Abstract
Pharmacological doses of glucocorticoids (GCs), acting via the glucocorticoid receptor (GR) to repress inflammation and immune function, remain the most effective therapy in the treatment of inflammatory and immune diseases. Since many patients on GC therapy exhibit GC-resistance and severe side-effects, much research is focussed on developing more selective GCs and combination therapies, with greater anti-inflammatory potency. GCs mediate their classical genomic transcriptional effects by binding to the cytoplasmic GR, followed by nuclear translocation and modulation of transcription of target genes by direct DNA-binding of the GR or its tethering to other transcription factors. Recent evidence suggests, however, that the responses mediated by the GR are much more complex and involve multiple parallel mechanisms integrating simultaneous signals from other receptors, both in the absence and presence of GCs, to shift the sensitivity of a target cell to GCs. The level of cellular stress, immune activation status, or the cell cycle phase may be crucial for determining GC sensitivity and GC responsiveness as well as subcellular localization of the GR and GR levels. Central to the development of new drugs that target GR signalling alone or as add-on therapies, is an in-depth understanding of the molecular mechanisms of GC-independent GR desensitization, priming and activation of the unliganded GR, as well as synergy and cross-talk with other signalling pathways. This review will discuss the information currently available on these topics and their relevance to immunotherapy, as well as identify unanswered questions and future areas of research.
Keywords: Ligand-independent activation, glucocorticoid receptor, priming, resistance, synergy
1. Introduction
Glucocorticoids (GCs) like cortisol, acting via the near ubiquitous glucocorticoid receptor (GR), play a central role in most physiological functions including homeostasis, inflammation, behaviour, metabolism, fat deposition and obesity, immune function and associated diseases in humans (Baschant & Tuckermann, 2010; Coutinho & Chapman, 2011; Lee et al., 2014; Silverman & Sternberg, 2012). GCs regulate all aspects of immune function and inflammation, including cellular trafficking, proliferation, cytokine and chemokine secretion, cytolytic activity, differentiation, effector function and antibody production in immune function cells (Padgett & Glaser, 2003). Infection with pathogens such as viruses and bacteria activates the adaptive and innate immune responses and causes an increase in secretion of inflammatory mediators. In addition, infection activates the hypothalamic-pituitary-adrenal (HPA) axis and induces the secretion of GCs from the adrenals, which in turn desensitize inflammation and immune function and prevent overshooting the immune response, which can be damaging and even lethal, due to cell death and tissue damage. GCs suppress cellular immunity and production of Th1 cytokines and stimulate humoral immunity by secretion of Th2-type anti-inflammatory cytokines (Ng et al., 2013). An appropriate immune response relies on a delicate balance between appropriate activation of the immune system and prevention of chronic immune activation by feedback mechanisms, which are tightly controlled in a GR-dependent manner.
Due to the key role in immune function and inflammation, dysregulation or inappropriate GR signalling is intimately linked with inflammatory and immune diseases such as asthma, rheumatoid arthritis, inflammatory bowel disease, chronic obstructive pulmonary disease (COPD), acute respiratory distress syndrome, cystic fibrosis, metabolic and autoimmune diseases (Barnes & Adcock, 2009; Silverman & Sternberg, 2012). These diseases are usually associated with chronic inflammation and increased cytokine production. Chronic stress is also associated with an increase in GC production, which can cause immune function dysregulation, diminished vaccine responses, increased viral and bacterial pathogenesis, and slower wound healing, due to repression of innate and adaptive immune function (Padgett & Glaser, 2003). Enhanced inflammation and hypercortisolism are associated with several stress-related illnesses including cardiovascular disease, osteoporosis and metabolic syndrome, which may also be related to increased GC resistance (Silverman & Sternberg, 2012). The pathophysiology of major depression has been linked to activation of immune responses, increased pro-inflammatory cytokines and GC resistance (Pace & Miller, 2009). Additionally, inflammation and the GR play a central role in many cancers, for example playing a causal role in the progression and recurrence of breast cancer (Ling & Kumar, 2012). Several cancers are treated with GCs in combination with other drugs, to induce apoptosis and death of tumor cells. Pharmacological doses of GCs, which act to repress inflammation and immune function, remain the most effective therapy in the treatment of inflammatory and immune diseases. However many patients become resistant or insensitive to GCs, posing a problem for therapy (Barnes & Adcock, 2009), and requiring higher doses of GCs to treat. However, GC therapy causes several side-effects, such as insulin resistance, diabetes, osteoporosis and muscle wasting.
The dose of GC used in therapy depends on the type and severity of disease treated and the pharmacokinetics of the different preparations (Liu et al., 2013). There is no single consistent dose, but rather a range of doses is used to achieve different therapeutic effects for different diseases. For example, the categories of GC doses used in treating rheumatoid arthritis can be grouped as follows: low dose (≤7.5mg prednisolone equivalent per day), medium dose (>7.5mg, but ≤ 30mg) and high dose (> 30mg) (Buttgereit et al., 2002). Low doses of inhaled GCs are currently used to treat asthma (Bateman et al., 2008), while higher GC doses between 100 mg/day and 450 mg/day are used in acute severe asthma (Barnes & Adcock, 2009; Manser et al., 2001). While some conditions appear to require higher GC doses than others (Adams & Jones, 2006), the scientific basis for this is unclear. It has been argued that high dose GC therapy induces both genomic and non-genomic effects of the GR which are required to resolve exacerbated inflammation (Buttgereit et al., 2002). However, there is no consensus on whether high GC doses are better than low GC doses in terms of therapeutic efficacy. Some studies have found no difference in dose-response effects between high and low GC doses, suggesting low dose GC regimens could result in the same efficacy as high GC dose with the added advantage of reduced side-effects (Manser et al., 2001). Information about serum concentrations of GCs and receptor occupancy in relevant target cells for different doses should be useful in predicting the lowest doses required for efficacy with minimal side-effects, but such information is not readily available in the literature. However, a recent study in mice showed no correlation between receptor occupancy and serum GC concentrations (Boger et al., 2015). This study also established that a GC dose of 47nmol/kg was required to occupy 50% of receptor and that more of the receptor was occupied at higher doses. Clearly more research is required to address this important issue of GC doses for immunotherapy. Clinical awareness is evident from trials assessing the clinical efficacy of high versus low dose GC therapy (Pincus & Cutolo, 2015).
Given this central role of GCs in human disease and the side-effects of GC therapy, much research is focussed on developing more selective GCs with greater potency (Ayroldi et al., 2014; Sundahl et al., 2015). This is most well studied in inflammatory lung disease, where there is a drive to develop selective GR agonists to avoid targeting pathways that cause side effects, while still activating pathways that inhibit inflammation (Miner et al., 2007; Newton et al., 2010). Due to the problem of GC resistance, the use of higher doses or more potent ligands is also being explored. In addition, combination pharmacological strategies that target the GR as well as other targets are being actively explored for a variety of conditions (Clark & Belvisi, 2012; Durham et al., 2015; Newton et al., 2010). Most promising is the use of “add-on” therapies or combination drugs, where in addition to a GR agonist, another drug is added to potentiate the effects of the GC. These currently include the use of long-acting β2 adrenoreceptor agonists (LABAs) and phosphodiesterase 4 inhibitors, which enhance the clinical efficacy of inhaled GCs (Giembycz & Newton, 2015; Newton et al., 2010). Other strategies currently undergoing clinical investigation in treatment of inflammatory disease include direct targeting of pro-inflammatory mediators such as using antibodies to interleukin (IL)4 or IL13, or chemokine and cytokine antagonists (Durham et al., 2015). Central to the development of new drugs that target GR signalling alone or as add-on therapies, is an in-depth understanding of the molecular mechanisms of action of the GR and in particular how other signalling pathways, in particular for cytokines, modulate GR signalling. Several comprehensive reviews have been written on mechanisms of anti-inflammatory actions of GCs and cross-talk between the cytokines and GR signalling pathways (Baschant & Tuckermann, 2010; Busillo & Cidlowski, 2013; Coutinho & Chapman, 2011; Cruz-Topete & Cidlowski, 2015; Dejager et al., 2014; Giembycz & Newton, 2015; Van Bogaert et al., 2010). However these have focussed on the role of the liganded GR. In this review we focus on the role of subcellular localization and expression levels of the unliganded GR and the role of the unliganded GR in GC-independent GR activation, priming and desensitizing of responses to GCs, with a view to the relevance to immunotherapy.
2. Brief overview of mechanisms of GR action
GC production in the adrenal cortex is stress-induced and tightly regulated by the HPA axis. GCs exert their effects via binding to the intracellular GR and regulating gene expression. The bioavailability of GCs inside the cell is regulated at multiple levels, including levels of the GC carrier corticosteroid-binding-globulin, the 11-beta hydroxysteroid dehydrogenases (11βHSD) which regulate the relative levels of corticosterone and cortisol, and the P-glycoprotein transporter which regulates GC efflux from the cells (Dejager et al., 2014). Mechanisms that regulate GC bioavailability will not be reviewed here, but it should be borne in mind that they are relevant to GC therapy and most likely play an important role in inflammation and GC immunotherapy, especially since there are reports of their regulation by GCs and cytokines (Dejager et al., 2014).
The GR mechanism of action is most clearly described in terms of its “classical genomic” actions. “Classical genomic” GC actions are usually defined as those which involve GR-mediated modulation of transcription in the nucleus via recruitment of GR to promoters on the genome. “Non-genomic” effects are usually perceived as “non-classical”, since they have been relatively recently discovered, and are generally defined as transcription-independent effects. GCs are understood to mediate their classical genomic effects by binding to the cytoplasmic GR in target cells, resulting in the dissociation of inhibitory proteins, followed by dimerization, nuclear translocation and regulation of transcription of target genes in a cell- and gene-specific manner (Figure 1) (Baschant & Tuckermann, 2010; Busillo & Cidlowski, 2013; Cain & Cidlowski, 2015; Coutinho & Chapman, 2011; Dejager et al., 2014; Nicolaides et al., 2010; Oakley & Cidlowski, 2011; Van Bogaert et al., 2010). Whether the preformation of GR dimers is a prerequisite for DNA binding is controversial, with some reports suggesting it occurs on the DNA, after the two GR monomers bind DNA (Kleiman & Tuckermann, 2007; Nixon et al., 2013; Ong et al., 2010), while other reports suggest it occurs before DNA binding (Baschant & Tuckermann, 2010; Busillo & Cidlowski, 2013; Cain & Cidlowski, 2015; Coutinho & Chapman, 2011; Dejager et al., 2014; Nicolaides et al., 2010; Oakley & Cidlowski, 2011; Robertson et al., 2013; Van Bogaert et al., 2010). Binding of GCs to the GR also leads to GR phosphorylation at several residues in the N-terminus, a requirement for its optimal genomic actions. The GR can regulate transcription both positively and negatively via several different genomic mechanisms, including by direct binding of the GR to promoters of target genes and by tethering to other transcription factors, to modulate their activity (Newton et al., 2010). Transactivation is mediated by the GR binding to glucocorticoid response elements (GREs) in the promoters of target genes, followed by recruitment of other proteins such as co-activators resulting in increased gene transcription. Transrepression occurs without direct DNA binding by the GR, but rather via protein-protein interactions with other transcription factors such as NF-κB and AP-1, to repress their function. Several other transcriptional mechanisms have been identified, including positive effects on transcription by GR tethering to some transcription factors as well as repression via GR binding to negative GREs (Baschant & Tuckermann, 2010; Busillo & Cidlowski, 2013; Coutinho & Chapman, 2011; Surjit et al., 2011; Van Bogaert et al., 2010). In addition, recent studies also show that monomeric GR can bind at GRE half sites and regulate transcription (Lim et al., 2015; Schiller et al., 2014). Once associated with the promoter the GR serves as a platform for recruitment of a multiprotein complex, including co-factors and chromatin remodelling complexes (Lonard et al., 2007; Rosenfeld & Glass, 2001; Rosenfeld et al., 2006; Xu et al., 1999), that increase or decrease chromatin accessibility, providing additional opportunities for cell- and gene-specific transcriptional regulation (Chinenov et al., 2014; Chinenov et al., 2013; King et al., 2012; Wu et al., 2014). Chromatin dynamics, oscillatory mechanisms, specialized chromosome structures and DNA topology are additional levels of complexity that play a critical role in cell- and gene-specific genomic responses to GCs (Burd & Archer, 2013; Hager & Varticovski, 2012; Miranda et al., 2013). Transrepression via tethering is of particular relevance to inflammation and immune function, since most cytokine and chemokine genes are upregulated by NF-κB and/or AP-1, and transrepression by the GR is thus the basis for the use of GCs in anti-inflammation and immunosuppressive therapies. Additionally, several anti-inflammatory genes are upregulated by the GR, contributing to the anti-inflammatory response, due to the subsequent actions of their gene products on the GR, NFκB and AP-1 signalling pathways. For example, mitogen-activated protein kinase phosphatase-1 (MKP-1), dephosphorylates mitogen-activated protein kinases (MAPKs) to inhibit inflammation, since MAPK is required to activate several transcription factors of pro-inflammatory genes.
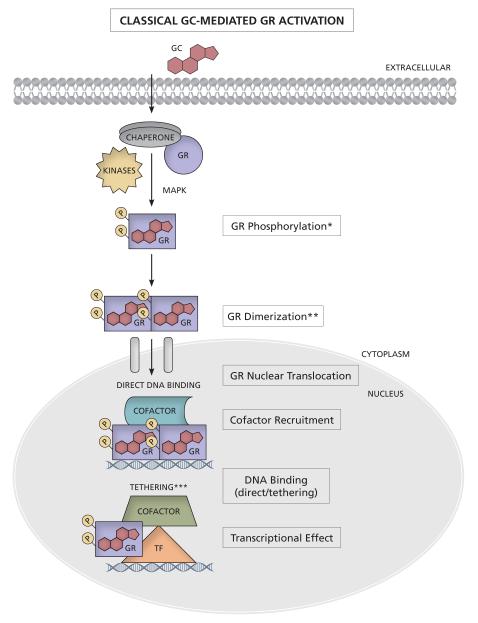
Figure 1. Classical GC-mediated GR activation.
GCs passively diffuse across the plasma membrane and bind to the unliganded GR, which resides in the cytoplasm in complex with various chaperone proteins, such as HSP90 and immunophilins. Once bound to GCs, the GR dissociates from some of the chaperones, undergoes a conformational change and becomes phosphorylated (indicated by Ps) by kinases, such as MAPK, at several serine residues. The GR translocates to the nucleus, where it can bind DNA (directly or via tethering), recruit cofactors and initiate transcription. * Recent reports indicate that HSP90 and some immunophilins translocate with the GR to the nucleus; however for simplicity this is not indicated. ** Whether GR dimerization occurs before or after nuclear translocation is still contentious. Recent studies suggest that whether preformed GR dimers are needed for DNA binding is controversial. *** The role of chromatin and HDACs is not indicated in this figure. TF = transcription factor.
Transcriptional responses to physiological and pharmacological doses of glucocorticoids are defined by dose response analysis which allows determination of biocharacter, potency and efficacy (Hapgood et al., 2014; Ong et al., 2010; Ronacher et al., 2009; van der Laan et al., 2008; Zhao et al., 2003). The shape of the dose response curve relative to reference agonists defines the biocharacter of the ligand i.e. whether a ligand is an agonist, partial agonist or antagonist (Hapgood et al., 2014). The potency is a measure of the sensitivity of the response to ligand and is defined as the concentration required for half maximal response, or EC50, while the efficacy is defined as the maximal response. Factors that affect the potency and/or efficacy of genomic responses to GCs include the receptor concentrations, the affinity of ligand for receptor, as well as the relative concentrations of co-activators, co-repressors, and co-modulators, for both transactivation and transrepression (Blackford et al., 2012; Chow et al., 2015; Chow et al., 2011; Hapgood et al., 2014; Simons, 2010; Simons & Chow, 2012; Szapary et al., 1996; van der Laan et al., 2008; Zhao et al., 2003).
To make matters more complicated, recent evidence suggests that the genomic responses mediated by the GR are modulated by multiple parallel mechanisms integrating signalling from several different hormones and involving cross-talk with other receptors and intracellular signalling pathways. In addition to exerting effects on transcription via the mechanisms described above, GCs have been shown to exert a wide array of rapid non-genomic effects. These in most cases involve modulation of kinase activity by the liganded GR to regulate other non-GR signalling pathways (Falkenstein et al., 2000). There is evidence that the GR can also cross-talk via rapid GC-dependent mechanisms with the T-cell receptor (TCR) (Bartis et al., 2007; Bartis et al., 2006; Harr et al., 2009; Lowenberg et al., 2006b), fibroblast growth factor receptor-1 (Croxtall et al., 2000), epidermal growth factor receptor (Croxtall et al., 2000) and insulin receptor pathways (Lowenberg et al., 2006a), as well as numerous pathways involved in neuroendocrine synaptic signalling (Di et al., 2009; Malcher-Lopes et al., 2008; Solito et al., 2003; Teng et al., 2013). Evidence is accumulating that the unliganded GR may associate with different plasma membrane receptor superfamilies in such a manner as to modulate their signalling both in the absence and presence of GCs. This non-genomic modulation by the GR of other signalling pathways is likely to be highly relevant to GC therapy and human diseases, including inflammatory, metabolic and cardiovascular disease. Besides classical genomic and rapid GC-induced non-genomic ligand-dependent steroid receptor actions and cross talk, there is increasing evidence that the unliganded GR can modulate cell signalling in the absence of GCs, adding another level of complexity.
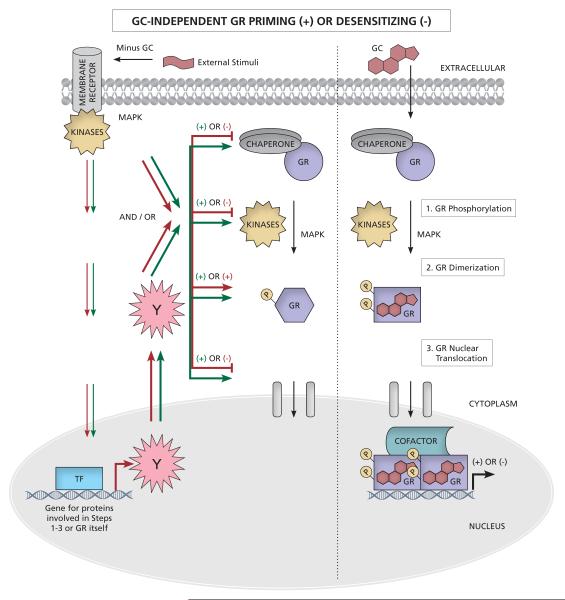
Several mechanisms exist to modulate the activity of the unliganded GR as well as the ability of the unliganded GR to respond to GCs. The GR has been reported to be activated in the absence of GCs, a form of signalling usually referred to as “ligand-independent activation” (Kotitschke et al., 2009; Matthews et al., 2011; Ritter et al., 2012; Ritter & Mueller, 2014; Robertson et al., 2013; Verhoog et al., 2011). However, this term is used to describe a variety of potentially different mechanisms. Changing unliganded GR expression levels has been reported to result in variable changes in traditional measures of GR activation, both without and in response to changes in external stimuli such as exposure to stressors or exogenous signalling molecules other than GCs. Some reports show an increase in unliganded GR phosphorylation and nuclear translocation, but no change in transcription of GR target genes (Eickelberg et al., 1999; Hu et al., 2013; Lambert et al., 2013; Pan et al., 2015), while others report a change in unliganded GR transcriptional activity on target reporter or endogenous genes, with or without demonstrated recruitment of the GR to the promoter (Matthews et al., 2011). Classical GC-mediated GR activation involves several steps, including GC binding to unliganded GR, receptor phosphorylation, nuclear translocation, recruitment of GR to promoters and DNA binding (directly or via tethering), changes in chromatin structure and cofactor and transcriptional machinery recruitment (Figure 1). For the purposes of this review we will use the terms “glucocorticoid (GC)-independent activation” of the GR, to refer to reports where GC independent, but GR-dependent changes in transcriptional activity have been demonstrated. However, when only some markers of GR activation increase, without detected changes in GR-mediated transcription, in the absence of GCs, we will refer to this as “GC-independent priming” of GR signalling, when these effects increase GR signalling in the presence of GCs. When only some markers of GR activation change, without detected changes in GR-mediated transcription, in the absence of GCs, we will refer to this as “GC-independent desensitizing” of GR signalling, when GR signalling decreases in the presence of GCs. Thus GC-independent priming and GC-independent desensitization, increases or decreases, respectively, the sensitivity of the unliganded GR to GCs. These mechanisms are especially relevant regarding GC-therapy and GC-resistance, most likely playing a major role in changing the potency and/or efficacy of the response. .
