Abstract
Peroxiredoxin-3 (Prdx3) is a mitochondrial protein of the thioredoxin family of antioxidant peroxidases and is the principal peroxidase responsible for metabolizing mitochondrial hydrogen peroxide. Recent reports have shown that mitochondrial reactive oxygen species (mROS) contribute to macrophage-mediated bactericidal activity in response to Toll-like receptors. Herein, we investigated the functional effect of Prdx3 in bactericidal activity. The mitochondrial localization of Prdx3 in HEK293T cells was confirmed by cell fractionation and confocal microscopy analyses. To investigate the functional role of Prdx3 in bactericidal activity, Prdx3-knockdown (Prdx3KD) THP-1 cells were generated. The mROS levels in Prdx3KD THP-1 cells were significantly higher than those in control THP-1 cells. Moreover, the mROS levels were markedly increased in response to lipopolysaccharide. Notably, the Salmonella enterica serovar Typhimurium infection assay revealed that the Prdx3KD THP-1 cells were significantly resistant to S. Typhimurium infection, as compared with control THP-1 cells. Taken together, these results indicate that Prdx3 is functionally important in bactericidal activity through the regulation of mROS.
Keywords: Peroxiredoxin-3, Mitochondrial reactive oxygen species, Bactericidal activity, Lipopolysaccharides, Toll like receptors
INTRODUCTION
Mitochondria are an important site for the production of mitochondria reactive oxygen species (mROS) that include hydrogen peroxide, superoxide, singlet oxygen, and the hydroxyl radical (1,2,3). The mitochondrial electron transport chain, located on the inner mitochondrial membrane and composed of four multiprotein complexes (I~IV), is involved in the generation of mROS (4,5). Low levels of mROS are important for various cellular functions and metabolic adaptation, as seen in hypoxia (6,7,8). Moderate levels of mROS, stimulated by lysophosphatidylcholine and the Toll-like receptor (TLR) 4 ligand, bacterial endotoxin lipopolysaccharide (LPS), are involved in regulating the inflammatory response (8,9). Recent studies indicate that mROS contribute to macrophage-mediated bactericidal activity. Engagement of TLRs, including TLR1, TLR2, and TLR4, leads to the recruitment of mitochondria to macrophage phagosomes and enhances mROS production (10,11). Upon TLR stimulation, the TLR signaling adapter, tumor necrosis factor receptor-associated factor 6 (TRAF6), translocates into mitochondria and engages evolutionarily conserved signaling intermediate in Toll (ECSIT) pathways. The interaction between TRAF6 and ECSIT leads to ECSIT ubiquitination, resulting in increased mitochondrial and cellular ROS generation (10). However, aberrant increases in mitochondrial and cellular ROS can induce apoptosis by causing the release of pro-apoptotic factors from the mitochondria, such as cytochrome c and apoptosis-inducing factor, through the opening of a nonselective mitochondrial permeability transition pore (12,13).
Peroxiredoxins (Prdxs) are a highly conserved and ubiquitous family of antioxidant enzymes found in most organisms, where they function primarily to scavenge ROS (14,15,16,17). Prdxs control cytokine-induced peroxide levels and thereby mediate signal transduction in mammalian cells (16,17,18). Its family members in humans are Prdxs 1~6 (14,15,16,17,18). Prdx3, a typical 2-Cys Prdx located exclusively in the mitochondrial matrix, is the principal peroxidase responsible for metabolizing mitochondrial hydrogen peroxide, a byproduct of cellular respiration originatinhg from the mitochondrial electron transport chain (19,20). Since increases in mitochondrial oxidant levels may activate stress signaling pathways and cause cellular damage when they reach a cytotoxic threshold (21,22), mROS levels need to be tightly regulated.
Here, we investigated whether Prdx3 was involved in bactericidal activity through the regulation of mROS. We confirmed the mitochondrial localization of Prdx3 in HEK293T cells by cell fractionation and confocal microscopy analyses. Notably, mROS levels in Prdx3-knockdown (Prdx3KD) THP-1 cells were significantly higher than those in control THP-1 cells. Moreover, the Salmonella enterica serovar Typhimurium infection assay revealed that the Prdx3KD THP-1 cells were significantly resistant to S. Typhimurium infection, as compared with control cells, indicating that Prdx3 is functionally involved in bactericidal activity through the regulation of mROS.
