Abstract
CD28 is a primary co-stimulatory receptor that is essential for successful T cell activation, proliferation, and survival. While ubiquitously expressed on naive T cells, the level of CD28 expression on memory T cells is largely dependent on the T-cell differentiation stage in humans. Expansion of circulating T cells lacking CD28 was originally considered a hallmark of age-associated immunological changes in humans, with a progressive loss of CD28 following replicative senescence with advancing age. However, an increasing body of evidence has revealed that there is a significant age-inappropriate expansion of CD4+CD28− T cells in patients with a variety of chronic inflammatory diseases, suggesting that these cells play a role in their pathogenesis. In fact, expanded CD4+CD28− T cells can produce large amounts of proinflammatory cytokines such as IFN-γ and TNF-α and also have cytotoxic potential, which may cause tissue damage and development of pathogenesis in many inflammatory disorders. Here we review the characteristics of CD4+CD28− T cells as well as the recent advances highlighting the contribution of these cells to several disease conditions.
Keywords: CD28, Co-stimulatory receptor, CD4+CD28− T cells, Chronic inflammatory diseases, Cytotoxic potential
INTRODUCTION
CD4 T cells play a critical role in orchestrating immune responses by helping humoral and cellular immune cells (1). Successful CD4 T-cell activation is guaranteed by two signals; TCR stimulation as the primary signal (signal 1) and antigen-independent costimulation as the secondary signal (signal 2) (2,3). CD28 is a primary co-signaling receptor that transduces a costimulatory signal 2 and is essential for successful T cell activation, proliferation, and survival (3,4). Furthermore, the CD28 molecule is constitutively expressed on naive and memory T cells. However, in humans there is a marked loss of CD28 expression on terminally-differentiated effector memory T cells (5). Repeated antigenic stimulation over a lifetime results in the generation of this terminally-differentiated T-cell subset following extensive division (6). Thus, CD28 is progressively lost after replicative senescence with advancing age (7). The molecular mechanisms controlling CD28 expression and loss appear to be similar in both T-cell subsets, but the age-associated accumulation of CD28− T cells is more prominent in CD8 T cells than CD4 T cells (8,9). Expansion of CD4+CD28− T cells in part reflects the infectious burden from latent viral infections such as CMV and EBV and leads to generating memory inflation in the elderly (10). Accumulating evidence reveals that there is a significant age-inappropriate expansion of cytotoxic CD4+CD28− T cells in patients with a variety of chronic inflammatory diseases, suggesting that these cells might play a role in the pathogenesis of these immune disorders (11,12,13,14,15). Despite the lack of CD28 expression on these cells, they are not anergic, but rather respond to stimulation in a costimulation independent nature. Moreover, loss of CD28 is closely linked to changes in the transcriptional program resulting in production of potent effector cells exhibiting atypical cytotoxic capacity and inflammatory cytokineproducing potential (7,11,16).
The biological role of CD4+CD28− T cells has been extensively reviewed elsewhere. Therefore, this review will focus on physiological and pathological characteristics of CD4+CD28− T cells and recent advances in the understanding of the role of CD4+CD28− T cells in several disease conditions.
CD4+CD28− T-CELL BIOLOGY
The mechanism underlying loss of CD28 molecules
CD28 is constitutively expressed on all T cells at birth in humans, but its expression level is changed during activation and differentiation of T cells in a lifetime (4). Considering that the majority of CD28− T cells are of the terminally-differentiated memory subset and that these cells accumulate gradually with age, replicative senescence caused by repeated antigenic stimulation, such as latent viral infection, is considered to be a primary cause of CD28 loss in T cells. Indeed, in vitro culture experiments clearly demonstrated that highly purified CD28+ T cells progressively lost CD28 expression after repeated TCR stimulation (9,17). In addition, the loss of CD28 also occurs when T cells are exposed to proinflammatory cytokines (18,19,20,21). Of interest, chronic exposure to TNF-α leads to downregulation of the CD28-specific initiator complex and consequently results in decreased CD28 expression in CD4+ T cells at the transcriptional level (20,22,23). Furthermore, repeated antigenic stimulation leads to chronic inflammation, making proinflammatory cytokines such as TNF-α abundant and therefore, loss of CD28 due to both replicative senescence and cytokine exposure may not be mutually exclusive in inflammatory disorders (10). Moreover, increased TNF-α is one of the features of inflammaging, which is a low-grade, chronic, systemic pro-inflammatory state frequently observed in the elderly that is associated with the unexpected upregulation of proinflammatory responses later in life (10,24,25,26). Although CD4+ T cells are more resistant to age-associated phenotypic and functional changes than CD8 T cells, CD4+CD28− T cells is also increased with advancing age in healthy individuals (9). Therefore, it will be an intriguing question whether increased TNF-α in the elderly affects the accumulation of CD4+CD28− T cells with advancing age.
