Cardiovascular Disease Magnetic Bead Panel (#HCVD3MAG-67K, Millipore) according to the manufacturer's protocol.
Assessment of cardiac structure and function
Echocardiography was performed in 23 subjects (71.9%). Comprehensive echocardiographic assessment was conducted by three experienced sonographers who had no knowledge of the clinical data using commercially available ultrasound systems (Sonos 5500, Philips Medical Systems, Andover, MA, USA; or Vivid Seven, GE Vingmed Ultrasound, Horten, Norway). All echocardiographic parameters were measured according to the recommendations of the American Society of Echocardiography [16]. Images were obtained in the parasternal (long and short axis) and apical views. The following parameters were evaluated: left ventricular end-diastolic diameter (LVEDD, mm), left ventricular end-systolic diameter (LVESD, mm), interventricular septum diameter (IVSd, mm) at end-diastole, left ventricular (LV) posterior wall diameter (PWd, mm) at end-diastole, and LV mass (g). LV mass was indexed for body surface area (left ventricular mass index [LVMI]). The left atrial volume index (LAVI) was calculated as the LA volume divided by body surface area. Left ventricular ejection fraction (LVEF) was calculated according to the biplane Simpson's method and apical four-and two-chamber views [17]. Relative wall thickness (RWT) was defined as 2×PWd divided by LVEDD. Early and late mitral flow velocities (E and A, m/sec) and deceleration time (DT) of E velocity (msec) were measured using the pulsed wave Doppler method by placing a sample volume at the mitral valve leaflet tips. In addition, early diastolic mitral annular velocity (E', m/sec) was measured at the septal corner of the mitral annulus using a tissue Doppler image in the apical four-chamber view. We then calculated the E/A and E/E' ratios, which mainly reflect diastolic function. The echocardiographic parameters were divided into two categories: structural abnormality and diastolic function. LVEF, LVEDD, LVESD, IVSd, PWd, LV mass, LVMI, LAVI, and RWT indicate structural abnormality, meanwhile E and A velocities, DT, E/A and E/E' ratios were regarded as representative factors related to diastolic function.
Statistical analysis
Statistical analyses were conducted using PASW version 21.0 (IBM Co., Armonk, NY, USA) and SAS version 9.2 (SAS Inc., Cary, NC, USA). Due to the small sample of patients, data were analyzed by nonparametric tests. The Mann-Whitney U test was used to compare differences in continuous variables between APA and BAH. Moreover, the Spearman partial correlation adjusted for age and sex was used to examine the relationships between inflammatory markers associated with oxidative stress and echocardiographic parameters. P values <0.05 were considered statistically significant.
RESULTS
The baseline characteristics of all patients are presented in Table 1. As noted earlier, females accounted for 56.3% of sample in this study. Mean HTN duration was approximately 8.0 years, and mean blood pressure was 149 over 92 mm Hg. In addition, mean ARR level was about 251. PAC level after the saline loading test was approximately 16.5 ng/dL.
Table 1. Baseline Characteristics of Patients (n=32).
| Characteristic | Value |
|---|---|
| Age, yr | 50.3±11.0 |
| Sex, male:female | 14:18 |
| Body mass index, kg/m2 | 25.5±4.2 |
| HTN duration, yr | 8.0±7.3 |
| History of previous vascular diseasesa | 4 |
| Systolic blood pressure, mm Hg | 149.1±19.7 |
| Diastolic blood pressure, mm Hg | 91.5±12.6 |
| Heart rate, beat/min | 78.6±21.9 |
| Serum potassium, mmol/L | 3.6±0.7 |
| PAC, ng/dL | 28.7±18.0 |
| PRA, ng/mL/hr | 0.2±0.2 |
| ARR | 250.5±433.4 |
| PAC after saline loading test, ng/dL | 16.5±13.1 |
Values are expressed as mean±SD.
HTN, hypertension; PAC, plasma aldosterone concentration; PRA, plasma renin activity; ARR, aldosterone-renin ratio.
aPrevious vascular diseases included angina pectoris, myocardial infarction, stroke or atrial fibrillation.
