Abstract
Background
Childhood early life stress (ELS) increases risk of adulthood Major Depressive Disorder (MDD) and is associated with altered brain structure and function. It is unclear whether specific ELSs affect depression risk, cognitive function and brain structure.
Methods
This cross-sectional study included 64 antidepressant-free depressed and 65 never depressed individuals. Both groups reported a range of ELSs on the Early Life Stress Questionnaire, completed neuropsychological testing and 3T MRI. Neuropsychological testing assessed domains of episodic memory, working memory, processing speed and executive function. MRI measures included cortical thickness and regional gray matter volumes, with a priori focus on cingulate cortex, orbitofrontal cortex (OFC), amygdala, caudate and hippocampus.
Results
Of 19 ELSs, only emotional abuse, sexual abuse and severe family conflict independently predicted adulthood MDD diagnosis. The effect of total ELS score differed between groups. Greater ELS exposure was associated with slower processing speed and smaller OFC volumes in depressed subjects, but faster speed and larger volumes in nondepressed subjects. In contrast, exposure to ELSs predictive of depression had similar effects in both diagnostic groups. Individuals reporting predictive ELSs exhibited poorer processing speed and working memory performance, smaller volumes of the lateral OFC and caudate, and decreased cortical thickness in multiple areas including the insula bilaterally. Predictive ELS exposure was also associated with smaller left hippocampal volume in depressed subjects.
Conclusion
Findings suggest an association between childhood trauma exposure and adulthood cognitive function and brain structure. These relationships appear to differ between individuals who do and do not develop depression.
INTRODUCTION
Childhood stress and trauma significantly influences the risk of adult psychopathology. Early life stress (ELS) exposure lowers the threshold for depressive reactions to stressors later in life (Harkness et al. 2006), while the intensity of ELS predicts symptom severity of mood episodes (Martins et al. 2014). ELS exposure can have long-lasting effects on hypothalamic-pituitary-adrenal (HPA) axis regulation (McEwen 2003), which may in part explain the relationship between ELSs and depression. It is currently unclear whether depression is associated with exposure to specific stressors or whether any childhood trauma could increase vulnerability to depression. Although stressors in childhood or adolescence contribute to a wide range of adulthood psychopathology, some studies associate major depressive disorder (MDD) with exposure to certain types of ELSs including sexual abuse (Kaplow and Widom 2007), emotional abuse (Martins et al. 2014), and family conflict (Kessler and Magee 1994). In contrast, others concluded there is insufficient evidence associating specific childhood adversities with specific psychiatric disorders (Gershon et al. 2013). Such potential ELS-disorder specificity is likely related to more than just the occurrence of the stressor and may be related to the intensity, duration, developmental stage of the victim, and physiological stress response at the time of the stressor. Presumably only stressors resulting in significant or sustained stress responses characterized by HPA axis or other immune system activity would influence vulnerability to psychiatric illnesses. Such stressors would also be expected to be associated with cognitive performance and MRI differences.
Poorer cognitive performance is increasingly recognized as an important aspect of MDD, characterized by poor performance on measures of executive function, processing speed and episodic memory (Snyder 2013). ELS exposure is associated with poorer adult cognitive function in memory domains and executive function in populations with and without psychopathology (Navalta et al. 2006). However, not all studies associate ELS exposure with poorer cognitive performance and much of this work focuses on post-traumatic stress disorder. It is thus unclear whether ELS exposure influences cognitive performance in MDD.
More consistently, even in individuals without psychiatric disorders neuroimaging studies associate ELS exposure with volumetric and functional alterations in brain regions including the anterior cingulate cortex (ACC) (Cohen et al. 2006; Udo et al. 2012), medial prefrontal cortex (van Harmelen et al. 2010), caudate (Cohen et al. 2006) and insula (Baker et al. 2013). In contrast, depressed patients exposed to ELSs exhibit smaller volumes of the orbitofrontal and prefrontal cortexes (Frodl et al. 2010; Udo et al. 2012) and the hippocampus (Cohen et al. 2006; Udo et al. 2012). Jointly, these findings suggest that ELS effects on brain structure may differ between healthy and depressed populations. Although it is challenging to disentangle the effects of ELS versus the effects of depression itself, such population-specific findings may provide clues related to depression vulnerability or resilience.
We hypothesized that specific ELSs are associated with a diagnosis of MDD in adulthood. We further hypothesized that those ELSs associated with MDD would also be associated with poorer performance on cognitive tests and structural alterations in brain regions involved in mood regulation. As those ELSs by definition would increase the risk of MDD, we also tested for statistical interactions between ELS and MDD diagnosis to determine whether the effect of ELS exposure on cognition and brain structure differed between depressed and nondepressed groups.
