Abstract
Background
Nonalbuminuric renal insufficiency is a unique category of diabetic kidney diseases. The objectives of the study were to evaluate prevalent rate of nonalbuminuric renal insufficiency and to investigate its relationship with previous cardiovascular disease (CVD) event in Korean patients with type 2 diabetes mellitus (T2DM).
Methods
Laboratory and clinical data of 1,067 subjects with T2DM were obtained and reviewed. Study subjects were allocated into four subgroups according to the CKD classification. Major CVD events were included with coronary, cerebrovascular, and peripheral vascular events.
Results
Nonalbuminuric stage ≥3 CKD group, when compared with albuminuric stage ≥3 CKD group, had shorter diabetic duration, lower concentrations of glycated hemoglobin, high density lipoprotein cholesterol, and high-sensitivity C-reactive protein, lower prevalent rates of retinopathy and previous CVD, and higher rate of treatment with angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers. Nonalbuminuric stage ≥3 CKD group showed a greater association with prior CVD events than no CKD group; however, albuminuric stage ≥3 CKD group made addition to increase prevalence of prior CVD events significantly when CKD categories were applied as covariates. Association of prior CVD events, when compared with normal estimated glomerular filtration rate (eGFR) and nonalbuminuria categories, became significant for declined eGFR, which was higher for eGFR of <30 mL/min/1.73 m2, and albuminuria.
Conclusion
The results show that subjects with nonalbuminuric stage ≥3 CKD is significantly interrelated with occurrence of prior CVD events than those with normal eGFR with or without albuminuria. Comparing with normal eGFR and nonalbuminuria categories, the combination of increased degree of albuminuria and declined eGFR is becoming significant for the association of prior CVD events.
Keywords: Nonalbuminuric renal insufficiency, Diabetes mellitus, Cardiovascular diseases
INTRODUCTION
In the current global epidemic, the high incidence of diabetes is dramatically related to an enlarged emerging of cardiovascular diseases (CVD) [1] and end-stage renal disease [2]. Preceding studies showed that both declined estimated glomerular filtration rate (eGFR) and increased degree of albuminuria presuppose CVD events in nondiabetics and subjects with type 2 diabetes mellitus (T2DM) [3,4,5]. Lately, Soveri et al. [6] showed that it may be possible to detect a rise in the risk of CVD development and cardiovascular death in those with eGFR of <90 mL/min/1.73 m2. On the average, 30% of diabetic patients will develop chronic renal disease [7], and nonalbuminuric form of renal insufficiency is also found [8,9]. The two large-scale clinical trials have analyzed and shown the self-determining influences of both increased degree of albuminuria and decreased renal function on the development of CVD and cardiovascular death [10,11]. A worsening of kidney function may not always be go together with high excretion rate of urinary albumin [12,13]. Therefore, nonalbuminuric renal insufficiency is a distinctive form of renal diseases in T2DM. But, its clinical progression and pathophysiology are still not conclusive. The recent study using meta-analysis showed that albuminuria and declined eGFR (<60 mL/min/1.73 m2) have both been confirmed—without any evidence of interaction—as independent predictors of cardiovascular death [14]. According to some studies in patients with T2DM, albuminuria predicts cardiovascular death better than declined eGFR [14,15]. Italian multi-center studies showed that nonalbuminuric renal insufficiency, compared with albuminuric renal insufficiency is significantly related to any CVD events in T2DM [16,17]. Still it is unclear whether nonalbuminuric form of renal insufficiency is linked with developing CVD, especially in Korean patients.
The purposes of the study were to evaluate prevalent rate of nonalbuminuric renal insufficiency in Korean diabetic patients and to investigate association of previous CVD events with nonalbuminuric renal insufficiency compared with albuminuric renal insufficiency or no CKD in the study subjects.
METHODS
Study subjects
The data were obtained for 1,067 patients with T2DM (561 men, 506 women) at the baseline visit attending the Diabetes Center at Pusan National University Yangsan Hospital for management of diabetes, between January 2011 and December 2013. We excluded patients under 18 or over 80 years of age, patients with receiving renal replacement therapy, patients with systemic conditions that may affect vascular glomerulonephritis or vasculitis, patients with other reasons of renal dysfunction, and patients with malignancy. The study was received the ethical approval from the Ethical Review Board of the Clinical Research Institute at Pusan National University Yangsan Hospital.