3. GR levels and isoforms
One mechanism to change sensitivity to GCs (discussed in more detail in section 2), is to change the levels of expression of the unliganded GR protein. Physiological GR levels exhibit a broad range of expression levels (4-900 fmol GR/mg protein) in different cells and disease states (Robertson et al., 2013). GR protein levels are known to be regulated by multiple mechanisms, including transcription of the GR gene, stability of GR mRNA and stability of GR protein (Cain & Cidlowski, 2015; Oakley & Cidlowski, 2011). The human GR gene is transcriptionally regulated by at least three different promoters in a cell-specific manner (Yudt & Cidlowski, 2002), but the signals and cell-specific factors that regulate transcription of the GR gene and GR protein expression levels are not well understood. GCs are known to downregulate GR levels in many but not all cell types, suggesting a possible mechanism of GC-resistance by lowering GR protein levels after prolonged exposure to GCs (Dong et al., 2015; Yudt & Cidlowski, 2002). Although both cAMP and GCs regulate expression of GR mRNA levels in resistant leukemic and multiple myeloma cells, changes in GR protein levels are, however, unlikely to be the cause of GC resistance in these and some other primary cells (Dong et al., 2015; Tissing et al., 2006). Interestingly, IL1β has also been shown to increase GR mRNA and protein in nasal polyps, via a mechanism involving p38 MAPK and c-Jun N-terminal kinases (JNK) (Wang et al., 2015), suggesting at least one example of GC-independent priming by changing GR mRNA levels. However, whether signalling molecules known to desensitize or prime GR signalling do this via mechanisms involving changes in transcription from the GR promoter, or changing stability of the GR mRNA or protein, is unclear in most cases.
Several transcriptional and translational isoforms of the GR exist, which appear to vary in their tissue distribution and gene-specific effects (Oakley & Cidlowski, 2011). Our current understanding of GR mechanism of action is mainly obtained from research on the almost ubiquitous and most abundant full length GRα isoform. This review is based on work assumed to be due mainly to the full length GRα isoform. However it should be noted that the contribution of other GR isoforms has not been investigated in most cases. Although the GR is derived from a single gene in humans, a large cohort of functionally distinct GR subtypes arise from alternative processing of the GR gene. While the GRα isoform is almost ubiquitously expressed, albeit at different levels in different tissues, the other isoforms are less widely and abundantly expressed and differ in their expression, functional and gene regulatory properties (Oakley & Cidlowski, 2011). For example, different GR isoforms have been shown to have distinct functions in Jurkat and probably in primary T cells (Wu et al., 2013). These authors concluded that cell-specific expression and function of GR isoforms may help to explain tissue- and individual-selective actions of GCs, which is likely to be relevant to GC therapy (Wu et al., 2013). Differential expression of GR translational isoforms has also been shown to be involved in differential effects on inflammation in immature versus mature dendritic cells (Cao et al., 2013), further suggesting a differential role of GR isoforms in immunotherapy. Furthermore these isoforms are subjected to a range of post-translational modifications which further increases their functional diversity. While differential site-specific unliganded GRα phosphorylation has been shown to play a role in GC-independent GR activation, as well as priming and desensitizing of GC responses, the role of other GR isoforms in these and other responses involving synergy and cross talk, have largely not been investigated. Interestingly, IL1β and tumor necrosis factor (TNF) have been shown to increase mRNA and protein expression of GRα and GRβ isoforms in cultures of HeLaS3 and CEM C7 cells lines (Webster et al., 2001). GRβ protein was upregulated much more than that of GRα, resulting in a disproportioned ratio of GRα to GRβ (Webster et al., 2001). IL1β appears to not only alter the GRα/GRβ ratio, but also the affinity of the unliganded GR for GCs (Wang et al., 2015). The GRβ differs from the GRα at the C terminus, resulting in a lack of binding to GCs, constitutive location in the nucleus and an inability to transactivate a GC-responsive reporter gene. However, it acts as a dominant-negative inhibitor of GRα genomic transactivation and transrepression when co-expressed with GRα (Oakley & Cidlowski, 2011). Interestingly GRβ has been implicated in playing an important role in GC resistance (Oakley & Cidlowski, 2011). Although higher expression levels of GRβ have been associated with a variety of inflammatory diseases and GC resistance (Oakley & Cidlowski, 2013), it is of note that in general GRβ appears to be expressed at much lower levels than GRα (Lewis-Tuffin & Cidlowski, 2006; Oakley & Cidlowski, 2013). Nevertheless, by increasing the GRβ/GRα ratio, it is possible to mimic GC resistance in cell line models (Wang et al., 2015). Altering the expression levels of unliganded GRα relative to the GRβ is a possible mechanism to either increase or decrease GC sensitivity, which could potentially be induced by various stimuli or signalling molecules.
4. Glucocorticoid-independent activation of GR signalling
The classical paradigm of GC-dependent signalling places the unliganded GR in the cytoplasm prior to ligand binding (Boardman et al., 2014; Nicolaides et al., 2010). However, some reports suggest that not only can the unliganded GR be nuclear, but that it can be transcriptionally active, modulating basal transcription of several endogenous as well as reporter genes (Kotitschke et al., 2009; Matthews et al., 2011; Ritter et al., 2012; Ritter & Mueller, 2014; Robertson et al., 2013; Verhoog et al., 2011). For example, there is evidence that the unliganded GR plays a protective role in breast cancer. The unliganded GR is associated with the promoter of a tumor suppressor gene, BRCA1, in non-malignant mammary cells, while exposure to GCs inhibits this interaction, suggesting a role of GCs in promoting breast cancer (Ritter et al., 2012). A recent study comparing gene expression by microarray analysis of a stable GR deficient (GR shRNA) epithelial (EPH-4) cell line with its parent GR-expressing cell line, found that 260 genes were negatively regulated and 343 genes were positively regulated by the unliganded GR in the absence of any exogenous GCs (Ritter & Mueller, 2014). Additionally, chromatin immunoprecipitation assays showed that the unliganded GR was present on the promoter of the cholesterol 25-hydroxylase (Ch25h) gene. Interestingly, upon the addition of the GR agonist dexamethasone (Dex), the GR moved away from the Ch25h promoter, resulting in decreased transcription. Furthermore, by transiently transfecting GR into the GR deficient cells, the authors showed that the unliganded GR dose-dependently increased transactivation of a Ch25h-promoter reporter construct in the absence of exogenous GCs (Ritter & Mueller, 2014). Whether other genes are activated by the unliganded GR, but repressed by the liganded GR, in these or other cells, remains to be investigated. In agreement with a role for the unliganded GR in transcriptional regulation, Robertson et al. showed that by increasing the amount of unliganded GR in the COS-1 GR-deficient cell line by transient transfection, they were able to enhance nuclear import, GR dimerization, GR binding to DNA and transcription, in the absence of exogenous GCs (Robertson et al., 2013). In this study, the levels of GR shown to result in GC-independent GR activation were determined by saturation binding and are about 760 fmol/mg protein, and about 60000 GR molecules/ cell (Robertson et al., 2013). GR levels have been quantified in only a few reports. They have been reported for example as 893 fmol GR/mg protein in healthy skin, 2777 fmol GR/mg protein in skin from AIDS patients (Guo et al., 1996), 16200 fmol GR/cell in normal epithelial stem cells (Driver et al., 2001) and 81000 molecules GR/cell in uterine cervical cancer cells (Lu et al., 2005). Thus where levels of GR shown to result in GC-independent GR activation have been quantified, these appear to be within the physiologically relevant range (Robertson et al., 2013), arguing that these effects are likely to occur in vivo. However, whether such effects occur in vivo remains to be determined.
Taken together, the results obtained for unliganded GR suggest that the GR does not always require binding of GCs for nuclear localization and transcriptional activation. Rather the findings suggest that an equilibrium exists between cytoplasmic and nuclear GR, which can be shifted by GCs. Given high enough GR levels, enough GR could be shifted to the nucleus to obtain a GR-dependent transcriptional response in the absence of GCs, most likely on select genes (Figure 2). When cells are cultured in serum-starved or charcoal-stripped serum, it appears that most of the endogenous GR resides in the cytoplasm and requires GC binding for activation. However this may not be the case in some cells with higher GR levels, or in many primary cells (discussed in section 9). It should also be noted that the reports of transcriptionally active GR in the absence of exogenously added GCs in vitro upon increasing the levels of GR may be due to the presence of very low concentrations of GCs still present in the medium, or even GCs produced within the cells. A transcriptional response detected only with higher GR levels would be possible, since it is known that increasing GR levels can shift the dose response to the left, resulting in a greater potency (see section 2) (Hapgood et al., 2014; Ong et al., 2010; Szapary et al., 1996; Zhao et al., 2003). This occurs when GR levels are not limiting, even without a change in receptor affinity. When GR levels are limiting, increasing GR levels will result in an increase in maximal response or efficacy. Thus potency and/or efficacy will decrease with decreasing unliganded GR levels and increase with increasing unliganded GR levels. It follows that factors that merely decrease or increase the expression levels and/or subcellular distribution of the unliganded GR are likely to affect the potency and/or efficacy of GR responses to GCs, resulting in physiological consequences ranging from the extreme cases of GC insensitivity, to GC hypersensitivity, and GC-independent activation.
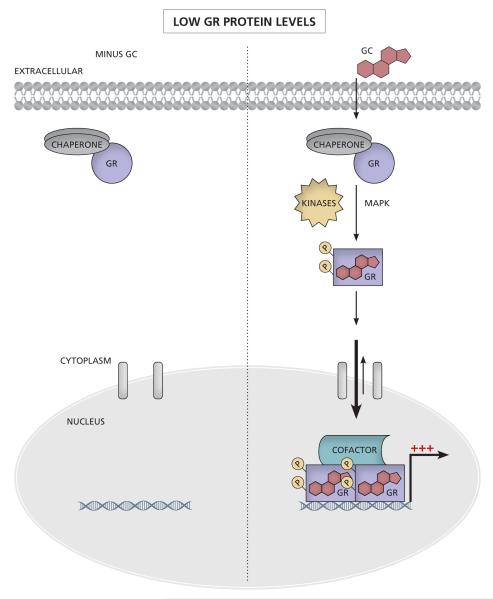
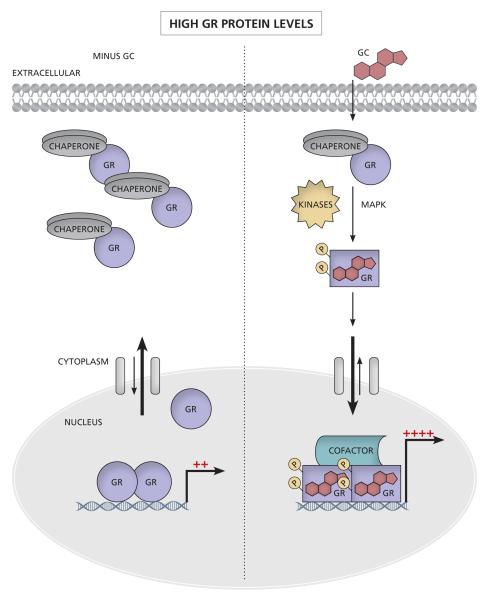
Figure 2. Changes in unliganded GR protein levels affect sensitivity to GCs.
(A) At low GR levels, there is a small amount of GR present in the cytoplasm. Upon GC binding, the GR is activated and the equilibrium shifts towards the nucleus as the GR translocates, binds DNA and initiates transcription. (B) At high GR levels, in the absence of GCs, the equilibrium between cytoplasmic and nuclear GR is still directed towards the cytoplasm. However due to more nuclear GR than at low GR levels, some genes can be transcriptionally regulated by the unliganded nuclear GR and there is likely a higher basal amount of transcription (see section 4 and Table 1). Upon GC binding the activated GR again translocates to the nucleus, binds DNA and initiates transcription. (C) Increasing GR levels shift the dose response curve to the left, resulting in a greater response with a lower concentration of GCs (lower EC50 and greater potency) and/or a greater maximal response (efficacy). Increasing numbers of red crosses indicate increasing efficacy of transcription.
However, changes in GR expression levels cannot account for some reports of GC-independent endogenous GR transcriptional activation by exogenous stimuli. This was shown for the first time by our laboratory, in response to the peptide gonadotropin-releasing hormone (GnRH) (Kotitschke et al., 2009), and subsequently in response to the cytokine TNF (Verhoog et al., 2011). In a mouse gonadotrope LβT2 cell line, Kotitschke et al. showed that GnRH can rapidly activate the endogenous human GR (hGR) via GnRH receptor, protein kinase C- and MAPK-dependent phosphorylation of the mouse GR at Ser-234 (equivalent to hGR Ser-226), without increasing total GR levels, resulting in transactivation of a GRE-reporter gene (Kotitschke et al., 2009). Furthermore GnRH treatment resulted in enhanced recruitment of the endogenous unliganded GR, as compared to vehicle, to the AP-1 region of the endogenous GnRH receptor promoter and transactivation thereof, in the absence of exogenous GCs (Kotitschke et al., 2009). Using GR siRNA, we established a requirement for the endogenous GR for the transcriptional response. Dex similarly resulted in enhanced GR recruitment to and transactivation of the GnRH receptor promoter. Similarly, Verhoog et al. demonstrated GC-independent activation of the endogenous GR in the End1/E6E7 endocervical cell line by TNF (Verhoog et al., 2011). We showed that TNF induced phosphorylation of the endogenous GR at Ser-226 and enhanced recruitment of the GR and coactivator glucocorticoid receptor interacting protein-1 (GRIP-1) to the IL6 promoter, in the absence of exogenously added GCs. This effect appears to be highly cell-specific (Hapgood et al., unpublished data). Furthermore, GR knockdown as well as co-stimulation with the GR antagonist RU486, increased TNF-mediated transactivation of the IL6 promoter, indicating that in this model, GC-independent GR activation by TNF repressed IL6 transcription (Verhoog et al., 2011). Dex similarly resulted in enhanced GR recruitment to the IL6 promoter and transrepression thereof, but interestingly did not induce recruitment of GRIP-1. Co-treatment with TNF and the GC resulted in repression of IL6 in a GR dependent manner, coupled with an enhanced recruitment of GRIP-1, more so than with TNF alone (Verhoog et al., 2011). It is possible that other examples of GC-independent activation occur in the literature, but it is difficult to find them if the authors do not specifically look for proof of or comment on detected GC-independent activation. One such example where GC-independent GR activation is indicated, but not reported, is the finding of recruitment of the GR to the proopiomelanocortin promoter upon stimulation with leukemia inhibitory factor (a member of the IL6 family) (Langlais et al., 2008). However these authors did not investigate phosphorylation, nuclear translocation of the GR or changes in transcription in response to stimulation with leukemia inhibitory factor. In the above-mentioned studies showing GC-independent GR activation by GnRH and TNF, these stimuli did not enhance total GR levels, suggesting a different mechanism compared to that involving increasing GR levels without exogenous stimuli. Further support that GC-independent activation occurs for the GR in the absence of changes in GR levels, are the findings by Matthews et al. (Matthews et al., 2011). These authors reported a cell cycle-dependent increased phosphorylation at Ser-203 and Ser-211, GC-independent nuclear localization and increased transactivation in a gene specific manner in G2/M-enriched HeLa cells, of the unliganded GR (Matthews et al., 2011). Furthermore, the authors showed that total GR levels were slightly lower under these circumstances. However, whether the nocodazole compound used to disrupt microtubules and force the cells into the G2/M phase, or other cell cycle signals induce GC-independent GR activation, is unclear.
Several earlier reports suggest that the GR can be “activated” in the absence of GCs by ursodeoxycholic acid (UDCA) and β-adrenergic receptor agonists, but insufficient evidence is available to determine whether the unliganded GR was transcriptionally activated or primed. Tanaka et al. found that UDCA promotes nuclear translocation of exogenously expressed GR and resulted in a very low extent of transactivation of a GRE-reporter gene in hamster ovary cells (Tanaka et al., 1996). Using electrophoretic mobility shift assays (EMSAs) the authors also showed that UDCA promoted in vitro DNA binding capability of the GR. Furthermore, using Northern blot analysis, the authors found that UDCA could increase basal HLA-Dra mRNA in a human B cell lymphoma-derived IM-9 cell line (Tanaka et al., 1996). Two additional papers showed that UDCA could increase nuclear translocation of the unliganded GR (Miura et al., 2001; Sola et al., 2004). In HeLa cells, Miura et al. showed increased nuclear translocation of the unliganded GR was accompanied by repression of a phorbol myristate acetate-induced NF-κB-luc reporter promoter. Sola et al. showed that UDCA-induced nuclear translocation of the unliganded GR in hepatocytes could prevent transforming growth factor-beta (TGFβ) 1 induced apoptosis (Sola et al., 2004). However, UDCA also increased GR mRNA and protein levels. Despite structural similarities between UDCA and GCs, various reports have shown that UDCA does not bind to the GR (Sharma et al., 2011; Weitzel et al., 2005). Thus the effects of UDCA on the unliganded GR are unlikely to be due to its direct actions as a GR ligand, but rather due to indirect effects via other target proteins. Similar effects to those of UDCA have been observed with the β-adrenergic receptor agonists salmeterol and salbutamol (Eickelberg et al., 1999). However, additional data (see section 6), suggest that the effects of β-adrenergic receptor agonists on the GR are due to priming rather than GC-independent activation. None of the above studies investigated a requirement for the GR in the transcriptional assays, effects on GR phosphorylation, or recruitment of the GR to a promoter. For one study GR levels increased (Sola et al., 2004) but this was not investigated in the other studies. It is difficult to assess whether changes in DNA binding as measured in vitro are a marker of transcriptionally active GR, since GR DNA binding in vitro is GC-independent (Ronacher et al., 2009; Willmann & Beato, 1986). In the above examples with UDCA and LABAs, it is unclear whether the unliganded GR was transcriptionally active or whether the transcriptional effects were indirect and not via the GR, or due to increased GR levels. Nevertheless, the increased nuclear translocation certainly suggests that the unliganded GR appears to be at least primed for increased transcription. Further investigation of GR levels and GR recruitment to endogenous promoters and GR-dependent changes in mRNA levels may yet reveal that some of these effects of UDCA and LABAs occur by GC-independent activation.
The mechanisms of GC-independent GR activation in the absence of changes in GR levels are not fully understood (Figure 3). Whether site-specific changes in unliganded GR phosphorylation alone are sufficient for GC-independent activation has not been established in most cases. By using GR phosphorylation deficient mutants, Matthews et al. showed that phosphorylation at Ser-211 was required for GC-independent transcription. In addition, a GR mutant that mimics GR phosphorylation (D-211) was enough to result in GC-independent transcription, implying that GR phosphorylation at Ser-211 alone is sufficient for GC-independent activation (Matthews et al., 2011). Unfortunately the authors did not investigate GR expression levels with the different GR mutants. Based on theoretical considerations, changes induced by stimuli other than GCs, in the levels or activities of any of the proteins involved in the GR signalling pathway (Figure 3), including the GR, could also potentially affect GR activation by shifting the equilibrium between transcriptionally inactive and transcriptionally active GR. These could include changes in proteins that regulate posttranslational modification of the GR or other proteins involved in GR signalling, including cofactors (section 2) and chaperones such as the immunophilins FKBP51 and FKBP52 (Tatro et al., 2009), heat-shock protein 90 (HSP90) and proteins involved in nuclear translocation and nuclear trafficking.