MATERIALS AND METHODS
Cell lines and reagents
HEK293T cells (American Type Culture Collection, Rockville, MD, USA) were cultured in Dulbecco's modified Eagle's medium (Gibco, Detroit, MI, USA) supplemented with 10% fetal bovine serum (FBS, Gibco). THP-1 human monocytic leukemia cells (American Type Culture Collection) were maintained in RPMI 1640 supplemented with 10% FBS, penicillin–streptomycin, and β-mercaptoethanol. Lentiviruscontaining small hairpin RNA (shRNA) targeting human Prdx3 (sc-40833-V) and control shRNA lentivirus (sc-108080) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). THP-1 cells were cultured in 24-well plates (2×105 cells/well) and infected with lentivirus according to the manufacturer's protocol. Control and Prdx3KD THP-1 cells were generated and maintained in puromycin-containing (4 to 8 mg/mL) medium. MitoTracker Green FM was obtained from Thermo Fisher Scientific (Waltham, MA, USA). The following antibodies and reagents were used: Prdx3 (Abcam, Cambridge, MA, USA), GRIM19 (Abcam), Myc (Cell Signaling Technology, Beverley, MA, USA), Flag, (Cell Signaling Technology), TRAF6 (Cell Signaling Technology), IκB-α (Cell Signaling Technology), 4’,6-diamidino-2-phenylindole (Thermo Fisher Scientific), MitoSOX Red (Molecular Probes, Invitrogen, Carlsbad, CA, USA), and LPS (Sigma-Aldrich, St. Louis, MO, USA).
Cell fractionation and mitochondria isolation
Cytoplasmic and mitochondrial fractions were isolated from HEK293T cells. The isolation protocol was carried out as described previously (10). Each fraction was confirmed by reaction to antibodies against GRIM19 for mitochondria or IκB-α for cytoplasm.
Immunofluorescence confocal microscopy
For all microscopy images, HEK293T cells were grown on coverslips and stained with MitoTracker FM (Invitrogen, Paisley, UK). After washing, cells were fixed with 4% paraformaldehyde for 20 min, permeabilized with 0.1% Triton X-100 in phosphate buffered saline (PBS) for 5 min, blocked with PBS containing 10% FBS for 30 min, and stained with the primary anti-Prdx3 antibody. Cells were then stained with the Alexa Fluor® 568 anti-rabbit IgG secondary antibody (Invitrogen). Cells were counterstained with 4’,6-diamidino-2-phenylindole (Sigma-Aldrich) and mounted with Prolong Gold anti-fade reagent (Molecular Probes). Cells were imaged under an LSM 710 laser-scanning confocal microscope (Carl Zeiss, Jena, Germany). Overlap coefficients were calculated using the ZEN 2011 program, which evaluated more than 10 cells from three images for each condition.
ROS measurements and staining
Control and Prdx3KD THP-1 cells were treated with or without 500 ng/mL LPS for 60 min. Culture medium was removed and cells were washed with PBS, then incubated in serum-free RPMI 1640 medium for 15 to 30 min at 37℃ with MitoSOX Red (2.5 µM final concentration) to measure mitochondrial superoxide. Cells were washed with warmed PBS (37℃), removed from plates by pipetting with cold PBS containing 1 mM EDTA, pelleted at 1500 rpm for 3 min, immediately resuspended in cold PBS containing 1% FBS, and subjected to fluorescence-activated cell sorting analysis using a FACScalibur apparatus (BD Biosciences, San Diego, CA, USA). All ROS experiments shown are representative of three independent experiments. For immunofluorescence microscopy, cells were mounted and imaged as described above.