Clinical information and biochemical data analysis
We retrospectively reviewed the records to evaluate patients' sex, age, body mass index, smoking status, diabetic duration, blood pressure, and previously documented history of major CVD events, including coronary diseases (myocardial infarction and/or coronary artery revascularization), cerebrovascular diseases (cerebral infarction and/or carotid artery revascularization), and peripheral artery diseases (peripheral artery occlusive disease and/or lower limb revascularization). Occurrence of retinopathy was determined by an ophthalmoscope examination by an expert ophthalmologist. Medication histories regarding antihypertensive drugs, including renin-angiotensin system (RAS) inhibitors, and lipid-lowering drugs were also reviewed. We measured the concentrations of blood urea nitrogen, creatinine, total cholesterol, triglyceride, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), and high-sensitivity C-reactive protein (hsCRP) using an autoanalyzer and an enzymatic colorimetric method (Hitachi 7600, Hitachi Ltd., Tokyo, Japan). Glycated hemoglobin (HbA1c) was measured using high performance liquid chromatography (Bio-Rad Laboratories, Hercules, CA, USA).
We calculated eGFR with the Modification of Diet in Renal Disease (MDRD) study equation [eGFR=175×(SCr)–1.154×(age)–0.203×0.742 (if female)×1.212 (if black)] [18], and renal insufficiency was defined as eGFR <60 mL/min/1.73 m2 (i.e., stage ≥3 CKD) according to the guidelines from the National Kidney Foundation (NKF) Kidney Disease Outcomes Quality Initiative (KDOQI) [19]. Moreover, we also measured eGFR using Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation [GFR=141×minimum (Scr/κ, 1)α×maximum (Scr/κ, 1)–1.209×0.993Age×1.018 (if female)×1.159 (if black), where: Scr is serum creatinine in mg/dL, κ is 0.7 (females) and 0.9 (males), α is –0.329 (females) and –0.411 (males), minimum of Scr/κ or 1, and maximum of Scr/κ or 1], which was recently developed to provide a lower estimate of CKD prevalence than the MDRD study formula [20].
Urinary albumin levels were calculated using Roche/Hitachi MODULAR analyzers (Roche Diagnostic GmbH, Mannheim, Germany) and urine creatinine concentrations were measured by the Jaffe method (Hitachi 7170, Hitachi, Tokyo, Japan). Albumin excretion rate was recalculated from the albumin-creatinine ratio (ACR) in the single spot urine samples using an adaptation formula developed in patients with type 1 diabetes mellitus (T1DM) [19]. Study subjects were then allotted to the subsequent groups according to the NKF KDOQI [19]: group 1 is no CKD (eGFR ≥60 mL/min/1.73 m2 and ACR <30 mg/g), group 2 is stages 1 to 2 CKD (eGFR ≥60 mL/min/1.73 m2 and ACR ≥30 mg/g), group 3 is nonalbuminuric stage ≥3 CKD (eGFR <60 mL/min/1.73 m2 and ACR <30 mg/g), and group 4 is albuminuric stage ≥3 CKD (eGFR <60 mL/min/1.73 m2 and ACR ≥30 mg/g).
Statistical analyses
Data were described as the mean±SD and median, or as the percentages and number of cases. The Kruskal-Wallis one-way analysis of variance for continuous variables or Pearson chi-square test for categorical variables were applied.
To detect the factors related to previous CVD events, logistic regression analyses were done. Individual values, deciles, or categories of eGFR and albuminuria or CKD types, such as nonalbuminuric stage ≥3 CKD, albuminuric stage ≥3 CKD, or stages 1 to 2 CKD and no CKD as a reference category, as covariates were together with age, sex, smoking, duration of diabetes, HbA1c, levels of triglycerides, HDL-C, LDL-C, and hsCRP, lipid-lowering treatment, antihypertensive medication, and retinopathy. The analyzed results were described as the odds ratios with their 95% confidence interval. A P<0.05 was considered statistically significant. Statistical analyses were executed using SPSS version 21.0 (IBM Co., Armonk, NY, USA).