Differences in inflammatory markers associated with oxidative stress and aldosterone levels between APA and BAH
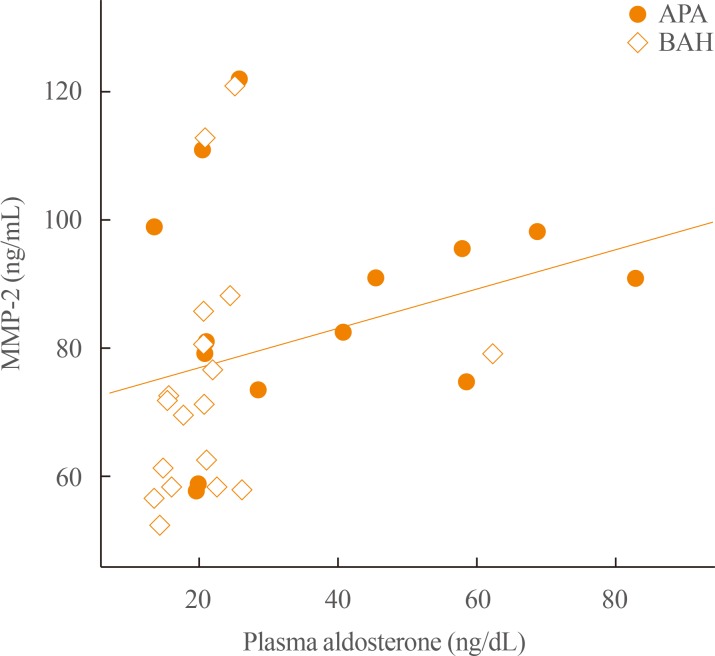
Table 2 demonstrates differences in inflammatory markers related to oxidative stress and hormone levels between APA and BAH patients. In this study, the number of APA patients was similar to that of BAH patients (43.7% and 56.3%, respectively), and no significant differences in blood pressure and heart rate between the two groups were found. As expected, there were significant differences in both serum potassium (3.3 mmol/L vs. 3.9 mmol/L) and PAC (25.9 ng/dL vs. 20.7 ng/dL) between APA and BAH; ARR was significantly higher in the former than in the latter (163.3 vs. 68.6). In terms of inflammatory markers associated with oxidative stress, the APA group showed significantly higher MMP-2 levels than the BAH group (86.8 ng/mL vs. 71.5 ng/mL); however, there were no differences in other cytokine levels between the two groups. We also found a linear relationship between MMP-2 levels and plasma aldosterone level (R=0.448, P=0.010) (Fig. 1).
Table 2. Differences in Inflammatory Markers Associated with Oxidative Stress and Aldosterone Levels between Patients with Aldosterone-Producing Adenoma and Bilateral Adrenal Hyperplasia.
| Variable | APA (n=14) | BAH (n=18) | P value |
|---|---|---|---|
| Age, yr | 51.5 (29.0–70.0) | 54.0 (27.0–64.0) | 0.909 |
| Systolic blood pressure, mm Hg | 156.0 (122.0–195.0) | 141.5 (119.0–200.0) | 0.200 |
| Diastolic blood pressure, mm Hg | 90.0 (65.0–124.0) | 88.5 (80.0–110.0) | 0.733 |
| Heart rate, beat/min | 68.0 (60.0–75.0) | 81.5 (57.0–93.0) | 0.061 |
| Serum potassium, mmol/L | 3.3 (2.4–4.4) | 3.9 (2.9–4.6) | 0.006 |
| PAC, ng/dL | 25.9 (13.5–82.8) | 20.7 (13.5–62.3) | 0.040 |
| PRA, ng/mL/hr | 0.1 (0.0–1.0) | 0.3 (0.0–0.6) | 0.188 |
| ARR | 163.3 (45.9–1,655.3) | 68.6 (28.9–1,053.8) | 0.006 |
| PAC after saline loading test, ng/dL | 20.4 (10.8–67.4) | 8.1 (5.4–21.6) | <0.001 |
| IL-1β, pg/mL | 0.4 (0.1–12.5) | 0.3 (0.1–1.1) | 0.201 |
| IL-6, pg/mL | 1.6 (0.2–73.7) | 0.6 (0.3–5.8) | 0.217 |
| IL-8, pg/mL | 22.4 (5.9–773.0) | 34.2 (4.8–262.0) | 0.761 |
| MCP-1, pg/mL | 384.5 (85.1–811.0) | 378.5 (76.1–770.0) | 0.970 |
| TNF-α, pg/mL | 9.3 (2.5–64.6) | 8.0 (2.8–18.9) | 0.287 |
| MMP-2, ng/mL | 86.8 (57.8–122.0) | 71.5 (52.4–121.0) | 0.028 |
| MMP-9, ng/mL | 121.0 (31.1–839.0) | 166.5 (38.5–902.0) | 0.323 |
Values are expressed as median (range). The Mann-Whitney U test was used to compare differences in continuous variables between APA and BAH. APA, aldosterone-producing adenoma; BAH, bilateral adrenal hyperplasia; PAC, plasma aldosterone concentration; PRA, plasma renin activity; ARR, aldosterone-renin ratio; IL, interleukin; MCP-1, monocyte chemoattractant protein 1; TNF-α, tumor necrosis factor α; MMP, matrix metalloproteinase.