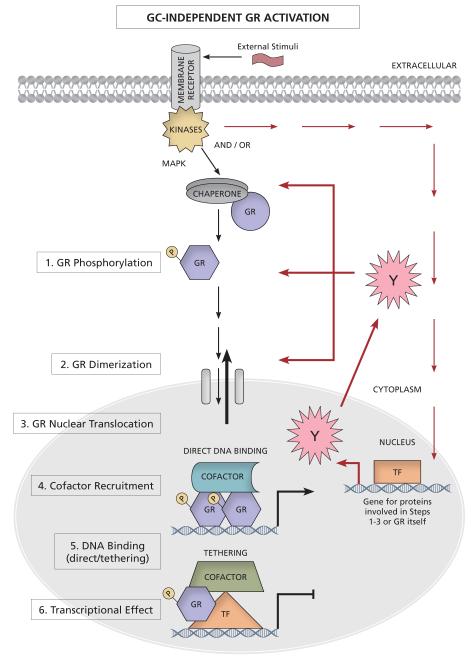
Figure 3. GC-independent GR activation.
External non-GC signals, bind to and activate their receptors, resulting in a signaling cascade which results in site-specific GR phosphorylation, nuclear translocation, DNA binding, cofactor recruitment and a GR-mediated transcriptional effect (see section 4 and Table 1 for details on the specific external signals). Whether the signaling cascade activated by the external stimulus directly activates the GR, or whether and to what extent this involves transcription and new protein synthesis of additional factors (Y) has not been determined in most cases. Labels are as for Figures 1 and 2.
5. Glucocorticoid-independent desensitizing of GR signalling
Decreasing GR levels without any changes in levels or activity of other downstream proteins in the GR signalling pathway is predicted to desensitize GR signalling, due to right-shifting of the dose response curve, as discussed above. Depending on the extent of decreased expression, this could contribute to GC-resistance. Decreased GR levels could be achieved by multiple mechanisms, including GC-mediated downregulation of GR transcription, decreases in synthesis or increases in turnover or degradation of the GR protein or decreases in stability or transport of the GR mRNA. One such mechanism was recently reported whereby GCs increase cleavage of the full length GR (Paugh et al., 2015). Primary leukemia cells from 444 patients newly diagnosed with acute lymphoblastic leukemia were found to express significantly higher levels of encoding caspase 1 (CASP1) and its activator NLRP3 in GC-resistant leukemia cells, resulting from significantly lower somatic methylation of the CASP1 and NLRP3 promoters (Paugh et al., 2015). Overexpression of CASP1 resulted in cleavage of the GR and decreased the GC-induced transcriptional response and increased GC resistance (Paugh et al., 2015). Evidently, the unliganded GR that is not cleaved is still capable of binding to the ligand and mediating ligand-dependent nuclear translocation, DNA binding and transcriptional modulation and is not in any way incapacitated to perform these functions. The right-shifting of the dose response curve and the accompanying drop in maximum response is therefore the consequence of less liganded GR being recruited to the promoters of target genes. In this example, sensitivity to GCs could be restored by simply raising cellular GR levels.
Cellular stressors have been reported to modulate the activity of the unliganded GR, without lowering GR levels, in a manner involving site-specific GR phosphorylation, to modulate GC-dependent transcriptional responses in a gene-specific manner (Galliher-Beckley et al., 2011). The unliganded GR is phosphorylated at Ser-134 in response to several cellular stressors but not in response to GCs (Galliher-Beckley et al., 2011), in a p38 MAPK-dependent manner. However in this report cellular stressors alone did not result in changes in GR nuclear translocation, DNA binding or GR-mediated transcription. Interestingly, upon co-treatment with stressors and GCs, the range of genes regulated by the GR was significantly different from those regulated by GCs alone, while GC-dependent nuclear translocation, stability, total GR levels or the GC-induced phosphorylation pattern of the GR remained unchanged. The data further show that increased interaction of Ser-134 phosphorylated GR with 14-3-3 proteins resulted in a lack of GR promoter recruitment and decreased GC-mediated upregulation of ladinin-1 and insulin-like growth factor binding protein 1 (IGFBP-1) mRNA but had no effect on the GC-induced leucine zipper (GILZ) mRNA (Galliher-Beckley et al., 2011). Taken together, these results indicate that these cellular stressors did not activate the GR in a GC-independent manner, but that phosphorylation at Ser-134 induced by these stressors rather desensitized the unliganded GR to elicit a gene-specific desensitizing of the response to GCs. Reactive oxygen species produced during a cytokine storm (Morgan et al., 2008) appear to also desensitize GR signalling by decreasing ligand binding of the unliganded GR and DNA binding affinities of the liganded GR (Hutchison et al., 1991).
Several cytokines have also been shown to desensitize GR signalling, via mechanisms involving various cytokine-induced kinases. Individual cytokines (e.g. IL13, TNF) and combinations of cytokines (e.g. IL2 and IL4) have been shown to reduce GR ligand binding affinity (Kam et al., 1993; Spahn et al., 1996; Szatmary et al., 2004). Others like IL1α (Wang et al., 2005) and interferon-α (Hu et al., 2009) appear to reduce the DNA binding affinity of the liganded GR. In addition, others have been shown to prevent the nuclear translocation of the liganded GR [IL2 (Goleva et al., 2002), IL2 and IL4 (Mercado et al., 2011), IL1β (Escoll et al., 2015) and IL1α (Pariante et al., 1999; Wang et al., 2005)]. The majority of these studies investigated the effects of cytokines on the liganded GR and hence it remains unclear how they affect the unliganded GR to desensitize it towards GC activation. Two studies (Kam et al., 1993; Wang et al., 2005) however reported increases in GR levels after treatment with cytokines (as assessed by GR total binding and mRNA qPCR assays) and yet reported diminished GR function. Although not investigated in these studies, increases in GRβ isoform expression rather than transcriptionally active GRα could explain the decrease in GC-mediated effects described above. Such an effect is seen with IL1β, as discussed in section 3. Additionally, IL1β was shown to reduce GR levels and inhibit GC-dependent GR nuclear translocation and site specific phosphorylation of the unliganded GR at Ser-203 and Ser-211 in A549 cells, resulting in a gene-specific reduction in GC-induced GR mediated transactivation (Escoll et al., 2015). Escoll et al. reported that sustained IL1β treatment alone resulted in a GC-independent reduction in total GR levels, with cytoplasmic GR rather than nuclear GR levels being affected. Although Escoll et al. did not investigate the effect of IL1β on the phosphorylation of the unliganded GR, their data indicate that IL1β can desensitize the unliganded GR as well as decrease the ability of liganded GR to mediate transcription. Whether effects on GRβ isoform expression were involved in this report was not investigated. Cytokines thus appear to desensitize GR signalling by a variety of mechanisms that may be cell-, gene- and context-specific, and depend on the extent of basal immune activation.
Kinases play an important role in desensitizing GR signalling and they can be activated by numerous exogenous as well as endogenous signals. The desensitizing effects of cytokines described above have been linked to the activation of MAPK signalling pathways. The activated MAPKs, interacting directly or indirectly with the GR, can phosphorylate the unliganded GR at specific residues affecting properties such as nuclear translocation and DNA binding affinity (Pace & Miller, 2009). JNK can phosphorylate the unliganded and liganded rat GR at Ser-246 (hGR Ser-226) resulting in increased nuclear export of the liganded GR (Itoh et al., 2002) and reduced GC-dependent transactivation (Rogatsky et al., 1998). Similarly, p38 MAPK activation is involved in the IL2-mediated inhibition of GC-dependent GR nuclear translocation in HT-2 cells (Goleva et al., 2002). Furthermore, other studies have implicated p38 and JNK in decreasing GC-dependent GR DNA binding affinity (Hu et al., 2009; Wang et al., 2005) and consequently reducing GC-mediated GR transactivation. Taken together the results suggest a general mechanism whereby cytokines can desensitize the unliganded GR by activation of kinases, in particular by MAPK-mediated site-specific phosphorylation of the unliganded GR, to decrease its ligand binding affinity and GC-induced nuclear translocation and DNA binding affinity, in a cell-specific and context-dependent manner.
Other kinases such as glycogen synthase kinase 3 (GSK3) can also modulate GC-dependent GR nuclear and/or mitochondrial translocation by preventing its dissociation from the multiprotein complex that keeps the unliganded GR in the cytoplasm. GSK3 is a serine/threonine kinase and exists as two isoforms, GKS3α and GSK3β, and aberrant regulation of both isoforms can result in GC-insensitivity. The active GSK3α (nonphosphorylated) is known to associate with the unliganded GR in the cytoplasm (Spokoini et al., 2010). In the presence of GCs, GSK3α dissociates from the GR in a GC-dependent manner and the liberated liganded GR then translocates to the mitochondria to induce the release of pro-apoptotic mediators, cytochrome c and Smac/DIABLO (Sionov et al., 2006; Spokoini et al., 2010; Talaber et al., 2009). Phosphatidylinositol 3-kinase/protein kinase-B (PI3K/Akt) can inhibit this process by phosphorylating GSK3α, resulting in its inactivation. GSK3β on the other hand is not associated with the cytoplasmic unliganded GR (Spokoini et al., 2010), but has been shown to be involved in the GC-dependent activation of the GR (Rubio-Patino et al., 2012). Rubio-Patino and colleagues showed that GC-induced GR transcriptional activity can be suppressed by either inhibiting or knocking down GSK3β. They further showed reduced recruitment of the GR to the GILZ promoter. Pharmacological inhibition of GSK3β impairs the ability of GCs to repress lipopolysaccharide (LPS)-induction of pro-inflammatory cytokines in primary bronchial epithelial cells and monocytes (Ngkelo et al., 2015). Individuals with COPD show resistance to GCs and using tissue biopsies from a cohort of COPD patients, Ngkelo et al. demonstrated that GSK3β is hyperphosphorylated in these samples. Ngkelo et al. also established the requirement for PI3K/Akt kinase in phosphorylating GSK3β and resulting in its inactivation. Conversely, activating GSK3β may also desensitize GR signalling. GSK3β has been shown to phosphorylate the GR at Ser-404 in a ligand-dependent manner (Galliher-Beckley et al., 2008), enhancing nuclear export of the liganded GR and thus reducing GC-dependent GR transcriptional activity. Thus any exogenous signals that lead to the modulation of GSK3 activity could potentially desensitize GR signalling. Whether this results in changes in phosphorylation or levels of the unliganded GR remains to be determined.
Another kinase, the type 1 TGFβ receptor (ALK5) kinase has been shown to be involved in TGFβ-mediated inhibition of GR transcriptional activity in human bronchial epithelial cell lines (Keenan et al., 2014). The mechanism by which this occurs remains unknown, but it is possible that any of the mechanisms described above might be involved.
Taken together, it appears that GR signalling can be desensitized by decreasing unliganded GR levels, or increased sequestering of the GR in the cytoplasm. Additionally a range of signals, including cellular stressors and cytokines, can desensitize the GR via mechanisms including decreased binding affinity for GCs, changes in kinase activity, in particular MAPKs and changes in site-specific phosphorylation of the unliganded GR (Figure 4).
Figure 4. GC-independent GR priming or desensitizing.
Upon binding to their respective receptors, external stimuli result in a signaling cascade that either directly, or indirectly via upregulation of another protein (Y), result in changes in the GR signalling pathway. In the absence of GCs, these include changes in unliganded GR affinity for GCs, binding to chaperones, immunophillins and/or site-specific GR phosphorylation (see Table 1, as well as section 5 for desensitizing and section 6 for priming, for details on the specific external signals). These changes, however, do not result in a GR-mediated transcriptional effect. Only upon co-stimulation with GCs, does the GR get recruited to promoters and elicit a transcriptional effect. In the case of priming, the modifications are all enhanced (green arrows), for example to increase GR phosphorylation or nuclear translocation, whereas in the case of desensitizing (red arrows), the modifications result in, for example, less GR nuclear translocation or decreased GC binding. Increased GR phosphorylation, can be involved in both priming and desensitization, depending on context. Labels are as for Figures 1 and 2.
Interestingly, several viral proteins have also been shown to modulate GR signalling. Respiratory syncytical virus (RSV) non-structural proteins and the Poxvirus molluscum contagiosum (MC) protein MC013C have been shown to inhibit GC-mediated GR transactivation (Chen et al., 2000; Webster Marketon et al., 2014). The precise mechanism whereby this occurs has not yet been established; however the poxvirus protein MC013C has been shown to bind directly to the unliganded and GC-activated GR, thereby possibly acting as a GR cofactor (Chen et al., 2000). Interestingly it appears that viral proteins can not only decrease the activity of the liganded GR, but also increase its activity, and possibly also act via the unliganded GR. The HIV accessory protein viral protein R (VPR), has been shown to bind to the GC-activated GR, but in this case to increase GR transactivation (Hapgood & Tomasicchio, 2010; Kino et al., 1999). This GR-VPR interaction could explain the increased muscle wasting symptoms in AIDS patients in the absence of increased GC levels (Kino et al., 1999). VPR also appears to act via the unliganded GR by repressing TNFα-induced upregulation of IL6 expression via the unliganded GR (Hapgood et al., unpublished data). Further research is required to investigate these mechanisms, which may have important implications for disease.
Desensitized GR exhibits reduced or abrogated response to GCs, as indicated by reduced nuclear translocation and reduced transcriptional activity. However whether changes in site-specific phosphorylation, or any other posttranslational modifications of the unliganded GR, are a requirement for some or all of the above examples of desensitization, and the extent to which decreases in unliganded GR levels are involved, remains to be determined. In addition, whether these mechanisms involve genomic actions of the cytokines and stressors, alone or in combination with non-genomic actions such as kinase activity, is currently unclear. While there is evidence that some desensitizing effects are gene-specific and context-specific, further research is required to investigate the selectivity of these responses.
6. Glucocorticoid-independent sensitizing of GR signalling
Several reports in the literature suggest mechanisms whereby exposure of cells to various stimuli or exogenous ligands other that GCs, while not activating the unliganded GR transcriptionally, can prime or sensitize the unliganded GR for increased transcriptional responses to GCs in the presence of the priming stimulus, compared to GCs alone. The finding that merely increasing GR levels in the absence of exogenous stimuli, as discussed in section 4, can result in GC-independent GR activation, suggests one possible mechanism whereby exogenous ligands could prime the GR. This could occur by increasing GR levels, albeit to levels below those needed to observe transcriptional responses by the unliganded GR. The GR levels needed to observe transcriptional responses by the unliganded GR are likely to be cell- and gene-specific, but where this has been investigated for expressed GR in COS cells, the levels appear to be about 760 fmol/mg protein (Robertson et al., 2013). The subsequent increase in responses to GCs could be due to a left shift in the GC dose response curve and/or an increased efficacy, due to increased unliganded GR levels. This mechanism appears to be at least in part involved in the priming of GR signalling by cocaine in rats, which exhibited increased GR mRNA and protein levels, via an unknown mechanism (Caffino et al., 2015). These authors also however showed increased GR phosphorylation at Ser-232 (the rat equivalent to hGR Ser-211), accumulation of nuclear GR and an increase in expression of the GR steroid receptor coactivator-1 (SRC-1) in the prefrontal cortex of rats three days after cocaine administration. Although it was not established in the rat model whether these effects of cocaine occurred via the unliganded GR, given that endogenous GCs would be present in the rats, the findings suggest that this is an example of priming of unliganded GR signalling by cocaine to increase GR levels as well as by increasing several other markers of activation in the GR pathway.
Some cytokines (e.g. TGFβ1 and IL13) can either desensitize GR signalling (as discussed in section 5) or sensitize GR signalling, depending on context (Hu et al., 2013; Keenan et al., 2014; Pan et al., 2015; Salem et al., 2012; Spahn et al., 1996). In human airway smooth muscle cells, Hu et al. showed that IL13 increased GR nuclear translocation, without changing GR levels (Hu et al., 2013). Furthermore, IL13 alone resulted in GC-independent GR phosphorylation at Ser-203 and Ser-211 in an extracellular signal-regulated kinase-1/2 (ERK1/2)- and JNK-dependent manner. Interestingly, treatment with IL13 alone resulted in unliganded GR binding to the mediator complex subunit 14 (MED14) co-activator, but did not render the unliganded GR capable of binding DNA, as shown by EMSAs, and did not result in a transcriptional response on a synthetic reporter promoter. Co-treatment with the GR agonist cortisone resulted in enhanced GR phosphorylation at Ser-211 and enhanced GR nuclear translocation, more so than either stimulus alone. Furthermore IL13 could dose-dependently increase cortisone-induced GR binding to DNA and co-treatment resulted in more transactivation of the synthetic reporter than treatment with the GC alone (Hu et al., 2013). Growth factors are also known to potentiate GR signalling. A recent study reported that TGFβ1, through the activation of p38 MAPK and ERK1/2 pathways, enhances the Dex-induced phosphorylation of the GR at Ser-211, resulting in the enhancement of GR transcriptional activity in ovarian cancer cells (Pan et al., 2015). However, TGFβ1 alone does not affect unliganded GR phosphorylation at Ser-211, nor expression levels or nuclear translocation of unliganded GR.
Interestingly GC-independent priming by brain derived neurotropic factor (BDNF) has been implicated in brain function and response to GCs (Lambert et al., 2013). BDNF treatment in the absence of GCs resulted in hGR phosphorylation at Ser-134 and Ser-267, as well as minimal phosphorylation at Ser-203, Ser-211 and Ser-226. This increase in GR phosphorylation by BDNF was not due to changes in GR levels, and BDNF treatment alone did not result in GR nuclear translocation or in a transcriptional response on a TAT-GRE reporter promoter construct. Interestingly, BDNF was shown to enhance GC-mediated transcription of the serum/glucocorticoid regulated kinase (SGK) promoter, indicating that BDNF primes the unliganded GR (Lambert et al., 2013).
LABAs have been shown to enhance the clinical efficacy of inhaled GCs (Chung et al., 2009; Giembycz et al., 2008; Giembycz & Newton, 2015; Newton et al., 2010). Several reports have shown that LABAs and GCs can regulate transcription in an additive or synergistic manner (as reviewed in (Giembycz & Newton, 2015; Newton et al., 2010). LABAs can modulate genes concurrent with, but independently of the GR, via activation of the cAMP pathway, or they can modulate GR signalling. However, very little mechanistic detail of how this occurs is known. As previously mentioned (section 4), two β2-adrenoreceptor agonists, salbutamol and salmeterol, enhance nuclear translocation of the unliganded GR (Eickelberg et al., 1999). Similarly, formoterol and salmeterol were shown to enhance nuclear translocation of the unliganded GR in primary cell cultures of human bronchial smooth muscle cells, primary macrophages and epithelial cells (Roth et al., 2002; Usmani et al., 2005). Salmeterol also increased nuclear translocation of the unliganded GR in H937 and BEAS-2B cell lines, which was accompanied by increased transcription from a GRE-luc reporter promoter (Usmani et al., 2005). Interestingly, in the same study, salmeterol did not increase transcription of the endogenous secretory leuko-proteinase inhibitor (SLP1) gene (Usmani et al., 2005). Formoterol and salmeterol have also been shown to increase nuclear translocation of the unliganded GR in peripheral blood mononuclear cells (PBMCs) (Mercado et al., 2011). In the same study, Mercado et al. showed that formoterol decreased activation of JNK and p38, which was accompanied by a decrease in total GR phosphorylation. Given that GR phosphorylation by JNK and p38 has been shown to result in increased nuclear export of the GR, the authors hypothesized that the decrease in activity of these kinases by formoterol could explain the increased GR nuclear translocation by the LABAs, indicating a possible mechanism of GR activation or priming by the LABAs (Mercado et al., 2011). A more recent study in primary macrophages by Haque et al. showed that salmeterol alone had no significant effect on nuclear translocation and binding of the unliganded GR to DNA (Haque et al., 2013). Furthermore, salmeterol did not enhance transactivation of the MKP-1 gene or a GRE-promoter reporter in U937 cells. However, salmeterol significantly increased GC-induced GR nuclear translocation, DNA binding and transactivation of both the MKP-1 gene and the GRE-luc reporter, indicating that salmeterol treatment primes the unliganded GR, rather than activating the unliganded receptor (Haque et al., 2013). It should be mentioned that LABAs have been shown to increase MKP-1 mRNA and protein expression in a manner involving the cAMP-PKA pathway (Kaur et al., 2008; Manetsch et al., 2013). However, whether activation of the cAMP-PKA pathway via LABAs is responsible or plays a role in the above-mentioned increase in GR nuclear translocation and DNA binding is not known. Similar to the situation with LABAs, epinephrine also appears to prime the GR by modulating its response to GCs, but via an apparently different mechanism. A study by Schmidt and colleagues demonstrated that epinephrine, acting via the ß2-adrenergic receptor, enhances GR-mediated transactivation on a GRE-reporter construct in a strictly GC-dependent fashion in a hippocampus-derived cell line (Schmidt et al., 2001). The liganded cytosolic GR directly interacts with Gβ and this interaction results in their co-migration to the plasma membrane after activation of the somatostatin receptor by somatostatin in HCT116 cells in a GC-dependent fashion (Kino et al., 2005). Thus, it is likely that G-protein associated GR might explain some of the non-genomic effects of GCs at the plasma membrane. Since changes in total GR levels were not investigated in the abovementioned studies, the role thereof in mediating the response to LABAs cannot be excluded. An early report by Korn et al. showed in a human bronchial epithelial cell line (Bet1A), that the LABA terbutaline could prevent GC-mediated downregulation of GR mRNA, resulting in increased GR levels as compared to the effects of GCs alone (Korn et al., 1998). This suggests that an increase in unliganded GR levels by LABAs may contribute to their effects on sensitizing the unliganded GR.