Of interest, loss of CD28 by T cells is exclusively observed in humans and non-human primates, and not in mice (7,27,28). Murine CD28 is expressed throughout the lifetime and in some strains its expression is even higher in geriatric mice than in younger mice (29). It should be noted that telomeres are 5~10 fold longer in mice than in humans and mice have much shorter lifespan (30), suggesting that telomere erosion may not play a significant role in regulating murine T cell memory, especially loss of CD28. Telomerase is the enzyme that extends telomeres and is induced in T cells by repeated antigenic stimulation. It has been demonstrated that antigen-specific telomerase inducibility is dependent on CD28 expression and the decline in telomerase activity parallels the loss of CD28 expression in human T cells (17). In humans, induction of telomerase, the enzyme that extends telomeres, accompanies Therefore, a limited number of CD28− T cell studies have utilized human and non-human primate cells in vitro (31).
Characteristics of CD4+CD28− T cells
Functionally, CD4+CD28− T cells can be defined as typical Th1 cells, which produce a large amount of IFN-γ and TNF-α and express increased levels of the T-bet transcription factor (32,33,34)(manuscripts in preparation). Moreover, unlike conventional Th1 cells these cells also express the cytotoxic molecules perforin and granzyme B (33), and it has been suggested that their cytotoxic features are responsible for tissue damage and development of pathogenesis in many inflammatory diseases. There are still debates on antigen specificity and possible activation mechanism of CD4+CD28− T cell. Given their oligoclonality, ubiquitous antigens including auto-antigens have been suggested as specific antigens for CD4+CD28− T cell. Alternatively, other molecules such as ligands for receptor, cytokine, adhesion molecules rather than antigen also have been suggested as their activation cues (35). CD4+CD28− T cells exhibit distinct surface expression profiles different from their CD28+ counterparts (36). Furthermore, like CD4+CD28− T cells, CD4+CD28− T cells also exhibit a phenotype typical of senescent T cells. In addition, the expression of many natural killer cell receptors (NKRs), including CD11b, CD57, CD85j, NKG2D and KIR2DS2, is an important characteristic of CD4+CD28− T cells (9,12,37,38,39) and might be involved in modulating their functional activity. Loss of CD28 on CD4+ T cells also coincides with decreased expression of CD40L and ICAM-1, implying a dysregulation of B-cell differentiation and immunoglobulin secretion and enhanced migratory potential, respectively (32). Virtually all CD4+CD28− T cells are lacking of the costimulatory CD27 receptor but overexpress CD70, the ligand for CD27, suggesting a possible role in modulating T cell activation (40). Moreover, recent studies demonstrated that CD4+CD28− T cells exhibit constitutive activation of p38 through the unconventional AMPK-TAB-1 signaling pathway. This finding suggests that DNA damage and dysregulation of glucose metabolism might be associated with characteristics of CD4+CD28− T cells (41,42).
CLINICAL RELEVANCE OF CD4+CD28− T CELLS
CD4+CD28− T cells in rheumatoid arthritis (RA)
CD4+CD28− T cells were initially identified and primarily characterized in patients with RA, a prototype systemic autoimmune disease that manifests through massive and chronic inflammatory infiltrates and bone destruction in joints (12,43,44,45). The frequency of circulating CD4+CD28− T cells in RA is positively correlated with disease severity and the presence of extra-articular manifestation (46). In early studies, the pathogenic role of these cells in RA was suggested by the strong cytotoxic potential, resistance to apoptosis, and tissue-infiltrating capacity of CD4+CD28− T cells (38,47,48,49,50). Furthermore, CD4+CD28− T cells are oligoclonal with limited TCR diversity and have shortened telomeres, implying that an expansion of these cells is a consequence of repeated exposure to the same antigen, possibly an autoantigen (35). However, it is difficult to prove whether the CD4+CD28− T-cell subset is a product of the autoreactive response because RA is a typical systemic autoimmune disease and its autoantigen is not yet defined (7). Rather, several studies have suggested that CD4+CD28− T cells in RA are probably not just autoreactive T cells. First, CD4+CD28− T cells are observed more infrequently in synovial fluid and extra-articular tissue than in peripheral blood of RA patients (43). Secondly, the dominant TCR-Vβ subsets of circulating CD4+CD28− T cells are often missing in synovial fluid (43). Finally, these cells tend to be more robustly stimulated by ubiquitous antigens, such as heat shock proteins or CMV antigens, than collagen, a putative autoantigen in RA. CD4+CD28− T cells also show no association with antibodies to anti-citrullinated protein, another putative autoantigen in RA, in the serum or synovial fluid (43,51,52). Moreover, accumulating evidence demonstrates that chronic stimulation with proinflammatory cytokines such as TNF-α and IL-15 is responsible for the expansion of CD4+CD28− T cells in humans (20,53,54).