METHODS
Subjects
Subjects were between 20 and 50 years of age and enrolled at Duke University (N=112) and Vanderbilt University (N=17) between April 2008 and December 2013. Depressed Subjects had a DSM-IV diagnosis of recurrent MDD, as assessed by the Mini-International Neuropsychiatric Interview (MINI, version 5.0) (Sheehan et al. 1998) and interview with a psychiatrist. Additional inclusion criteria included onset of first depressive episode before age 35 years and a Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg 1979) score of 15 or greater. Inclusion criteria specified no psychotropic medication use in the last month; most subjects reported no use for at least three months or longer. Eligible control subjects had neither a history of psychiatric disorders nor a history of psychotropic medication use. Although not an entry criterion, medical comorbidity was quantified using the Cumulative Illness Rating Scale (CIRS) (Miller et al. 1992).
Exclusion criteria included other lifetime DSM-IV Axis I disorders including substance abuse or dependence, although comorbid anxiety symptoms occurring in context of depressive episodes were allowable. Subjects were excluded for Axis II disorders assessed by the Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) (First et al. 1997). Additional exclusion criteria included: history of psychosis, acute suicidality, use of illicit substances in the last month, ECT in the last 6 months, a family history of bipolar disorder, any unstable medical condition, any history of neurological illness or head injury, or MRI contraindications.
Both the Duke University and the Vanderbilt University Institutional Review Boards approved this study. All subjects provided written informed consent.
Assessment of Early Life Stress and Estimation of Total Time Depressed
Exposure to childhood stressors was assessed using the self-report Early Life Stress Questionnaire (ELSQ). The ELSQ was developed from the Child Abuse and Trauma Scale (Sanders and Becker-Lausen 1995), which has strong internal consistency, validity, and test-retest reliability. The ELSQ consists of 19 traumatic items answered yes or no occurring during childhood and adolescent ages up to 17 years. We modified the ELSQ to ask about the ages during the time exposed for each stressor, allowing us to estimate the duration of exposure in years.
Following procedures similar to past reports (Sheline et al. 1999), duration of lifetime depression was assessed through detailed clinical interview and acquisition of medical records. We used a life charting approach to anchor each episode, applying diagnostic criteria to each episode.
Neuropsychological Testing
A trained psychometric technician supervised by a licensed neuropsychologist administered neuropsychological testing. Similar to our approach in geriatric depression (Sheline et al. 2010), we created rationally constructed composite domain variables from a broad test battery. To combine tasks, we created Z-scores for each measure based on the performance of all subjects, then averaged the Z-scores for all tests within each domain. Internal consistency for each domain was assessed using Cronbach's coefficient alpha (CoA). This resulted in four composite neuropsychological measures: a) episodic memory (Logical Memory 1 and 2; Benton Visual Retention Test, number correct; Rey's Verbal Learning Test, total I–V and total VII; CoA=0.87); b) executive function (Controlled Oral Word Association test (total score); Trail Making B time (reverse scored time to completion); verbal fluency (total phonological and semantic); Stroop Color-Word interference condition (number completed); CoA=0.75); c) processing speed (Symbol-Digit Modality (number completed); Trail Making A (reverse scored time to completion); Stroop Color Naming condition (number completed); CoA=0.70); and d) working memory (Digit Span forward (number of trials correctly completed); Digit Span backward (number of trials correctly completed); CoA=0.75).
MRI Acquisition
Due to differences in MRI manufacturers, only MRI data acquired at Duke University was included in analyses. Cranial MRI was performed using the eight-channel parallel imaging head coil on a whole-body MRI system (Trio, Siemens Medical Systems, Malvern, PA). Parallel imaging was employed with an acceleration factor of 2. Duplicate T1-weighted image sets were acquired during the scan session using a sagittal MPRAGE sequence with TR/TE = 2300/3.46 msec, a 240 Hz/pixel bandwidth, a 256 × 256 matrix, a 240 mm diameter field-of-view, 160 slices with a 1.2 mm slice thickness, yielding an image with voxel sizes of 0.9 × 0.9 × 1.2mm. In eight cases, subjects did not complete MRI or scan quality was not suitable for image processing.