Salmonella infection assay
The salmonella infection protocol was described previously (23). Briefly, 5×105 THP-1 cells were cultured in fresh RPMI 1640 complete medium without antibiotics and infected with wild type Salmonella enterica serovar Typhimurium (14028s strain) at a multiplicity of infection of 10 bacteria/cell. Culture plates were centrifuged at 200×g for 5 min and incubated at 37℃ for 30 min to allow phagocytosis to occur. The medium was then replaced with fresh medium containing gentamicin (20 mg/mL) and incubated for different times. The total cell population in the well was harvested. An aliquot of the harvested cell population was centrifuged, the cells were lysed by 0.5% deoxycholate in Dulbecco's PBS, and the bacteria were diluted and plated on Luria-Bertani agar. All experiments were done in duplicate on at least three independent occasions.
Plasmids
The following plasmids were used: Flag-tagged TRAF6, Myc-tagged ECSIT, and Myc-tagged Prdx3. Flag-tagged TRAF6 truncated mutants were generated with specific primers: Flag-tagged 110-522, forward 5’-GCGAAGCTTATGGAA ATACTGCTGGAAAATC AACT-3’ and reverse 5’-AACTCGAGCTATACCCCTG CATCAGTACT-3’; Flag-tagged 260-522, forward 5’-AT AAGCTTATGCGCCACCTACAAGAGAACA-3’ and reverse 5’-AACTCGAGCTATACCCCTGCATCAGTA CT-3’; Flag-tagged 349-522 TRAF6, forward 5’-GCGC AAGCTTATGTGCAATGGAATTTATATTTGGAAG-3’ and reverse 5’-AACTCGAGCTATACCCCTGCATCAGT ACT-3’.
Western blotting and immunoprecipitation assay
Immunoprecipitation and western blotting were performed as described previously (23,24,25,26,27,28,29). In brief, HEK293T cells were co-transfected with mock, Flag-TRAF6, Myc-ECSIT, or Myc-Prdx3 as indicated in the Figure. After 38 h, the cells were extracted and immunoprecipitated with an anti-Myc antibody, followed by immune blotting with antibodies to Myc or Flag. HEK293T cells were transfected with mock, Flag-TRAF6 wild type, Flag-TRAF6 110-522, Flag-TRAF6 260-522, or Flag-TRAF6 349-522, along with Myc-Prdx3. After 38 h, the cells were extracted and immunoprecipitated with an anti-Flag antibody, followed by immune blotting with antibodies to Myc or Flag.
RESULTS AND DISCUSSION
Prdx3 is involved in the generation of mROS
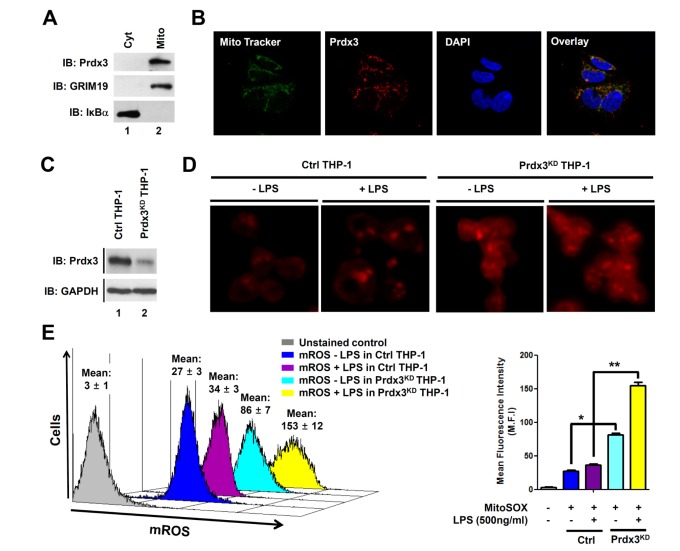
The generation of ROS in mammalian mitochondria is important because they may elicit oxidative damage in many pathologies and contribute to retrograde redox signaling from the organelle to the cytosol and nucleus (6,7,8,9,10,11,12,13). In addition, recent reports have shown that mROS play a pivotal role in macrophage-mediated bactericidal activity (10,11). Prdx3 is synthesized with a mitochondrial targeting sequence and then transferred to mitochondria where it might provide a primary line of defense against hydrogen peroxide produced by the mitochondrial respiratory chain (19,20). Nevertheless, it remains unclear whether Prdx3 is involved in bactericidal activity through the regulation of mROS. To investigate this issue, we first confirmed the localization of Prdx3 in mitochondria. The cytoplasmic and mitochondrial fractions from HEK293T cells were isolated and the localization of Prdx3 verified by western blotting. As expected, Prdx3 existed predominately in the mitochondria, but not in the cytoplasm (Fig. 1A, lane 2). The localization of Prdx3 in the mitochondria was clearly confirmed by confocal microscopy where the co-localization between Prdx3 and mitochondria could be seen (Fig. 1B). These data indicated that Prdx3 was localized exclusively in the mitochondria.
