Abstract
Background
Apolipoprotein A-II (apoA-II) is the second most abundant protein in high-density lipoprotein (HDL) particles. However, it exists in plasma in multiple forms. The effect of diabetes on apoA-II proteoforms is not known.
Objective
Our objective was to characterize plasma apoA-II proteoforms in participants with and without type 2 diabetes.
Methods
Using a novel mass spectrometric immunoassay, the relative abundance of apoA-II proteoforms was examined in plasma of 30 participants with type 2 diabetes and 25 participants without diabetes.
Results
Six apoA-II proteoforms were identified (monomer, truncated TQ monomer, truncated Q monomer, dimer, truncated Q dimer, and truncated 2Qs dimer), as well as their oxidized proteoforms. The ratios of oxidized monomer and all oxidized proteoforms to the native apoA-II were significantly greater in the diabetic group (p=0.004 and p= 0.005, respectively) compared to the non-diabetic group.
Conclusion
The relative abundance of oxidized apoA-II is significantly increased in type 2 diabetes.
Keywords: Apolipoprotein A-II, oxidations, mass spectrometry, diabetes
Introduction
High-density lipoprotein (HDL) is a protein lipid complex that participates in reverse cholesterol transport from the periphery to the liver, maintaining healthy vasculature and lipid metabolism1. The main protein components of HDL are apolipoprotein A-I (apoA-I) and A-II (apoA-II). Both apoA-I and apoA-II induce cholesterol efflux from cells through the ATP binding cassette transporter-A1 (ABCA1), although apoA-II is less efficient1. While many cardio-protective roles have been identified for HDL, the role of apoA-II in lipid metabolism remains less clear.1
Type 2 diabetes mellitus is associated with abnormal lipid metabolism and increased oxidative stress2–4. These perturbations in metabolism are reflected by low plasma levels of HDL cholesterol and high levels of lipid peroxidation products5. Oxidative stress in diabetes is thought to be caused by the overproduction of free radicals from abundant glucose auto-oxidation and diminished antioxidant capacity3, 4. We recently reported increased levels of methionine oxidized apoA-I6 in type 2 diabetes. Oxidized apoA-I has a limited capacity to mediate cholesterol efflux in plasma7. Oxidation of apoA-II is known to alter the protein’s lipid binding capacity in vitro and may contribute to the development of aberrant lipid metabolism and atherosclerosis8. Due to technical limitations, apoA-II oxidations in plasma have not yet been characterized. Current methods for analysis of apoA-II are based on conventional immunoassay techniques and provide information about the total protein concentration. Mass Spectrometric Immunoassay (MSIA) is a high throughput methodology that is well suited to identify and quantify molecular variants and posttranslational modifications of plasma proteins9, 10. This technique is based on immunoaffinity capture of target proteins, followed by mass spectrometry detection.
We developed a qualitative MSIA for apoA-II analysis, and applied it to plasma from five healthy males. Six major apoA-II proteoforms were identified: monomer (M), truncated monomer missing C-terminal glutamine (MQ), truncated monomer missing C-terminal tryptophan and glutamine (MTQ), dimer (D), truncated dimer missing C-terminal glutamine (DQ), and truncated dimer missing two glutamines (D2Q), as well as their oxidized proteoforms11. These proteoforms were later confirmed in 96 healthy male and female participants ranging in age from 18 to 6512. In the present study, we used MSIA to characterize plasma apoA-II proteoforms in the setting of type 2 diabetes, by analyzing 30 individuals with type 2 diabetes vs. a control group.
Methods
Clinical Samples
The study was approved by the University of Arizona Institutional Review Board, and all participants provided written informed consent prior to testing. Two groups of adult participants (>18 years of age) were recruited: 30 participants with type 2 diabetes and 25 non-diabetic controls. Participants reported to the Center for Clinical and Translational Sciences after an overnight fast. Blood was collected for laboratory measurements: lipid profile, hemoglobin A1c (HbA1c), C-reactive protein (CRP), and fasting insulin. Additional samples were collected in EDTA tubes and plasma was separated and immediately frozen at −80°C for all other assays. Demographic information (age, sex, ethnicity), physical exam measurements (blood pressure, waist circumference, weight, height, body mass index (BMI), medication use, and medical history (hypertension, hyperlipidemia, smoking, type and duration of diabetes) were also recorded. Exclusion criteria included any of the following: type 1 diabetes, participation in an active weight loss program, history of cancer, HIV or current steroid use. Non-diabetics were classified based on clinical and medication history, and had glycated hemoglobin less than 6%. None of the participants had cardiovascular events such as heart attacks, strokes, amputations or coronary angioplasty.