Structural MRI Analyses
Volumetric MRI Analyses
Regional volumes and cortical thickness were calculated using FreeSurfer (version 5.1) software. The FreeSurfer methods used to derive cortical and subcortical brain volumes have been previously described (Dale et al. 1999; Fischl et al. 2002; Fischl et al. 2004a; Fischl et al. 2004b). Cortical parcellation used an anatomical mask derived from the Desikan-Killiany Atlas (Desikan et al. 2006); in each hemisphere, this method identified 33 cortical and 7 subcortical gray matter regions (nucleus accumbens, amygdala, caudate, hippocampus, pallidum, putamen, and thalamus). Intracranial volume was assessed using the method implemented in FreeSurfer. We visually inspected the data by overlaying the surfaces and subcortical segmentations over the T1 data. Individual slices in each orientation were assessed for errors. No manual corrections were needed.
Thickness Analyses
This was an exploratory approach to test for differences not captured using our atlas-based comparisons. We tested for differences in cortical thickness using FreeSurfer's QDEC module. We used a general linear model (GLM) to test for differences in cortical thickness between groups exposed or not exposed to ELSs, including age as a nuisance variable. In this method, cortical thickness is computed as the shortest distance between any point on the pial surface and the gray/white boundary and vice-versa; these two values are averaged (Fischl and Dale 2000). Maps were smoothed using the standard Gaussian kernel of 10mm. We used a general linear model to test for differences in cortical thickness between diagnostic groups, including age as a nuisance variable. Correction for multiple comparisons was carried out using the Monte Carlo simulation method using an initial cluster threshold set at p < 0.01. Data were tested against an empirical null distribution of maximum cluster size by running 10,000 synthesized Gaussian noise simulations, producing clusters fully corrected for multiple comparisons. Right and left hemispheres were tested separately.
Statistical Analyses
All analyses were conducted using SAS 9.4 (Cary, NC). We tested for univariate differences between diagnostic groups in demographic and clinical variables using chi-square tests for categorical variables and two-tailed t-tests for continuous variables. Initial tests for differences in the report of early life stressors between depressed and nondepressed cohorts were conducted using chi-square tests, or Fisher's exact test when cell sizes were low.
ELSs that differed between groups in univariate tests were incorporated into general linear models predicting diagnosis (MDD or nondepressed) while controlling for age, sex, education and medical morbidity (CIRS score). Retaining these demographic variables, we conducted backward regression to develop a parsimonious model, removing each ELS item based on its statistical significance level of p=0.05. The remaining ELS items that subsequently predicted a MDD diagnosis were termed “predictive ELSs.”
We next planned a hypothesis-driven approach to reduce the number of comparisons. For neuropsychological analyses, we consolidated individual test results into domain scores as described above. For MRI analyses, we selected a priori regions associated with ELS in existent literature: the ACC (Cohen et al. 2006; Udo et al. 2012), OFC (Frodl et al. 2010; Udo et al. 2012), amygdala (Tottenham et al. 2010), hippocampus (Cole et al. 2011; Sheline et al. 1996; Teicher et al. 2012) and caudate (Cohen et al. 2006; Udo et al. 2012). For exploratory analyses of ELS effects on other regions identified by FreeSurfer, we controlled for multiple comparisons using FDR (false discovery rate), implemented within SAS.
We first examined the effect of the total ELS exposure (defined as total ELSQ score) on cognitive domains and brain volumes. For models examining cognitive domains, we controlled for diagnosis, age, sex, and education. For models examining MRI variables, we controlled for diagnosis, age, sex, and intracranial volume. A similar approach was used for examining the effects of predictive ELSs on cognition and brain structure, with participants dichotomized as exposed or not exposed.
To determine whether ELS effects differed between diagnostic groups, we added a term coding for an interaction between ELS and diagnosis. This was done for analyses of both total ELSQ score and predictive ELS exposure. If the interaction did not reach statistical significance, this suggested that there was no significant difference in the relationship between ELSs, cognition, and brain morphology between diagnostic groups.
Finally, for predictive ELSs, we conducted exploratory analyses using models similar to those described above, but examining the effect of duration of stressor exposure. For these analyses, individuals who denied each stressor were scored as a duration of zero. Due to the high number of people reporting the absence of trauma, we utilized a log transformation of the years of reported duration plus one.
RESULTS
We examined 129 subjects: 64 with MDD and 65 nondepressed controls. The diagnostic groups differed in age and medical morbidity (Table 1), with the depressed group being older, having more medical illnesses, and higher total ELSQ score (range: depressed 0–11, nondepressed 0–8). In univariate analyses, depressed groups exhibited poorer performance in episodic memory, executive function and processing speed. However, these were no longer statistically significant after controlling for age and education level (Table 1).
Table 1.