The loss of CD28 is closely associated with the acquisition of multiple regulatory molecules on the T cell surface (9,16,55). Utilizing these regulatory molecules, the CD4+CD28− T cell subset on its own or through its interactions with other immune cells might contribute to the perpetuation and amplification of inflammatory responses. It has recently been shown that a variety of NKRs, such as NKG2D, KIR2DS2, 2B4, and DNAM-1, are upregulated by CD4+CD28− T cells, and activation of these NKRs can provide co-stimulatory signals during TCR activation (56). In addition, CD4+CD28− T cells in RA patients aberrantly sustain CD70 express for a longer time after T cell activation than do counterpart CD28+ T cells. When expressed on CD4+CD28− T cells, CD70 can act as a bystander costimulatory signal to naive CD27+CD4+ T cells and facilitate their activation and proliferation though the CD70-CD27 interaction. This causes lowering of the activation threshold of CD4+CD28− T cells themselves as well as other immune cells, rendering cell activation completely independent of recognition of the appropriate antigenic peptide (40). Moreover, a majority of cytotoxic CD4+CD28− T cells in RA patients express CX3CR1, a specific chemokine receptor for CX3CL1, which is abundantly produced by fibroblast-like synoviocytes (FLS) in the RA synovium. Thus, these cells can selectively migrate into inflamed tissue through the CX3CL1-CX3CR1 interaction, further supporting their pathogenic roles in patients with RA (57,58). In summary, several studies have provided substantial evidence for a contributing role of CD4+CD28− T cells in the pathogenesis of RA.
CD4+CD28− T cells in vascular-related diseases
The increased frequency of CD4+CD28− T cells has been associated with various types of cardiovascular diseases such as atherosclerosis and acute coronary syndromes (ACS). Expansion of CD4+CD28− T cells was initially identified in peripheral blood of patients with unstable angina (UA) (13), an ACS that is generally provoked by unstable atherosclerotic plaque rupture. Consistent with general features of CD4+CD28− T cells in RA, in UA these cells also produce significant amounts of IFN-γ and the cytotoxic components, perforin and granzyme (13). In a vascular disease setting, IFN-γ is associated with activation of macrophages to secrete metalloproteases, which can destabilize the fibrous cap surrounding the plaque (59). On the other hand, perforin and granzyme induce endothelial and vascular smooth muscle cell damage through direct lysis (60). Furthermore, a variety of extracellular proteins are cleaved by granzyme, which consequently, results in matrix remodeling and cell detachment. These mechanisms were clinically corroborated by the finding that CD4+CD28− T cells are preferentially accumulated in coronary atherosclerotic plaques, but not in stable lesions (61). The expansion of CD4+CD28− T cells has also been associated with the recurrence of acute coronary events, showing that the increased frequency of these cells remains relatively stable after the acute episode in patients with UA (62). These findings imply that CD4+CD28− T-cell expansion is not just a consequence of the inflammatory response triggered by acute events, but rather reflects the chronic inflammatory response to certain viral infections or autoantigens (e.g. human heat shock protein 60), which are present in atherosclerotic plaques (15). In addition, a multivariate logistic regression analysis of ACS patients showed that the frequency of CD4+CD28− T cells is an independent predictor of future acute coronary events (62). Moreover, recent data demonstrated that statins as well as anti-TNF-α treatment can decrease the circulating CD4+CD28− T-cell level, suggesting that these cells are a promising therapeutic target for the prevention of acute coronary events (15,63).
In addition to the direct role of these cells in vascular disease, Betjes and colleagues recently demonstrated that the relative and absolute numbers of circulating CD4+CD28− T cells is massively expanded in patients with end-stage renal diseases (ESRD) (14). In ESRD, the substantially elevated risk of cardiovascular diseases is closely linked to uremia-related immune activation such as hypercytokinemia and inflammation (64). Thus, this implies that the expanded CD4+CD28− T cell population also plays a pathogenic role in ESRD. Furthermore, patients with ESRD suffer a remarkably high risk for acute atherosclerotic vascular events shortly after kidney transplantation (65).