Mass Spectrometric Immunoassay (MSIA)
Initially, the affinity pipettes were derivatized with antibody against apoA-II (affinity purified polyclonal goat anti-human apoA-II antibody, Cat.No. 12A-G1b, Academy Biomedical Co., Houston, TX) as previously described13. The affinity capture consisted of 750 aspiration/dispense cycles of anti-apoA-II (3.75 µg/pipette in 10 mM MES buffer), followed by ETA and HBS-N washes (50 cycles, 100 µL aspiration/dispense volume each). The derivatized affinity pipettes were stored at 4°C until used. The apoA-IIMSIA was applied to extract and analyze apoA-II in all plasma samples. Prior to protein extraction, the affinity pipettes were pre-rinsed with assay buffer (PBS, 0.1%Tween, 10 aspiration/dispense cycles, 100 µL each). Next, the pipettes were immersed into a microplate containing the analytical samples (120 µL of 100-fold diluted plasma in PBS,0.1%Tween buffer) and 250 aspirations and dispense cycles were performed (100 µL each) allowing for affinity capture of apoA-II from the samples. The pipettes were then rinsed with PBS, 0.1%Tween (100 cycles, 100 µL aspiration/dispense each), and twice with water (10 cycles and 20 cycles respectively, 100 µL aspiration/dispense each). ApoA-II loaded tips were then exposed to six-microliter aliquots of MALDI matrix solution (25 g/L sinapic acid in aqueous solution containing 33% (v/v) acetonitrile, and 0.4 % (v/v) trifluoroacetic acid). After a 10 second delay (to allow for the dissociation of the protein from the capturing antibody), the eluents were dispensed directly onto a 96-well formatted MALDI target. Following drying, linear-mode mass spectra were acquired from each sample spot, each consisting of ten thousand laser shots using an Ultraflex III MALDI-TOF/TOF mass spectrometer (Bruker, Billerica, MA). The mass spectra were internally calibrated using protein calibration standard I, and further processed (baseline subtracted and smoothed) with the Flex Analysis software (Bruker Daltonics). Due to the high degree of overlap between the native proteoform signals and the corresponding oxidation derivative (Figure 1), integration of the signals was done using peak intensities as opposed to peak areas. Peak intensities from each protein signal were obtained in Flex Analysis and tabulated in an excel spreadsheet for further analysis. Initially, all mass spectra were baseline subtracted (Tophat algorithm) and smoothed (SavitzkyGolay algorithm; width = 0.2 m/z; cycles = 1). Peak intensities were tabulated for all signals that represented apoA-II proteoforms. The relative percent abundance of each proteoform was calculated by dividing the peak intensity of that proteoform with the sum of the peak intensities of all apoA-II proteoforms in that sample and multiplying the result by 100.
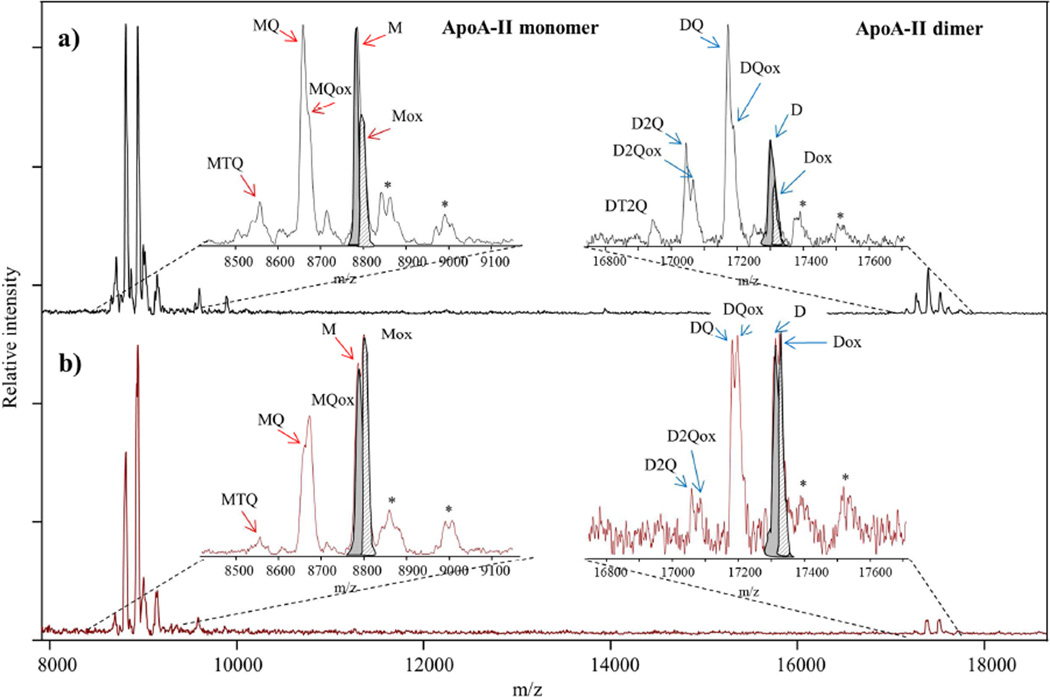
Figure 1. Example of MALDI TOF Mass Spectra of apoA-II proteoforms obtained with MSIA from: a) non-diabetic and b) diabetic human plasma sample.