RESULTS
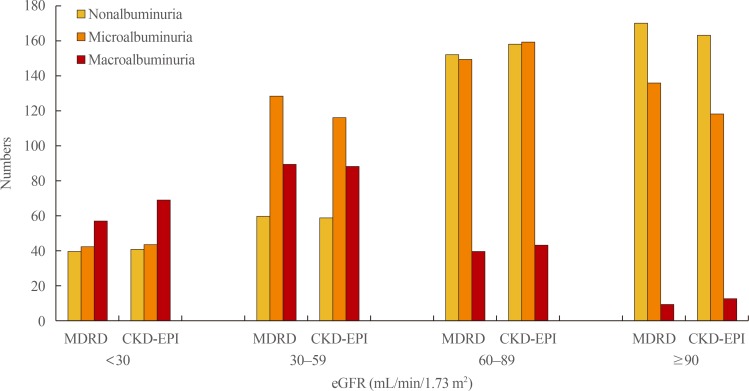
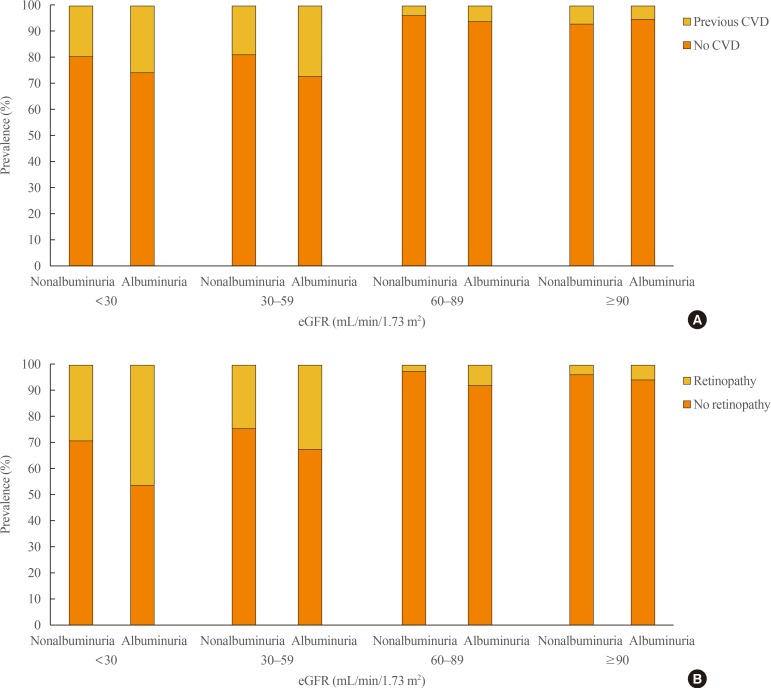
We enrolled 1,067 study subjects (561 males [52.5%] and 506 females [47.5%]). The mean enrolled patients' age was 59.5±15.9 year. Median diabetic duration was 6.2 years, and mean value of eGFR calculated by the CKD-EPI formula was greater than by the MDRD study formula in the study subjects (66.9 mL/min/1.73 m2 vs. 64.9 mL/min/1.73 m2, correspondingly). Study subjects were allotted to one of the following eGFR classes: class 1 (≥90), class 2 (60 to 89), class 3 (30 to 59), and class 4 (<30). When calculated by the MDRD study equation, prevalent rates of classes 1, 2, 3, and 4 were 37.2%, 23.9%, 24.3%, and 14.5%, respectively. The prevalent rates of classes 1, 2, 3, and 4 were 38.1%, 25.8%, 24.4%, and 11.7%, respectively, when measured by the CKD-EPI formula. Prevalent rates of non-, micro-, and macroalbuminuria were 39.3%, 42.5%, and 18.2%, respectively (Fig. 1). The occurrences of previous CVD events and retinopathy significantly increased according to eGFR classes and degree of albuminuria (Fig. 2). Of the 414 patients with declined eGFR, 98 subjects were nonalbuminuric and 316 subjects were albuminuric. Among those with declined eGFR, 310 subjects did not have any history of CVD events and 384 subjects presented without albuminuria and previous CVD events, whereas any CVD history were presented in 20 subjects with stages 1 to 2 CKD, 105 subjects having both albuminuria and previous CVD events. Amongst those with declined eGFR, 153 subjects showed retinopathy and the other 261 subjects lacked retinopathy. Additionally, 383 subjects had neither albuminuria nor retinopathy, and only 149 subjects had both albuminuria and retinopathy (Table 1).
Fig. 1. Prevalence of non-, micro-, and macroalbuminuria according to classes of estimated glomerular filtration rate (eGFR) by the Modification of Diet in Renal Disease (MDRD) study and the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) formulas.
Fig. 2. Prevalence of (A) previous cardiovascular disease (CVD) and (B) retinopathy according to degree of albuminuria and classes of estimated glomerular filtration rate (eGFR) by the Modification of Diet in Renal Disease study equation.