Fig. 1. Linear correlation between matrix metalloproteinase 2 (MMP-2) and aldosterone levels. In the current study, there was a significant linear relationship between MMP-2 and plasma aldosterone levels in patients with primary aldosteronism (R=0.448, P=0.010). The Spearman correlation was used to evaluate the linear relationship between MMP-2 and aldosterone levels. APA, aldosterone-producing adenoma; BAH, bilateral adrenal hyperplasia.
Differences in echocardiographic parameters between APA and BAH
Differences in echocardiographic parameters between APA and BAH patients are reported in Table 3. Some of parameters showed different values between APA and BAH groups. Regarding parameters describing structural abnormalities of the heart, patients with APA had significantly higher LV mass and LVMI values compared to BAH, even though there was no significant difference in RWT between the two groups. LVEF, LVEDD, LVESD, IVSd, and PWd of the two groups were similar. Moreover, there were no differences in parameters for diastolic function such as E velocity, DT, or E/E' ratio between the two groups except for A velocity, which was significantly higher in APA than that in BAH (P=0.047).
Table 3. Differences in Echocardiographic Parameters between Patients with Aldosterone-producing Adenoma and Bilateral Adrenal Hyperplasia.
| Parameter | APA (n=8) | BAH (n=15) | P value |
|---|---|---|---|
| Parameters for structural abnormality | |||
| LVEF, % | 68.5 (61.0–76.0) | 71.0 (61.0–76.0) | 0.112 |
| LVEDD, mm | 48.0 (44.0–53.0) | 50.0 (43.0–57.0) | 0.516 |
| LVESD, mm | 30.5 (27.0–36.0) | 30.0 (26.0–36.0) | 0.795 |
| IVSd, mm | 12.0 (8.0–13.0) | 11.0 (8.0–20.0) | 0.265 |
| PWd, mm | 11.0 (7.0–14.0) | 11.0 (7.0–16.0) | 0.578 |
| LV mass, g | 234.6 (187.5–259.6) | 174.5 (133.6–294.4) | 0.034 |
| LVMI, g/m2 | 127.3 (101.9–153.6) | 99.8 (74.7–136.2) | 0.043 |
| LAVI, mL/m2 | 38.7 (20.6–54.0) | 30.2 (22.1–50.4) | 0.029 |
| RWT | 0.45 (0.32–0.58) | 0.44 (0.27–0.70) | 0.561 |
| Parameters for diastolic function | |||
| E velocity, m/sec | 0.8 (0.5–1.0) | 0.7 (0.4–1.0) | 0.260 |
| A velocity, m/sec | 0.69 (0.72–1.11) | 0.65 (0.47–0.84) | 0.047 |
| E/A ratio | 1.0 (0.7–1.4) | 1.2 (0.5–2.0) | 0.458 |
| Deceleration time, msec | 181.0 (141.0–237.0) | 194.0 (151.0–260.0) | 0.183 |
| E’, m/sec | 0.07 (0.04–0.11) | 0.06 (0.03–0.11) | 0.945 |
| E/E’ ratio | 12.3 (6.8–20.0) | 10.5 (6.0–20.0) | 0.516 |
Values are expressed as median (range). The Mann-Whitney U test was used to compare differences in continuous variables between APA and BAH. APA, aldosterone-producing adenoma; BAH, bilateral adrenal hyperplasia; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; IVSd, interventricular septum diameter; PWd, posterior wall diameter; LV, left ventricular; LVMI, left ventricular mass index; LAVI, left atrial volume index; RWT, relative wall thickness.
Association between inflammatory markers associated with oxidative stress and echocardiographic parameters
As shown in Table 4, we also evaluated whether the linear relationships were present between each level of inflammatory markers related to oxidative stress and echocardiographic parameters after adjustments for age and sex, because circulating cytokine levels can be modified by age and sex. IL-1β was positively correlated with LAVI, which is a structural factor reflecting diastolic dysfunction (R=0.694, P=0.018). IL-6 also had a positive linear correlation with E velocity (R=0.680, P=0.011). MCP-1 was positively correlated with A velocity (R=0.615, P=0.019), but showed a negative correlation with E' velocity (R=–0.517, P=0.048). Both TNF-α and MMP-2 had positive linear correlations with A velocity (R=0.819, P<0.001; and R=0.751, P=0.002, respectively). MMP-9 was positively correlated with IVSd (R=0.538, P=0.047) as well as LV mass (R=0.553, P=0.040), whereas it showed a negative correlation with LVESD (R=–0.622, P=0.018). However, no significant linear relationships were detected between IL-8 and these echocardiographic parameters. In addition, there were no linear correlations between any of these inflammatory markers associated with oxidative stress and RWT in patients with PA.