Demographics and Cognitive Function
| Variable | Depressed (N = 64) | Control (N = 65) | Test Statistic | df | p value |
|---|---|---|---|---|---|
| Age (years) | 35.1 (8.9) | 29.7 (9.2) | t=3.40 | 127 | 0.0009 |
| Sex, Women % (n) | 60.9% (N=39) | 66.2% (N=43) | X2= 0.38 | 1 | 0.5382 |
| Education (years) | 15.2 (2.4) | 16.0 (1.9) | t=1.94 | 127 | 0.0550 |
| CIRS total | 0.6 (1.0) | 0.022 (0.5) | t=2.89 | 127 | 0.0043 |
| MADRS total score | 25.1 (4.5) | 0.7 (0.9) | t=41.97 | 64.15 | <.0001 |
| Depression duration (lifetime, years) | 5.8 (4.5) | - | - | - | - |
| ELSQ total score | 3.5 (2.7) | 1.8 (1.7) | t=4.36 | 106.13 | <.0001 |
| Race, white % (n) | 67.2% (N=43) | 55.4% (N=36) | X2=1.89 | 1 | 0.1689 |
| Processing Speed | −0.13 (0.73) | 0.31 (0.73) | t = 3.41 | 127 | 0.0009 |
| • Adjusted | F=3.50 | 1,125 | 0.0639 | ||
| Working memory | −0.09 (0.86) | 0.17 (0.95) | t = 1.60 | 127 | 0.1123 |
| • Adjusted | F=0.21 | 1,125 | 0.6453 | ||
| Episodic memory | −0.21 (0.82) | 0.26 (0.66) | t = 3.55 | 127 | 0.0006 |
| • Adjusted | F=2.82 | 1,125 | 0.0954 | ||
| Executive function | −0.06 (0.78) | 0.26 (0.70) | t = 2.45 | 127 | 0.0158 |
| • Adjusted | F=0.94 | 1,125 | 0.3350 |
Data presented as mean (standard deviation) for continuous variables or percentage (N) for categorical variables. Comparison of demographic variables utilized pooled, two-tailed t-tests for continuous measures with equal variances and Satterthwaite's t-tests for unequal variances. Comparison of categorical variables utilized chi-square tests. Cognitive measures were z-transformed and presented both as unadjusted (pooled t-tests) and adjusted for age and education level (general linear models with F-values). Note that N=52 for depression duration as not all participants could provide data for past episodes. CIRS = Cumulative Illness Rating Scale; ELSQ = Early Life Stress Questionnaire; MADRS = Montgomery-Asberg Depression Rating Scale
Early Life Stressors: Predicting MDD Diagnosis
Depressed patients reported significantly higher rates of six ELSs (Table 2). While controlling for covariates, we incorporated those ELSs into a model predicting MDD diagnosis. After backwards regression, in the final parsimonious model three ELS variables significantly and independently predicted a diagnosis of MDD: emotional trauma (F1,121= 6.79, p= 0.0103), sexual abuse (F1,121=6.00, p=0.0157) and severe family conflict (F1,121= 7.85, p=0.0059).
Table 2.
Reported ELS Exposure between Depressed and Nondepressed Participants
| Reported trauma | Depressed (n=64) | Control (n=65) | p value |
|---|---|---|---|
| Emotional Trauma | 37.5 % (n=24) | 7.7 % (n=5) | < 0.0001 |
| Physical abuse | 18.7 % (n=12) | 3.0 % (n=2) | 0.0045 |
| Sexual abuse | 28.1 % (n=18) | 4.6 % (n=3) | 0.0003 |
| Domestic violence | 9.4 % (n=6) | 6.1 % (n=4) | 0.5305 |
| Severe family conflict | 39.1 % (n=25) | 12.3 % (n=8) | 0.0006 |
| Neglect | 15.6 % (n=10) | 1.5 % (n=1) | 0.0043 |
| Divorce | 21.9 % (n=14) | 13.9 % (n=9) | 0.2581 |
| Separated | 18.8 % (n=12) | 12.3 % (n=8) | 0.3407 |
| Death in family | 39.1 % (n=25) | 44.6 % (n=29) | 0.5227 |
| Major illness in family | 28.1 % (n=18) | 13.9 % (n=9) | 0.0536 |
| Fire destroyed home | 3.1 % (n=2) | 1.5 % (n=1) | 0.6191 |
| War | 3.1 % (n=2) | 3.1 % (n=2) | 1.0000 |
| Natural disaster | 1.6 % (n=1) | 3.1 % (n=2) | 1.0000 |
| Major personal illness | 6.3 % (n=4) | 7.7 % (n=5) | 1.0000 |
| Hospitalization/surgery | 21.9 % (n=14) | 18.5 % (n=12) | 0.6290 |
| Bullied | 37.5 % (n=24) | 13.9 % (n=9) | 0.0025 |
| Premature birth | 10.9 % (n=7) | 3.1 % (n=2) | 0.0958 |
| Adoption | 1.6 % (n=1) | 1.5 % (n=1) | 1.0000 |
| Other events | 9.4 % (n=6) | 6.2 % (n=4) | 0.5305 |
Percentile (number) of subjects exposed to each trauma type. Due to small cell sizes, ELSs were compared using Fisher's exact test except chi-square tests were used for death in family (x2 = 0.41, 1df) and hospitalization/surgery (x2=0.23, 1df).