CD4+CD28− T cells in solid organ transplantation
Of interest, the clinical relevance of CD4+CD28− T cells was first explored in the late 1980s in renal transplant recipients by monitoring of their frequency (66). It was noted that there was an increase of CD4+CD28− T cells in patients after renal or liver transplantation and their expansion is associated with chronic graft rejection (66,67,68). Considering the primary role of CD28 as a co-stimulatory molecule for T cell activation, it was reasonable to target the CD28-B7 interaction for costimulation blockade therapies as an alternative to calcineurin inhibitor (CNI)-based therapy. Consequently, functional blockade of CD28 by belatacept, a human CTLA-4-Ig fusion protein, has recently become a clinically important immunosuppressive strategy. However, co-stimulation blockade resistant rejection (CoBRR) has increasingly been observed in kidney transplant patients and therefore, efforts to identify potential explanations for CoBRR are ongoing. It should be noted that the loss of CD28 on CD4 T cells has been implicated in promoting immunosuppression resistance and allograft rejection (69,70) due to the cytotoxic capacity of these cells. Very recently it was reported that increases in circulating CD4+CD28− CD57+ T cells prior to transplantation is associated with belatacept resistant rejection (BRR) post-transplantation (71,72). Furthermore, cytomegalovirus-related, cytotoxic CD4+CD28− T cells potentiate kidney allograft dysfunction by glomerular endothelial injury in an NKG2D-dependent manner. The two above-mentioned populations (CD4+CD57+ cells in BRR and CD4+CD28− T cells in chronic CMV infection) exhibit remarkable overlap both phenotypically and functionally. Therefore, given their expansion in patients with ESRD under the influence of a previous CMV infection and the chronic inflammatory cytokine milieu, CD4+CD28− T cells are likely a nonclassical risk factor for atherosclerotic disease pre- and post-renal transplantation (73,74). However, further investigation regarding their contribution to graft rejection or tolerance is still necessary.
CONCLUDING REMARKS
Accumulation of CD28− T cells was initially considered a hallmark of age-associated changes in the human immune system. However, loss of CD28 on CD4 T cells also occurs in patients with chronic autoimmune diseases in an age-inappropriate manner (Fig. 1). Despite their restricted TCR diversity, shorter telomeres and abundance at the inflamed site, it remains questionable whether the autoreactive T cell response is a major contributing factor for expansion of CD4+CD28− T cells. Rather, recent studies suggest that repeated antigenic stimulation of T cells by chronic inflammation or latent CMV infections causes them to proliferate more rapidly and extensively resulting in the loss of CD28. Although most studies of CD28− T cells have been conducted in humans, which are much more sensitive to loss of CD28 than mice are, development of an appropriate animal model will be required in order to investigate the underlying mechanisms of this phenomenon and its biological relevance in vivo. In this context, recent CD28 co-stimulation blockade therapy might provide invaluable information regarding the biological role of CD4+CD28− T cells in physiological settings. Moreover, it is necessary to understand how CD28 loss is linked to the transcriptomic shift into pathogenic T cells. Better understanding of the molecular and functional features of CD4+CD28− T cells will open new avenues to explore potential targets for intervention in a variety of chronic inflammatory diseases. Future research will be needed to investigate whether the accumulation of CD4+CD28− T cells is generalized for various chronic inflammatory disorders and to evaluate their usefulness as a biomarker or a prognostic factor. Recovery of CD28 expression by TNF-α inhibition or selective depletion of CD4+CD28− T cells by targeting specific surrogate molecules on their surface will be promising approaches for therapeutic intervention in various inflammatory disorders.
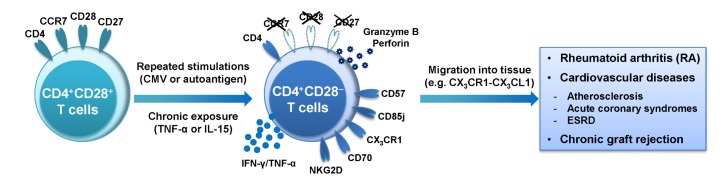
Figure 1. Immunological role of expanded CD4+CD28− T cells in chronic inflammatory disorders. CD28+ T cells lose CD28 expression after repeated stimulation with latent viral infections or autoantigen. Additionally, the loss of CD28 occurs when T cells are exposed to proinflammatory cytokines. Expanded CD4+CD28− T cells produce large amounts of proinflammatory cytokines (e.g. IFN-γ and TNF-α) and cytotoxic mediators (e.g. granzyme B and perforin), which cause tissue damage and development of pathogenesis in many inflammatory disorders such as RA, cardiovascular diseases and chronic graft rejection of solid organ transplantation.
ACKNOWLEDGEMENTS
This research was supported by grants from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI13C0715 and HI13C0954 to W.W. L.).
Abbreviations
- CMV
Cytomegalovirus
- EBV
Epstein–Barr virus
- NKRs
Natural killer cell receptors
- AMPK
AMP-activated protein kinase
- TAB-1
TGF-β activated kinase 1 (MAP3K7) binding protein 1
- RA
Rheumatoid arthritis
- CX3CR1
CX3C chemokine receptor 1
- ACS
Acute coronary syndromes
- UA
Unstable angina
- ESRD
End-stage renal diseases
- BRR
Belatacept resistant rejection
Footnotes
CONFLICTS OF INTEREST: The authors declare that they have no conflicts of interest with the contents of this article.