Thus a trend is emerging whereby several signals including growth factors, cytokines and β2-adrenoreceptor agonists appear to prime the unliganded GR to result in an increased sensitivity to GCs. Taken together, this priming appears to involve site-specific increases in phosphorylation and/or increased nuclear translocation of the unliganded GR, in some cases in combination with effects on activity or levels of signalling proteins in the GR pathway, without detected GR-dependent transcriptional activity of the unliganded GR (Figure 4). However whether there are cell- and gene- specific effects, whether these mechanisms occur with all members of these families of signalling molecules, and the detailed mechanisms involved, are unknown in most cases. In particular, whether the mechanisms involve or require changes in GR levels and/or post-translational modification and changes in nuclear translocation of the unliganded GR, has not been established in most cases. Nevertheless several reports suggest that priming of the unliganded GR without full GC-independent activation does involve site-specific GR phosphorylation, similar to what occurs for GC-independent GR activation (section 3). What determines whether the unliganded GR is primed but not transcriptionally activated or whether full GC-independent GR activation occurs, remains to be investigated, but most likely involves integration of several mechanisms. The extent to which non-GC signals that affect changes in levels and activity of cofactors, other transcription factors or proteins involved in chromatin structure or components of the basal transcriptional machinery, contribute to enhanced GC-responses of the primed GR, also remains to be determined. It has been shown that changing expression levels of cofactors can modulate maximal activity and potency of steroid receptors in response to ligands (see section 2), suggesting at least one mechanism whereby genomic responses of primed unliganded GR can be further affected by non-GC ligands. The outcome is likely to be determined by the extent of changes in relative levels of the GR, alone or in combination with changes in levels of other receptors and downstream signalling proteins, as well as changes in their posttranslational modifications, via both genomic and non-genomic mechanisms.
7. Synergy between GCs and other ligands
Synergism is defined as a response obtained by a combination of two ligands or signals that is greater than the sum of the individual responses, where each signal alone also induces a response (Chou, 2006). When one signal alone does not induce a response but rather increases the response to the second signal, we have referred to this as priming or sensitizing, as discussed in section 6 above. Several drugs and endogenous signalling molecules have been shown to result in synergistic responses with GCs, as well as to prime GC responses, providing the possibility of enhancing the therapeutic benefits of GCs (Giembycz & Newton, 2015). The net effect would be to improve the therapeutic outcome of drugs while limiting or eliminating the onset of GC resistance/insensitivity. These mechanisms appear to be predominantly genomic. In this section we discuss examples of some of these mechanisms, with a focus on synergism which appears to also involve GC-independent activation of the unliganded GR.
Co-activation of the GR and pro-inflammatory transcription factors like NF-κB and AP-1, has been shown to enhance the acute phase inflammatory response (Busillo & Cidlowski, 2013). ChIP-Seq and microarray data have revealed that a subset of genes, including NFκB-regulated pro-inflammatory genes, are synergistically upregulated by both GCs and TNF (Langlais et al., 2008; Lannan et al., 2012; Rao et al., 2011). This synergism may or may not involve changes in GR levels. For example, TNF and Dex have been reported to synergize in inducing the expression of epithelial sodium channels in GR-expressing HT-29/B6 cells (Bergann et al., 2009). Bergann and colleagues reported that TNF on its own caused an increase in GR mRNA and protein levels and that this TNF effect was unaffected in the presence of Dex, suggesting TNF increased GR levels in a GC-independent manner. The authors also found no evidence for TNF induction of unliganded GR translocation to the nucleus. While this study by Bergann et al. suggests the TNF-mediated increase in GR levels may account for the synergism with Dex on epithelial sodium channel expression, it is not always clear if this is a requirement for synergism between GCs and other stimuli. LPS has been shown to act synergistically with GCs to upregulate sphingosine kinase 1 in macrophages to inhibit inflammation (Vettorazzi et al., 2015). Although changes in GR expression level were not investigated in this study, Vettorazzi et al. showed more GR was recruited to the sphingosine kinase 1 promoter with LPS and Dex than with Dex alone. A recent study demonstrated that Dex and the non-typeable Haemophilus influenza synergize in the induction of interleukin-1 receptor associated kinase (IRAK-M) in human bronchial epithelial cells (Miyata et al., 2015) in a manner requiring both p65 and GR recruitment to the IRAK-M promoter. Miyata and colleagues showed that an inhibitor of kappa B kinase (IκB) inhibitor can prevent the GC-dependent recruitment of the GR to the IRAK-M promoter in the presence of H. influenza. RU486 also prevented the H. influenza-induced recruitment of p65 to the IRAK-M promoter, demonstrating that the liganded GR was needed for p65 recruitment (and vice versa). Although, H. influenza stabilised GR protein levels in this study, it did not induce the recruitment of the unliganded GR to the IRAK-M promoter. However, the recruitment of both p65 and the GR to the promoter of targeted genes is required for the synergistic upregulation by Dex and TNF of the TNFAIP3 gene in Beas-2B cells (Altonsy et al., 2014), which may also be required for the upregulation of serpinA3 in A549 cells (Lannan et al., 2012). A requirement for the liganded GR in NF-κB DNA binding has also been demonstrated in the synergistic induction of IL1β by Dex and phorbol myristate acetate (PMA) in THP-1 cells (Wang et al., 1997). Besides NF-κB, the Smad3 transcription factor activated by TGFβ has been shown to be partially involved with the GR in the synergistic upregulation of plasmogen activator inhibitor (PAI)-1 gene by Dex and TGFβ (Pan et al., 2015). Pan and colleagues reported that TGFβ treatment of ovarian cancer cell lines had no effect on GR expression and phosphorylation, but enhanced the GC-dependent phosphorylation of the GR at Ser-211 in a p38 dependent manner. Thus these synergistic mechanisms appear to mainly involve genomic effects, with a trend emerging whereby signalling from the receptors of each ligand appear to converge independently on transcription factors recruited to the promoter, to result in cooperative interactions on the promoter. However, the possibility that these pro-inflammatory signals also increase GR levels or activate the unliganded GR in some of these examples to contribute to the synergism requires further investigation.
GC-independent activation of the unliganded GR has been shown to be involved in some examples of synergy. This appears to contribute to gene-specific synergistic effects in response to GnRH (Kotitschke et al., 2009; Wehmeyer et al., 2014). One of the downstream effects of the GC-independent activation of the GR by GnRH is the synergistic induction of the GnRH receptor gene by Dex and GnRH, via an AP-1 site (Kotitschke et al., 2009). Furthermore, the GR has been shown to co-localise with the GnRH receptor and flotillin-1 (Flot-1) and all three proteins are required for the synergistic induction of serum/glucocorticoid regulated kinase-1 (SGK-1) mRNA in LβT2 cells (Wehmeyer et al., 2014), implicating lipid-raft-associated GR and GnRH receptor in the synergism.
As previously discussed, several LABAs potentiate GR signalling, involving priming mechanisms. Some of these effects are due to true synergism (Holden et al., 2011; Holden et al., 2014; Moodley et al., 2013). In BEAS-2B cells, stimulation with formoterol, or the GC fluticasone propionate, was shown to enhance expression of RGS-2, CD200 and CRISPLD2 gene expression, while co-stimulation resulted in synergistic activation of those genes (Moodley et al., 2013). Similarly Holden et al., showed that salmeterol, formoterol and GCs alone enhance expression of RGS-2, while co-stimulation with either LABA, together with GCs, resulted in synergistic activation (Holden et al., 2011; Holden et al., 2014). In reports where treatment with either GCs or LABAs alone results in a response that is too small to establish statistical significance, it is difficult to distinguish between synergism and priming. Nevertheless, both effects allow for higher biological effects to be obtained as compared to when a high dose of GCs is used alone. Whether the above-mentioned synergism between LABAs and GCs requires GC-independent activation or priming is not known. Although enhanced repression of transcription by LABAs and GCs has been shown (Giembycz & Newton, 2015), synergistic effects on transrepression of transcription with LABAs in combination with other ligands have not, to our knowledge, been previously reported. This may be because synergistic transactivation is easier to quantify than transrepression, but this remains to be investigated. Given the importance of using LABAs in combination therapy with GCs, much more research on these mechanisms is required.
8. Role of GR phosphorylation in modulating activity of the unliganded GR
A theme that emerges strongly is that changing the phosphorylation status of the unliganded GR plays a key role in GC-independent activation, sensitizing and desensitizing of the unliganded GR to GC responses, as well as in synergistic responses. Changing the site-specific phosphorylation status of the unliganded GR is an attractive mechanism for modulating unliganded GR activity by other signalling pathways, since GR phosphorylation has been shown to modulate several steps in the GR signalling pathway. These include stability and subcellular localization of the unliganded GR (Oakley & Cidlowski, 2011), as well as transcriptional activity in response to GCs. Interestingly Ser-211 phosphorylation enhances GR-mediated transactivation and appears to be required for apoptosis in lymphoid cells (Chen et al., 2008; Galliher-Beckley & Cidlowski, 2009; Miller et al., 2007; Miller et al., 2005). In contrast, phosphorylation of Ser-226 has been linked to impaired GR signalling capability, presumably via enhancement of GR nuclear export (Blind & Garabedian, 2008; Chen et al., 2008; Galliher-Beckley & Cidlowski, 2009; Wang et al., 2002). Differential GR signalling due to differential phosphorylation status is most likely due to differential interactions with other proteins in the GR signalling cascade, such as the co-activators mediator complex subunit 14 (MED14) (Chen et al., 2008) and cAMP-responsive element-binding protein-binding protein (p300/CBP), the p65 subunit of NF-κB (Chen et al., 2008; Galliher-Beckley et al., 2008; Kino et al., 2007) and GRIP-1 (Avenant et al., 2010). Receptor phosphorylation appears to increase binding of several cofactors (Garza et al., 2010), suggesting a mechanism for the observed effects. The residues of the unliganded GR that are phosphorylated in response to other signalling pathways, as compared to those phosphorylated in response to GC binding, differ for different reports. Additionally, it is not known in most cases whether other residues in addition to those investigated are also modified. Although it is becoming clear that GC-independent GR activation, GC-independent GR priming and desensitizing involve GR phosphorylation, the specific residues modified in each case seem to differ. Furthermore, in most cases not all of the phosphorylation sites were examined in the same study, making it impossible at this point to state which sites are important for a specific mode of GR signalling. It is possible that phosphorylation at multiple sites is involved in fine tuning the responses. The MAPKs, ERK1/2, JNK and p38, which have been implicated in GR phosphorylation, have also been implicated in GR priming (ERK1/2 and JNK) and GR desensitization (predominantly p38, but also JNK). In line with a role for p38 in desensitization, inhibition of p38 (by means of inhibitors and siRNA knockdown), resulted in an increase in GC-independent and GC-dependent GR nuclear translocation and transcription of GILZ (Bouazza et al., 2014). The authors also showed that inhibition of p38 resulted in an increase in phosphorylation of the unliganded GR at Ser-211 and Ser-203, without changing total GR levels. This study shows that changing the phosphorylation status of the unliganded GR, dramatically alters the transcriptional response via the unliganded GR. Taken together with the finding that cells expressing a mutant GR incapable of phosphorylation at Ser-134 (S134A GR) had significantly altered and gene-specific hormone-dependent genome-wide transcriptional responses (Galliher-Beckley et al., 2011), these data suggest that effects of GR phosphorylation may be gene-specific and different depending on which GR residues are phosphorylated. However, it should be noted that in many reports suggesting a role for phosphorylation of the unliganded GR in activation, priming or desensitization, whether the site-specific phosphorylation alone is a requirement for the differential response has not been established. Furthermore, whether other forms of GR post-translational modifications such as acetylation, ubiquitination and sumoylation are involved in GC-independent effects by other signalling pathways remains to be determined, but is likely given their capability to change GR expression levels and activities (Oakley & Cidlowski, 2011).
9. Role of unliganded GR subcellular localization and membrane-associated GR
Subcellular localization of the unliganded GR is likely to play an important role in GC-independent GR signalling. Subcellular localization of the GR appears to be a dynamic process whereby factors such as hormone binding influence the relative activity of nuclear import or export signals contained within the receptor (Madan & DeFranco, 1993). As mentioned in section 4, changing levels of nuclear versus cytoplasmic versus membrane-associated unliganded GR are possible mechanisms whereby GC-independent GR signalling could be modulated, although this has not been much investigated. Relatively little is known about signals that regulate subcellular localization of the unliganded GR; however evidence suggests a role for cell cycle phase and developmental stage (Matthews et al., 2011; Matthews et al., 2015; Tsiarli et al., 2013). Matthews et al. reported that the heterogeneity in unliganded GR subcellular distribution is reflective of cell cycle phase and that mitotic index contributes to tissue GC sensitivity (Matthews et al., 2011). Recent work in neural stem/progenitor cells of the developing mouse ventral telencephalon suggests that the unliganded GR may be subjected to region and neurodevelopmental stage-specific regulation, with the GR being mostly nuclear in some neurons in the absence of GCs (Tsiarli et al., 2013). A recent study has reported a requirement for the unliganded GR in chromosome segregation (Matthews et al., 2015). Matthews et al. showed that the unliganded GR localizes to the centrosomes of interphase and mitotic cells, enabling equal numbers of centrosomes to migrate to opposite poles of the cells. They further demonstrated, via GR knockdown, that reduced GR levels negatively affect this process, resulting in daughter cells with unequal numbers of chromosomes (aneuploidy) if the cells successfully complete mitosis. They also showed that ligand-independent phosphorylation of the GR at Ser-203 and Ser-211 by Aurora kinase was not required for the response, and occurs downstream of chromosome segregation. Interestingly, the unliganded nuclear GR was minimally active for transcription, suggesting that cell cycle phase may play a role in GC-resistance.
The above findings and those in section 4 raise the issue of whether the unliganded GR is predominantly cytoplasmic or nuclear in primary cells. Interestingly, there are some reports showing that the GR is mainly nuclear in primary untransformed cells, such as cortical astrocytes (Unemura et al., 2012), some neural stem and progenitor cell populations (Tsiarli et al., 2013), human monocytes and mouse gonadotropes (Hapgood et al., unpublished data) as well as in some transformed cell lines (Verhoog et al., 2011), even in the absence of serum. Moreover, a substantial proportion of unliganded GR is nuclear in the absence of serum in primary normal human bronchial epithelial (NHBE) cells (Hakim et al., 2013) and other cells isolated from the sputum of healthy patients (macrophages, epithelial cells, neutrophils and Eosinophils) (Usmani et al., 2005), all key targets for immunotherapy. Taken together it seems that the classical model of exclusive cytoplasmic localization of the unliganded GR may require revision, especially in primary cells relevant to GC therapy. Whether the unliganded GR is predominantly nuclear or cytoplasmic could determine its ability to be activated independent of GCs, or primed or desensitized, in its responses to GCs.
Shuttling between the cytoplasm and nucleus has emerged as a critical control point for nuclear receptors such as the thyroid hormone receptor (Mavinakere et al., 2012). Both ligand-dependent and ligand-independent nuclear localization signals (NLSs) have been identified in nuclear receptors. While a bipartite NLS has been identified in the GR DNA-binding domain and a ligand-dependent NLS in its ligand-binding domain (Cadepond et al., 1992; LaCasse et al., 1993; Picard & Yamamoto, 1987), the role of NLSs in GC-independent nuclear trafficking of the GR remains to be investigated. The immunophilins FKBP51, FKBP52, CyP40 and PP5 associated with steroid receptors, including the GR, have been implicated in playing a role in steroid hormone-binding affinity, nucleocytoplasmic shuttling and transcriptional activation of target genes, in a tissue-specific manner (Ratajczak et al., 2015). It has been reported that the GR accumulates in the nucleus as an intact receptor-Hsp90 complex associated with FKBP52, after GC exposure (Echeverria et al., 2009; Galigniana et al., 2010). However, there is evidence that both the unliganded and liganded GR shuttle freely between the nucleus and the cytoplasm (Echeverria et al., 2009; Hache et al., 1999; Madan & DeFranco, 1993). Another question that remains to be explored is whether all nuclear unliganded GR is transcriptionally active. Based on work done on other nuclear receptors which are predominantly nuclear in the absence of cognate ligand, as well as changing patterns of nuclear distribution and nuclear foci observed for the nuclear receptors in response to cognate ligands (Kumar et al., 2006), this would seem unlikely. Another intriguing question is to what extent the GR is unliganded in adults in vivo, given that the basal free GC concentrations are about 5 nM (Henley & Lightman, 2011) and the KD of the hGR for cortisol ranges between 10-17 nM (Gaeggeler et al., 2005; Huizenga et al., 2000; Mulatero et al., 1997). Under these conditions, about 60% of the GR would be unliganded, suggesting that unliganded GR is available to mediate responses in the basal state.