Using MSIA, we detected signals for the native monomer and dimer of apoA-II, along with several apoA-II proteoforms in plasma. Monomer apoA-II (M) represents cysteinylation on C in position 6 and cyclization on N-terminal Q residue. Truncated TQ monomer (MTQ) represents the monomer apoA-II lacking the C-terminal TQ dipeptide. The truncated Q monomer (MQ) is monomeric apoA-II missing the C-terminal Q residue. The dimer (D) comprises 2 monomers linked by a disulfide bond. The truncated Q dimer (DQ) is an apoA-II dimer missing the Q residue on only one monomer. The truncated 2Qs dimer (D2Q) represents the Dimer apoA-II lacking 2 × C-terminal Q residues on both monomers. Ox refers to the corresponding oxidized proteoforms (signals in the mass spectra are detected at a mass shift of +16). The most abundant proteoforms in our samples were M and Mox. Signals labeled with (*) in the mass spectra correspond to the matrix adducts. Note that for apoA-II M and D proteoforms in both mass spectra, native vs oxidation peaks are highlighted in grey (M and D) and in grey stripes (Mox and Dox). Because of the peak overlap, integration was done using peak intensity as oppose to peak area. The relative percent abundance (RPA) of the oxidized proteoforms is calculated as percentage of the total apoA-II (sum of oxidized and non-oxidized proteoforms).
Sample mass spectra from control (Fig 1a) and diabetic (Fig 1b) subjects show the difference in oxidation ratios between the two groups. The relative abundance of ox proteoforms was greater in the mass spectra from the diabetic sample compared to the non-diabetic sample. MSIA: Mass Spectrometric Immunoassay.
Statistical Analysis
Mean (SD) and median (25th and 75th percentiles) were used to describe the distribution of the continuous variables. The control and type 2 diabetes groups were compared using independent t-tests (normally distributed variables) or Wilcoxon rank sum tests (non-normally distributed variables). Categorical variables were compared using chi-square test. The relationship between apoA-II proteoforms and diabetes status was measured by logistic regression, with diabetes status as the binary dependent variable. BMI, sex and HDL were considered as possible confounders. All statistical analyses were performed using R program version 3.3; a p-value of 0.025 (0.05 divided by 2) was used to define significance levels in the ratios apoA-II oxidized monomer and total oxidations (sum of all oxidized proteoforms) to non-oxidized monomer and non-oxidized total apoA-II respectively, between the diabetes and non-diabetes groups. All other statistical tests used a p-value of 0.05.
Results
ApoA-II proteoforms were measured in plasma of two groups of participants: persons with diabetes (n=30) and non-diabetic controls (n=25). Participants with diabetes had, on average, higher BMI and demonstrated several metabolic characteristics of type 2 diabetes, including elevated levels of HbA1c and fasting insulin, as well as low HDL cholesterol concentrations. In addition, participants with diabetes displayed greater urine micro albumin excretion and lower estimated GFR compared to the non-diabetic controls. Additional data on participants’ demographic and clinical characteristics are summarized in Table 1.
Table. 1.
Demographic, clinical and biochemical characteristics for the study participants
| Characteristics | N1/N2 | Non diabetics | Diabetics | p-value |
|---|---|---|---|---|
| Age, years | 25/30 | 47 ±15 | 51 ±15 | 0.25 |
| Sex | 25/30 | 0.36 | ||
| Male | 6 (25%) | 11 (36.67%) | ||
| Female | 19 (75%) | 19 (63.33%) | ||
| Race | 25/30 | 0.33 | ||
| Caucasians | 18 (71%) | 16 (53%) | ||
| Hispanics | 7 (29%) | 13 (43%) | ||
| Others | 0 (0%) | 1(3%) | ||
| Body mass index, kg/m2 | 25/27 | 28 (24, 32) | 37 (29, 44) | 0.006 |
| Waist circumference, cm | 25/27 | 95 ± 20 | 117 ± 18 | <0.001 |
| Glucose, mg/dL | 25/27 | 100 (93, 110) | 156 (118, 259) | <0.0001 |
| Hemoglobin AIC, (%) | 24/29 | 5.3 (5.0, 5.6) | 7.5 (6, 11) | <0.0001 |
| LDL cholesterol, mg/dL | 25/30 | 110 ± 30 | 106 ±32 | 0.47 |
| HDL cholesterol, mg/dL | 25/30 | 56 ±17 | 46 ±13 | 0.02 |
| Total cholesterol, mg/dL | 25/30 | 190 ± 35 | 186 ± 36 | 0.50 |
| Triglycerides, mg/dL | 25/30 | 123 (83, 175) | 151(103, 195) | 0.17 |
| Glomerular Filtration Rate (GFR), L/min/1.73m2 | 21/19 | 102.4 ± 25 | 73.87 ± 35 | <0.01 |
| Fasting Insulin, IU | 23/21 | 8 (7, 12) | 16 (9, 31) | <0.01 |
| Urine Microalbumin, mg/mg creatinine | 24/25 | 5 (5, 7) | 10 (5, 27) | 0.03 |
| CRP, mg/dL | 16/25 | 5 (3, 7) | 5 (2, 10) | 0.47 |
Mean ± SD or median (25th percentile, 75th percentile) shown for continuous variables (normally distributed or non-normally distributed, respectively).