Table 1. Clinical Characteristics of Study Subjects with No CKD, Stages 1 to 2 CKD, Nonalbuminuric, and Albuminuric Stage ≥3 CKD.
| Variable | No CKD | Stages 1–2 CKD | Nonalbuminuric stage ≥3 CKD | Albuminuric stage ≥3 CKD | P value |
|---|---|---|---|---|---|
| Number | 321 (30) | 332 (31) | 98 (9) | 316 (30) | |
| Age, yr | 55.5±11.1 | 55.3±11.6 | 66.4±11.4 | 60.9±12.8 | <0.001 |
| Male sex | 189 (59) | 186 (56) | 60 (61) | 126 (40) | 0.016 |
| DM duration, yr | 3.1±2.2 | 4.6±3.0 | 6.8 ±4.1 | 10.5±6.2 | <0.001 |
| BMI, kg/m2 | 24.6±3.8 | 25.6±4.1 | 24.1±4.2 | 24.7±4.7 | 0.752 |
| SBP, mm Hg | 126±17 | 128±17 | 134±17 | 138±25 | <0.001 |
| DBP, mm Hg | 72±11 | 72±12 | 85±14 | 86±15 | <0.001 |
| BUN, mg/dL | 15.0±4.1 | 15.2±4.5 | 29.4±8.4 | 32.1±11.9 | <0.001 |
| Serum creatinine, mg/dL | 0.81±0.18 | 0.83±0.18 | 2.12±0.63 | 2.43±0.85 | <0.001 |
| HbA1c, % | 7.4±1.1 | 7.7±1.5 | 8.1±1.9 | 8.8±1.1 | 0.012 |
| Total cholesterol, mg/dL | 177±31 | 179±34 | 189±25 | 190±37 | 0.051 |
| LDL-C, mg/dL | 91±19 | 94±28 | 110±30 | 109±28 | 0.109 |
| Triglyceride, mg/dL | 185±99 | 188±83 | 192±103 | 190±93 | 0.256 |
| HDL-C, mg/dL | 49±7 | 48±6 | 41±7 | 43±10 | 0.010 |
| eGFR, mL/min/1.73 m2 | 88.4±10.7 | 90.2±11.3 | 42.1±10.0 | 39.1±11.1 | <0.001 |
| Urine ACR, mg/g | 16.9±4.5 | 223.2±81.3 | 18.7±5.9 | 1,032.5±88.1 | <0.001 |
| hsCRP, mg/dL | 0.10±0.06 | 0.10±0.08 | 0.11±0.35 | 0.15±0.29 | <0.001 |
| Smoking | 144 (45) | 143 (43) | 38 (39) | 133 (42) | 0.249 |
| Retinopathy | 9 (3) | 23 (7) | 27 (28) | 126 (40) | <0.001 |
| Previous CVD events | 16 (5) | 20 (6) | 19 (19) | 85 (27) | 0.002 |
| Coronary artery diseases | 9 | 10 | 12 | 65 | |
| Cerebrovascular diseases | 7 | 8 | 7 | 16 | |
| Peripheral vascular diseases | 0 | 2 | 0 | 4 | |
| Statin | 144 (45) | 156 (47) | 59 (60) | 205 (65) | <0.001 |
| Antihypertensive drugs | 202 (63) | 225 (68) | 71 (72) | 246 (78) | <0.001 |
| ACEIs/ARBs | 173 (54) | 189 (57) | 67 (68) | 186 (59) | <0.001 |
Values are expressed as number (%) or mean±SD. eGFR was estimated by the Modification of Diet in Renal Disease study equation. Patients who co-use ACEIs/ARBs and other antihypertensive drugs were counted in duplicate.
CKD, chronic kidney disease; DM, diabetes mellitus; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; BUN, blood urea nitrogen; HbA1c, glycated hemoglobin; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; ACR, albumin-creatinine ratio; hsCRP, high-sensitivity C-reactive protein; CVD, cardiovascular disease; ACEI, angiotensinconverting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Nonalbuminuric stage ≥3 CKD group had shorter diabetic duration, lower concentrations of HbA1c, HDL-C, and hsCRP, lower prevalent rates of retinopathy and previous CVD, and higher rate of treatment with angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers (ACEIs/ARBs), than albuminuric stage ≥3 CKD group (Table 1). Likewise, when comparing no CKD group, nonalbuminuric stage ≥3 CKD group was more nonsmokers and had lower concentration of HDL-C, higher rates of treatment with ACEIs/ARBs and statin, and higher rates of previous CVD events and retinopathy (Table 1).