We next dichotomized the sample based on whether they reported one or more of those three ELSs: emotional abuse, sexual abuse and severe family conflict (“predictive” ELSs). Forty percent of the study population, approximately 75% of the depressed sample and 33% of the nondepressed sample reported one or more of these predictive ELSs (χ2=24.34, 1df, p<0.0001). Women were more highly represented in the group reporting predictive ELSs (78.4%, compared with 53.9% of those denying predictive ELSs; χ2=8.05, 1df, p=0.0046). These groups did not otherwise significantly differ on age, education, medical morbidity, or duration of depression (Supplement eTable 1).
Effect of ELS on Cognition
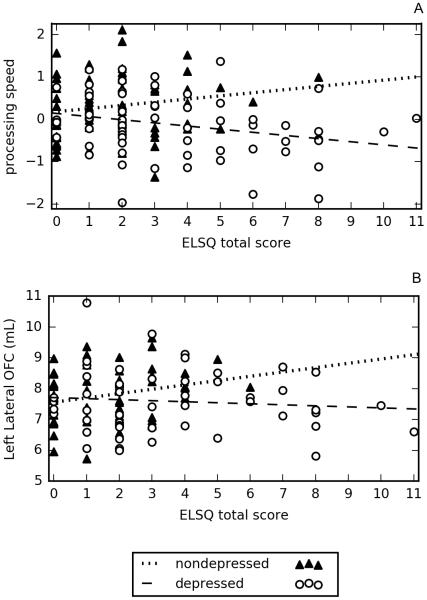
We found no significant main effect of total ELSQ score on any z-transformed cognitive domain. After controlling for covariates of age, sex, and education, we observed an interaction between total ELSQ score and diagnosis on processing speed (F1,122=5.28, p=0.0232) and a trend for an effect on working memory (F1,122=3.64, p=0.0588), but not episodic memory or executive function. On analyzing the interaction, depressed patients exhibited worsening processing speed with increasing ELSQ score (Figure 1.A.), while nondepressed subjects exhibited faster processing speed performance with increased ELSQ score.
Figure 1.
Relationship of ELSQ total score with processing speed and lateral OFC volume
Figures show how exposure to increasing numbers of early life stressors (as total ELSQ score) have different effects on processing speed performance and lateral OFC volume between individuals with and without depression. Figure 1.A., While increased number of ELSs resulted in progressively poorer performance in the z-transformed process speed domain in depressed patients, greater numbers of ELSs is associated with better process speed performance in nondepressed controls. Figure 1.B., With increasing ELS exposure, nondepressed subjects showed relative increases in OFC volume. Conversely, depressed subjects exhibited a slight decline in OFC volume with increasing ELS exposure.
In contrast, exposure to predictive ELSs was associated with progressively poorer performance on working memory (F1,123=5.08, p=0.0260) and processing speed (F1,123=7.74, p=0.0062), but not executive function or episodic memory. To better elucidate these relationships, we found that increasing predictive ELS exposure was associated with worsening performance on mean z-transformed cognitive domain scores of working memory (0 predictive ELS = 0.24, SD=0.93; 1 ELS = −0.13, SD=0.96; 2 ELS = −0.28, SD=0.64; 3 ELS = −0.70, SD = 0.50) and processing speed mean (0 predictive ELS = 0.24, SD=0.68; 1 ELS = 0.09, SD=0.77; 2 ELS = −0.26, SD=0.83; 3 ELS = −0.70, SD = 0.68). Importantly, we found no statistically significant interactions between diagnosis and predictive ELS exposure, suggesting that predictive ELSs affect cognition comparably regardless of depression.
Finally, in exploratory analyses, we examined the relationship between domain scores and log-transformed duration of predictive ELS exposure. Poorer processing speed performance was associated with longer childhood exposure to emotional (F1,121=5.74, p=0.0183) and sexual abuse (F1,121=4.98, p=0.0277) in both cohorts. We did not find statistically significant associations between predictive ELS duration and other cognitive domains.