Besides shuttling between the cytoplasm and nucleus, there is increasing evidence that location of unliganded GR with membrane-associated signalling complexes could be a mechanism whereby reciprocal cross-talk occurs between the GR and other membrane-associated receptors, to facilitate non-genomic reciprocal cross-talk. For example, it was found that unliganded GR was essential for the formation of a complex between the plasma membrane-associated T-cell receptor (TCR) and the heat-shock protein 90 (Hsp90), leukocyte-specific protein tyrosine kinase (LCK), tyrosine kinase FYN and zeta-chain-associated protein kinase 70 (Bartis et al., 2007; Bartis et al., 2006), implicating levels of unliganded GR in immune cell signalling. While several groups have reported the presence of a membrane-associated GR (mGR), the identity of such a protein was contentious until quite recently, when strong evidence emerged for the presence of the classical GRα protein associated with membrane fractions (Deng et al., 2015; Strehl & Buttgereit, 2014; Wehmeyer et al., 2014). mGR may represent only a small population of the total pool of steroid receptor protein present in a cell, as suggested by the finding that a subset of the GR resides in the plasma membrane of human leukemic cells (Gametchu et al., 1999; Gametchu et al., 1993), mouse monocytes and B-cells (Bartholome et al., 2004), further suggesting a role for mGR in immune function. Several reports suggest that the GR associates with lipid rafts, which are specialized plasma membrane microdomains that recruit various signalling proteins involved in coordinating cellular responses (Jain et al., 2005; Matthews et al., 2008; Samarasinghe et al., 2011; Wehmeyer et al., 2014). Jain et al. showed that the classical GR localizes with HSP90 and STAT3 in caveolin-1-containing membrane microdomains, known as caveolae, in human liver Hep3B cells (Jain et al., 2005). This study further provided evidence for a functional role of the mGR in Dex-mediated transcription, since Dex-induced GRE transactivation was significantly repressed in the presence of a lipid raft disrupter (Jain et al., 2005). The unliganded GR has been shown to localize with c-src in caveolae to facilitate the rapid Dex-induced phosphorylation of Akt and caveolin-1 (Cav-1) in A549 cells. Furthermore, the same study showed the abolishment of Dex-induced phosphorylation of Akt and Cav-1 in the presence of a lipid raft disruptor, while the knockdown of Cav-1 protein reduced the Dex-induced activation of Akt, but had no effect on GRE transactivation (Matthews et al., 2008). Samarasinghe et al. showed that the GR localizes to caveolae, residing in a complex with Cav-1 in a ligand-independent manner in embryonic mouse neural progenitor cells (Samarasinghe et al., 2011). The same study further provided physiologically relevant evidence for the mGR by showing the Cav-1-dependent activation of ERK-1/2 by GCs inhibited gap junction intercellular communication, which resulted in decreased cell proliferation (Samarasinghe et al., 2011). While the abovementioned reports certainly provide support for a role for mGR in modulating GC responses via interaction with kinases in a membrane complex, it was not until recently that a role for mGR in cross-talk with other receptor pathways has emerged (Wehmeyer et al., 2014). Both the GnRH receptor and a small proportion of the GR were shown to co-localize with the lipid raft protein Flot-1 at the plasma membrane in a mouse pituitary gonadotrope precursor cell line. The GR was found to be present in a complex with Flot-1, independent of the presence of GCs or GnRH. Using siRNA-mediated knockdown and antagonist strategies, the authors showed that a gene-specific synergistic transcriptional response requires the GR, GnRH receptor, and Flot-1 as well as the protein kinase C pathway. The results suggest that lipid raft association of the GR and the GnRH receptor has a role in enhancing transcriptional output in the nucleus in a gene-specific manner, as compared to that obtained with either GC or GnRH alone (Wehmeyer et al., 2014). The mGR can thus potentially crosstalk with several intracellular signalling pathways via a mechanism involving its association with a multi-protein signalling complex in membrane microdomains (Strehl & Buttgereit, 2014).
Besides the GR, other classical steroid receptors have also been localized to the plasma membrane in both the absence and presence of steroids (Bennesch & Picard, 2015). In the absence of estradiol, the estrogen receptor (ER) has been shown to co-localize with Cav-1 in association with plasma membranes in endothelial cells (Razandi et al., 2002). Cav-1 has been shown to augment both estrogen-independent and estrogen-dependent ERα signaling (Schlegel et al., 1999). Interestingly, growth factor receptors such as receptor tyrosine kinases (RTKs) have also been shown to form signalling complexes with caveolin (Liu et al., 1997). Similarly, G protein–coupled receptors (GPCRs), including the GnRH receptor, have been shown to localize to lipid rafts (Navratil et al., 2003). The same study showed that the GnRH receptor is associated with lipid rafts in a complex with ERK and c-raf. A more recent report verified this association and also showed the presence of Gαq in the raft complex (Bliss et al., 2007). The same study reported that the GnRH receptor is associated with lipid rafts in association with Gαq and raf (Bliss et al., 2007). The localization of both steroid receptors, RTKs, GPCRs, G-proteins, kinases and chaperone proteins in membrane domains, together with the finding of dependence of an integrated signal by the GR and GnRH receptor on lipid raft proteins, suggests that membrane domains may provide a platform for non-genomic reciprocal modulation of unliganded and liganded GR signalling and signalling via other membrane receptors. Further research is required to determine to what extent mGR is involved in GC-independent GR signalling and cross-talk, especially in immune function cells, to obtain insights into the role in immunotherapy.
10. Physiological significance and implications of unliganded GR for therapy
Cross-talk between the GR and other signalling pathways to activate, desensitize or prime the unliganded GR may be common mechanisms for functional integration between neuroendocrine, stress, reproductive and immune functions. A fully functioning GR with an intact negative feedback mechanism for inhibiting excessive inflammation plays a protective role against a number of diseases involving inflammation (Silverman & Sternberg, 2012). The extent to which non-classical mechanisms of GR action contribute to this protective effect is not fully understood, but is likely to be significant. Data suggest that classical GR dimerization and genomic transactivation mechanisms involving direct DNA binding of the GR to DNA are essential for proper regulation and HPA axis recovery. However the dependence of appropriate responses to immune challenges on this mechanism, varies depending on the type of immune challenge (Silverman & Sternberg, 2012). Genomic tethering mechanisms on the other hand appear to be insufficient for the GR to exert its full anti-inflammatory effects (Silverman & Sternberg, 2012).
Diseases related to abnormal inflammatory and immune responses are characterized by changes in expression of a broad range of target genes, especially cytokine genes. For example, upregulation of TGFβ, IκBα, MKP1, GILZ, IL10 and IL12 (Barnes, 2011; Baschant & Tuckermann, 2010) and repression of IL2, IL3, IL4, IL6, TNFα, IL8, VCAM1 and ICAM1 genes, has been linked to the use of GCs to treat asthma (Barnes, 2011). In addition, the levels of Th17 cytokines IL21, IL22 and IL23 are elevated in some forms of asthma (Zhao et al., 2010). Several genes including IL1β, IL6, IL8, IL10, IL12p70 and TNFα are upregulated in COPD (Gessner et al., 2005). GC immunotherapy does not target a single or a few key genes in restricted target cell types, but is a rather blunt approach, targeting all genes regulated by the GR, including those regulated by transactivation via GREs and those regulated by transrepression via NF-κB and AP-1 cis elements in their promoters, as is the case for most of the promoters of the above-mentioned genes. In addition, activation of several kinases including MAPKs (p38 and JNK) and transcription factors NF-κB, AP-1 is strongly implicated in inflammatory diseases (Barnes & Adcock, 2009; Baschant & Tuckermann, 2010; Dejager et al., 2014; Durham et al., 2016; Giembycz & Newton, 2015). While a direct role for activation, potentiation and desensitization of the unliganded GR in regulating all key genes, such as those listed above, in relevant target cells in immunotherapy has not been established, much of the data suggest that this is likely to occur for many. Several of the above-mentioned cytokines (TGFβ, TNF, IL1B, IL2, IL4) have been shown to activate, potentiate and/or desensitization the unliganded GR, while MAPKs have been shown to be integral to these processes via phosphorylating the GR, also discussed in previous sections. There is also strong evidence for cross-talk between the GR and activated AP-1 and NF-κB transcription factors, most likely involving the activated or potentiated unliganded GR, as discussed in previous sections. The unliganded GR has also been shown to be substantially nuclear in several primary target cells relevant to immunotherapy (Hakim et al., 2013; Usmani et al., 2005), consistent with an important transcriptional regulatory role in immunotherapy. Direct effects by activation, potentiation or desensitization of the unliganded GR on synthetic and an endogenous GRE-containing promoter (Bouazza et al., 2014), have been demonstrated. Strong evidence exists for a direct role of the unliganded GR in potentiation of GR responses in immunotherapy in combination with LABAs, including in primary cell models relevant to asthma and COPD (Mercado et al., 2011; Roth et al., 2002; Usmani et al., 2005), as discussed in previous sections. Further research is required to establish the mechanisms and gene- and cell-specific roles of the unliganded and liganded GR in immunotherapy on key target genes and how this is modulated by endogenous cytokines and other immune function modulators, and exogenous LABAs, in relevant target cells.
The mechanisms of GC resistance in disease are the subject of intense research. While they appear to involve inhibition or desensitising of GR function due to chronic inflammation, many unanswered questions remain (Newton et al., 2010). While the molecular basis of GC resistance is not fully understood, desensitizing of the unliganded GR is likely to play an important role. In addition, priming of desensitized unliganded GR by other ligands is likely to be an important mechanism to increase GC-sensitivity in GC-resistant patients. Current combination treatments for asthma and COPD using inhaled LABA’s or phosphodiesterase 4 inhibitors are widely believed to enhance the anti-inflammatory actions of inhaled GCs via mechanisms that involve cAMP-dependent pathways (Giembycz & Newton, 2015). Some of the beneficial actions of LABA/GC combination therapy are most likely due to synergistic actions in upregulating anti-inflammatory genes containing GREs in their promotes. While these most likely involve genomic actions, the findings that several LABAs induce unliganded GR nuclear translocation suggest that priming and even possibly GC-independent activation contribute to the effects. Many cytokines have been shown to desensitize the unliganded GR towards GC responses, which may lead to insensitivity to GC therapy. However, many of the same signals also appear to be able to prime or activate the unliganded GR under other conditions, enhancing its sensitivity to GCs, via mechanisms requiring p38. Synergistic responses that occur with combinations of cytokines and GCs on pro-inflammatory genes would at first appear to contradict the established anti-inflammatory role of GCs. However it is emerging that GCs can exert both pro- and anti-inflammatory responses. Pro-inflammatory responses to GCs appear to be involved in reinforcing initial pro-inflammatory effects of the innate immune system, to allow it to respond quickly and effectively to infection and tissue damage (Busillo & Cidlowski, 2013). Anti-inflammatory effects are required to resolve the response and restore homeostasis. In this context, synergy between GCs and pro-inflammatory cytokines may be a useful mechanism for rapidly reenforcing initial pro-inflammatory responses. The unliganded GR also appears to desensitize TNF signalling and be involved in an auto-regulatory mechanism that may prevent overproduction of IL6 in the endocervix, possibly protecting against negative effects of excessive inflammation in this tissue (Verhoog et al., 2011). GCs acting via the GR play an important role in adipose tissue biology and fat deposition (Lee et al., 2014). GCs regulate adipocyte metabolic, endocrine and immune function, and desensitize adipose tissue inflammation. The finding that GCs act synergistically with insulin, to increase the expression of numerous genes involved in fat deposition (Lee et al., 2014), suggests an important role for non classical GR mediated mechanisms in obesity. It has been proposed that high inflammatory cytokine levels in obese adipose tissues may increase GR phosphorylation at Ser-226 and lead to GC resistance (Lee et al., 2014), but this remains to be investigated Thus the level of cellular stress, immune activation status, or the cell cycle phase may be crucial for determining GC sensitivity and GC responsiveness. These responses are likely to be dose-, time-, cell-, gene- and context-dependent. A better understanding of these mechanisms may lead to identification of new targets design of new drugs for metabolic disorders and immunotherapy.
Whether GC-independent activation of the GR is physiologically relevant is supported by similar findings for other members of the steroid receptor (SR) family, given the large degree of structural and functional similarity between the steroid receptors (Lu et al., 2006). A large body of evidence supports the paradigm that the estrogen receptor (ER), androgen receptor (AR), mineralocorticoid receptor (MR) and progesterone receptor (PR) exhibit cognate ligand-independent activation, to result in similar but not identical outputs to those in response to cognate ligand, a topic recently reviewed by Bennesch and Picard (Bennesch & Picard, 2015). This cognate ligand-independent activation involves activation to a form that is transcriptionally activation and thus is analogous to our definition of GC-independent activation. Although the precise mechanisms are unknown, substantial evidence suggest that these steroid receptors can be activated by other non-steroid signals acting via their own membrane-associated receptors, via mechanisms that involve phosphorylation of the SRs and/or other targets involved in the SR signalling pathway, such as co-activators. Membrane-associated receptors that have been implicated in cognate ligand-independent activation of the ER, PR, MR or AR include RTKs for epidermal growth factor, histidine-rich glycoprotein, insulin and insulin-like growth factor 1, neurotransmitter receptors like dopamine, cytokine receptors such as the IL6 receptor and GPCRs such as the prolactin receptor (Bennesch & Picard, 2015). Additionally amino acids, cyclin D1, and cyclin–dependent kinase 2 have also been implicated. The findings suggest that non-steroid signals acting via their own membrane-associated receptors can ligand-independently activate SRs, via activation of downstream pathways involved in RTK, GPCR and cytokine signalling, such as cAMP, protein kinase A, PI3K/Akt (Bennesch & Picard, 2015). Thus cognate ligand-independent activation of SRs by other receptor signalling pathways appears to be a common mechanism for all steroid receptors, including the GR. Physiologically relevant roles for cognate ligand-independent activation have been demonstrated, in most cases for the ER, which has been the most intensively investigated SR in this context. Ligand-independent activation of the ER has been implicated in playing a role in cell proliferation in the reproductive tract, in brain signalling, breast cancer, mammary gland development and signalling, uterine growth and integration of signals for food availability with reproduction in the liver (Bennesch & Picard, 2015). For the PR, significant roles have been implicated for brain signalling and breast cancer while androgen-independent activation of the AR is implicated in prostate cancer (Bennesch & Picard, 2015). The extent to which changes in ER, MR, AR and PR levels and indirect genomic effects are involved in some of these mechanisms has, however, not been investigated in most cases. GC-independent activation has been much less widely investigated, but to date has been implicated in playing several diverse physiological roles, including in cell proliferation (Wehmeyer et al., 2014), cell cycle control (Matthews et al., 2011) and repression of inflammation (Verhoog et al., 2011).
11. Conclusions
GC-independent effects on GR signalling are implicated in playing an important role in development, cell cycle, cell proliferation, apoptosis, immune and inflammatory responses, neuronal function, cancer and inflammatory diseases. These roles are consistent with the key role of the GR in many physiological functions. The terminology used to indicate GC-independent activation, priming, desensitization and synergy, as defined in this review, is not uniform in the literature, and hence requires standardization. The unliganded GR has been shown to be transcriptionally active at physiologically significant, albeit relatively high levels, and in response to cell cycle, developmental and other signals like cytokines and peptide hormones, without changes in GR levels. Detection of GC-independent GR activation may be more widespread than reported in the literature, since many investigators normalize basal activities in the absence of GCs, but in the presence of other signals, to a value of one, masking potential differences compared to effects in the absence of any ligand. Evidence to date further suggests that the GR does not absolutely require binding of GCs for nuclear translocation and transcriptional activation, nor is it always cytoplasmic in the absence of GCs, challenging the classical paradigm of GR mechanism of action. GC-independent GR activation has in some cases been linked to examples of synergistic gene expression between GCs and other signals. The unliganded GR can also be primed by external signals to result in increased nuclear translocation, without a change in detectable transcriptional activity or GR levels, but rather to involve changes in kinase activity. Experimental evidence taken together with theoretical considerations suggests that similar mechanism may be involved in GC-independent GR activation, desensitization and priming, where the outcome is dependent on degrees. These mechanisms are likely to include changes in GR protein levels and/or posttranslational modification, in particular site-specific phosphorylation of the unliganded GR and/or other proteins involved in GR signalling. Kinases, in particular MAPKs induced by non-GC signals, are strongly implicated in many of these effects. Proteins downstream to the GR shown to be involved in unliganded GR activation, priming or desensitizing, include proteins involved in determining GR subcellular localization, kinases, and transcription cofactors. Membrane-associated unliganded GR is implicated in playing a role in cross-talk between the GR and other signalling pathways and synergism, and requires further investigation to determine the functional interplay between and differential roles for membrane and intracellular GR. Whether the response is to increase or decrease sensitivity of the unliganded GR to GCs, or even to activate or completely inactivate the GR (as in some cases of GC-resistance), most likely depends on the relative levels and identities of the specific targeted proteins and the extent to which their levels or post-translational modifications are affected. Furthermore, the same signal can prime or desensitize the unliganded GR, depending on context. The specific GR residues linked to these responses do not appear to dictate the type of response, but rather to be cell- and signal-specific. Where investigated, many of the transcriptional responses involving the unliganded GR in the absence or subsequent presence of GCs are highly gene-specific for endogenous genes and cell-specific, suggesting that proteins downstream from the GR in the GR signalling pathway must be involved, to explain why these effects are not observed for all GR-regulated genes. These downstream proteins most likely include other transcription factors and co-factors and or proteins involved in gene-specific chromatin structure, to explain the levels of gene and cell-specificity. Increases or decreases in unliganded GR levels without changes in external signals can alone account for increased or decreased sensitivity to GCs, respectively, due to shifts in the position of dose response curve. GC-independent GR activation and synergy, as well as or priming, provide the possibility of enhancing the therapeutic benefits of GCs, as has been shown most convincingly with LABAs used to treat inflammatory lung disease. Besides LABAs, several other signals like cytokines and peptide hormones have been shown to potentiate the unliganded GR or synergize with GCs. While synergistic effects have mostly been shown to involve genomic mechanisms, the extent to which GC-independent activation are involved remains to be determined. Emerging evidence for an important role for GC-independent GR activation and mGR is consistent with more established roles for other steroid receptors, in particular the membrane-associated ER. Furthermore, a role for unliganded GR in modulating transcription is consistent with established roles for other nuclear receptors. Further experiments including investigation of GR levels, GR recruitment to promoters, investigation of site-specific posttranslational modifications of the GR and other proteins involved in the signalling, establishing a requirement for the GR in the response, and genome wide investigation of target genes, and kinetics of responses, are required to understand the extent and specificity of signalling via the unliganded GR. A greater understanding of mechanisms of GC-independent effects on GR signalling will enhance our understanding of cross-talk between neuroendocrine, stress, reproductive and immune functions as well as diseases associated with inflammation. Furthermore it will likely lead to new strategies for design of more potent and selective anti-inflammatory drugs for combination immunomodulatory therapies and to treat GC-resistance.
Table 1. The effects of different signals on unliganded glucocorticoid receptor (GR) function.
Several signals such as cytokines, growth factors, cellular stressors, pathogens and pharmacological compounds can modulate the activity of the unliganded GR to decrease or increase GC-dependent GR function. The table summarizes current knowledge on signals that affect unliganded GR to result in GC-independent activation, desensitization or priming.
| Mechanism | Signal | Levels of unliganded GR |
GC-independent phosphorylation (hGR numbering) |
GC-independent GR nuclear translocation |
GC-independent GR-dependent effects on transcription |
Refs |
|---|---|---|---|---|---|---|
|
GC-
independent activation |
GnRH | ↔ endog GR | ↑ ser226, ↔ser211 endog GR | ↑ | Yes, siRNA, GR promoter recruitment (ChIP) |
Kotitschke et al., 2009
Wehmeyer et al., 2014 |
| TNF | ↔ endog GR | ↑ ser226, ↔ser211 endog GR | n/a as unliganded GR is nuclear |
Yes, siRNA, GR promoter recruitment (ChIP) | Verhoog et al., 2009 | |
| Overexpressed GR |
↑ | n/d | ↑ | Yes, Transactivation of GRE promoter, GR promoter recruitment (ChIP) |
Robertson et al., 2013 | |
| Pharmaco- logical induced changes cell cycle |
Unclear, possible ↓ during mitosis |
↑ ser211 but not ser203 expressed and endog GR |
Nuclear during interphase and cytoplasmic during mitosis |
Yes, ↑TAT-GRE in mitosis, ↓response to GCs. ↔ NFKB reporter, ↓GC-induced expression of endog genes in mitosis |
Matthews et al., 2011 | |
| n/d | n/d | n/d | n/d | Yes, GR recruitment to endog. BRAC1 promoter |
Ritter et al., 2012
Ritter & Mueller, 2014 |
|
|
GC-
independent desensitising |
Stressors | ↔ overexpressed & endog GR |
↑ser134 | No | Few genes i.e. 40/4000 GC-regulated | Galiiher-Beckley et al., 2011 |
| JNK activation | ↔ overexpressed GR | ↑ser226 | Not clear, but ↑ nuclear export of liganded GR |
N/d, but ↓transactivation of a GRE promoter | Itoh et al., 2002 | |
| p38 activation | ↑endog GR | n/d, but ligand-binding domain may be targeted by p38 |
n/d | ↓ basal luciferase activity of MMTV-promoter | Szartmary et al., 2004 | |
| IL2 | n/d | n/d | n/d, but ↓ Dex-induced GR nuclear translocation |
n/d | Goleva et al., 2002 | |
| IL1β | ↓total endog GR | n/d, but ↓ dex-induced phosphorylation at ser203 & ser211 |
No | n/d | Escoll et al., 2015 | |
|
GC-
independent priming |
TNF | ↑ endog cytosolic GR | ↔ ser211 phosphorylation | No | ↑ dex-induction of epithelial sodium channels | Bergmann et al., 2009 |
| n/d | n/d | Apparent | Apparent, siRNA, GR promoter recruitment (ChIP) | Lannan et al., 2012 | ||
| n/d | n/d | n/d | Yes, GR recruited to the endog. TNFAIP3 promoter | Altonsy et al., 2014 | ||
| IL13 | ↔ total endog GR | ↑ser203, ser211 | Yes | No, but ↑dex transactivation of a GRE-promoter | Hu et al., 2013 | |
| NTHi | ↑ endog GR | n/d | n/d | No, siRNA, GR promoter recruitment (ChIP) | Miyata et al., 2015 | |
| LPS | n/d | n/d | n/d | n/d | Vettorazzi et al., 2015 | |
| TGFβ1 | ↔ endog total GR | No effect on ser211 phosphorylation, but ↑dex-induced phosphorylation at ser211 |
n/d | No, RU486 did not alter TGFβ1 induction of PAI-1 | Pan et al., 2015 | |
| BNDF | ↔ GR | ↑ ser134, ser267, minimal ↑ser203, ser211, ser226 |
No | ↔TAT-GRE, but ↑ GC transactivation on SGK promoter |
Lambert et al., 2013 | |
| LABAs | n/d | n/d | No | No, but ↑ GC transactivation | Haque et al., 2013 | |
| n/d | n/d | Yes | n/d | Roth et al., 2002 | ||
| n/d | n/d | Yes | Yes on GRE promoter, ↔ on endog genes | Usmani et al., 2005 | ||
| ↔ endog GR | ↓ | Yes | n/d | Mercado et al., 2011 | ||
| Cocaine | ↑ ser-211 | Yes | ↑ | Caffino et al., 2015 |
Key: ↑ = increased/enhanced, ↓ = decreased/inhibit, ↔ = no change, n/d = not determined, n/a = not applicable, endog = endogenous, NTHi = non-typeable H. influenza, LPS = lipopolysaccharide
Acknowledgements
The financial assistance of the National Research Foundation (NRF) towards this research is hereby acknowledged. Opinions expressed and conclusions arrived at, are those of the author and are not necessarily to be attributed to the NRF.