Figure 1. Peroxiredoxin-3 (Prdx3) is implicated in the generation of mitochondrial reactive oxygen species. (A) HEK293T cells were fractionated and protein extracts were analyzed by western-blotting with antibodies for Prdx3, IκB-α for cytoplasmic marker or GRIM19 for mitochondrial marker. The western blotting shown is representative of three independent experiments. (B) HEK293T cells were fixed and immunostained with MitoTracker (green) and an anti-Prdx3 antibody (red), and counterstained with 4’,6-diamidino-2-phenylindole (blue). Data are representative of three independent experiments. (C) THP-1 cells were infected with lentivirus containing shRNA targeted to human Prdx3, or control lentivirus, according to the manufacture's protocol. Control THP-1 (Ctrl) and Prdx3-knockdown THP-1 (Prdx3KD THP-1) cells were cultured in puromycin-containing medium (4 µg/mL) for 2 weeks to select stable clones. Immunoblotting with anti-Prdx3 or anti-GAPDH antibodies was performed to evaluate the knockdown efficacy. The western blotting shown is representative of three independent experiments. (D) Ctrl and Prdx3KD THP-1 cells were cultured without or with 500 ng/mLlipopolysaccharide (LPS) for 60 min, stained with MitoSOX, and analyzed by immunofluorescence microscopy. Data are representative of three independent replicates. (E) Ctrl and Prdx3KD THP-1 cells were cultured without or with 500 ng/mL LPS for 60 min, stained with MitoSOX, and analyzed by flow cytometry. Data are presented as the mean fluorescence intensity (M.F.I)±SEM from triplicate samples. *p<0.05,**p<0.01.
We next determined whether Prdx3 was able to affect the generation of mROS. Control and Prdx3KD THP-1 cells were generated using control and Prdx3 shRNA-containing lentiviral particles, respectively. The endogenous expression of Prdx3 was markedly attenuated in Prdx3KD THP-1 as compared with control cells (Fig. 1C, lane 1 versus lane 2). We next examined whether the knockdown of Prdx3 affected mROS levels. Control and Prdx3KD THP-1 cells were treated with or without LPS, stained with MitoSOX Red, and the levels of mROS evaluated by fluorescence microscopy and flow cytometry. The fluorescent intensity of mROS was higher in Prdx3KD than in control THP-1 cells in the absence of LPS (Fig. 1D). Since Prdx3 is an important cellular antioxidant capable of eliminating ROS (14,15,16,17,18,19,20), the increased mROS in Prdx3KD THP-1 cells might be due to the decrease of Prdx3 expression in Prdx3KD THP-1 cells. Upon LPS stimulation, the intensity was increased significantly in Prdx3KD compared with control THP-1 cells (Fig. 1D). To verify the results, flow cytometry analysis was performed. Consistently, the levels of mROS in Prdx3KD THP-1 cells were significantly higher in the absence of LPS, and markedly increased in the presence of LPS, as compared with control THP-1 cells (Fig. 1D, E). These results strongly indicate that Prdx3 is critically involved in maintaining and producing mROS.