Table 4. Associations between Inflammatory Markers Associated with Oxidative Stress and Echocardiographic Parameters.
| Parameter | Correlation coefficient | ||||||
|---|---|---|---|---|---|---|---|
| IL-1β | IL-6 | IL-8 | MCP-1 | TNF-α | MMP-2 | MMP-9 | |
| Structural abnormality | |||||||
| LVEF, % | –0.162 | 0.023 | –0.021 | 0.442 | 0.309 | 0.200 | 0.459 |
| LVEDD, mm | 0.343 | 0.032 | 0.177 | 0.290 | 0.035 | 0.095 | –0.313 |
| LVESD, mm | 0.411 | 0.106 | 0.196 | –0.083 | –0.192 | –0.099 | –0.622a |
| IVSd, mm | 0.424 | 0.276 | –0.060 | –0.206 | –0.008 | –0.086 | 0.538a |
| PWd, mm | 0.099 | –0.054 | –0.315 | –0.242 | –0.205 | –0.145 | –0.061 |
| LV mass, g | 0.386 | 0.478 | –0.040 | 0.186 | 0.246 | 0.206 | 0.553a |
| LVMI, g/m2 | 0.487 | 0.444 | –0.101 | –0.043 | 0.032 | –0.037 | 0.329 |
| LAVI, mL/m2 | 0.694a | 0.060 | –0.001 | –0.165 | 0.077 | –0.115 | 0.040 |
| RWT | –0.402 | 0.127 | 0.218 | 0.164 | 0.223 | 0.114 | 0.182 |
| Function | |||||||
| E velocity, m/sec | 0.587 | 0.680a | 0.071 | –0.214 | –0.086 | –0.160 | 0.265 |
| A velocity, m/sec | –0.172 | 0.401 | 0.425 | 0.615a | 0.819a | 0.751a | 0.502 |
| E/A ratio | 0.52 | 0.402 | 0.029 | –0.472 | –0.436 | –0.472 | –0.133 |
| Deceleration time, msec | 0.002 | –0.100 | 0.053 | 0.446 | 0.120 | 0.267 | –0.131 |
| E’, m/sec | 0.335 | 0.127 | –0.354 | –0.517a | –0.422 | –0.371 | –0.046 |
| E/E’ ratio | 0.300 | 0.418 | 0.512 | 0.179 | 0.297 | 0.505 | –0.031 |
Spearman partial correlation adjusted for age and sex was used to examine the relationships between inflammatory markers associated with oxidative stress and echocardiographic parameters.
IL, interleukin; MCP, monocyte chemoattractant protein; TNF-α, tumor necrosis factor α; MMP, matrix metalloproteinase; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; IVSd, interventricular septum diameter; PWd, posterior wall diameter; LV, left ventricular; LVMI, left ventricular mass index; LAVI, left atrial volume index; RWT, relative wall thickness.
aP<0.05, after adjustment for age and sex.
DISCUSSION
High aldosterone status was identified as an independent cardiovascular risk factor [18]. Previous study has shown that cardiovascular complications such as myocardial infarction, stroke, and sustained arrhythmia, are more prevalent in patients with PA than in those with essential HTN, for which the odds ratio for overall cardiovascular events was 4.61 [19]. The increased risk of cardiovascular morbidity is associated with cardiac remodeling and LV changes that can be reversible through long-term control with surgical or medical treatment for PA [20]. However, the mechanism underlying the adverse cardiovascular effects of aldosterone remains unclear.
Freel et al. [5] demonstrated that blood pressure-independent non-infarct myocardial fibrosis is frequent in those with PA. This phenomenon may be in part mediated through oxidative stress and inflammation. Oxidative stress markers such as NADPH oxidase plasma levels and urinary isoprostanes were decreased after laparoscopic adrenalectomy of APA. Furthermore, aldosterone can lead to oxidative damage to the DNA and chromosomes [21] and promote the induction of reactive oxygen formation via non-genomic effects [22]. Calo et al. [23] stated that oxidative stress and the associated signaling may be involved in causing oxidative damage to hot spot sequences in the Kir3.4 gene, thereby contributing to the autonomy of aldosterone overproduction in PA. These results also support the theory that reactive oxidant species play an essential role in the development of endothelial dysfunction and vascular remodeling of atherosclerotic processes, as well as progression of multi-organ damage in high cardiovascular risk conditions [24]. Nevertheless, there are limited data regarding the influence of oxidative stress on cardiac structure and function in patients with PA, although a variety of studies have examined the effects of oxidative stress or inflammation markers on the heart disease [25].