We thank Calvin Kemp, Lance Wehmeyer and Andrea Du Toit for intellectual contributions. We also thank all the other current and past members of the Hapgood laboratory for intellectual input and discussions over the years. Thanks to Karen van der Merwe for editorial aspects and assistance with referencing.
Abbreviations
- Akt
protein kinase B
- AR
androgen receptor
- BDNF
brain derived neurotropic factor
- CASP1
encoding caspase 1
- Cav-1
caveolin-1
- Ch25h
cholesterol 25-hydroxylase
- COPD
chronic obstructive pulmonary disease
- Dex
dexamethasone
- EMSA
electrophoretic mobility shift assay
- ER
estrogen receptor
- ERK
extracellular signal-regulated kinases
- Flot-1
Flotillin-1
- GC
glucocorticoid
- GILZ
GC-induced leucine zipper
- GnRH
Gonadotropin-releasing hormone
- GPCR
G protein–coupled receptor
- GR
glucocorticoid receptor
- GREs
glucocorticoid response elements
- GRIP-1
glucocorticoid receptor interacting protein-1
- GSK3
glycogen synthase kinase 3
- hGR
human GR
- HPA
hypothalamic–pituitary–adrenal
- HSP90
heat shock protein 90
- IL
interleukin
- IRAK-M
interleukin-1 receptor associated kinase
- JNK
c-Jun N-terminal kinases
- LABA
long-acting β2 adrenoreceptor agonist
- LPS
lipopolysaccharide
- mGR
membrane-associated GR
- MAPK
mitogen-activated protein kinase
- MKP-1
mitogen-activated protein kinase phosphatase-1
- MR
mineralocorticoid receptor
- NLS
nuclear localization signals
- PI3K/Akt
phosphatidylinositol 3-kinase/protein kinase-B
- PR
progesterone receptor
- RTKs
receptor tyrosine kinases
- SR
steroid receptor
- TGFβ
transforming growth factor-beta
- TNF
tumor necrosis factor
- UDCA
ursodeoxycholic acid
Footnotes
Conflict of Interest Statement: The authors declare that there are no conflicts of interest.
Reference List
- Adams NP, Jones PW. The dose-response characteristics of inhaled corticosteroids when used to treat asthma: an overview of Cochrane systematic reviews. Respiratory Medicine. 2006;100:1297–1306. doi: 10.1016/j.rmed.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Altonsy MO, Sasse SK, Phang TL, Gerber AN. Context-dependent cooperation between nuclear factor kappaB (NF-kappaB) and the glucocorticoid receptor at a TNFAIP3 intronic enhancer: a mechanism to maintain negative feedback control of inflammation. Journal of Biological Chemistry. 2014;289:8231–8239. doi: 10.1074/jbc.M113.545178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenant C, Kotitschke A, Hapgood JP. Glucocorticoid receptor phosphorylation modulates transcription efficacy through GRIP-1 recruitment. Biochemistry. 2010;49:972–985. doi: 10.1021/bi901956s. [DOI] [PubMed] [Google Scholar]
- Ayroldi E, Macchiarulo A, Riccardi C. Targeting glucocorticoid side effects: selective glucocorticoid receptor modulator or glucocorticoid-induced leucine zipper? A perspective. FASEB Journal. 2014;28:5055–5070. doi: 10.1096/fj.14-254755. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Glucocorticosteroids: current and future directions. British Journal of Pharmacology. 2011;163:29–43. doi: 10.1111/j.1476-5381.2010.01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373:1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- Bartholome B, Spies CM, Gaber T, Schuchmann S, Berki T, Kunkel D, et al. Membrane glucocorticoid receptors (mGCR) are expressed in normal human peripheral blood mononuclear cells and up-regulated after in vitro stimulation and in patients with rheumatoid arthritis. FASEB Journal. 2004;18:70–80. doi: 10.1096/fj.03-0328com. [DOI] [PubMed] [Google Scholar]
- Bartis D, Boldizsar F, Kvell K, Szabo M, Palinkas L, Nemeth P, et al. Intermolecular relations between the glucocorticoid receptor, ZAP-70 kinase, and Hsp-90. Biochemical and Biophysical Research Communications. 2007;354:253–258. doi: 10.1016/j.bbrc.2006.12.211. [DOI] [PubMed] [Google Scholar]
- Bartis D, Boldizsar F, Szabo M, Palinkas L, Nemeth P, Berki T. Dexamethasone induces rapid tyrosine-phosphorylation of ZAP-70 in Jurkat cells. Journal of Steroid Biochemistry and Molecular Biology. 2006;98:147–154. doi: 10.1016/j.jsbmb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Baschant U, Tuckermann J. The role of the glucocorticoid receptor in inflammation and immunity. Journal of Steroid Biochemistry and Molecular Biology. 2010;120:69–75. doi: 10.1016/j.jsbmb.2010.03.058. [DOI] [PubMed] [Google Scholar]
- Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, et al. Global strategy for asthma management and prevention: GINA executive summary. European Respiratory Journal. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- Bennesch MA, Picard D. Minireview: Tipping the balance: ligand-independent activation of steroid receptors. Molecular Endocrinology. 2015;29:349–363. doi: 10.1210/me.2014-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergann T, Zeissig S, Fromm A, Richter JF, Fromm M, Schulzke JD. Glucocorticoids and tumor necrosis factor-alpha synergize to induce absorption by the epithelial sodium channel in the colon. Gastroenterology. 2009;136:933–942. doi: 10.1053/j.gastro.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Blackford JA, Jr., Guo C, Zhu R, Dougherty EJ, Chow CC, Simons SS., Jr. Identification of location and kinetically defined mechanism of cofactors and reporter genes in the cascade of steroid-regulated transactivation. Journal of Biological Chemistry. 2012;287:40982–40995. doi: 10.1074/jbc.M112.414805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blind RD, Garabedian MJ. Differential recruitment of glucocorticoid receptor phospho-isoforms to glucocorticoid-induced genes. Journal of Steroid Biochemistry and Molecular Biology. 2008;109:150–157. doi: 10.1016/j.jsbmb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss SP, Navratil AM, Breed M, Skinner DC, Clay CM, Roberson MS. Signaling complexes associated with the type I gonadotropin-releasing hormone (GnRH) receptor: colocalization of extracellularly regulated kinase 2 and GnRH receptor within membrane rafts. Molecular Endocrinology. 2007;21:538–549. doi: 10.1210/me.2006-0289. [DOI] [PubMed] [Google Scholar]
- Boardman C, Chachi L, Gavrila A, Keenan CR, Perry MM, Xia YC, et al. Mechanisms of glucocorticoid action and insensitivity in airways disease. Pulm Pharmacol Ther. 2014;29:129–143. doi: 10.1016/j.pupt.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Boger E, Ewing P, Eriksson UG, Fihn BM, Chappell M, Evans N, et al. A novel in vivo receptor occupancy methodology for the glucocorticoid receptor: toward an improved understanding of lung pharmacokinetic/pharmacodynamic relationships. Journal of Pharmacology and Experimental Therapeutics. 2015;353:279–287. doi: 10.1124/jpet.114.221226. [DOI] [PubMed] [Google Scholar]
- Bouazza B, Debba-Pavard M, Amrani Y, Isaacs L, O'Connell D, Ahamed S, et al. Basal p38 mitogen-activated protein kinase regulates unliganded glucocorticoid receptor function in airway smooth muscle cells. Am J Respir Cell Mol Biol. 2014;50:301–315. doi: 10.1165/rcmb.2012-0522OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd CJ, Archer TK. Chromatin architecture defines the glucocorticoid response. Molecular and Cellular Endocrinology. 2013;380:25–31. doi: 10.1016/j.mce.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busillo JM, Cidlowski JA. The five Rs of glucocorticoid action during inflammation: ready, reinforce, repress, resolve, and restore. Trends in Endocrinology and Metabolism. 2013;24:109–119. doi: 10.1016/j.tem.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttgereit F, da Silva JA, Boers M, Burmester GR, Cutolo M, Jacobs J, et al. Standardised nomenclature for glucocorticoid dosages and glucocorticoid treatment regimens: current questions and tentative answers in rheumatology. Annals of the Rheumatic Diseases. 2002;61:718–722. doi: 10.1136/ard.61.8.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadepond F, Gasc JM, Delahaye F, Jibard N, Schweizer-Groyer G, Segard-Maurel I, et al. Hormonal regulation of the nuclear localization signals of the human glucocorticosteroid receptor. Experimental Cell Research. 1992;201:99–108. doi: 10.1016/0014-4827(92)90352-9. [DOI] [PubMed] [Google Scholar]
- Caffino L, Giannotti G, Malpighi C, Racagni G, Fumagalli F. Short-term withdrawal from developmental exposure to cocaine activates the glucocorticoid receptor and alters spine dynamics. Eur Neuropsychopharmacol. 2015;25:1832–1841. doi: 10.1016/j.euroneuro.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Cain DW, Cidlowski JA. Specificity and sensitivity of glucocorticoid signaling in health and disease. Best Pract Res Clin Endocrinol Metab. 2015;29:545–556. doi: 10.1016/j.beem.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Bender IK, Konstantinidis AK, Shin SC, Jewell CM, Cidlowski JA, et al. Glucocorticoid receptor translational isoforms underlie maturational stage-specific glucocorticoid sensitivities of dendritic cells in mice and humans. Blood. 2013;121:1553–1562. doi: 10.1182/blood-2012-05-432336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Baudino T, MacDonald PN, Green M, Kelley WL, Burnett JW, et al. Selective inhibition of nuclear steroid receptor function by a protein from a human tumorigenic poxvirus. Virology. 2000;274:17–25. doi: 10.1006/viro.2000.0410. [DOI] [PubMed] [Google Scholar]
- Chen W, Dang T, Blind RD, Wang Z, Cavasotto CN, Hittelman AB, et al. Glucocorticoid receptor phosphorylation differentially affects target gene expression. Molecular Endocrinology. 2008;22:1754–1766. doi: 10.1210/me.2007-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinenov Y, Coppo M, Gupte R, Sacta MA, Rogatsky I. Glucocorticoid receptor coordinates transcription factor-dominated regulatory network in macrophages. BMC Genomics. 2014;15:656. doi: 10.1186/1471-2164-15-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinenov Y, Gupte R, Rogatsky I. Nuclear receptors in inflammation control: repression by GR and beyond. Molecular and Cellular Endocrinology. 2013;380:55–64. doi: 10.1016/j.mce.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacological Reviews. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- Chow CC, Finn KK, Storchan GB, Lu X, Sheng X, Simons SS., Jr. Kinetically-defined component actions in gene repression. PLoS Comput Biol. 2015;11:e1004122. doi: 10.1371/journal.pcbi.1004122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CC, Ong KM, Dougherty EJ, Simons SS., Jr. Inferring mechanisms from dose-response curves. Methods in Enzymology. 2011;487:465–483. doi: 10.1016/B978-0-12-381270-4.00016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KF, Caramori G, Adcock IM. Inhaled corticosteroids as combination therapy with beta-adrenergic agonists in airways disease: present and future. European Journal of Clinical Pharmacology. 2009;65:853–871. doi: 10.1007/s00228-009-0682-z. [DOI] [PubMed] [Google Scholar]
- Clark AR, Belvisi MG. Maps and legends: the quest for dissociated ligands of the glucocorticoid receptor. Pharmacology & Therapeutics. 2012;134:54–67. doi: 10.1016/j.pharmthera.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Molecular and Cellular Endocrinology. 2011;335:2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxtall JD, Choudhury Q, Flower RJ. Glucocorticoids act within minutes to inhibit recruitment of signalling factors to activated EGF receptors through a receptor-dependent, transcription-independent mechanism. British Journal of Pharmacology. 2000;130:289–298. doi: 10.1038/sj.bjp.0703272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Topete D, Cidlowski JA. One hormone, two actions: anti- and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation. 2015;22:20–32. doi: 10.1159/000362724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejager L, Vandevyver S, Petta I, Libert C. Dominance of the strongest: inflammatory cytokines versus glucocorticoids. Cytokine & Growth Factor Reviews. 2014;25:21–33. doi: 10.1016/j.cytogfr.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Deng Q, Riquelme D, Trinh L, Low MJ, Tomic M, Stojilkovic S, et al. Rapid Glucocorticoid Feedback Inhibition of ACTH Secretion Involves Ligand-Dependent Membrane Association of Glucocorticoid Receptors. Endocrinology. 2015;156:3215–3227. doi: 10.1210/EN.2015-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di S, Maxson MM, Franco A, Tasker JG. Glucocorticoids regulate glutamate and GABA synapse-specific retrograde transmission via divergent nongenomic signaling pathways. Journal of Neuroscience. 2009;29:393–401. doi: 10.1523/JNEUROSCI.4546-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Carlton ME, Lerner A, Epstein PM. Effect of cAMP signaling on expression of glucocorticoid receptor, Bim and Bad in glucocorticoid-sensitive and resistant leukemic and multiple myeloma cells. Front Pharmacol. 2015;6:230. doi: 10.3389/fphar.2015.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver PM, Kilby MD, Bujalska I, Walker EA, Hewison M, Stewart PM. Expression of 11 beta-hydroxysteroid dehydrogenase isozymes and corticosteroid hormone receptors in primary cultures of human trophoblast and placental bed biopsies. Mol Hum Reprod. 2001;7:357–363. doi: 10.1093/molehr/7.4.357. [DOI] [PubMed] [Google Scholar]
- Durham AL, Caramori G, Chung KF, Adcock IM. Targeted anti-inflammatory therapeutics in asthma and chronic obstructive lung disease. Transl Res. 2015 doi: 10.1016/j.trsl.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham AL, Caramori G, Chung KF, Adcock IM. Targeted anti-inflammatory therapeutics in asthma and chronic obstructive lung disease. Transl Res. 2016;167:192–203. doi: 10.1016/j.trsl.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria PC, Mazaira G, Erlejman A, Gomez-Sanchez C, Piwien Pilipuk G, Galigniana MD. Nuclear import of the glucocorticoid receptor-hsp90 complex through the nuclear pore complex is mediated by its interaction with Nup62 and importin beta. Molecular and Cellular Biology. 2009;29:4788–4797. doi: 10.1128/MCB.00649-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickelberg O, Roth M, Lorx R, Bruce V, Rudiger J, Johnson M, et al. Ligand-independent activation of the glucocorticoid receptor by beta2-adrenergic receptor agonists in primary human lung fibroblasts and vascular smooth muscle cells. Journal of Biological Chemistry. 1999;274:1005–1010. doi: 10.1074/jbc.274.2.1005. [DOI] [PubMed] [Google Scholar]
- Escoll P, Ranz I, Munoz-Anton N, van-den-Rym A, Alvarez-Mon M, Martinez-Alonso C, et al. Sustained interleukin-1beta exposure modulates multiple steps in glucocorticoid receptor signaling, promoting split-resistance to the transactivation of prominent anti-inflammatory genes by glucocorticoids. Mediators Inflamm. 2015;2015:347965. doi: 10.1155/2015/347965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones--a focus on rapid, nongenomic effects. Pharmacological Reviews. 2000;52:513–556. [PubMed] [Google Scholar]
- Gaeggeler HP, Gonzalez-Rodriguez E, Jaeger NF, Loffing-Cueni D, Norregaard R, Loffing J, et al. Mineralocorticoid versus glucocorticoid receptor occupancy mediating aldosterone-stimulated sodium transport in a novel renal cell line. J Am Soc Nephrol. 2005;16:878–891. doi: 10.1681/ASN.2004121110. [DOI] [PubMed] [Google Scholar]
- Galigniana MD, Erlejman AG, Monte M, Gomez-Sanchez C, Piwien-Pilipuk G. The hsp90-FKBP52 complex links the mineralocorticoid receptor to motor proteins and persists bound to the receptor in early nuclear events. Molecular and Cellular Biology. 2010;30:1285–1298. doi: 10.1128/MCB.01190-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliher-Beckley AJ, Cidlowski JA. Emerging roles of glucocorticoid receptor phosphorylation in modulating glucocorticoid hormone action in health and disease. IUBMB Life. 2009;61:979–986. doi: 10.1002/iub.245. [DOI] [PubMed] [Google Scholar]
- Galliher-Beckley AJ, Williams JG, Cidlowski JA. Ligand-independent phosphorylation of the glucocorticoid receptor integrates cellular stress pathways with nuclear receptor signaling. Molecular and Cellular Biology. 2011;31:4663–4675. doi: 10.1128/MCB.05866-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliher-Beckley AJ, Williams JG, Collins JB, Cidlowski JA. Glycogen synthase kinase 3beta-mediated serine phosphorylation of the human glucocorticoid receptor redirects gene expression profiles. Molecular and Cellular Biology. 2008;28:7309–7322. doi: 10.1128/MCB.00808-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gametchu B, Chen F, Sackey F, Powell C, Watson CS. Plasma membrane-resident glucocorticoid receptors in rodent lymphoma and human leukemia models. Steroids. 1999;64:107–119. doi: 10.1016/s0039-128x(98)00097-x. [DOI] [PubMed] [Google Scholar]
- Gametchu B, Watson CS, Wu S. Use of receptor antibodies to demonstrate membrane glucocorticoid receptor in cells from human leukemic patients. FASEB Journal. 1993;7:1283–1292. doi: 10.1096/fasebj.7.13.8405814. [DOI] [PubMed] [Google Scholar]
- Garza AM, Khan SH, Kumar R. Site-specific phosphorylation induces functionally active conformation in the intrinsically disordered N-terminal activation function (AF1) domain of the glucocorticoid receptor. Molecular and Cellular Biology. 2010;30:220–230. doi: 10.1128/MCB.00552-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessner C, Scheibe R, Wotzel M, Hammerschmidt S, Kuhn H, Engelmann L, et al. Exhaled breath condensate cytokine patterns in chronic obstructive pulmonary disease. Respiratory Medicine. 2005;99:1229–1240. doi: 10.1016/j.rmed.2005.02.041. [DOI] [PubMed] [Google Scholar]
- Giembycz MA, Kaur M, Leigh R, Newton R. A Holy Grail of asthma management: toward understanding how long-acting beta(2)-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids. British Journal of Pharmacology. 2008;153:1090–1104. doi: 10.1038/sj.bjp.0707627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giembycz MA, Newton R. Potential mechanisms to explain how LABAs and PDE4 inhibitors enhance the clinical efficacy of glucocorticoids in inflammatory lung diseases. F1000Prime Rep. 2015;7:16. doi: 10.12703/P7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goleva E, Kisich KO, Leung DY. A role for STAT5 in the pathogenesis of IL-2-induced glucocorticoid resistance. Journal of Immunology. 2002;169:5934–5940. doi: 10.4049/jimmunol.169.10.5934. [DOI] [PubMed] [Google Scholar]
- Guo WX, Antakly T, Cadotte M, Kachra Z, Kunkel L, Masood R, et al. Expression and cytokine regulation of glucocorticoid receptors in Kaposi's sarcoma. Am J Pathol. 1996;148:1999–2008. [PMC free article] [PubMed] [Google Scholar]