Frequency (percentage) shown for categorical variables.
GFR was calculated by the MDRD formula.
N1 = number of non-diabetic subjects
N2 = number of diabetic subjects
Presented in Figure 1 are representative mass spectra of apoA-II from plasma of a control participant (Fig 1a) and a diabetic patient (Fig 1b). ApoA-II was detected in its monomeric (M) and dimeric (D) native forms in addition to 10 posttranslationally modified proteoforms derived by C-terminal truncation and/or oxidization. The characteristics of these apoA-II proteoforms are summarized in Table 2. Among the 55 participants, there was significant heterogeneity of the proteoforms that were detected in plasma. Of the participants in the study, 91% had detectable M (monomer), 85% had Mox (M oxidized), 31% had MQ (monomer missing C-terminal glutamine), 94.5% had MQox (oxidized MQ), 43.6% had MTQ (monomer missing C-terminal tryptophan and glutamine), 9% had MTQox (oxidized MTQ), 87% had D (dimer), 27% had Dox (oxidized dimer), 85% had DQ (dimer missing C-terminal glutamine), 27% had DQox (DQ oxidized), 34.5% had D2Q (dimer missing two C-terminal glutamines), and 9% had D2Qox (oxidized DQ). The frequency of the native and oxidized proteoforms detected in plasma of both groups is summarized in Table 3. Eighty percent of participants with diabetes presented with detectable native monomeric apoA-II in plasma compared to controls where the monomer was detected in all (p=0.03). We also observed a trend for apoA-II dimer and its truncated proteoforms to be less commonly detected in plasma of participants with diabetes. On the other hand, only participants with diabetes expressed oxidized dimer missing the C-terminus MTQ (p=0.03). In participants with detectable monomer in plasma, the relative abundance of the native monomer to total apoA-II did not differ between the diabetic and control groups.
Table 2.
ApoA-II proteoforms detected by MSIA
| Label | Proteoform | Theoretical m/z value |
|---|---|---|
| M | Monomer apoA-II– cysteinylation on C in position 6 & cyclization on N-terminal Q residue | 8807.9 |
| Mox | Monomer apoA-II– cysteinylation on C in position 6 & cyclization on N-terminal Q residue oxidized | 8823.9 |
| MQ | Monomer apoA-II– cysteinylation on C in position 6 & cyclization on N-terminal Q residue missing C-terminal Q residue | 8679.8 |
| MQox | Monomer apoA-II– cysteinylation on C in position 6 & cyclization on N-terminal Q residue missing C-terminal Q residue oxidized | 8695.8 |
| MTQ | Monomer apoA-II– cysteinylation on C in position 6 & cyclization on N-terminal Q residue missing C-terminal TQ dipeptide | 8578.7 |
| MTQox | Monomer apoA-II– cysteinylation on C in position 6 & cyclization on N-terminal Q residue missing C-terminal TQ dipeptide oxidized | 8594.7 |
| D | Dimer apoA-II– 2 × monomer (through S-S bond) + 2 × cyclization on N-terminal Q residues | 17377.7 |
| Dox | Dimer apoA-II– 2 × monomer (through S-S bond) + 2 × cyclization on N-terminal Q residues oxidized | 17393.7 |
| DQ | Dimer apoA-II– lacking 1 × C-terminal Q residue (on one monomer) | 17249.5 |
| DQox | Dimer apoA-II– lacking 1 × C-terminal Q residue oxidized | 17265.6 |
| D2Q | Dimer apoA-II– lacking 2 × C-terminal Q residues (on both monomers) | 17121.4 |
| D2Qox | Dimer apoA-II– lacking 2 × C-terminal Q residues oxidized | 17137.5 |
MSIA: Mass Spectrometric Immunoassay.
Table 3.
Frequency of oxidized and native apoA-II proteoforms
| Proteoform | Percent of control participants with native proteoform |
Percent of diabetic participants with native proteoform |
p-value | Percent of control participants with oxidized proteoform |
Percent of diabetic participants with oxidized proteoform |
p-value |
|---|---|---|---|---|---|---|
| Monomer (M) | 100 (25/25) | 80 (25/30) | 0.03 | 80 (20/25) | 90 (27/30) | 0.3 |
| Monomer missing C-terminal glutamine (MQ) | 28 (7/25) | 33 (10/30) | 0.67 | 92 (23/25) | 97 (29/30) | 0.45 |
| Monomer missing C-terminal tryptophan and glutamine (MTQ) | 52 (13/25) | 37 (11/30) | 0.04 | 0 (0/25) | 17 (5/30) | 0.03 |
| Dimer (D) | 96 (24/25) | 80 (24/30) | 0.08 | 20 (5/25) | 33 (10/30) | 0.3 |
| Dimer missing C-terminal glutamine (DQ) | 92 (23/25) | 80 (24/30) | 0.21 | 16 (4/25) | 37 (11/30) | 0.09 |
| Dimer missing two C-terminal glutamines (D2Q) | 48 (12/25) | 23 (7/30) | 0.06 | 4 (1/25) | 13 (4/30) | 0.23 |
Percent (frequency). The frequency of native and oxidized proteoforms detected in plama was compared between the diabetic and non-diabetic groups by a Chi-square test.