Our findings indicate that some inflammatory markers associated with oxidative stress play an important role in developing diastolic dysfunction accompanied by concentric left ventricular hypertrophy (LVH), especially in patient with APA. In the current study, only MMP-2 level was higher in patients with APA than in those with BAH. However, we found that LV mass and LVMI in the APA were significantly higher than in the BAH, although there were no significant differences in blood pressure and heart rate between the two groups. Generally, when both LVMI and RWT are increased, concentric LVH can be defined [26]. Although the difference in RWT between the two groups was not significant, the pattern of concentric LVH was observed in all patients because the RWT was elevated in both groups, considering that the reference cut-off point for increased RWT is usually accepted to be 0.43 [26]. The results of our study are consistent with those of previous studies demonstrating that PA patients have frequent LVH and diastolic dysfunction [27]. Excess LVH appears to be an important factor increasing risk of cardiovascular events in patients with PA, compared with those with essential HTN [28].
Interestingly, LAVI was significantly elevated in APA patients compared to BAH. Since LA enlargement is attributable to sustained elevation of LV filling pressure and atrial volume overload caused by diastolic dysfunction in hypertensive patients [29], LAVI is strongly associated with LV diastolic dysfunction, and this relationship is independent of age, sex, LVEF, and cardiovascular risk score [30]. In this study, A velocity was also elevated in patients with APA compared to those with BAH, suggesting the possibility of impact on diastolic function, although differences in E/A and E/E' ratios between the two groups were not found.
Moreover, a considerable number of inflammatory markers related to oxidative stress had linear correlations with echocardiographic parameters in patients with PA, even after adjustments for age and sex. In this study, only IL-1β was positively correlated with LAVI (R=0.694, P=0.018), indicating that IL-1β can worsen LV diastolic function in patients with PA. IL-1β, which is the main form of circulating IL-1, is known to play an important role in the amplification of tissue inflammatory re-sponse; thereby, affecting the progression of atherosclerosis and cardiac remodeling [31]. IL-1β, as well as IL-6 and TNF-α, are usually increased in patients with chronic heart failure (HF), and can therefore be used to predict adverse outcome in these patients [31,32,33]. Thus, our results are consistent with those of previous studies related to IL-1β. In the case of IL-6, Fredj et al. [34] suggested that IL-6 signaling may be the key to promote cardiomyocyte hypertrophy, while Huang et al. [35] stated that IL-6 can tune myocardial remodeling through suppression of innate immune signals to prevent the catastrophic consequences of uncontrolled inflammation on cardiac geometry and function. However, we did not observe evidence that IL-6 has an influence on cardiac structure or function, although there was a positive linear correlation between IL-6 and E velocity in our study (R=0.680, P=0.011). We found no significant linear relationships between IL-8 and any echocardiographic parameters. In this study, we did not measure IL-18 levels; however, Toldo et al. [33] demonstrated that IL-1 leads to a release of active IL-18 in the experiments using mice, but not the induction of IL-6, indicating the potential of IL-18 blockade as a novel therapeutic target of HF.
The correlation coefficient between TNF-α and A velocity was high (R=0.819, P<0.001), but there were no relationships between TNF-α and other parameters. TNF-α is thought to have pleiotrophic effects on cardiovascular disease. Elevated TNF-α levels are positively correlated with the progression of HF, and have cytoprotective effects in myocardium of acute ischemic injury [36]. Furthermore, MCP-1 was positively correlated with A velocity (R=0.615, P=0.019), while it had a negative correlation with E' velocity (R=–0.517, P=0.048), which is relatively insensitive to change of preload in the presence of LV systolic dysfunction [37]. Thus, we speculated that MCP-1 also affects the development of functional abnormality in the diastolic phase. According to previous results described by Blasi et al. [6], renal expression levels of proinflammatory gene, such as MCP-1, IL-1β, or IL-6, were significantly increased following 28 days of aldosterone administration, and this phenomenon was attenuated by eplerenone treatment. In addition, MCP-1 contributes to the recruitment of monocytes to injury sites by interacting with chemokine receptor 2 expressed on the surface of monocytes, and IL-1β plays a role in enhancing the expression of adhesion molecules by immune cells through the interaction with IL-1β receptor [9].