References
- 1.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lafferty KJ, Cunningham AJ. A new analysis of allogeneic interactions. Aust J Exp Biol Med Sci. 1975;53:27–42. doi: 10.1038/icb.1975.3. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol. 2003;3:939–951. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- 5.Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975–983. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- 6.Derhovanessian E, Maier AB, Hahnel K, Beck R, de Craen AJ, Slagboom EP, Westendorp RG, Pawelec G. Infection with cytomegalovirus but not herpes simplex virus induces the accumulation of late-differentiated CD4+ and CD8+ T-cells in humans. J Gen Virol. 2011;92:2746–2756. doi: 10.1099/vir.0.036004-0. [DOI] [PubMed] [Google Scholar]
- 7.Weng NP, Akbar AN, Goronzy J. CD28(−) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vallejo AN, Bryl E, Klarskov K, Naylor S, Weyand CM, Goronzy JJ. Molecular basis for the loss of CD28 expression in senescent T cells. J Biol Chem. 2002;277:46940–46949. doi: 10.1074/jbc.M207352200. [DOI] [PubMed] [Google Scholar]
- 9.Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, Ouslander JG, Weyand CM, Goronzy JJ. T cell subset-specific susceptibility to aging. Clin Immunol. 2008;127:107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu HT, Park S, Shin EC, Lee WW. T cell senescence and cardiovascular diseases. Clin Exp Med. 2016;16:257–263. doi: 10.1007/s10238-015-0376-z. [DOI] [PubMed] [Google Scholar]
- 11.Broux B, Markovic-Plese S, Stinissen P, Hellings N. Pathogenic features of CD4+ Trends Mol Med. 2012;18:446–453. doi: 10.1016/j.molmed.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt D, Goronzy JJ, Weyand CM. CD4+ CD7−. J Clin Invest. 1996;97:2027–2037. doi: 10.1172/JCI118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liuzzo G, Kopecky SL, Frye RL, O'Fallon WM, Maseri A, Goronzy JJ, Weyand CM. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation. 1999;100:2135–2139. doi: 10.1161/01.cir.100.21.2135. [DOI] [PubMed] [Google Scholar]
- 14.Betjes MG, Huisman M, Weimar W, Litjens NH. Expansion of cytolytic CD4+ Kidney Int. 2008;74:760–767. doi: 10.1038/ki.2008.301. [DOI] [PubMed] [Google Scholar]
- 15.Dumitriu IE, Araguas ET, Baboonian C, Kaski JC. CD4+ CD28 null T cells in coronary artery disease: when helpers become killers. Cardiovasc Res. 2009;81:11–19. doi: 10.1093/cvr/cvn248. [DOI] [PubMed] [Google Scholar]
- 16.Fann M, Chiu WK, Wood WH, III, Levine BL, Becker KG, Weng NP. Gene expression characteristics of CD28null memory phenotype CD8+ T cells and its implication in T-cell aging. Immunol Rev. 2005;205:190–206. doi: 10.1111/j.0105-2896.2005.00262.x. [DOI] [PubMed] [Google Scholar]
- 17.Valenzuela HF, Effros RB. Divergent telomerase and CD28 expression patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clin Immunol. 2002;105:117–125. doi: 10.1006/clim.2002.5271. [DOI] [PubMed] [Google Scholar]
- 18.Chiu WK, Fann M, Weng NP. Generation and growth of CD28nullCD8+ memory T cells mediated by IL-15 and its induced cytokines. J Immunol. 2006;177:7802–7810. doi: 10.4049/jimmunol.177.11.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borthwick NJ, Lowdell M, Salmon M, Akbar AN. Loss of CD28 expression on CD8(+) T cells is induced by IL-2 receptor gamma chain signalling cytokines and type I IFN, and increases susceptibility to activation-induced apoptosis. Int Immunol. 2000;12:1005–1013. doi: 10.1093/intimm/12.7.1005. [DOI] [PubMed] [Google Scholar]
- 20.Bryl E, Vallejo AN, Weyand CM, Goronzy JJ. Down-regulation of CD28 expression by TNF-alpha. J Immunol. 2001;167:3231–3238. doi: 10.4049/jimmunol.167.6.3231. [DOI] [PubMed] [Google Scholar]