Subjects with nonalbuminuria and subnormal eGFR categories (60 to 89 mL/min/1.73 m2), when comparing with normal renal function (≥90 mL/min/1.73 m2), were not significantly related with previous CVD events. However, its relevance was significantly increasing for those with declined renal function (<60 mL/min/1.73 m2), which was greater for eGFR of <30 mL/min/1.73 m2, and albuminuria (Table 2).
Table 2. Logistic Regression Analysis of Previous Cardiovascular Disease Events with Categories of eGFR and Albuminuria as Covariates.
| Variable | OR | 95% CI |
|---|---|---|
| Albuminuria categories, mg/g | ||
| <30 | 1.0 | - |
| 30–299 | 1.199 | 1.126–1.272 |
| ≥300 | 1.329 | 1.215–1.443 |
| eGFR categories, mL/min/1.73 m2 | ||
| ≥90 | 1.0 | - |
| 60–89 | 1.085 | 0.968–1.202 |
| 30–59 | 1.438 | 1.285–1.591 |
| <30 | 1.739 | 1.486–1.992 |
eGFR was estimated by the Modification of Diet in Renal Disease study equation.
eGFR, estimated glomerular filtration rate; OR, odds ratio; CI, confidence interval.
According to the logistic regression analyses, there were positive correlations of male sex, diabetic duration, current smoking, and hypertension with occurrence of any CVD events; conversely, HDL-C level was negatively correlated (Table 3). Non-albuminuric stage ≥3 CKD group showed a stronger association with previous CVD events than no CKD group when CKD categories were considered as covariates; however, albuminuric stage ≥3 CKD group showed an augmented increase in the prevalent rate of any CVD events (Table 3).
Table 3. Logistic Regression Analysis of Previous CVD Events with Individual Categories as Covariates.
| Variable | OR | 95% CI |
|---|---|---|
| Age (1 year) | 1.065 | 1.047–1.083 |
| Male sex | 1.167 | 1.138–1.196 |
| DM duration (1 year) | 1.127 | 1.087–1.167 |
| Smoking status | ||
| Never | 1.000 | - |
| Ex/Current | 1.188 | 1.024–1.352 |
| Hypertension | 1.998 | 1.785–2.211 |
| Triglyceride (10 mg/dL) | 1.015 | 0.998–1.032 |
| HDL-C (5 mg/dL) | 0.928 | 0.909–0.947 |
| Retinopathy | ||
| Absent | 1.000 | - |
| Present | 1.403 | 1.223–1.582 |
| CKD categories | ||
| No CKD | 1.000 | - |
| Stages 1–2 CKD | 1.217 | 1.091–1.343 |
| Nonalbuminuric stage ≥3 CKD | 1.499 | 1.349–1.649 |
| Albuminuric stage ≥3 CKD | 1.918 | 1.695–2.141 |
CVD, cardiovascular disease; OR, odds ratio; CI, confidence interval; DM, diabetes mellitus; HDL-C, high density lipoprotein cholesterol; CKD, chronic kidney disease.
DISCUSSION
We found that nonalbuminuric stage ≥3 CKD group had lower prevalent rates of retinopathy and previous CVD than albuminuric stage ≥3 CKD group. In addition, albuminuric stage ≥3 CKD group showed a stronger association with retinopathy than those with the normal eGFR with or without albuminuria. We also showed that nonalbuminuric stage ≥3 CKD group was significantly related with the incidence of any CVD events than no CKD. There is a consistency between the higher CVD prevalent rate observed in albuminuric stage ≥3 CKD group and the additional cardiovascular risk associated with combination of declined eGFR and increased degree of albuminuria [14]. Therefore, nonalbuminuric form of renal insufficiency is the clinically distinctive entity of renal diseases in T2DM, and it should be considered in the management of these patients. There is a weak association between previous CVD events and patients with albuminuria alone because patients with increased degree of albuminuria and normal/subnormal eGFR were assigned to the early stage of CKD than patients with declined eGFR, without regard to albuminuria. But on the other, when compared with nonalbuminuric stage ≥3 CKD group, the additively higher cardiovascular burden associated with the integration of declined eGFR and albuminuria bears out that patients with albuminuric stage ≥3 CKD should be considered at higher cardiovascular risk, which is in line with previous studies [8,10]. Microalbuminuria is a first sign of renal dysfunction based on the clinical progression of diabetic kidney diseases [21]. Thus, in diabetic patients, it is important to measure and monitor excretion of urine albumin, as a predictive clinical marker of chronic renal complications, for preventing overt diabetic nephropathy [22,23]. Measuring excretion of albumin in 24 hours collected urine sample is the standard method [24], although a substitute way is measuring excretion of albumin and creatinine in single spot urine sample [25,26]. Ratio of albumin and creatinine excretion was accepted as a reliable screening method for selecting study population in many trials [25,27]. However, the early loss of renal function