The relative abundance of the oxidized to total (both oxidized and non-oxidized) proteoforms was calculated. In the samples assayed, an average of 48% of M, 83% of MQ, 8.5% of MTQ, 18.7% of D, 21% of DQ and 8% of D2Q was oxidized. We also calculated the sum of the all the oxidized peak areas of the detected proteoforms with their corresponding non-oxidized proteoforms. The total oxidation ratio (sum of all oxidized/sum of both non-oxidized and oxidized), together with the ratio of oxidized to native proteoforms, were compared between participants with and without diabetes (Table 4). The relative abundance of the oxidized monomer and the total oxidized ratio were significantly greater in plasma of participants with type 2 diabetes. The relative abundance of oxidized apoA-II monomer was not influenced by sex (p=0.5). Using a multivariate linear regression analysis with oxidized monomer as the dependent variable and diabetes as the independent variable, the association of diabetes status with the oxidized monomer persisted after adjusting for BMI or HDL cholesterol (p=0.02). The coefficient and standard error of the multivariate model is presented in Table 5. We did not find a significant correlation between the relative abundance of any of the native or oxidized proteoforms with glycated hemoglobin or fasting glucose levels.
Table 4.
ApoA-II oxidation ratios in diabetic and non-diabetic samples
| Proteoform | No-Diabetes Oxidized ratio |
Diabetes Oxidized ratio |
p-value |
|---|---|---|---|
| Monomer (M) | 0.38 ± 0.02 | 0.56 ± 0.26 | 0.004 |
| Monomer missing C - terminal glutamine (MQ) | 0.83 ± 0.31 | 0.83 ± 0.26 | 0.94 |
| Monomer missing C-terminal tryptophan and glutamine (MTQ) | 0 | 0.186 ± 0.22 | 0.02 |
| Dimer (D) | 0.11 ± 0.25 | 0.26 ± 0.39 | 0.11 |
| Dimer missing C-terminal glutamine (DQ) | 0.12 ± 0.3 | 0.28 ± 0.41 | 0.09 |
| Dimer missing two C-terminal glutamines (D2Q) | 0.04 ± 0.14 | 0.36 ± 0.5 | 0.063 |
| Total oxidized ratio | 0.45 ± 0.15 | 0.59 ± 0.2 | 0.005 |
The oxidation ratio represented by the relative abundance of the oxidized to total (oxidized/sum of non-oxidized and oxidized). The total oxidation ratio represents the sum of all oxidized forms by the sum of total.
Means ±SD are presented. The two groups were compared by a Wilcoxon t-test.
Table 5.
Estimates (standard error) of the multivariate model describing the association of oxidized apoA-II monomer with diabetes adjusted for body mass index and HDL cholesterol levels
| (predictor: Relative abundance of oxidized apoA-II monomer) | |||
|---|---|---|---|
| Covariates: | Estimate | Std. Error | p-value |
| (Intercept) | 0.51 | 0.23 | 0.03 |
| diabetes | 0.12 | 0.08 | 0.013 |
| BMI | −0.0021 | 0.004 | 0.62 |
| HDL-C | −0.0014 | 0.002 | 0.60 |
Multiple R2: 0.15, p-value of the overall model: 0.05
In a subgroup of participants, some proteoforms were found to be 100% oxidized (100% of the detected proteoform was oxidized, and no native form was detected). The frequency of totally oxidized monomer was greater in plasma of participants with type 2 diabetes (p=0.04). Similar trends were found for other proteoforms (Table 6). Persons with diabetes made up 75% of participants lacking native DQ. All patients lacking DQ had detectable Dox, while only 14.6% of patients with detectable DQ also had DQox. The same trend occurred in detection of D and Dox. There was a greater frequency of Dox occurring in patients missing the native form (85.7%) compared to those who had native proteoform (16.3%). Of those missing D proteoform, 85.7% were also diabetic. All participants with 100% oxidized D also had 100% oxidized DQ. We did not find any unifying demographic characteristics due to the small number of participants where all of D and DQ proteoforms were oxidized (n=5).
Table 6.