- 21.Liaskou E, Jeffery LE, Trivedi PJ, Reynolds GM, Suresh S, Bruns T, Adams DH, Sansom DM, Hirschfield GM. Loss of CD28 expression by liver-infiltrating T cells contributes to pathogenesis of primary sclerosing cholangitis. Gastroenterology. 2014;147:221–232. doi: 10.1053/j.gastro.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vallejo AN, Weyand CM, Goronzy JJ. Functional disruption of the CD28 gene transcriptional initiator in senescent T cells. J Biol Chem. 2001;276:2565–2570. doi: 10.1074/jbc.M005503200. [DOI] [PubMed] [Google Scholar]
- 23.Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev. 2005;205:158–169. doi: 10.1111/j.0105-2896.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 24.Cavanagh MM, Weyand CM, Goronzy JJ. Chronic inflammation and aging: DNA damage tips the balance. Curr Opin Immunol. 2012;24:488–493. doi: 10.1016/j.coi.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Curr Opin Immunol. 2010;22:507–513. doi: 10.1016/j.coi.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franceschi C, Bonafe M, Valensin S, Olivieri F, De LM, Ottaviani E, De BG. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 27.Ortiz-Suarez A, Miller RA. A subset of CD8 memory T cells from old mice have high levels of CD28 and produce IFN-gamma. Clin Immunol. 2002;104:282–292. doi: 10.1006/clim.2002.5221. [DOI] [PubMed] [Google Scholar]
- 28.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, Axthelm MK, Picker LJ. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 29.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 30.Kipling D, Cooke HJ. Hypervariable ultra-long telomeres in mice. Nature. 1990;347:400–402. doi: 10.1038/347400a0. [DOI] [PubMed] [Google Scholar]
- 31.High KP, Akbar AN, Nikolich-Zugich J. Translational research in immune senescence: assessing the relevance of current models. Semin Immunol. 2012;24:373–382. doi: 10.1016/j.smim.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weyand CM, Brandes JC, Schmidt D, Fulbright JW, Goronzy JJ. Functional properties of CD4+ Mech Ageing Dev. 1998;102:131–147. doi: 10.1016/s0047-6374(97)00161-9. [DOI] [PubMed] [Google Scholar]
- 33.Dumitriu IE, Baruah P, Finlayson CJ, Loftus IM, RF Antunes, Lim P, Bunce N, Kaski JC. High levels of costimulatory receptors OX40 and 4-1BB characterize CD4+CD28null T cells in patients with acute coronary syndrome. Circ Res. 2012;110:857–869. doi: 10.1161/CIRCRESAHA.111.261933. [DOI] [PubMed] [Google Scholar]
- 34.Chanouzas D, Dyall L, Dale J, Moss P, Morgan M, Harper L. CD4+ Lancet. 2015;385(Suppl 1):S30. doi: 10.1016/S0140-6736(15)60345-2. [DOI] [PubMed] [Google Scholar]
- 35.Dumitriu IE. The life (and death) of CD4+ CD28(null) T cells in inflammatory diseases. Immunology. 2015;146:185–193. doi: 10.1111/imm.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maly K, Schirmer M. The story of CD4+ J Immunol Res. 2015;2015:348746. doi: 10.1155/2015/348746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Groh V, Bruhl A, El-Gabalawy H, Nelson JL, Spies T. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2003;100:9452–9457. doi: 10.1073/pnas.1632807100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Namekawa T, Snyder MR, Yen JH, Goehring BE, Leibson PJ, Weyand CM, Goronzy JJ. Killer cell activating receptors function as costimulatory molecules on CD4+CD28null T cells clonally expanded in rheumatoid arthritis. J Immunol. 2000;165:1138–1145. doi: 10.4049/jimmunol.165.2.1138. [DOI] [PubMed] [Google Scholar]
- 39.Yen JH, Moore BE, Nakajima T, Scholl D, Schaid DJ, Weyand CM, Goronzy JJ. Major histocompatibility complex class I-recognizing receptors are disease risk genes in rheumatoid arthritis. J Exp Med. 2001;193:1159–1167. doi: 10.1084/jem.193.10.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee WW, Yang ZZ, Li G, Weyand CM, Goronzy JJ. Unchecked CD70 expression on T cells lowers threshold for T cell activation in rheumatoid arthritis. J Immunol. 2007;179:2609–2615. doi: 10.4049/jimmunol.179.4.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Mitri D, Azevedo RI, Henson SM, Libri V, Riddell NE, Macaulay R, Kipling D, Soares MV, Battistini L, Akbar AN. Reversible senescence in human CD4+CD45RA+ J Immunol. 2011;187:2093–2100. doi: 10.4049/jimmunol.1100978. [DOI] [PubMed] [Google Scholar]