may occur even in the condition without albuminuria in some T1DM with a long duration, and this is more of a common occurrence than in T2DM. The mechanism of this is not clear; however, possible factors of nonalbuminuric form of renal insufficiency are renal nephron loss according to aging, interstitial fibrosis, cholesterol emboli, and atherosclerotic changes in renal vasculature [28]. To interpret the growing prevalent rate of nonalbuminuric renal insufficiency, the impact of recent treatment trends should be also considered. For example, during the last two decades, there has been an intense rise in hypertension and/or renal insufficiency treated with RAS inhibitors in patients with T2DM [8]. The role of RAS blocking agents have distinct effects on degrees of both GFR and albuminuria [10,23]. In our study subjects, 72% of patients were used with such a treatment; and a greater proportion has been described previously [8]. Moreover, the rising prevalent rate of nonalbuminuric renal insufficiency may reveal alterations in the fundamental renal pathology with macrovascular lesions prevailing against microvascular lesions [24]. Such an alteration may also be attributed to the recent changes in treatment modality, especially to the intensive management of blood pressure, glucose, and lipids, which has been achieved in diabetic patients established on results of previous trials [25,27].
Matsushita et al. [28] has proposed that CKD-EPI formula was able to more accurately detect patients in respect to risk of cardiovascular events and death compared to MDRD study equation. When we computed eGFR using CKD-EPI formula in the study, the proportions of patients were nearly equivalent to those calculated using MDRD study equation, and prevalent rate of previous CVD events also did not change significantly.
According to the preceding survey, about 30% of patients with T2DM had stage ≥3 CKD without both retinopathy and albuminuria [29]. In several studies, when compared with the albuminuric renal insufficiency group, nonalbuminuric renal insufficiency group was marked by showing a preponderance of female sex, older age, higher HDL-C, and possibly better prognosis [7,30,31]. In our study, nonalbuminuric stage ≥3 CKD group had shorter diabetic duration, lower concentrations of HbA1c, HDL-C, and hsCRP, lower prevalent rates of retinopathy and previous CVD, and higher rate of treatment with ACEIs/ARBs compared with albuminuric stage ≥3 CKD group. Furthermore, when compared with no CKD group, non-albuminuric stage ≥3 CKD group was more often nonsmokers, and had lower concentration of HDL-C, higher rates of therapeutic intervention with statin and ACEIs/ARBs, but higher prevalent rates of retinopathy and prior CVD events. Our results are somewhat similar to those from studies of Caucasian population, but also different in other aspects with respect to non-smoker status and HDL-C level.
When CKD categories were applied to the statistical model as covariates, nonalbuminuric stage ≥3 CKD group has a stronger statistical significance with prior CVD events than no CKD group; however, the prevalent rate of prior CVD events in albuminuric stage ≥3 CKD group was markedly increased in additional manner than no CKD group. These results show a somewhat similar but more powerful relations than those shown in previous report [17]. We also showed that albuminuric stage ≥3 CKD group had the strongest prevalent rate of prior CVD events. This is concomitant with the perception that a high-powered predictor of cardiovascular death in the public and in diabetes is a decreased renal function, irrespective of conventional CVD risk factors including albuminuria [10,11]. A more powerful correlation between cardiovascular events and declined eGFR than albuminuria can be explained by the distinctions in the kidney pathophysiology that underlies the albuminuric and nonalbuminuric forms of renal insufficiency. Although it may occur even if not have significant renal damages, microalbuminiuria is considered to be a clinically reliable marker for renal microvascular lesions [32,33]. On the other hand, eGFR had an inverse association with the indicators of both extrarenal and intrarenal atherosclerotic lesions [7,34]. Vascular cell dysfunction and resulting atherosclerotic changes are specious pathophysiologic mechanism for the connection between declined renal function with albuminuria and cardiovascular death [25,33]. Therefore, albuminuric renal insufficiency, as a prevalent feature of renal macrovascular lesions, would be more commonly related with cardiovascular events.