Distribution of oxidized apoA-II proteoforms in diabetic and non-diabetic samples
| Proteoform | No-Diabetes with 100% oxidized |
Diabetes With 100% oxidized |
p-value |
|---|---|---|---|
| Monomer (M) | 0% | 16.6% | 0.04 |
| Monomer missing C - terminal glutamine (MQ) | 72% | 66.6% | 0.67 |
| Monomer missing C-terminal tryptophan and glutamine (MTQ) | 0% | 0% | - |
| Dimer (D) | 4% | 17% | 0.12 |
| Dimer missing C-terminal glutamine (DQ) | 8% | 20% | 0.21 |
| Dimer missing two C-terminal glutamines (D2Q) | 0% | 36% | 0.022 |
In some samples, only the oxidized version of the proteoform was detected without any detectable levels of the native form. In these cases, 100% of the available proteoform was oxidized (100% oxidized). This occurred more often in samples from patients with diabetes.
Discussion
In this study, we applied a novel high throughput mass spectrometric immunoassay to characterize the heterogeneity of apoA-II in plasma in type 2 diabetes. We observed six plasma apoA-II proteoforms and their relevant oxidized forms. The native apoA-II monomer and its oxidized proteoforms were detected in highest frequency. This is the first report to demonstrate a relative increase in oxidized apoA-II in the setting of type 2 diabetes. In a subgroup (16.6%) of participants with type 2 diabetes, all of the M proteoforms were oxidized (p=0.04). This was not apparent in samples from any of the control participants. These findings reflect a metabolic environment in type 2 diabetes that favors oxidative damage of apoA-II.
Examining the heterogeneity of apoA-II proteoforms in diabetes can enhance our understanding of its role in lipid metabolism. Known functions of apoA-II include increasing the affinity of hepatic lipase for HDL, impairing lipid hydrolysis by the enzyme, and disrupting HDL recycling14. Overexpression of human apoA-II in mice increases plasma triglyceride levels and is associated with insulin resistance15–17. However, apoA-II concentrations are decreased in plasma from human patients with type 2 diabetes18.
In the present study, the relative abundance of oxidized apoA-II in plasma was found to be 45% in non-diabetic patients compared to 59% in patients with diabetes, and less monomeric native apoA-II was detected in plasma of participants with diabetes. Oxidation of apoA-II at methionine residues can alter protein functionality by impacting its alpha helical structure8. Amphipathic alpha-helices on lipoproteins have the ability to bind lipids through polar and non-polar interactions on opposing faces of the helix19. Oxidation of methionine alters the helical structure of these functional regions and reduces their lipid binding affinity8. Because the hydrophobic face of the helix associates with non-polar fatty acids, the more hydrophilic oxidized form of methionine will disrupt the protein lipid-binding ability. This alteration in lipid binding affinity is clearly demonstrated in studies by Anantharamaiah et al. in which native forms of lipoproteins instantly bind and clear lipid vesicles, while methionine-oxidized analogues perform the action less rapidly8.
Among the apoA-II proteoforms identified in this study, six were monomeric versions of the protein. While previous studies on apoA-II have focused on the dimeric protein, our detection of the monomer in human samples suggests different monomer proteoforms may exist, with less monomeric apoA-II detected in plasma of participants with diabetes. Brewer et al. previously reported that apoA-II appears to be a homodimer bridged by a disulfide bond at cysteine 6 of each monomer20. However, this characterization was limited to plasma from only one patient. Our studies11, 12 suggest that apoA-II is present in both monomeric and dimeric forms in plasma. It is also unlikely that the monomers detected were simply degraded dimers, as sample preparation for MSIA does not include any reducing or denaturing agents. Expression of the human dimeric or monomeric apoA-II (through a cystine mutation that prevents the formation of a dimer) in transgenic mice does not induce a difference in HDL number, size, or plasma cholesterol levels21, 22. Additional studies on the roles apoA-II proteoforms will be required to fully understand their physiological relevance of less abundance of monomeric apoA-II compared to the other proteoforms in diabetes.
One potential concern for the greater apoA-II oxidations is the possibility of ex vivo oxidations. The samples were randomly pre-aliquoted in the 96-well tray, and thawed to room temperature approximately at the same time prior to analysis. The time from sample dilution to mass spectrometry using an 8-channel pipettor was less than 10 min. Any ex vivo oxidation would have affected all the samples in the same manner. In addition, a control sample was analyzed with every plate to test the between run variability. The control sample did not present with significant differences in the oxidation state in different runs. One limitation of this study was that we did not measure apoA-II concentrations.
We conclude that the oxidation ratio of apoA-II proteoforms is increased in the setting of type 2 diabetes. The ratio of oxidized apoA-II monomer to native apoA-II monomer can be used as an index for this oxidation. Additional studies are needed to further investigate the role of oxidized apoA-II as a biomarker for diabetes complications and to understand the functional significance of apoA-II oxidations.