Prdx3 does not interrupt formation of the TRAF6-ECSIT complex
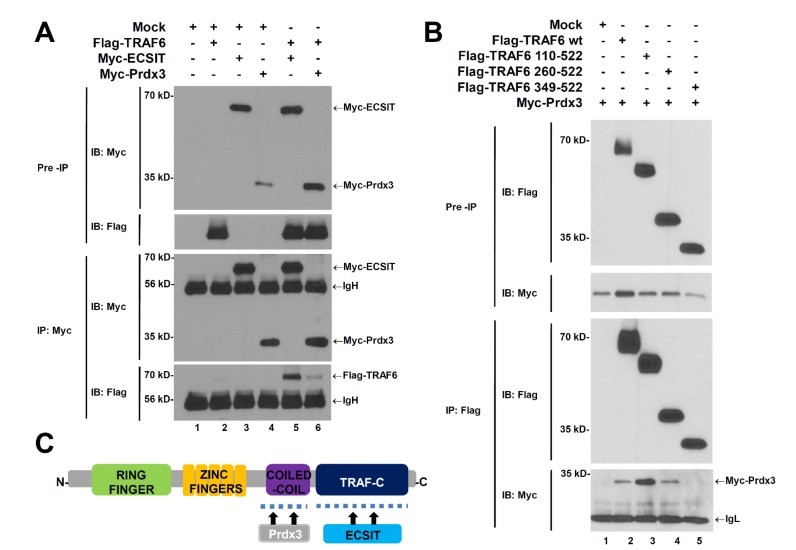
Previous studies have shown that the TRAF6-ECSIT complex induced by TLR4 is critical for macrophage-mediated bactericidal activity through the regulation of mROS (10). Therefore, we investigated whether Prdx3 was involved in this process. We first examined whether Prdx3 affected the association of the TRAF6-ECSIT complex. Flag-TRAF6, Myc-ECSIT, or Myc-Prdx3 was transiently transfected into HEK293T cells and then immunoprecipitated using an anti-Myc antibody (Fig. 2A). Consistent with previous work (24,30), Myc-ECSIT was precipitated with Flag-TRAF6 (Fig. 2A, lane 5). Moreover, Myc-Prdx3 was precipitated specifically with Flag-TRAF6 (Fig. 2A, lane 6), indicating that Prdx3 interacted with TRAF6.
Figure 2. Prdx3 may not interrupt formation of the TRAF6-ECSIT complex. (A) HEK293T cells were transiently transfected with mock, Flag-TRAF6, Myc-ECSIT, or Myc-Prdx3 as indicated. After 38 h, an immunoprecipitation (IP) assay with an anti-Myc antibody was performed, followed by immune blotting (IB) with anti-Myc or anti-Flag antibodies. The western blotting shown is representative of three independent experiments. (B) HEK293T cells were transfected with mock, Myc-Prdx3, Flag-TRAF6 wild type (wt), Flag-TRAF6 110-522, Flag-TRAF6 260-522, or Flag-TRAF6 349-522, as indicated. At 38 h after transfection, cells were extracted, immunoprecipitated with an anti-Flag antibody, and an immune blotting (IB) assay performed with anti-Flag or anti-Myc antibodies. The western blotting shown is representative of three independent experiments. (C) A schematic model for how TRAF6 interacts with Prdx3 and ECSIT.
Based on the results in Fig. 2A, we identified the specific sites where TRAF6 interacted with Prdx3. Myc-Prdx3 was transiently transfected into HEK293T cells along with wild type Flag-TRAF6 and TRAF6 truncated mutants (Flag-TRAF6 110-522, Flag-TRAF6 260-522, and Flag-TRAF6 349-522), and then immunoprecipitated using an anti-Flag antibody. Consistent with Fig. 2A, wild type Flag-TRAF6 was precipitated specifically with Myc-Prdx3 (Fig. 2B, lane 2). In addition, two truncated mutants(Flag-TRAF6 110-522 and Flag-TRAF6 260-522) were precipitated specifically with Myc-Prdx3 (Fig. 2B, lanes 3 and 4), whereas no significant interaction between Myc-Prdx3 and Flag-TRAF6 349-522 could be seen (Fig. 2B, lane 5). This indicates that Prdx3 interacts with the coiled-coil domain of TRAF6. Because previous studies show that ECSIT interacts with the TRAF-C domain of TRAF6 (24,30), our results also suggest that Prdx3 and ECSIT interact with different domains of TRAF6, as depicted in Fig. 2C, therefore indicating that Prdx3 does not interrupt formation of the TRAF6-ECSIT complex.