The limitations of the study are as follows. First, the cross-sectional design and small number of study population limit the inferences of the observed causal relations between relevant factors of nonalbuminuric renal insufficiency and occurrence of any CVD events. Second, this study was conducted at a single-center hospital. Therefore, the making generalization from our results to the entire the Korean patients with T2DM is weak. Third, the assessment of degree of albuminuria was done with only one spot urine sample. A single urine specimen might not exactly estimate albuminuria compared with 24 hours urine collection.
In conclusion, we confirm that nonalbuminuric stage ≥3 CKD group is significantly interrelated with occurrence of prior CVD events than group with normal eGFR with or without albuminuria. Comparing with normal eGFR and nonalbuminuria categories, the combination of increased degree of albuminuria and declined eGFR is becoming significant for the association of prior CVD events. Additional large prospective studies will be required in order to clear up the relevant causes for the development of any CVD events in nonalbuminuric renal insufficiency in patients with T2DM.
ACKNOWLEDGMENTS
This study was supported for 2 years by Pusan National University Research Grant.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332:73–78. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brancati FL, Whelton PK, Randall BL, Neaton JD, Stamler J, Klag MJ. Risk of end-stage renal disease in diabetes mellitus: a prospective cohort study of men screened for MRFIT. Multiple Risk Factor Intervention Trial. JAMA. 1997;278:2069–2074. [PubMed] [Google Scholar]
- 3.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 5.Anavekar NS, Gans DJ, Berl T, Rohde RD, Cooper W, Bhaumik A, et al. Predictors of cardiovascular events in patients with type 2 diabetic nephropathy and hypertension: a case for albuminuria. Kidney Int Suppl. 2004;92:S50–S55. doi: 10.1111/j.1523-1755.2004.09213.x. [DOI] [PubMed] [Google Scholar]
- 6.Soveri I, Arnlov J, Berglund L, Lind L, Fellstrom B, Sundstrom J. Kidney function and discrimination of cardiovascular risk in middle-aged men. J Intern Med. 2009;266:406–413. doi: 10.1111/j.1365-2796.2009.02122.x. [DOI] [PubMed] [Google Scholar]
- 7.Thomas MC, Weekes AJ, Broadley OJ, Cooper ME, Mathew TH. The burden of chronic kidney disease in Australian patients with type 2 diabetes (the NEFRON study) Med J Aust. 2006;185:140–144. doi: 10.5694/j.1326-5377.2006.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 8.Thomas MC, Macisaac RJ, Jerums G, Weekes A, Moran J, Shaw JE, et al. Nonalbuminuric renal impairment in type 2 diabetic patients and in the general population (national evaluation of the frequency of renal impairment co-existing with NIDDM [NEFRON] 11) Diabetes Care. 2009;32:1497–1502. doi: 10.2337/dc08-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afghahi H, Cederholm J, Eliasson B, Zethelius B, Gudbjornsdottir S, Hadimeri H, et al. Risk factors for the development of albuminuria and renal impairment in type 2 diabetes: the Swedish National Diabetes Register (NDR) Nephrol Dial Transplant. 2011;26:1236–1243. doi: 10.1093/ndt/gfq535. [DOI] [PubMed] [Google Scholar]
- 10.Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20:1813–1821. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drury PL, Ting R, Zannino D, Ehnholm C, Flack J, Whiting M, et al. Estimated glomerular filtration rate and albuminuria are independent predictors of cardiovascular events and death in type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia. 2011;54:32–43. doi: 10.1007/s00125-010-1854-1. [DOI] [PubMed] [Google Scholar]
- 12.Lane PH, Steffes MW, Mauer SM. Glomerular structure in IDDM women with low glomerular filtration rate and normal urinary albumin excretion. Diabetes. 1992;41:581–586. doi: 10.2337/diab.41.5.581. [DOI] [PubMed] [Google Scholar]
- 13.Tsalamandris C, Allen TJ, Gilbert RE, Sinha A, Panagiotopoulos S, Cooper ME, et al. Progressive decline in renal function in diabetic patients with and without albuminuria. Diabetes. 1994;43:649–655. doi: 10.2337/diab.43.5.649. [DOI] [PubMed] [Google Scholar]