Effect of ELS on Brain Structure
Due to differences in MRI manufacturers, MRI analyses included only data gathered at Duke University, resulting in a sample of 51 depressed and 53 nondepressed individuals. After controlling for age, sex, and intracranial volume, we found no significant differences between diagnostic groups in a priori brain regions (Supplement eTable 2). We also found no direct effects of total ELSQ score on a priori brain regions. However, we observed an interactive effect between total ELSQ score and diagnosis on the left lateral OFC (F1,97=4.05, p=0.0469). Greater numbers of ELSs are associated with increasing OFC volumes in nondepressed subjects, but minimal differences in depressed subjects (Figure 1.B.). In exploratory analyses, after controlling for multiple comparisons we did not observe significant direct or interactive effects of total ELSQ score on other brain regions.
Forty percent of the MRI sample (32 depressed and 10 nondepressed subjects) reported one or more predictive ELSs. In analyses of a priori regions, predictive ELSs were associated with smaller left lateral OFC (F1,98= 5.11, p=0.0260) and smaller right caudate volumes (F1,98=6.19, p=0.0145). We found a significant interaction between diagnosis and predictive ELS exposure only for the hippocampus, with predictive ELSs being associated with smaller left hippocampus volume (F1,98=4.98, p=0.0280) and a trend for smaller right hippocampus volume (F1,98=3.35, p=0.0705), but only in the depressed group. After controlling for multiple comparisons there were no statistically significant findings in other brain regions.
In exploratory analyses, we examined the relationship between the volumes of the a priori regions and duration of exposure to the predictive ELSs. A statistically significant relationship was only observed between exposure to sexual abuse and caudate volume, wherein a greater log-transformed duration of exposure was associated with smaller caudate volumes (Left: F1,96 =4.92, p=0.0292; Right: F1,96 =5.02, p=0.0276).
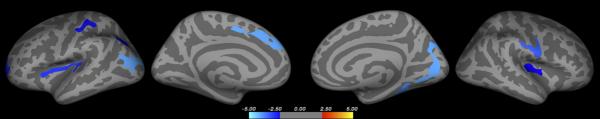
Finally, we tested for relationships between predictive ELS exposure and cortical thickness. After controlling for sex and diagnosis, predictive ELS exposure was associated with reduced cortical thickness in several regions, including the bilateral insula, frontal and parietal lobes (Figure 2 and Supplement eTable 3). We found no association between predictive ELS exposure and increased cortical thickness.
Figure 2.
Cortical thickness differences related to predictive ELS exposure
Whole brain vertex-wise display shows the direct effect of reported predictive ELSs (emotional abuse, sexual abuse, or severe family strife) on cortical thickness. Analyses controlled for diagnosis (MDD or nondepressed) and sex. Lighter blue color reflects areas where ELS exposure is associated with thinner cortex. ELS exposure was not significantly associated with increased cortical thickness in any region.
DISCUSSION
Similar to past literature (Wise et al. 2001), depressed individuals report more ELSs than nondepressed individuals. However, only emotional abuse, sexual abuse and severe family conflict significantly predicted adult MDD. Exposure to these childhood stressors is associated with poorer cognitive performance and alterations in brain morphology that did not differ between diagnostic groups. Additionally, we found that exposure to predictive ELSs was associated with smaller hippocampal volumes, but only in depressed individuals. In contrast, when defined broadly, overall ELS exposure has a different relationship with processing speed and OFC volumes between depressed and nondepressed adults.
Childhood trauma has prominent effects on adult mental health (Navalta et al. 2006). Past work shows altered HPA axis regulation and secondary regional brain structure changes in children exposed to emotional abuse, sexual abuse and aggressive families (McEwen 2003). Emotional abuse is common in childhood and adversely affects self-esteem, interpersonal skills and personal autonomy and integrity (Vietze et al. 1980). Childhood sexual abuse has a worldwide prevalence of 20% (Jakubczyk et al. 2014) and is associated with multiple psychiatric disorders including MDD, addictions, and increased suicide risk (Jakubczyk et al. 2014). Significant family strife also interferes with normal development and creates a vulnerability to maladjustment and internalizing personal problems (Luebbe and Bell 2014). Admittedly, while these specific stresses predicted adulthood MDD, others propose that any significant childhood stress may increase the risk of depression (Brown and Harris 1978). Such effects may depend on stressor severity and chronicity, age of exposure and positive support (Brown and Harris 1978).