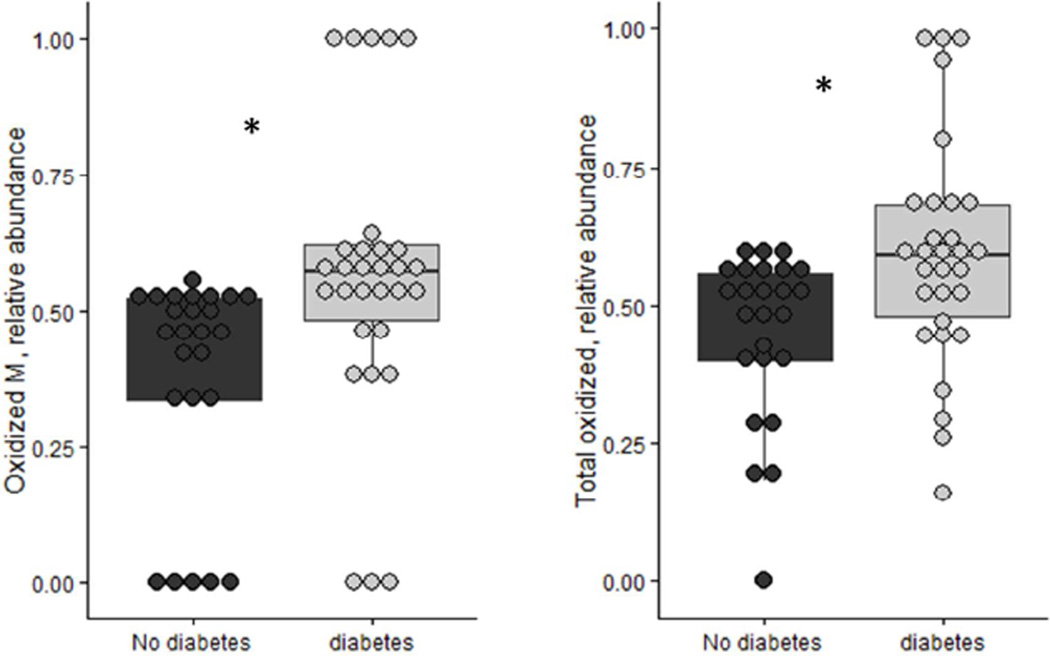
Figure 2. Distribution of the relative abundance of apoA-II oxidized monomer and total oxidized apoA-II in diabetes versus control samples.
The ratio of apoA-II Mox/M was significantly increased in participants with diabetes (n=30) compared to participants without diabetes (n=25). The differences persisted after adjusting for HDL cholesterol levels and BMI (p=0.003). The total oxidation ratio computed for all proteoforms was also higher in participants with diabetes (p=0.005) M: apoA-II monomer. Mox: apoA-II monomer oxidized (+16). *p<0.01
In plasma, the heterogeneity of apolipoprotein A-II has not been investigated in the setting of type 2 diabetes.
Apolipoprotein A-II proteoforms were measured using a novel mass spectrometric immunoassay.
The relative abundance of oxidized apolipoprotein A-II was increased in type 2 diabetes.
Acknowledgments
Sources of support: Dr. Yassine was supported by K23HL107389 from National Institute of Heart, Lung and Blood, 15BGIA25690024 from the American Heart Association and USC CTSI pilot UL1TR000130. Mass spectrometry work was supported by Awards R01DK082542 and R24DK090958 from the National Institute of Diabetes And Digestive and Kidney Diseases.
Abbreviations
- MSIA
Mass Spectrometric Immunoassay
- ApoA-II
Apolipoprotein A-II
- ApoA-I
Apolipoprotein A-I
- HDL
High density lipoprotein
- LDL
Low density lipoprotein
- M
Monomer
- MQ
Monomer missing C-terminal glutamine
- MTQ
truncated monomer missing C-terminal tryptophan and glutamine
- D
dimer
- DQ
truncated dimer missing C-terminal glutamine
- D2Q
truncated dimer missing two glutamines
- Ox
oxidized
- CRP
C-reactive protein
- HbA1c
hemoglobin A1c
- BMI
body mass index
- GFR
glomerular filtration rate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interests to disclose for any of the authors.
Disclosures: IA drafted the manuscript. OT ran the MSIA assays and verified the mass spectra data. YB and JH analyzed the data. JK, PR, RN and DN edited the manuscript. HY designed the study, analyzed the data and drafted the manuscript. All authors have approved the final article.