- 14.Chronic Kidney Disease Prognosis Consortium. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruno G, Merletti F, Bargero G, Novelli G, Melis D, Soddu A, et al. Estimated glomerular filtration rate, albuminuria and mortality in type 2 diabetes: the Casale Monferrato study. Diabetologia. 2007;50:941–948. doi: 10.1007/s00125-007-0616-1. [DOI] [PubMed] [Google Scholar]
- 16.Penno G, Solini A, Bonora E, Fondelli C, Orsi E, Zerbini G, et al. Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J Hypertens. 2011;29:1802–1809. doi: 10.1097/HJH.0b013e3283495cd6. [DOI] [PubMed] [Google Scholar]
- 17.Solini A, Penno G, Bonora E, Fondelli C, Orsi E, Arosio M, et al. Diverging association of reduced glomerular filtration rate and albuminuria with coronary and noncoronary events in patients with type 2 diabetes: the renal insufficiency and cardiovascular events (RIACE) Italian multicenter study. Diabetes Care. 2012;35:143–149. doi: 10.2337/dc11-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hostetter TH. Prevention of the development and progression of renal disease. J Am Soc Nephrol. 2003;14(7 Suppl 2):S144–S147. doi: 10.1097/01.asn.0000070150.60928.06. [DOI] [PubMed] [Google Scholar]
- 22.Heerspink HJ, Holtkamp FA, de Zeeuw D, Ravid M. Monitoring kidney function and albuminuria in patients with diabetes. Diabetes Care. 2011;34(Suppl 2):S325–S329. doi: 10.2337/dc11-s247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int. 2004;65:2309–2320. doi: 10.1111/j.1523-1755.2004.00653.x. [DOI] [PubMed] [Google Scholar]
- 24.Rowe DJ, Dawnay A, Watts GF. Microalbuminuria in diabetes mellitus: review and recommendations for the measurement of albumin in urine. Ann Clin Biochem. 1990;27(Pt 4):297–312. doi: 10.1177/000456329002700404. [DOI] [PubMed] [Google Scholar]
- 25.Croal BL, Mutch WJ, Clark BM, Dickie A, Church J, Noble D, et al. The clinical application of a urine albumin: creatinine ratio point-of-care device. Clin Chim Acta. 2001;307:15–21. doi: 10.1016/s0009-8981(01)00450-8. [DOI] [PubMed] [Google Scholar]
- 26.Marshall SM. Screening for microalbuminuria: which measurement? Diabet Med. 1991;8:706–711. doi: 10.1111/j.1464-5491.1991.tb01688.x. [DOI] [PubMed] [Google Scholar]
- 27.Rychlik I, Jancova E, Tesar V, Kolsky A, Lacha J, Stejskal J, et al. The Czech registry of renal biopsies. Occurrence of renal diseases in the years 1994-2000. Nephrol Dial Transplant. 2004;19:3040–3049. doi: 10.1093/ndt/gfh521. [DOI] [PubMed] [Google Scholar]
- 28.Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307:1941–1951. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kramer HJ, Nguyen QD, Curhan G, Hsu CY. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA. 2003;289:3273–3277. doi: 10.1001/jama.289.24.3273. [DOI] [PubMed] [Google Scholar]
- 30.MacIsaac RJ, Panagiotopoulos S, McNeil KJ, Smith TJ, Tsalamandris C, Hao H, et al. Is nonalbuminuric renal insufficiency in type 2 diabetes related to an increase in intrarenal vascular disease? Diabetes Care. 2006;29:1560–1566. doi: 10.2337/dc05-1788. [DOI] [PubMed] [Google Scholar]
- 31.Rigalleau V, Lasseur C, Raffaitin C, Beauvieux MC, Barthe N, Chauveau P, et al. Normoalbuminuric renal-insufficient diabetic patients: a lower-risk group. Diabetes Care. 2007;30:2034–2039. doi: 10.2337/dc07-0140. [DOI] [PubMed] [Google Scholar]
- 32.Futrakul N, Sridama V, Futrakul P. Microalbuminuria: a biomarker of renal microvascular disease. Ren Fail. 2009;31:140–143. doi: 10.1080/08860220802595948. [DOI] [PubMed] [Google Scholar]
- 33.Fioretto P, Mauer M, Brocco E, Velussi M, Frigato F, Muollo B, et al. Patterns of renal injury in NIDDM patients with microalbuminuria. Diabetologia. 1996;39:1569–1576. doi: 10.1007/s001250050616. [DOI] [PubMed] [Google Scholar]
- 34.Taniwaki H, Nishizawa Y, Kawagishi T, Ishimura E, Emoto M, Okamura T, et al. Decrease in glomerular filtration rate in Japanese patients with type 2 diabetes is linked to atherosclerosis. Diabetes Care. 1998;21:1848–1855. doi: 10.2337/diacare.21.11.1848. [DOI] [PubMed] [Google Scholar]