Effects of ELS on Cognition
ELS exposure also affected cognitive performance. When defined broadly using the total ELSQ score, ELS exhibited different effects between depressed and nondepressed subjects on processing speed (Figure 1.A.). We propose that these group differences reflect different long-term adaptations to stress that are related to either vulnerability to or resilience to developing later depression. In contrast, the effect of predictive ELS exposure on processing speed and working memory was independent of diagnosis. Similarly, duration of emotional and sexual abuse exposure was associated with slower processing speed.
Our results did not support prior literature associating poorer performance on executive function measure or episodic memory with ELSs (Brewin 2014; Polak et al. 2012). The difference between our results and past work associating ELS with episodic and semantic memory impairment (Parks and Balon 1995), could be related to heterogeneity in samples or measures used to examine episodic memory. While prior studies used the word-cueing technique and Logical Memory test (Parks and Balon 1995), we additionally used the Benton Visual Retention and Rey's Verbal learning tests.
When considering the relationship between ELS and processing speed, we may be observing an inverted U-shaped curve. In this model, stress exposure broadly defined is associated with improved processing speed, but exposure to more severe (and potentially more chronic) ELSs results in impaired performance and vulnerability to depression. This is concordant with studies in older adults associating childhood trauma with better processing speed (Feeney et al. 2013). In our nondepressed population, it is possible that less severe stresses result in improved processing speed and contribute to a resiliency mechanism (Wu et al. 2013). However, subjects predisposed to depression may have pre-existing circuit dysfunction where neurobiological changes related to even milder stresses may result in poorer cognitive performance.
A similar model may apply to working memory, although we observed a relationship only with the more severe predictive stressors that did not differ between diagnostic groups. Past work supports negative effects of childhood stressors on working memory (Navalta et al. 2006). This may be related to altered function of stress-sensitive systems, as working memory deteriorates with increased allostatic stress load (Evans and Schamberg 2009).
Effects of ELS on Brain Structure
ELS exposure was also associated with altered volumes of several regions involved in emotional regulation (Udo et al. 2012). In parallel with our observations on processing speed, we observed diagnostic group differences on the relationship between total ELSQ score and lateral OFC volume (Figure 1.B.). A similar inverted U-shaped model may also apply to this relationship. This theory is concordant with a primate study examining early life maternal separation (Parker et al. 2005). This study associated separation with increased adult OFC volume, a finding thought to be related to stress resiliency by learning extinction of fear through top-down regulation (Lyons et al. 2009). Although the underlying mechanism is unclear, the localization of our OFC finding to the left hemisphere is supported by prior studies suggesting higher left hemisphere sensitivity to emotional neglect during brain development (Frodl et al. 2010)
Exposure to more severe predictive ELSs was associated with smaller OFC and caudate volumes in both cohorts. This is concordant with past work showing that physically and emotionally abused children exhibit smaller OFC volumes (De Brito et al. 2013) while domestic violence and sexual abuse are associated with smaller caudate volumes (Cohen et al. 2006). Our results from cortical thickness analyses are in line with a study associating decreased insula thickness with ELS exposure (Baker et al. 2013). As the insula plays a role in salience network regulation, reported abnormalities may explain deterioration in working memory and processing speed (Krishnadas et al. 2014).
Smaller hippocampal volumes are reported in MDD (MacQueen and Frodl 2011). We found that predictive ELSs were associated with hippocampal volume, but only in depressed individuals. Animal models demonstrate that controlled maternal separation results in decreased hippocampal volumes, however those volumes may normalize in adulthood (Herpfer et al. 2012). Extending that finding to our data, we may be observing a vulnerability mechanism wherein subjects who do not experience recovery of hippocampal neurogenesis are at increased risk of adult depression. This theory is concordant with past work demonstrating that depression itself contributes to hippocampal volume reduction (Sheline et al. 1996) while smaller hippocampal volumes are also a risk factor for depression (Cole et al. 2011). Moreover, our finding is consistent with past observations that the left hippocampus is more sensitive to stressful events than the right hippocampus (Teicher et al. 2012). Although the underlying cause for this difference is unclear, it is hypothesized that it is related to cortisol's effect on NMDA receptor function and hemispheric differences in NMDA subunit distributions (Teicher et al. 2012).
Limitations and Conclusions
Despite the strength of a large, well-characterized sample, the study also has weaknesses. These include using a retrospective, self-report scale for ELS, which does not measure severity, chronicity, or include stressful events with positive connotations. Self-report is subject to a memory bias, which may be significant in depressed individuals, although others report consistency between retrospective accounts and documented events (Martins et al. 2014). Further, our study is cross sectional so cannot address longitudinal developmental effects of ELS exposure on brain volume or cognition. It also does not inform us if the observed cognitive and volumetric differences observed in the MDD population persist with successful depression treatment.