References
- 1.Chan DC, Ng TW, Watts GF. Apolipoprotein A-II: evaluating its significance in dyslipidaemia, insulin resistance, and atherosclerosis. Annals of medicine. 2012;44:313–324. doi: 10.3109/07853890.2011.573498. [DOI] [PubMed] [Google Scholar]
- 2.Dierckx N, Horvath G, Van Gils C, et al. Oxidative stress status in patients with diabetes mellitus: relationship to diet. European journal of clinical nutrition. 2003;57:999–1008. doi: 10.1038/sj.ejcn.1601635. [DOI] [PubMed] [Google Scholar]
- 3.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 4.Maritim A, Sanders R, Watkins rJ. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17 doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 5.Davì G, Falco A, Patrono C. Lipid peroxidation in diabetes mellitus. Antioxidants & redox signaling. 2005;7:256–268. doi: 10.1089/ars.2005.7.256. [DOI] [PubMed] [Google Scholar]
- 6.Yassine HN, Jackson AM, Reaven PD, et al. The application of multiple reaction monitoring to assess ApoA-I methionine oxidations in diabetes and cardiovascular disease. Translational Proteomics. 2014;4–5:18–24. doi: 10.1016/j.trprot.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shao B, Tang C, Sinha A, et al. Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase. Circulation research. 2014;114:1733–1742. doi: 10.1161/CIRCRESAHA.114.303454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anantharamaiah G, Hughes TA, Iqbal M, et al. Effect of oxidation on the properties of apolipoproteins AI and A-II. Journal of lipid research. 1988;29:309–318. [PubMed] [Google Scholar]
- 9.Nelson RW, Borges CR. Mass spectrometric immunoassay revisited. J Am Soc Mass Spectrom. 2011;22:960–968. doi: 10.1007/s13361-011-0094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson RW, Krone JR, Bieber AL, Williams P. Mass spectrometric immunoassay. Anal Chem. 1995;67:1153–1158. doi: 10.1021/ac00103a003. [DOI] [PubMed] [Google Scholar]
- 11.Niederkofler EE, Tubbs KA, Kiernan UA, Nedelkov D, Nelson RW. Novel mass spectrometric immunoassays for the rapid structural characterization of plasma apolipoproteins. Journal of lipid research. 2003;44:630–639. doi: 10.1194/jlr.D200034-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Nedelkov D, Kiernan UA, Niederkofler EE, Tubbs KA, Nelson RW. Investigating diversity in human plasma proteins. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10852–10857. doi: 10.1073/pnas.0500426102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yassine HN, Trenchevska O, He H, et al. Serum amyloid a truncations in type 2 diabetes mellitus. PloS one. 2015;10:e0115320. doi: 10.1371/journal.pone.0115320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahn CE, Osborne JC, Schaefer EJ, Brewer HB. In vitro activation of the enzymic activity of hepatic lipase by apoA-II. FEBS letters. 1981;131:366–368. doi: 10.1016/0014-5793(81)80405-x. [DOI] [PubMed] [Google Scholar]
- 15.Julve J, Marzal-Casacuberta À, Ordóñez-Llanos J, González-Sastre F, Blanco-Vaca F. ApoA-II expression in CETP transgenic mice increases VLDL production and impairs VLDL clearance. Journal of lipid research. 2001;42:241–248. [PubMed] [Google Scholar]
- 16.Koike T, Kitajima S, Yu Y, et al. Expression of Human ApoAII in Transgenic Rabbits Leads to Dyslipidemia A New Model for Combined Hyperlipidemia. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:2047–2053. doi: 10.1161/ATVBAHA.109.190264. [DOI] [PubMed] [Google Scholar]
- 17.Boisfer E, Lambert G, Atger V, et al. Overexpression of human apolipoprotein A-II in mice induces hypertriglyceridemia due to defective very low density lipoprotein hydrolysis. Journal of Biological Chemistry. 1999;274:11564–11572. doi: 10.1074/jbc.274.17.11564. [DOI] [PubMed] [Google Scholar]
- 18.Hedrick C, Castellani L, Warden CH, Puppione DL, Lusis AJ. Influence of mouse apolipoprotein A-II on plasma lipoproteins in transgenic mice. Journal of Biological Chemistry. 1993;268:20676–20682. [PubMed] [Google Scholar]
- 19.Segrest J, Jones M, De Loof H, Brouillette C, Venkatachalapathi Y, Anantharamaiah G. The amphipathic helix in the exchangeable apolipoproteins: a review of secondary structure and function. Journal of lipid research. 1992;33:141–166. [PubMed] [Google Scholar]
- 20.Brewer H, Lux S, Ronan R, John K. Amino acid sequence of human apoLp-Gln-II (apoA-II), an apolipoprotein isolated from the high-density lipoprotein complex. Proceedings of the National Academy of Sciences. 1972;69:1304–1308. doi: 10.1073/pnas.69.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong EL, Stoltzfus LJ, Brion CM, Murugesh D, Rubin EM. Contrasting in Vivo Effects of Murine and Human Apolipoprotein A-II ROLE OF MONOMER VERSUS DIMER. Journal of Biological Chemistry. 1996;271:5984–5987. doi: 10.1074/jbc.271.11.5984. [DOI] [PubMed] [Google Scholar]
- 22.Lund-Katz S, Murley YM, Yon E, Gillotte KL, Davidson WS. Comparison of the structural and functional effects of monomeric and dimeric human apolipoprotein A-II in high density lipoprotein particles. Lipids. 1996;31:1107–1113. doi: 10.1007/BF02524284. [DOI] [PubMed] [Google Scholar]