- 42.Lanna A, Henson SM, Escors D, Akbar AN. The kinase p38 activated by the metabolic regulator AMPK and scaffold TAB1 drives the senescence of human T cells. Nat Immunol. 2014;15:965–972. doi: 10.1038/ni.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fasth AE, Snir O, Johansson AA, Nordmark B, Rahbar A, Af KE, Bjorkstrom NK, Ulfgren AK, van Vollenhoven RF, Malmstrom V, Trollmo C. Skewed distribution of proinflammatory CD4+CD28null T cells in rheumatoid arthritis. Arthritis Res Ther. 2007;9:R87. doi: 10.1186/ar2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McInnes IB, O'Dell JR. State-of-the-art: rheumatoid arthritis. Ann Rheum Dis. 2010;69:1898–1906. doi: 10.1136/ard.2010.134684. [DOI] [PubMed] [Google Scholar]
- 45.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 46.Martens PB, Goronzy JJ, Schaid D, Weyand CM. Expansion of unusual CD4+ T cells in severe rheumatoid arthritis. Arthritis Rheum. 1997;40:1106–1114. doi: 10.1002/art.1780400615. [DOI] [PubMed] [Google Scholar]
- 47.Namekawa T, Wagner UG, Goronzy JJ, Weyand CM. Functional subsets of CD4 T cells in rheumatoid synovitis. Arthritis Rheum. 1998;41:2108–2116. doi: 10.1002/1529-0131(199812)41:12<2108::AID-ART5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 48.Schirmer M, Vallejo AN, Weyand CM, Goronzy JJ. Resistance to apoptosis and elevated expression of Bcl-2 in clonally expanded CD4+ J Immunol. 1998;161:1018–1025. [PubMed] [Google Scholar]
- 49.Vallejo AN, Schirmer M, Weyand CM, Goronzy JJ. Clonality and longevity of CD4+CD28null T cells are associated with defects in apoptotic pathways. J Immunol. 2000;165:6301–6307. doi: 10.4049/jimmunol.165.11.6301. [DOI] [PubMed] [Google Scholar]
- 50.Warrington KJ, Takemura S, Goronzy JJ, Weyand CM. CD4+ Arthritis Rheum. 2001;44:13–20. doi: 10.1002/1529-0131(200101)44:1<13::AID-ANR3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 51.Thewissen M, Somers V, Hellings N, Fraussen J, Damoiseaux J, Stinissen P. CD4+CD28null T cells in autoimmune disease: pathogenic features and decreased susceptibility to immunoregulation. J Immunol. 2007;179:6514–6523. doi: 10.4049/jimmunol.179.10.6514. [DOI] [PubMed] [Google Scholar]
- 52.Zal B, Kaski JC, Arno G, Akiyu JP, Xu Q, Cole D, Whelan M, Russell N, Madrigal JA, Dodi IA, Baboonian C. Heat-shock protein 60-reactive CD4+CD28null T cells in patients with acute coronary syndromes. Circulation. 2004;109:1230–1235. doi: 10.1161/01.CIR.0000118476.29352.2A. [DOI] [PubMed] [Google Scholar]
- 53.Alonso-Arias R, Moro-Garcia MA, Vidal-Castineira JR, Solano-Jaurrieta JJ, Suarez-Garcia FM, Coto E, Lopez-Larrea C. IL-15 preferentially enhances functional properties and antigen-specific responses of CD4+CD28(null) compared to CD4+CD28+ T cells. Aging Cell. 2011;10:844–852. doi: 10.1111/j.1474-9726.2011.00725.x. [DOI] [PubMed] [Google Scholar]
- 54.Broux B, Mizee MR, Vanheusden M, van der PS, van HJ, Van WB, Somers V, de Vries HE, Stinissen P, Hellings N. IL-15 amplifies the pathogenic properties of CD4+ J Immunol. 2015;194:2099–2109. doi: 10.4049/jimmunol.1401547. [DOI] [PubMed] [Google Scholar]
- 55.Goronzy JJ, Weyand CM. T-cell co-stimulatory pathways in autoimmunity. Arthritis Res Ther. 2008;10(Suppl 1):S3. doi: 10.1186/ar2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fasth AE, Bjorkstrom NK, Anthoni M, Malmberg KJ, Malmstrom V. Activating NK-cell receptors co-stimulate CD4(+)CD28(−) T cells in patients with rheumatoid arthritis. Eur J Immunol. 2010;40:378–387. doi: 10.1002/eji.200939399. [DOI] [PubMed] [Google Scholar]
- 57.Goronzy JJ, Henel G, Sawai H, Singh K, Lee EB, Pryshchep S, Weyand CM. Costimulatory pathways in rheumatoid synovitis and T-cell senescence. Ann N Y Acad Sci. 2005;1062:182–194. doi: 10.1196/annals.1358.022. [DOI] [PubMed] [Google Scholar]