In the current study, MMP-2 levels were significantly higher in the APA group than in the BAH group. We observed a linear relationship between MMP-2 and plasma aldosterone levels (R=0.448, P=0.010) (Fig. 1). The activities of MMP, which is known as a family of proteolytic enzymes, have been examined as candidate biomarkers or therapeutic targets for cardiovascular disease, including myocardial infarction and congestive HF [38]. MMP activation mediates myocardial remodeling in cardiac pathogenesis [38]. Hughes et al. [39] demonstrated that MMP-2 localized in mitochondria-associated heart membrane could affect mitochondria function by influencing endoplasmic reticulum Ca2+ signaling. We also observed a positive linear correlation between MMP-2 level and A velocity (R=0.751, P=0.002). Therefore, we speculate that MMP-2 can be an aggravating factor for the cardiac structure or diastolic function in the APA patients with higher aldosterone levels than BAH patients. In addition, MMP-9 was positively correlated with LV mass (R=0.553, P=0.040) and IVSd (R=0.538, P=0.047), whereas it showed a negative correlation with LVESD (R=–0.622, P=0.018), suggesting that MMP-9 may be associated with cardiac remodeling. According to Halade et al. [40], MMP-9 can serve as a proximal biomarker for cardiac remodeling and a distal biomarker for inflammation. Thus, in the future, more research is needed to explain the roles or interactions of these cytokines in the development of adverse outcomes in patients with PA.
To our knowledge, this is the first clinical study to analyze differences in inflammatory markers associated with oxidative stress according to subtypes of PA. Although previous research addressed increases in oxidative stress, such as NADPH oxidase plasma levels [22], malondialdehyde or amino terminal propeptides of type I [12] in patients with PA, they compared patients with PA and essential HTN. We further investigated cardiac structure and function with echocardiography in order to evaluate whether aldosterone-induced oxidative stress has an impact on heart condition, detecting strong correlations between each marker and echocardiographic parameter in PA even after adjustments for age and sex.
However, this study also has some limitations. First, this is cross-sectional study, and it is therefore difficult to infer causality. Secondly, the study sample was small. That may be why we could not reconfirm the linear relationship between inflammatory markers such as MMP-2 and plasma aldosterone levels after the adjustment for confounding factors. Thirdly, this study had no control group matched to PA patients in age, sex, body mass index, HTN duration, blood pressure, smoking and so on. Fourthly, echocardiographic assessment was performed by three sonographers. So, there might be inter-individual variations among them. Finally, when blood sample volume is insufficient or circulating cytokine levels are low, analytical variation is likely. Hence, assessing the additional effects of inflammatory markers related to oxidative stress on adverse cardiovascular outcomes in patients with PA requires a large well-designed research study with long-term follow-up.
In summary, our results suggest that inflammatory markers associated with oxidative stress are involved in developing diastolic dysfunction as well as LV hypertrophy in patients with PA. However, we have little explanation to fully answer the mechanism underlying the role of oxidative stress in PA, because the roles of some of these cytokines in the development of cardiovascular disease remain controversial. Nevertheless, aldosterone seems to promote the recruitment or activation of immune cells by several mechanisms [9]. For this reason, better understanding the mechanisms of aldosterone-induced target organ damage by enhanced oxidative stress may be a new avenue for developing a promising therapeutic target for PA. Further investigations are needed to clarify the role of oxidative stress in the course of cardiac remodeling.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Zennaro MC, Boulkroun S, Fernandes-Rosa F. An update on novel mechanisms of primary aldosteronism. J Endocrinol. 2015;224:R63–R77. doi: 10.1530/JOE-14-0597. [DOI] [PubMed] [Google Scholar]
- 2.Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:3266–3281. doi: 10.1210/jc.2008-0104. [DOI] [PubMed] [Google Scholar]