- Hache RJ, Tse R, Reich T, Savory JG, Lefebvre YA. Nucleocytoplasmic trafficking of steroid-free glucocorticoid receptor. Journal of Biological Chemistry. 1999;274:1432–1439. doi: 10.1074/jbc.274.3.1432. [DOI] [PubMed] [Google Scholar]
- Hager GL, Varticovski L. Chromatin in time and space. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2012;1819:631. doi: 10.1016/j.bbagrm.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim A, Barnes PJ, Adcock IM, Usmani OS. Importin-7 mediates glucocorticoid receptor nuclear import and is impaired by oxidative stress, leading to glucocorticoid insensitivity. FASEB Journal. 2013;27:4510–4519. doi: 10.1096/fj.12-222604. [DOI] [PubMed] [Google Scholar]
- Hapgood JP, Africander D, Louw R, Ray RM, Rohwer JM. Potency of progestogens used in hormonal therapy: toward understanding differential actions. Journal of Steroid Biochemistry and Molecular Biology. 2014;142:39–47. doi: 10.1016/j.jsbmb.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Hapgood JP, Tomasicchio M. Modulation of HIV-1 virulence via the host glucocorticoid receptor: towards further understanding the molecular mechanisms of HIV-1 pathogenesis. Archives of Virology. 2010;155:1009–1019. doi: 10.1007/s00705-010-0678-0. [DOI] [PubMed] [Google Scholar]
- Haque R, Hakim A, Moodley T, Torrego A, Essilfie-Quaye S, Jazrawi E, et al. Inhaled long-acting beta2 agonists enhance glucocorticoid receptor nuclear translocation and efficacy in sputum macrophages in COPD. Journal of Allergy and Clinical Immunology. 2013;132:1166–1173. doi: 10.1016/j.jaci.2013.07.038. [DOI] [PubMed] [Google Scholar]
- Harr MW, Rong Y, Bootman MD, Roderick HL, Distelhorst CW. Glucocorticoid-mediated inhibition of Lck modulates the pattern of T cell receptor-induced calcium signals by down-regulating inositol 1,4,5-trisphosphate receptors. Journal of Biological Chemistry. 2009;284:31860–31871. doi: 10.1074/jbc.M109.005579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley DE, Lightman SL. New insights into corticosteroid-binding globulin and glucocorticoid delivery. Neuroscience. 2011;180:1–8. doi: 10.1016/j.neuroscience.2011.02.053. [DOI] [PubMed] [Google Scholar]
- Holden NS, Bell MJ, Rider CF, King EM, Gaunt DD, Leigh R, et al. beta2-Adrenoceptor agonist-induced RGS2 expression is a genomic mechanism of bronchoprotection that is enhanced by glucocorticoids. Proc Natl Acad Sci U S A. 2011;108:19713–19718. doi: 10.1073/pnas.1110226108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden NS, George T, Rider CF, Chandrasekhar A, Shah S, Kaur M, et al. Induction of regulator of G-protein signaling 2 expression by long-acting beta2-adrenoceptor agonists and glucocorticoids in human airway epithelial cells. Journal of Pharmacology and Experimental Therapeutics. 2014;348:12–24. doi: 10.1124/jpet.113.204586. [DOI] [PubMed] [Google Scholar]
- Hu A, Josephson MB, Diener BL, Nino G, Xu S, Paranjape C, et al. Proasthmatic cytokines regulate unliganded and ligand-dependent glucocorticoid receptor signaling in airway smooth muscle. PLoS One. 2013;8:e60452. doi: 10.1371/journal.pone.0060452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Pace TW, Miller AH. Interferon-alpha inhibits glucocorticoid receptor-mediated gene transcription via STAT5 activation in mouse HT22 cells. Brain Behav Immun. 2009;23:455–463. doi: 10.1016/j.bbi.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizenga NA, De Herder WW, Koper JW, de Lange P, v DLAJ, Brinkmann AO, et al. Decreased ligand affinity rather than glucocorticoid receptor down-regulation in patients with endogenous Cushing's syndrome. European Journal of Endocrinology. Oslo. 2000;142:472–476. doi: 10.1530/eje.0.1420472. [DOI] [PubMed] [Google Scholar]
- Hutchison KA, Matic G, Meshinchi S, Bresnick EH, Pratt WB. Redox manipulation of DNA binding activity and BuGR epitope reactivity of the glucocorticoid receptor. Journal of Biological Chemistry. 1991;266:10505–10509. [PubMed] [Google Scholar]
- Itoh M, Adachi M, Yasui H, Takekawa M, Tanaka H, Imai K. Nuclear export of glucocorticoid receptor is enhanced by c-Jun N-terminal kinase-mediated phosphorylation. Molecular Endocrinology. 2002;16:2382–2392. doi: 10.1210/me.2002-0144. [DOI] [PubMed] [Google Scholar]
- Jain S, Li Y, Kumar A, Sehgal PB. Transcriptional signaling from membrane raft-associated glucocorticoid receptor. Biochemical and Biophysical Research Communications. 2005;336:3–8. doi: 10.1016/j.bbrc.2005.08.057. [DOI] [PubMed] [Google Scholar]
- Kam JC, Szefler SJ, Surs W, Sher ER, Leung DY. Combination IL-2 and IL-4 reduces glucocorticoid receptor-binding affinity and T cell response to glucocorticoids. Journal of Immunology. 1993;151:3460–3466. [PubMed] [Google Scholar]
- Kaur M, Chivers JE, Giembycz MA, Newton R. Long-acting beta2-adrenoceptor agonists synergistically enhance glucocorticoid-dependent transcription in human airway epithelial and smooth muscle cells. Molecular Pharmacology. 2008;73:203–214. doi: 10.1124/mol.107.040121. [DOI] [PubMed] [Google Scholar]
- Keenan CR, Mok JS, Harris T, Xia Y, Salem S, Stewart AG. Bronchial epithelial cells are rendered insensitive to glucocorticoid transactivation by transforming growth factor-beta1. Respir Res. 2014;15:55. doi: 10.1186/1465-9921-15-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HA, Trotter KW, Archer TK. Chromatin remodeling during glucocorticoid receptor regulated transactivation. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2012;1819:716–726. doi: 10.1016/j.bbagrm.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T, Gragerov A, Kopp JB, Stauber RH, Pavlakis GN, Chrousos GP. The HIV-1 virion-associated protein vpr is a coactivator of the human glucocorticoid receptor. Journal of Experimental Medicine. 1999;189:51–62. doi: 10.1084/jem.189.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T, Ichijo T, Amin ND, Kesavapany S, Wang Y, Kim N, et al. Cyclin-dependent kinase 5 differentially regulates the transcriptional activity of the glucocorticoid receptor through phosphorylation: clinical implications for the nervous system response to glucocorticoids and stress. Molecular Endocrinology. 2007;21:1552–1568. doi: 10.1210/me.2006-0345. [DOI] [PubMed] [Google Scholar]
- Kino T, Tiulpakov A, Ichijo T, Chheng L, Kozasa T, Chrousos GP. G protein beta interacts with the glucocorticoid receptor and suppresses its transcriptional activity in the nucleus. Journal of Cell Biology. 2005;169:885–896. doi: 10.1083/jcb.200409150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman A, Tuckermann JP. Glucocorticoid receptor action in beneficial and side effects of steroid therapy: lessons from conditional knockout mice. Molecular and Cellular Endocrinology. 2007;275:98–108. doi: 10.1016/j.mce.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Korn SH, Wouters EF, Wesseling G, Arends JW, Thunnissen FB. Interaction between glucocorticoids and beta2-agonists: alpha and beta glucocorticoid-receptor mRNA expression in human bronchial epithelial cells. Biochemical Pharmacology. 1998;56:1561–1569. doi: 10.1016/s0006-2952(98)00179-8. [DOI] [PubMed] [Google Scholar]
- Kotitschke A, Sadie-Van Gijsen H, Avenant C, Fernandes S, Hapgood JP. Genomic and nongenomic cross talk between the gonadotropin-releasing hormone receptor and glucocorticoid receptor signaling pathways. Molecular Endocrinology. 2009;23:1726–1745. doi: 10.1210/me.2008-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Saradhi M, Chaturvedi NK, Tyagi RK. Intracellular localization and nucleocytoplasmic trafficking of steroid receptors: an overview. Molecular and Cellular Endocrinology. 2006;246:147–156. doi: 10.1016/j.mce.2005.11.028. [DOI] [PubMed] [Google Scholar]
- LaCasse EC, Lochnan HA, Walker P, Lefebvre YA. Identification of binding proteins for nuclear localization signals of the glucocorticoid and thyroid hormone receptors. Endocrinology. 1993;132:1017–1025. doi: 10.1210/endo.132.3.8440170. [DOI] [PubMed] [Google Scholar]
- Lambert WM, Xu CF, Neubert TA, Chao MV, Garabedian MJ, Jeanneteau FD. Brain-derived neurotrophic factor signaling rewrites the glucocorticoid transcriptome via glucocorticoid receptor phosphorylation. Molecular and Cellular Biology. 2013;33:3700–3714. doi: 10.1128/MCB.00150-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlais D, Couture C, Balsalobre A, Drouin J. Regulatory network analyses reveal genome-wide potentiation of LIF signaling by glucocorticoids and define an innate cell defense response. PLoS Genetics. 2008;4:e1000224. doi: 10.1371/journal.pgen.1000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannan EA, Galliher-Beckley AJ, Scoltock AB, Cidlowski JA. Proinflammatory actions of glucocorticoids: glucocorticoids and TNFalpha coregulate gene expression in vitro and in vivo. Endocrinology. 2012;153:3701–3712. doi: 10.1210/en.2012-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Pramyothin P, Karastergiou K, Fried SK. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2014;1842:473–481. doi: 10.1016/j.bbadis.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis-Tuffin LJ, Cidlowski JA. The physiology of human glucocorticoid receptor beta (hGRbeta) and glucocorticoid resistance. Annals of the New York Academy of Sciences. 2006;1069:1–9. doi: 10.1196/annals.1351.001. [DOI] [PubMed] [Google Scholar]
- Lim HW, Uhlenhaut NH, Rauch A, Weiner J, Hubner S, Hubner N, et al. Genomic redistribution of GR monomers and dimers mediates transcriptional response to exogenous glucocorticoid in vivo. Genome Research. 2015;25:836–844. doi: 10.1101/gr.188581.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling J, Kumar R. Crosstalk between NFkB and glucocorticoid signaling: a potential target of breast cancer therapy. Cancer Letters. 2012;322:119–126. doi: 10.1016/j.canlet.2012.02.033. [DOI] [PubMed] [Google Scholar]
- Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9:30. doi: 10.1186/1710-1492-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Oh P, Horner T, Rogers RA, Schnitzer JE. Organized endothelial cell surface signal transduction in caveolae distinct from glycosylphosphatidylinositol-anchored protein microdomains. Journal of Biological Chemistry. 1997;272:7211–7222. doi: 10.1074/jbc.272.11.7211. [DOI] [PubMed] [Google Scholar]
- Lonard DM, Lanz RB, O'Malley BW. Nuclear receptor coregulators and human disease. Endocrine Reviews. 2007;28:575–587. doi: 10.1210/er.2007-0012. [DOI] [PubMed] [Google Scholar]
- Lowenberg M, Tuynman J, Scheffer M, Verhaar A, Vermeulen L, van Deventer S, et al. Kinome analysis reveals nongenomic glucocorticoid receptor-dependent inhibition of insulin signaling. Endocrinology. 2006a;147:3555–3562. doi: 10.1210/en.2005-1602. [DOI] [PubMed] [Google Scholar]
- Lowenberg M, Verhaar AP, Bilderbeek J, Marle J, Buttgereit F, Peppelenbosch MP, et al. Glucocorticoids cause rapid dissociation of a T-cell-receptor-associated protein complex containing LCK and FYN. EMBO Rep. 2006b;7:1023–1029. doi: 10.1038/sj.embor.7400775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu NZ, Wardell SE, Burnstein KL, Defranco D, Fuller PJ, Giguere V, et al. International Union of Pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacological Reviews. 2006;58:782–797. doi: 10.1124/pr.58.4.9. [DOI] [PubMed] [Google Scholar]
- Lu YS, Lien HC, Yeh PY, Yeh KH, Kuo ML, Kuo SH, et al. Effects of glucocorticoids on the growth and chemosensitivity of carcinoma cells are heterogeneous and require high concentration of functional glucocorticoid receptors. World J Gastroenterol. 2005;11:6373–6380. doi: 10.3748/wjg.v11.i40.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan AP, DeFranco DB. Bidirectional transport of glucocorticoid receptors across the nuclear envelope. Proc Natl Acad Sci U S A. 1993;90:3588–3592. doi: 10.1073/pnas.90.8.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcher-Lopes R, Franco A, Tasker JG. Glucocorticoids shift arachidonic acid metabolism toward endocannabinoid synthesis: a non-genomic anti-inflammatory switch. European Journal of Pharmacology. 2008;583:322–339. doi: 10.1016/j.ejphar.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manetsch M, Rahman MM, Patel BS, Ramsay EE, Rumzhum NN, Alkhouri H, et al. Long-acting beta2-agonists increase fluticasone propionate-induced mitogen-activated protein kinase phosphatase 1 (MKP-1) in airway smooth muscle cells. PLoS One. 2013;8:e59635. doi: 10.1371/journal.pone.0059635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser R, Reid D, Abramson M. Corticosteroids for acute severe asthma in hospitalised patients. Cochrane Database Syst Rev. 2001 doi: 10.1002/14651858.CD001740. CD001740. [DOI] [PubMed] [Google Scholar]
- Matthews L, Berry A, Ohanian V, Ohanian J, Garside H, Ray D. Caveolin mediates rapid glucocorticoid effects and couples glucocorticoid action to the antiproliferative program. Molecular Endocrinology. 2008;22:1320–1330. doi: 10.1210/me.2007-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews L, Johnson J, Berry A, Trebble P, Cookson A, Spiller D, et al. Cell cycle phase regulates glucocorticoid receptor function. PLoS One. 2011;6:e22289. doi: 10.1371/journal.pone.0022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews LC, Berry AA, Morgan DJ, Poolman TM, Bauer K, Kramer F, et al. Glucocorticoid receptor regulates accurate chromosome segregation and is associated with malignancy. Proc Natl Acad Sci U S A. 2015;112:5479–5484. doi: 10.1073/pnas.1411356112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavinakere MS, Powers JM, Subramanian KS, Roggero VR, Allison LA. Multiple novel signals mediate thyroid hormone receptor nuclear import and export. Journal of Biological Chemistry. 2012;287:31280–31297. doi: 10.1074/jbc.M112.397745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado N, To Y, Kobayashi Y, Adcock IM, Barnes PJ, Ito K. p38 mitogen-activated protein kinase-gamma inhibition by long-acting beta2 adrenergic agonists reversed steroid insensitivity in severe asthma. Molecular Pharmacology. 2011;80:1128–1135. doi: 10.1124/mol.111.071993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL, Garza AS, Johnson BH, Thompson EB. Pathway interactions between MAPKs, mTOR, PKA, and the glucocorticoid receptor in lymphoid cells. Cancer Cell Int. 2007;7:3. doi: 10.1186/1475-2867-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL, Webb MS, Copik AJ, Wang Y, Johnson BH, Kumar R, et al. p38 Mitogen-activated protein kinase (MAPK) is a key mediator in glucocorticoid-induced apoptosis of lymphoid cells: correlation between p38 MAPK activation and site-specific phosphorylation of the human glucocorticoid receptor at serine 211. Molecular Endocrinology. 2005;19:1569–1583. doi: 10.1210/me.2004-0528. [DOI] [PubMed] [Google Scholar]
- Miner JN, Ardecky B, Benbatoul K, Griffiths K, Larson CJ, Mais DE, et al. Antiinflammatory glucocorticoid receptor ligand with reduced side effects exhibits an altered protein-protein interaction profile. Proc Natl Acad Sci U S A. 2007;104:19244–19249. doi: 10.1073/pnas.0705517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda TB, Morris SA, Hager GL. Complex genomic interactions in the dynamic regulation of transcription by the glucocorticoid receptor. Molecular and Cellular Endocrinology. 2013;380:16–24. doi: 10.1016/j.mce.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura T, Ouchida R, Yoshikawa N, Okamoto K, Makino Y, Nakamura T, et al. Functional modulation of the glucocorticoid receptor and suppression of NF-kappaB-dependent transcription by ursodeoxycholic acid. Journal of Biological Chemistry. 2001;276:47371–47378. doi: 10.1074/jbc.M107098200. [DOI] [PubMed] [Google Scholar]
- Miyata M, Lee JY, Susuki-Miyata S, Wang WY, Xu H, Kai H, et al. Glucocorticoids suppress inflammation via the upregulation of negative regulator IRAK-M. Nat Commun. 2015;6:6062. doi: 10.1038/ncomms7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodley T, Wilson SM, Joshi T, Rider CF, Sharma P, Yan D, et al. Phosphodiesterase 4 inhibitors augment the ability of formoterol to enhance glucocorticoid-dependent gene transcription in human airway epithelial cells: a novel mechanism for the clinical efficacy of roflumilast in severe chronic obstructive pulmonary disease. Molecular Pharmacology. 2013;83:894–906. doi: 10.1124/mol.112.083493. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Kim YS, Liu ZG. TNFalpha and reactive oxygen species in necrotic cell death. Cell Research. 2008;18:343–349. doi: 10.1038/cr.2008.31. [DOI] [PubMed] [Google Scholar]
- Mulatero P, Panarelli M, Schiavone D, Rossi A, Mengozzi G, Kenyon CJ, et al. Impaired cortisol binding to glucocorticoid receptors in hypertensive patients. Hypertension. 1997;30:1274–1278. doi: 10.1161/01.hyp.30.5.1274. [DOI] [PubMed] [Google Scholar]
- Navratil AM, Bliss SP, Berghorn KA, Haughian JM, Farmerie TA, Graham JK, et al. Constitutive localization of the gonadotropin-releasing hormone (GnRH) receptor to low density membrane microdomains is necessary for GnRH signaling to ERK. Journal of Biological Chemistry. 2003;278:31593–31602. doi: 10.1074/jbc.M304273200. [DOI] [PubMed] [Google Scholar]
- Newton R, Leigh R, Giembycz MA. Pharmacological strategies for improving the efficacy and therapeutic ratio of glucocorticoids in inflammatory lung diseases. Pharmacology & Therapeutics. 2010;125:286–327. doi: 10.1016/j.pharmthera.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Ng SS, Li A, Pavlakis GN, Ozato K, Kino T. Viral infection increases glucocorticoid-induced interleukin-10 production through ERK-mediated phosphorylation of the glucocorticoid receptor in dendritic cells: potential clinical implications. PLoS One. 2013;8:e63587. doi: 10.1371/journal.pone.0063587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngkelo A, Hoffmann RF, Durham AL, Marwick JA, Brandenburg SM, de Bruin HG, et al. Glycogen synthase kinase-3beta modulation of glucocorticoid responsiveness in COPD. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1112–1123. doi: 10.1152/ajplung.00077.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaides NC, Galata Z, Kino T, Chrousos GP, Charmandari E. The human glucocorticoid receptor: molecular basis of biologic function. Steroids. 2010;75:1–12. doi: 10.1016/j.steroids.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon M, Andrew R, Chapman KE. It takes two to tango: dimerisation of glucocorticoid receptor and its anti-inflammatory functions. Steroids. 2013;78:59–68. doi: 10.1016/j.steroids.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Cidlowski JA. Cellular processing of the glucocorticoid receptor gene and protein: new mechanisms for generating tissue-specific actions of glucocorticoids. Journal of Biological Chemistry. 2011;286:3177–3184. doi: 10.1074/jbc.R110.179325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. Journal of Allergy and Clinical Immunology. 2013;132:1033–1044. doi: 10.1016/j.jaci.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong KM, Blackford JA, Jr., Kagan BL, Simons SS, Jr., Chow CC. A theoretical framework for gene induction and experimental comparisons. Proc Natl Acad Sci U S A. 2010;107:7107–7112. doi: 10.1073/pnas.0911095107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Miller AH. Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Annals of the New York Academy of Sciences. 2009;1179:86–105. doi: 10.1111/j.1749-6632.2009.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett DA, Glaser R. How stress influences the immune response. Trends in Immunology. 2003;24:444–448. doi: 10.1016/s1471-4906(03)00173-x. [DOI] [PubMed] [Google Scholar]