- 58.Sawai H, Park YW, Roberson J, Imai T, Goronzy JJ, Weyand CM. T cell costimulation by fractalkine-expressing synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2005;52:1392–1401. doi: 10.1002/art.21140. [DOI] [PubMed] [Google Scholar]
- 59.Leon ML, Zuckerman SH. Gamma interferon: a central mediator in atherosclerosis. Inflamm Res. 2005;54:395–411. doi: 10.1007/s00011-005-1377-2. [DOI] [PubMed] [Google Scholar]
- 60.Nakajima T, Schulte S, Warrington KJ, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. T-cell-mediated lysis of endothelial cells in acute coronary syndromes. Circulation. 2002;105:570–575. doi: 10.1161/hc0502.103348. [DOI] [PubMed] [Google Scholar]
- 61.Liuzzo G, Goronzy JJ, Yang H, Kopecky SL, Holmes DR, Frye RL, Weyand CM. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation. 2000;101:2883–2888. doi: 10.1161/01.cir.101.25.2883. [DOI] [PubMed] [Google Scholar]
- 62.Liuzzo G, Biasucci LM, Trotta G, Brugaletta S, Pinnelli M, Digianuario G, Rizzello V, Rebuzzi AG, Rumi C, Maseri A, Crea F. Unusual CD4+CD28null T lymphocytes and recurrence of acute coronary events. J Am Coll Cardiol. 2007;50:1450–1458. doi: 10.1016/j.jacc.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 63.Brugaletta S, Biasucci LM, Pinnelli M, Biondi-Zoccai G, Di GG, Trotta G, Liuzzo G, Crea F. Novel anti-inflammatory effect of statins: reduction of CD4+CD28null T lymphocyte frequency in patients with unstable angina. Heart. 2006;92:249–250. doi: 10.1136/hrt.2004.052282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Betjes MG. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol. 2013;9:255–265. doi: 10.1038/nrneph.2013.44. [DOI] [PubMed] [Google Scholar]
- 65.Betjes MG, Weimar W, Litjens NH. Circulating CD4(+)CD28null T Cells May Increase the risk of an atherosclerotic vascular event shortly after kidney transplantation. J Transplant. 2013;2013:841430. doi: 10.1155/2013/841430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brando B, Sommaruga E, Civati G, Busnach G, Broggi ML, Seveso M, Minetti L. Monitoring of CD4+ Transplant Proc. 1989;21:1192–1193. [PubMed] [Google Scholar]
- 67.Pawlik A, Florczak M, Masiuk M, Dutkiewicz G, Machalinski B, Rozanski J, Domanski L, Gawronska-Szklarz B. The expansion of CD4+ Transplant Proc. 2003;35:2902–2904. doi: 10.1016/j.transproceed.2003.10.061. [DOI] [PubMed] [Google Scholar]
- 68.Pawlik A, Florczak M, Masiuk M, Machalinski B, Syczewska M, Szych Z, Gawronska-Szklarz B. The increased number of CD4+ Ann Transplant. 2003;8:54–56. [PubMed] [Google Scholar]
- 69.Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, Swanson SJ, Mannon RB, Roederer M, Kirk AD. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5:465–474. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 70.Trzonkowski P, Zilvetti M, Friend P, Wood KJ. Recipient memory-like lymphocytes remain unresponsive to graft antigens after CAMPATH-1H induction with reduced maintenance immunosuppression. Transplantation. 2006;82:1342–1351. doi: 10.1097/01.tp.0000239268.64408.84. [DOI] [PubMed] [Google Scholar]
- 71.Espinosa J, Herr F, Tharp G, Bosinger S, Song M, Farris AB, III, George R, Cheeseman J, Stempora L, Townsend R, Durrbach A, Kirk AD. CD57(+) CD4 T Cells Underlie Belatacept-Resistant Allograft Rejection. Am J Transplant. 2016;16:1102–1112. doi: 10.1111/ajt.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shabir S, Smith H, Kaul B, Pachnio A, Jham S, Kuravi S, Ball S, Chand S, Moss P, Harper L, Borrows R. Cytomegalovirus-Associated CD4(+) CD28(null) Cells in NKG2D-Dependent Glomerular Endothelial Injury and Kidney Allograft Dysfunction. Am J Transplant. 2016;16:1113–1128. doi: 10.1111/ajt.13614. [DOI] [PubMed] [Google Scholar]
- 73.Mou D, Espinosa J, Lo DJ, Kirk AD. CD28 negative T cells: is their loss our gain? Am J Transplant. 2014;14:2460–2466. doi: 10.1111/ajt.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murakami N, Riella LV. CD4+ CD28-Negative Cells: Armed and Dangerous. Am J Transplant. 2016;16:1045–1046. doi: 10.1111/ajt.13646. [DOI] [PMC free article] [PubMed] [Google Scholar]