- 3.Douma S, Petidis K, Doumas M, Papaefthimiou P, Triantafyllou A, Kartali N, et al. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet. 2008;371:1921–1926. doi: 10.1016/S0140-6736(08)60834-X. [DOI] [PubMed] [Google Scholar]
- 4.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–1248. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Freel EM, Mark PB, Weir RA, McQuarrie EP, Allan K, Dargie HJ, et al. Demonstration of blood pressure-independent noninfarct myocardial fibrosis in primary aldosteronism: a cardiac magnetic resonance imaging study. Circ Cardiovasc Imaging. 2012;5:740–747. doi: 10.1161/CIRCIMAGING.112.974576. [DOI] [PubMed] [Google Scholar]
- 6.Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003;63:1791–1800. doi: 10.1046/j.1523-1755.2003.00929.x. [DOI] [PubMed] [Google Scholar]
- 7.Hidaka T, Shiwa T, Fujii Y, Nishioka K, Utsunomiya H, Ishibashi K, et al. Impact of aldosterone-producing adenoma on cardiac structures in echocardiography. J Echocardiogr. 2013;11:123–129. doi: 10.1007/s12574-013-0168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown NJ. Aldosterone and vascular inflammation. Hypertension. 2008;51:161–167. doi: 10.1161/HYPERTENSIONAHA.107.095489. [DOI] [PubMed] [Google Scholar]
- 9.Herrada AA, Campino C, Amador CA, Michea LF, Fardella CE, Kalergis AM. Aldosterone as a modulator of immunity: implications in the organ damage. J Hypertens. 2011;29:1684–1692. doi: 10.1097/HJH.0b013e32834a4c75. [DOI] [PubMed] [Google Scholar]
- 10.Zia AA, Kamalov G, Newman KP, McGee JE, Bhattacharya SK, Ahokas RA, et al. From aldosteronism to oxidative stress: the role of excessive intracellular calcium accumulation. Hypertens Res. 2010;33:1091–1101. doi: 10.1038/hr.2010.159. [DOI] [PubMed] [Google Scholar]
- 11.Petramala L, Violi F, Letizia C. Aldosterone-induced oxidative stress: a potential mechanism of aldosterone autonomy in primary aldosteronism. J Hypertens. 2014;32:2281. doi: 10.1097/HJH.0000000000000376. [DOI] [PubMed] [Google Scholar]
- 12.Stehr CB, Mellado R, Ocaranza MP, Carvajal CA, Mosso L, Becerra E, et al. Increased levels of oxidative stress, subclinical inflammation, and myocardial fibrosis markers in primary aldosteronism patients. J Hypertens. 2010;28:2120–2126. doi: 10.1097/HJH.0b013e32833d0177. [DOI] [PubMed] [Google Scholar]
- 13.Jin HM, Zhou DC, Gu HF, Qiao QY, Fu SK, Liu XL, et al. Antioxidant N-acetylcysteine protects pancreatic β-cells against aldosterone-induced oxidative stress and apoptosis in female db/db mice and insulin-producing MIN6 cells. Endocrinology. 2013;154:4068–4077. doi: 10.1210/en.2013-1115. [DOI] [PubMed] [Google Scholar]
- 14.Nishikawa T, Omura M, Satoh F, Shibata H, Takahashi K, Tamura N, et al. Guidelines for the diagnosis and treatment of primary aldosteronism: the Japan Endocrine Society 2009. Endocr J. 2011;58:711–721. doi: 10.1507/endocrj.ej11-0133. [DOI] [PubMed] [Google Scholar]
- 15.Park SI, Rhee Y, Lim JS, Park S, Kang SW, Lee MS, et al. Right adrenal venography findings correlated with C-arm CT for selection during C-arm CT-assisted adrenal vein sampling in primary aldosteronism. Cardiovasc Intervent Radiol. 2014;37:1469–1475. doi: 10.1007/s00270-013-0820-y. [DOI] [PubMed] [Google Scholar]
- 16.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Folland ED, Parisi AF, Moynihan PF, Jones DR, Feldman CL, Tow DE. Assessment of left ventricular ejection fraction and volumes by real-time, two-dimensional echocardiography. A comparison of cineangiographic and radionuclide techniques. Circulation. 1979;60:760–766. doi: 10.1161/01.cir.60.4.760. [DOI] [PubMed] [Google Scholar]
- 18.Rossi G, Boscaro M, Ronconi V, Funder JW. Aldosterone as a cardiovascular risk factor. Trends Endocrinol Metab. 2005;16:104–107. doi: 10.1016/j.tem.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Catena C, Colussi G, Nadalini E, Chiuch A, Baroselli S, Lapenna R, et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med. 2008;168:80–85. doi: 10.1001/archinternmed.2007.33. [DOI] [PubMed] [Google Scholar]
- 20.Rossi GP, Cesari M, Cuspidi C, Maiolino G, Cicala MV, Bisogni V, et al. Long-term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension. 2013;62:62–69. doi: 10.1161/HYPERTENSIONAHA.113.01316. [DOI] [PubMed] [Google Scholar]
- 21.Schupp N, Queisser N, Wolf M, Kolkhof P, Barfacker L, Schafer S, et al. Aldosterone causes DNA strand breaks and chromosomal damage in renal cells, which are prevented by mineralocorticoid receptor antagonists. Horm Metab Res. 2010;42:458–465. doi: 10.1055/s-0029-1243253. [DOI] [PubMed] [Google Scholar]