Prdx3 is involved in bactericidal activity
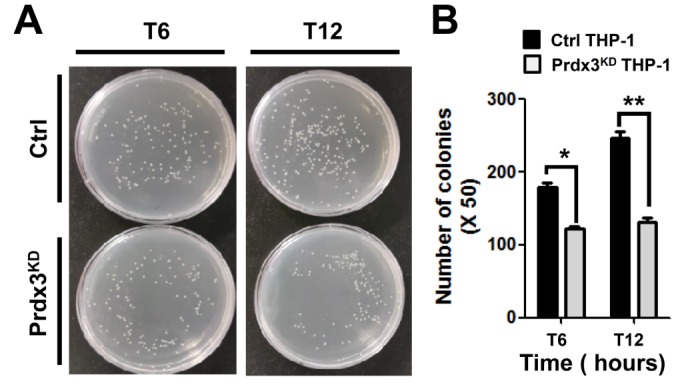
mROS are important for macrophage-mediated bactericidal activity (10,11). Having shown that Prdx3KD THP-1 cells had increased mROS (Fig. 1), we examined the functional effect of Prdx3 in bactericidal activity. Control or Prdx3KD THP-1 cells were infected with the Gram-negative, facultative intracellular pathogen Salmonella enterica serovar Typhimurium, and survival of the bacterium was measured. The number of colonies increased significantly in control THP-1 cells in a time-dependent manner (Fig. 3A, B). In contrast, significant decreases in the number of colonies were observed in Prdx3KD THP-1 cells (Fig. 3A, B), strongly indicating that Prdx3 was critically important in bactericidal activity through the regulation of mROS.
Figure 3. Salmonella survival assay in peroxiredoxin-3 (Prdx3)-knockdown THP-1 cells. (A) Control (Ctrl) and Prdx3-knockdown (Prdx3KD) THP-1 cells were infected with wild type Salmonella enterica serovar Typhimurium (14028s strain) at a multiplicity of infection of 10 bacteria/cell as described in Methods. Cells were lysed by 0.5% deoxycholate in Dulbecco's phosphate-buffered saline, and the bacteria were diluted (50:1) and plated on Luria-Bertani agar. (B) The number of colonies was counted. Data are presented as means±SEM of three independent experiments.*p<0.05,**p<0.01.
In the present study, we observed that Prdx3, a mitochondrion-specific peroxidase, was negatively involved in macrophage-mediated bactericidal activity. Consistent with previous reports (19,20), Prdx3 existed predominantly in mitochondria (Fig. 1). Importantly, Prdx3KD THP-1 cells generated by lentiviral particles containing shRNA targeted to Prdx3 exhibited increased mROS levels and significant resistance against S. Typhimurium infection. This strongly indicated that the regulation of mROS by Prdx3 in the mitochondria was involved in bactericidal activity.
The phagocytic response of the innate immune system involves the production of ROS via the phagosomal NADPH-oxidase-dependent respiratory burst and the mitochondrial oxidative phosphorylation machinery, and plays a pivotal role in the destruction of intracellular microbes (6,7,8,9,10,11). Although the molecular and cellular mechanisms linking innate immune signaling to mROS generation remain unclear, recent reports have proposed a novel pathway by which macrophages generate ROS in response to bacteria (10,11). Specifically, upon stimulation with TLR1/2/4, mitochondria are recruited to macrophage phagosomes where the production of mROS is enhanced (10). These researchers also found that the process of mROS production involves translocation of the TRAF6 protein to mitochondria, where TRAF6 engages the ECSIT protein. The interaction between TRAF6 and ECSIT leads to ubiquitination of the ECSIT protein, resulting in increased mitochondrial and cellular ROS generation.