- Pan XY, Wang Y, Su J, Huang GX, Cao DM, Qu S, et al. The mechanism and significance of synergistic induction of the expression of plasminogen activator inhibitor-1 by glucocorticoid and transforming growth factor beta in human ovarian cancer cells. Molecular and Cellular Endocrinology. 2015;407:37–45. doi: 10.1016/j.mce.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Pearce BD, Pisell TL, Sanchez CI, Po C, Su C, et al. The proinflammatory cytokine, interleukin-1alpha, reduces glucocorticoid receptor translocation and function. Endocrinology. 1999;140:4359–4366. doi: 10.1210/endo.140.9.6986. [DOI] [PubMed] [Google Scholar]
- Paugh SW, Bonten EJ, Savic D, Ramsey LB, Thierfelder WE, Gurung P, et al. NALP3 inflammasome upregulation and CASP1 cleavage of the glucocorticoid receptor cause glucocorticoid resistance in leukemia cells. Nature Genetics. 2015;47:607–614. doi: 10.1038/ng.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard D, Yamamoto KR. Two signals mediate hormone-dependent nuclear localization of the glucocorticoid receptor. EMBO Journal. 1987;6:3333–3340. doi: 10.1002/j.1460-2075.1987.tb02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus T, Cutolo M. Clinical trials documenting the efficacy of low-dose glucocorticoids in rheumatoid arthritis. Neuroimmunomodulation. 2015;22:46–50. doi: 10.1159/000362734. [DOI] [PubMed] [Google Scholar]
- Rao NA, McCalman MT, Moulos P, Francoijs KJ, Chatziioannou A, Kolisis FN, et al. Coactivation of GR and NFKB alters the repertoire of their binding sites and target genes. Genome Research. 2011;21:1404–1416. doi: 10.1101/gr.118042.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak T, Cluning C, Ward BK. Steroid Receptor-Associated Immunophilins: A Gateway to Steroid Signalling. Clin Biochem Rev. 2015;36:31–52. [PMC free article] [PubMed] [Google Scholar]
- Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER. ERs associate with and regulate the production of caveolin: implications for signaling and cellular actions. Molecular Endocrinology. 2002;16:100–115. doi: 10.1210/mend.16.1.0757. [DOI] [PubMed] [Google Scholar]
- Ritter HD, Antonova L, Mueller CR. The unliganded glucocorticoid receptor positively regulates the tumor suppressor gene BRCA1 through GABP beta. Molecular Cancer Research. 2012;10:558–569. doi: 10.1158/1541-7786.MCR-11-0423-T. [DOI] [PubMed] [Google Scholar]
- Ritter HD, Mueller CR. Expression microarray identifies the unliganded glucocorticoid receptor as a regulator of gene expression in mammary epithelial cells. BMC Cancer. 2014;14:275. doi: 10.1186/1471-2407-14-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S, Rohwer JM, Hapgood JP, Louw A. Impact of glucocorticoid receptor density on ligand-independent dimerization, cooperative ligand-binding and basal priming of transactivation: a cell culture model. PLoS One. 2013;8:e64831. doi: 10.1371/journal.pone.0064831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogatsky I, Logan SK, Garabedian MJ. Antagonism of glucocorticoid receptor transcriptional activation by the c-Jun N-terminal kinase. Proc Natl Acad Sci U S A. 1998;95:2050–2055. doi: 10.1073/pnas.95.5.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronacher K, Hadley K, Avenant C, Stubsrud E, Simons SS, Jr., Louw A, et al. Ligand-selective transactivation and transrepression via the glucocorticoid receptor: role of cofactor interaction. Molecular and Cellular Endocrinology. 2009;299:219–231. doi: 10.1016/j.mce.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Rosenfeld MG, Glass CK. Coregulator codes of transcriptional regulation by nuclear receptors. Journal of Biological Chemistry. 2001;276:36865–36868. doi: 10.1074/jbc.R100041200. [DOI] [PubMed] [Google Scholar]
- Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes & Development. 2006;20:1405–1428. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- Roth M, Johnson PR, Rudiger JJ, King GG, Ge Q, Burgess JK, et al. Interaction between glucocorticoids and beta2 agonists on bronchial airway smooth muscle cells through synchronised cellular signalling. Lancet. 2002;360:1293–1299. doi: 10.1016/S0140-6736(02)11319-5. [DOI] [PubMed] [Google Scholar]
- Rubio-Patino C, Palmeri CM, Perez-Perarnau A, Cosialls AM, Moncunill-Massaguer C, Gonzalez-Girones DM, et al. Glycogen synthase kinase-3beta is involved in ligand-dependent activation of transcription and cellular localization of the glucocorticoid receptor. Molecular Endocrinology. 2012;26:1508–1520. doi: 10.1210/me.2011-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem S, Harris T, Mok JS, Li MY, Keenan CR, Schuliga MJ, et al. Transforming growth factor-beta impairs glucocorticoid activity in the A549 lung adenocarcinoma cell line. British Journal of Pharmacology. 2012;166:2036–2048. doi: 10.1111/j.1476-5381.2012.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarasinghe RA, Di Maio R, Volonte D, Galbiati F, Lewis M, Romero G, et al. Nongenomic glucocorticoid receptor action regulates gap junction intercellular communication and neural progenitor cell proliferation. Proc Natl Acad Sci U S A. 2011;108:16657–16662. doi: 10.1073/pnas.1102821108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller BJ, Chodankar R, Watson LC, Stallcup MR, Yamamoto KR. Glucocorticoid receptor binds half sites as a monomer and regulates specific target genes. Genome Biol. 2014;15:418. doi: 10.1186/s13059-014-0418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel A, Wang C, Katzenellenbogen BS, Pestell RG, Lisanti MP. Caveolin-1 potentiates estrogen receptor alpha (ERalpha) signaling. caveolin-1 drives ligand-independent nuclear translocation and activation of ERalpha. Journal of Biological Chemistry. 1999;274:33551–33556. doi: 10.1074/jbc.274.47.33551. [DOI] [PubMed] [Google Scholar]
- Schmidt P, Holsboer F, Spengler D. Beta(2)-adrenergic receptors potentiate glucocorticoid receptor transactivation via G protein beta gamma-subunits and the phosphoinositide 3-kinase pathway. Molecular Endocrinology. 2001;15:553–564. doi: 10.1210/mend.15.4.0613. [DOI] [PubMed] [Google Scholar]
- Sharma R, Prichard D, Majer F, Byrne AM, Kelleher D, Long A, et al. Ursodeoxycholic acid amides as novel glucocorticoid receptor modulators. Journal of Medicinal Chemistry. 2011;54:122–130. doi: 10.1021/jm100860s. [DOI] [PubMed] [Google Scholar]
- Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its behavioral and metabolic correlates: from HPA axis to glucocorticoid receptor dysfunction. Annals of the New York Academy of Sciences. 2012;1261:55–63. doi: 10.1111/j.1749-6632.2012.06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons SS. Glucocorticoid receptor co-factors as therapeutic targets. Curr Opin Pharmacol. 2010;10:613–619. doi: 10.1016/j.coph.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons SS, Jr., Chow CC. The road less traveled: new views of steroid receptor action from the path of dose-response curves. Molecular and Cellular Endocrinology. 2012;348:373–382. doi: 10.1016/j.mce.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sionov RV, Cohen O, Kfir S, Zilberman Y, Yefenof E. Role of mitochondrial glucocorticoid receptor in glucocorticoid-induced apoptosis. Journal of Experimental Medicine. 2006;203:189–201. doi: 10.1084/jem.20050433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola S, Castro RE, Kren BT, Steer CJ, Rodrigues CMP. Modulation of nuclear steroid receptors by ursodeoxycholic acid inhibits TGF-beta 1-Induced E2F-1/p53-mediated apoptosis of rat hepatocytes. Biochemistry. 2004;43:8429–8438. doi: 10.1021/bi049781x. [DOI] [PubMed] [Google Scholar]
- Solito E, Mulla A, Morris JF, Christian HC, Flower RJ, Buckingham JC. Dexamethasone induces rapid serine-phosphorylation and membrane translocation of annexin 1 in a human folliculostellate cell line via a novel nongenomic mechanism involving the glucocorticoid receptor, protein kinase C, phosphatidylinositol 3-kinase, and mitogen-activated protein kinase. Endocrinology. 2003;144:1164–1174. doi: 10.1210/en.2002-220592. [DOI] [PubMed] [Google Scholar]
- Spahn JD, Szefler SJ, Surs W, Doherty DE, Nimmagadda SR, Leung DY. A novel action of IL-13: induction of diminished monocyte glucocorticoid receptor-binding affinity. Journal of Immunology. 1996;157:2654–2659. [PubMed] [Google Scholar]
- Spokoini R, Kfir-Erenfeld S, Yefenof E, Sionov RV. Glycogen synthase kinase-3 plays a central role in mediating glucocorticoid-induced apoptosis. Molecular Endocrinology. 2010;24:1136–1150. doi: 10.1210/me.2009-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehl C, Buttgereit F. Unraveling the functions of the membrane-bound glucocorticoid receptors: first clues on origin and functional activity. Annals of the New York Academy of Sciences. 2014;1318:1–6. doi: 10.1111/nyas.12364. [DOI] [PubMed] [Google Scholar]
- Sundahl N, Bridelance J, Libert C, De Bosscher K, Beck IM. Selective glucocorticoid receptor modulation: New directions with non-steroidal scaffolds. Pharmacology & Therapeutics. 2015;152:28–41. doi: 10.1016/j.pharmthera.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Surjit M, Ganti KP, Mukherji A, Ye T, Hua G, Metzger D, et al. Widespread negative response elements mediate direct repression by agonist-liganded glucocorticoid receptor. Cell. 2011;145:224–241. doi: 10.1016/j.cell.2011.03.027. [DOI] [PubMed] [Google Scholar]
- Szapary D, Xu M, Simons SS., Jr. Induction properties of a transiently transfected glucocorticoid-responsive gene vary with glucocorticoid receptor concentration. Journal of Biological Chemistry. 1996;271:30576–30582. doi: 10.1074/jbc.271.48.30576. [DOI] [PubMed] [Google Scholar]
- Szatmary Z, Garabedian MJ, Vilcek J. Inhibition of glucocorticoid receptor-mediated transcriptional activation by p38 mitogen-activated protein (MAP) kinase. Journal of Biological Chemistry. 2004;279:43708–43715. doi: 10.1074/jbc.M406568200. [DOI] [PubMed] [Google Scholar]
- Talaber G, Boldizsar F, Bartis D, Palinkas L, Szabo M, Berta G, et al. Mitochondrial translocation of the glucocorticoid receptor in double-positive thymocytes correlates with their sensitivity to glucocorticoid-induced apoptosis. International Immunology. 2009;21:1269–1276. doi: 10.1093/intimm/dxp093. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Makino Y, Miura T, Hirano F, Okamoto K, Komura K, et al. Ligand-independent activation of the glucocorticoid receptor by ursodeoxycholic acid. Repression of IFN-gamma-induced MHC class II gene expression via a glucocorticoid receptor-dependent pathway. Journal of Immunology. 1996;156:1601–1608. [PubMed] [Google Scholar]
- Tatro ET, Everall IP, Kaul M, Achim CL. Modulation of glucocorticoid receptor nuclear translocation in neurons by immunophilins FKBP51 and FKBP52: implications for major depressive disorder. Brain Research. 2009;1286:1–12. doi: 10.1016/j.brainres.2009.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng Z, Zhang M, Zhao M, Zhang W. Glucocorticoid exerts its non-genomic effect on IPSC by activation of a phospholipase C-dependent pathway in prefrontal cortex of rats. J Physiol. 2013;591:3341–3353. doi: 10.1113/jphysiol.2013.254961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissing WJ, Meijerink JP, Brinkhof B, Broekhuis MJ, Menezes RX, den Boer ML, et al. Glucocorticoid-induced glucocorticoid-receptor expression and promoter usage is not linked to glucocorticoid resistance in childhood ALL. Blood. 2006;108:1045–1049. doi: 10.1182/blood-2006-01-0261. [DOI] [PubMed] [Google Scholar]
- Tsiarli MA, Paula Monaghan A, Defranco DB. Differential subcellular localization of the glucocorticoid receptor in distinct neural stem and progenitor populations of the mouse telencephalon in vivo. Brain Research. 2013;1523:10–27. doi: 10.1016/j.brainres.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unemura K, Kume T, Kondo M, Maeda Y, Izumi Y, Akaike A. Glucocorticoids decrease astrocyte numbers by reducing glucocorticoid receptor expression in vitro and in vivo. J Pharmacol Sci. 2012;119:30–39. doi: 10.1254/jphs.12047fp. [DOI] [PubMed] [Google Scholar]
- Usmani OS, Ito K, Maneechotesuwan K, Ito M, Johnson M, Barnes PJ, et al. Glucocorticoid receptor nuclear translocation in airway cells after inhaled combination therapy. American Journal of Respiratory and Critical Care Medicine. 2005;172:704–712. doi: 10.1164/rccm.200408-1041OC. [DOI] [PubMed] [Google Scholar]
- Van Bogaert T, De Bosscher K, Libert C. Crosstalk between TNF and glucocorticoid receptor signaling pathways. Cytokine & Growth Factor Reviews. 2010;21:275–286. doi: 10.1016/j.cytogfr.2010.04.003. [DOI] [PubMed] [Google Scholar]
- van der Laan S, Lachize SB, Vreugdenhil E, de Kloet ER, Meijer OC. Nuclear receptor coregulators differentially modulate induction and glucocorticoid receptor-mediated repression of the corticotropin-releasing hormone gene. Endocrinology. 2008;149:725–732. doi: 10.1210/en.2007-1234. [DOI] [PubMed] [Google Scholar]
- Verhoog NJ, Du Toit A, Avenant C, Hapgood JP. Glucocorticoid-independent repression of tumor necrosis factor (TNF) alpha-stimulated interleukin (IL)-6 expression by the glucocorticoid receptor: a potential mechanism for protection against an excessive inflammatory response. Journal of Biological Chemistry. 2011;286:19297–19310. doi: 10.1074/jbc.M110.193672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vettorazzi S, Bode C, Dejager L, Frappart L, Shelest E, Klassen C, et al. Glucocorticoids limit acute lung inflammation in concert with inflammatory stimuli by induction of SphK1. Nat Commun. 2015;6:7796. doi: 10.1038/ncomms8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wu H, Lakdawala VS, Hu F, Hanson ND, Miller AH. Inhibition of Jun N-terminal kinase (JNK) enhances glucocorticoid receptor-mediated function in mouse hippocampal HT22 cells. Neuropsychopharmacology. 2005;30:242–249. doi: 10.1038/sj.npp.1300606. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang JJ, Dai W, Lei KY, Pike JW. Dexamethasone potently enhances phorbol ester-induced IL-1beta gene expression and nuclear factor NF-kappaB activation. Journal of Immunology. 1997;159:534–537. [PubMed] [Google Scholar]
- Wang Z, Frederick J, Garabedian MJ. Deciphering the phosphorylation "code" of the glucocorticoid receptor in vivo. Journal of Biological Chemistry. 2002;277:26573–26580. doi: 10.1074/jbc.M110530200. [DOI] [PubMed] [Google Scholar]
- Wang Z, Li P, Zhang Q, Lv H, Liu J, Si J. Interleukin-1beta regulates the expression of glucocorticoid receptor isoforms in nasal polyps in vitro via p38 MAPK and JNK signal transduction pathways. J Inflamm (Lond) 2015;12:3. doi: 10.1186/s12950-014-0046-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster JC, Oakley RH, Jewell CM, Cidlowski JA. Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative beta isoform: a mechanism for the generation of glucocorticoid resistance. Proc Natl Acad Sci U S A. 2001;98:6865–6870. doi: 10.1073/pnas.121455098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster Marketon JI, Corry J, Teng MN. The respiratory syncytial virus (RSV) nonstructural proteins mediate RSV suppression of glucocorticoid receptor transactivation. Virology. 2014;449:62–69. doi: 10.1016/j.virol.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehmeyer L, Du Toit A, Lang DM, Hapgood JP. Lipid raft- and protein kinase C-mediated synergism between glucocorticoid- and gonadotropin-releasing hormone signaling results in decreased cell proliferation. Journal of Biological Chemistry. 2014;289:10235–10251. doi: 10.1074/jbc.M113.544742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzel C, Stark D, Kullmann F, Scholmerich J, Holstege A, Falk W. Ursodeoxycholic acid induced activation of the glucocorticoid receptor in primary rat hepatocytes. Eur J Gastroenterol Hepatol. 2005;17:169–177. doi: 10.1097/00042737-200502000-00007. [DOI] [PubMed] [Google Scholar]
- Willmann T, Beato M. Steroid-free glucocorticoid receptor binds specifically to mouse mammary tumour virus DNA. Nature. 1986;324:688–691. doi: 10.1038/324688a0. [DOI] [PubMed] [Google Scholar]
- Wu DY, Ou CY, Chodankar R, Siegmund KD, Stallcup MR. Distinct, genome-wide, gene-specific selectivity patterns of four glucocorticoid receptor coregulators. Nucl Recept Signal. 2014;12:e002. doi: 10.1621/nrs.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu I, Shin SC, Cao Y, Bender IK, Jafari N, Feng G, et al. Selective glucocorticoid receptor translational isoforms reveal glucocorticoid-induced apoptotic transcriptomes. Cell Death Dis. 2013;4:e453. doi: 10.1038/cddis.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Glass CK, Rosenfeld MG. Coactivator and corepressor complexes in nuclear receptor function. Current Opinion in Genetics & Development. 1999;9:140–147. doi: 10.1016/S0959-437X(99)80021-5. [DOI] [PubMed] [Google Scholar]
- Yudt MR, Cidlowski JA. The glucocorticoid receptor: coding a diversity of proteins and responses through a single gene. Molecular Endocrinology. 2002;16:1719–1726. doi: 10.1210/me.2002-0106. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Pang J, Favata MF, Trzaskos JM. Receptor density dictates the behavior of a subset of steroid ligands in glucocorticoid receptor-mediated transrepression. International Immunopharmacology. 2003;3:1803–1817. doi: 10.1016/j.intimp.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Yang J, Gao YD, Guo W. Th17 immunity in patients with allergic asthma. International Archives of Allergy and Immunology. 2010;151:297–307. doi: 10.1159/000250438. [DOI] [PubMed] [Google Scholar]