- 22.Petramala L, Pignatelli P, Carnevale R, Zinnamosca L, Marinelli C, Settevendemmie A, et al. Oxidative stress in patients affected by primary aldosteronism. J Hypertens. 2014;32:2022–2029. doi: 10.1097/HJH.0000000000000284. discussion 2029. [DOI] [PubMed] [Google Scholar]
- 23.Calo LA, Lenzini L, Rossi GP. Aldosterone-induced oxidative stress: a potential mechanism of aldosterone autonomy in primary aldosteronism. J Hypertens. 2014;32:2280–2281. doi: 10.1097/HJH.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 24.Violi F, Basili S, Nigro C, Pignatelli P. Role of NADPH oxidase in atherosclerosis. Future Cardiol. 2009;5:83–92. doi: 10.2217/14796678.5.1.83. [DOI] [PubMed] [Google Scholar]
- 25.Gullestad L, Ueland T, Vinge LE, Finsen A, Yndestad A, Aukrust P. Inflammatory cytokines in heart failure: mediators and markers. Cardiology. 2012;122:23–35. doi: 10.1159/000338166. [DOI] [PubMed] [Google Scholar]
- 26.Ganau A, Devereux RB, Roman MJ, de Simone G, Pickering TG, Saba PS, et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550–1558. doi: 10.1016/0735-1097(92)90617-v. [DOI] [PubMed] [Google Scholar]
- 27.Chang YY, Lee HH, Hung CS, Wu XM, Lee JK, Wang SM, et al. Association between urine aldosterone and diastolic function in patients with primary aldosteronism and essential hypertension. Clin Biochem. 2014;47:1329–1332. doi: 10.1016/j.clinbiochem.2014.05.062. [DOI] [PubMed] [Google Scholar]
- 28.Savard S, Amar L, Plouin PF, Steichen O. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension. 2013;62:331–336. doi: 10.1161/HYPERTENSIONAHA.113.01060. [DOI] [PubMed] [Google Scholar]
- 29.Douglas PS. The left atrium: a biomarker of chronic diastolic dysfunction and cardiovascular disease risk. J Am Coll Cardiol. 2003;42:1206–1207. doi: 10.1016/s0735-1097(03)00956-2. [DOI] [PubMed] [Google Scholar]
- 30.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002;90:1284–1289. doi: 10.1016/s0002-9149(02)02864-3. [DOI] [PubMed] [Google Scholar]
- 31.Orus J, Roig E, Perez-Villa F, Pare C, Azqueta M, Filella X, et al. Prognostic value of serum cytokines in patients with congestive heart failure. J Heart Lung Transplant. 2000;19:419–425. doi: 10.1016/s1053-2498(00)00083-8. [DOI] [PubMed] [Google Scholar]
- 32.Frye RF, Schneider VM, Frye CS, Feldman AM. Plasma levels of TNF-alpha and IL-6 are inversely related to cytochrome P450-dependent drug metabolism in patients with congestive heart failure. J Card Fail. 2002;8:315–319. doi: 10.1054/jcaf.2002.127773. [DOI] [PubMed] [Google Scholar]
- 33.Toldo S, Mezzaroma E, O'Brien L, Marchetti C, Seropian IM, Voelkel NF, et al. Interleukin-18 mediates interleukin-1-induced cardiac dysfunction. Am J Physiol Heart Circ Physiol. 2014;306:H1025–H1031. doi: 10.1152/ajpheart.00795.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fredj S, Bescond J, Louault C, Potreau D. Interactions between cardiac cells enhance cardiomyocyte hypertrophy and increase fibroblast proliferation. J Cell Physiol. 2005;202:891–899. doi: 10.1002/jcp.20197. [DOI] [PubMed] [Google Scholar]
- 35.Huang M, Yang D, Xiang M, Wang J. Role of interleukin-6 in regulation of immune responses to remodeling after myocardial infarction. Heart Fail Rev. 2015;20:25–38. doi: 10.1007/s10741-014-9431-1. [DOI] [PubMed] [Google Scholar]
- 36.Nakano M, Knowlton AA, Dibbs Z, Mann DL. Tumor necrosis factor-alpha confers resistance to hypoxic injury in the adult mammalian cardiac myocyte. Circulation. 1998;97:1392–1400. doi: 10.1161/01.cir.97.14.1392. [DOI] [PubMed] [Google Scholar]
- 37.Mulatero P, Monticone S, Bertello C, Viola A, Tizzani D, Iannaccone A, et al. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013;98:4826–4833. doi: 10.1210/jc.2013-2805. [DOI] [PubMed] [Google Scholar]
- 38.Mishra A, Srivastava A, Mittal T, Garg N, Mittal B. Association of matrix metalloproteinases (MMP2, MMP7 and MMP9) genetic variants with left ventricular dysfunction in coronary artery disease patients. Clin Chim Acta. 2012;413:1668–1674. doi: 10.1016/j.cca.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Hughes BG, Fan X, Cho WJ, Schulz R. MMP-2 is localized to the mitochondria-associated membrane of the heart. Am J Physiol Heart Circ Physiol. 2014;306:H764–H770. doi: 10.1152/ajpheart.00909.2013. [DOI] [PubMed] [Google Scholar]
- 40.Halade GV, Jin YF, Lindsey ML. Matrix metalloproteinase (MMP)-9: a proximal biomarker for cardiac remodeling and a distal biomarker for inflammation. Pharmacol Ther. 2013;139:32–40. doi: 10.1016/j.pharmthera.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]