Based on these previous findings, we examined whether Prdx3 was associated with the TRAF6-ECSIT complex. Our studies showed that Prdx3 interacted with the coiled-coil domain of TRAF6 (Fig. 2). Since previous reports showed that ECSIT interacts specifically with the TRAF-C domain of TRAF6 (24,30), we speculated that the Prdx3 and ECSIT proteins were able to interact with different domains of TRAF6, as depicted in Fig. 2C, and the interaction between Prdx3 and TRAF6 may not interrupt association of the TRAF6-ECSIT complex. Therefore, our results demonstrate that Prdx3 may negatively regulate bactericidal activity through its the antioxidant activity, rather than interrupting formation of the TRAF6-ECSIT complex.
In summary, cellular oxidants contributed by mitochondria may activate stress signaling pathways and cause cellular damage when oxidant levels reach a cytotoxic threshold. The oxidative stress induced by cellular ROS production can be systemic and regulated locally by cellular antioxidant enzymes such as the superoxide dismutases, catalase, glutathione peroxidases, and Prdxs. Prdx3 may have a critical role in preventing mitochondrial dysfunction induced by ROS. In addition, the studies presented here demonstrate that Prdx3 is negatively implicated in bactericidal activity through the regulation of mROS. Although the physiological relationship between Prdx3-induced cellular protection against mROS and bactericidal activity remains unclear, our data may contribute to a better understanding of the phagocytic response for the regulation of intracellular microbes.
ACKNOWLEDGEMENTS
This work was supported by the Mid-career Researcher Program through an NRF grant (NRF-2014R1A2A1A 11053221).
Abbreviations
- Prdx3
Peroxiredoxin 3
- mROS
mitochondrial reactive oxygen species
- TRAF6
tumor necrosis factor receptor-associated factor 6
- ECSIT
evolutionarily conserved signaling intermediate in Toll pathways
Footnotes
CONFLICTS OF INTEREST: The authors declare no competing financial interests.
References
- 1.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mailloux RJ. Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species. Redox Biol. 2015;4:381–398. doi: 10.1016/j.redox.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mailloux RJ, Jin X, Willmore WG. Redox regulation of mitochondrial function with emphasis on cysteine oxidation reactions. Redox Biol. 2014;2:123–139. doi: 10.1016/j.redox.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- 5.Titov DV, Cracan V, Goodman RP, Peng J, Grabarek Z, Mootha VK. Complementation of mitochondrial electron transport chain by manipulation of the NAD+/NADH ratio. Science. 2016;352:231–235. doi: 10.1126/science.aad4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang X, Luo YX, Chen HZ, Liu DP. Mitochondria, endothelial cell function, and vascular diseases. Front Physiol. 2014;5:175. doi: 10.3389/fphys.2014.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Fang P, Li Y, Kuo YM, Andrews AJ, Nanayakkara G, Johnson C, Fu H, Shan H, Du F, Hoffman NE, Yu D, Eguchi S, Madesh M, Koch WJ, Sun J, Jiang X, Wang H, Yang X. Mitochondrial reactive oxygen species mediate lysophosphatidylcholine-induced endothelial cell activation. Arterioscler Thromb Vasc Biol. 2016;36:1090–1100. doi: 10.1161/ATVBAHA.115.306964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finkel T. Signal transduction by mitochondrial oxidants. J Biol Chem. 2012;287:4434–4440. doi: 10.1074/jbc.R111.271999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geng J, Sun X, Wang P, Zhang S, Wang X, Wu H, Hong L, Xie C, Li X, Zhao H, Liu Q, Jiang M, Chen Q, Zhang J, Li Y, Song S, Wang HR, Zhou R, Johnson RL, Chien KY, Lin SC, Han J, Avruch J, Chen L, Zhou D. Kinases Mst1 and Mst2 positively regulate phagocytic induction of reactive oxygen species and bactericidal activity. Nat Immunol. 2015;16:1142–1152. doi: 10.1038/ni.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 13.Susin SA, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, Daugas E, Geuskens M, Kroemer G. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med. 1996;184:1331–1341. doi: 10.1084/jem.184.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher AB. Peroxiredoxin 6: a bifunctional enzyme with glutathione peroxidase and phospholipase A(2) activities. Antioxid Redox Signal. 2011;15:831–844. doi: 10.1089/ars.2010.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- 17.Park MH, Jo M, Kim YR, Lee CK, Hong JT. Roles of peroxiredoxins in cancer, neurodegenerative diseases and inflammatory diseases. Pharmacol Ther. 2016;163:1–23. doi: 10.1016/j.pharmthera.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fatma N, Kubo E, Sharma P, Beier DR, Singh DP. Impaired homeostasis and phenotypic abnormalities in Prdx6-/-mice lens epithelial cells by reactive oxygen species: increased expression and activation of TGFbeta. Cell Death Differ. 2005;12:734–750. doi: 10.1038/sj.cdd.4401597. [DOI] [PubMed] [Google Scholar]
- 19.Chang TS, Cho CS, Park S, Yu S, Kang SW, Rhee SG. Peroxiredoxin III, a mitochondrion-specific peroxidase, regulates apoptotic signaling by mitochondria. J Biol Chem. 2004;279:41975–41984. doi: 10.1074/jbc.M407707200. [DOI] [PubMed] [Google Scholar]
- 20.Granville DJ, Gottlieb RA. Mitochondria: regulators of cell death and survival. Scientific World Journal. 2002;2:1569–1578. doi: 10.1100/tsw.2002.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunniff B. Peroxiredoxin 3 levels regulate a mitochondrial redox setpoint in malignant mesothelioma cells. Redox Biol. 2014;3:79–87. doi: 10.1016/j.redox.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L, Yu AQ. The functional role of peroxiredoxin 3 in reactive oxygen species, apoptosis, and chemoresistance of cancer cells. J Cancer Res Clin Oncol. 2015;141:2071–2077. doi: 10.1007/s00432-015-1916-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mi WS, Park J, Shim JH, Chun E, Lee KY. Ubiquitination of ECSIT is crucial for the activation of p65/p50 NF-kappaBs in Toll-like receptor 4 signaling. Mol Biol Cell. 2015;26:151–160. doi: 10.1091/mbc.E14-08-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wi SM, Moon G, Kim J, Kim ST, Shim JH, Chun E, Lee KY. TAK1-ECSIT-TRAF6 complex plays a key role in the TLR4 signal to activate NF-kappaB. J Biol Chem. 2014;289:35205–35214. doi: 10.1074/jbc.M114.597187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moon G, Kim J, Min Y, Wi SM, Shim JH, Chun E, Lee KY. Phosphoinositide-dependent kinase-1 inhibits TRAF6 ubiquitination by interrupting the formation of TAK1-TAB2 complex in TLR4 signaling. Cell Signal. 2015;27:2524–2533. doi: 10.1016/j.cellsig.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Kim SY, Baik KH, Baek KH, Chah KH, Kim KA, Moon G, Jung E, Kim ST, Shim JH, Greenblatt MB, Chun E, Lee KY. S6K1 negatively regulates TAK1 activity in the toll-like receptor signaling pathway. Mol Cell Biol. 2014;34:510–521. doi: 10.1128/MCB.01225-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yong KS, Jeong S, Chah KH, Jung E, Baek KH, Kim ST, Shim JH, Chun E, Lee KY. Salt-inducible kinases 1 and 3 negatively regulate Toll-like receptor 4-mediated signal. Mol Endocrinol. 2013;27:1958–1968. doi: 10.1210/me.2013-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SY, Jeong S, Jung E, Baik KH, Chang MH, Kim SA, Shim JH, Chun E, Lee KY. AMP-activated protein kinase-alpha1 as an activating kinase of TGF-beta-activated kinase 1 has a key role in inflammatory signals. Cell Death Dis. 2012;3:e357. doi: 10.1038/cddis.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wi SM, Lee KY. 5-aminoimidazole-4-carboxamide riboside induces apoptosis through AMP-activated protein kinase-independent and NADPH oxidase-dependent pathways. Immune Netw. 2014;14:241–248. doi: 10.4110/in.2014.14.5.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopp E, Medzhitov R, Carothers J, Xiao C, Douglas I, Janeway CA, Ghosh S. ECSIT is an evolutionarily conserved intermediate in the Toll/IL-1 signal transduction pathway. Genes Dev. 1999;13:2059–2071. doi: 10.1101/gad.13.16.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]