There are additional limitations specifically related to the depressed group. The depressed group is older than the never-depressed group, which is important as increased age is associated with changes on MRI and neuropsychological testing. Although this concern is ameliorated by controlling for age in statistical analyses and the lack of an observed age difference between individuals who were and were not exposed to predictive ELSs, study findings do need replication in a truly age-matched sample. Finally, it can be challenging to disentangle the effects of ELS from the occurrence of depression. As we did not observe significant differences in cognitive or MRI measures between groups after controlling for demographic variables (Table 1, eTable 2), the predictive ELSs do not appear to be serving as a surrogate marker for depression diagnosis. However, it is possible that predictive ELS exposure may influence the duration or recurrence of depressive episodes, or be related to early antidepressant treatment. The complexity of this relationship may require prospective longitudinal studies to disentangle these effects and identify potential benefits for early intervention.
It is important to note that analyses included numerous comparisons. This included analyses of 4 z-transformed cognitive domains, 7 a priori brain regions measured bilaterally, and numerous other regions examined in exploratory analyses. For analyses of exploratory brain regions, we controlled for multiple comparisons using the FDR method. However, as examination of cognitive domains and our a priori regions were based on specific hypotheses and past literature, we did not control for multiple comparisons in those analyses. Despite this hypothesis-driven approach, this does increase the risk for false positive findings. Adjusting those analyses for multiple comparisons would have limited cognitive findings to processing speed with no a priori brain region surviving correction. Thus our findings should be viewed cautiously and need replication in context of the broader literature.
This study supports that not all childhood stressors increase risk of depression in adulthood. We found a complex relationship between ELSs, cognitive function and regional brain structures that in some cases differed between diagnostic groups. As we defined predictive ELSs based on their relationship with MDD, these findings require replication in independent populations. Future longitudinal human studies are required to incorporate physiological measures of stress reactivity, investigate what factors contribute to the cognitive deficits observed with exposure to ELS, and to clarify the association with volumetric brain changes. Such studies should also examine the effect of emotional processes on cognitive performance, and how ELS exposure may influence those relationships.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Dr. Hakmook Kang for guidance on statistical analyses. This project was conducted using the resources of the Advanced Computing Center for Research and Education at Vanderbilt University, Nashville, TN.
Financial Support: This project was supported by the National Institute of Mental Health (NIMH) grant R01 MH077745; and the National Center for Advancing Translational Sciences (NCATS) award UL1TR000445.
Footnotes
Previous Presentation: Poster presentation at the American Psychiatric Association 168th annual meeting. Toronto, ON. Canada. March 2015
Conflicts: All authors deny any potential conflicts of interest.
References
- Baker LM, Williams LM, Korgaonkar MS, Cohen RA, Heaps JM, Paul RH. Impact of early vs. late childhood early life stress on brain morphometrics. Brain Imaging and Behavior. 2013;7:196–203. doi: 10.1007/s11682-012-9215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin CR. Episodic memory, perceptual memory, and their interaction: foundations for a theory of posttraumatic stress disorder. Psychological Bulletin. 2014;140:69–97. doi: 10.1037/a0033722. [DOI] [PubMed] [Google Scholar]
- Brown WG, Harris TO. Social Origins of Depression: A Study of Psychiatric Disorder in Women. The Free Press; New York: 1978. [Google Scholar]
- Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, Gunstad J, Stroud L, McCaffery J, Hitsman B, Niaura R, Clark CR, McFarlane A, Bryant R, Gordon E, Williams LM. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biological Psychiatry. 2006;59:975–982. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Cole J, Costafreda SG, McGuffin P, Fu CH. Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. Journal of Affective Disorders. 2011;134:483–487. doi: 10.1016/j.jad.2011.05.057. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- De Brito SA, Viding E, Sebastian CL, Kelly PA, Mechelli A, Maris H, McCrory EJ. Reduced orbitofrontal and temporal grey matter in a community sample of maltreated children. Journal of Child Psychology and Psychiatry. 2013;54:105–112. doi: 10.1111/j.1469-7610.2012.02597.x. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:6545–6549. doi: 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney J, Kamiya Y, Robertson IH, Kenny RA. Cognitive function is preserved in older adults with a reported history of childhood sexual abuse. Journal of Traumatic Stress. 2013;26:735–743. doi: 10.1002/jts.21861. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JB, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) American Psychiatric Press, Inc; Washington, D.C: 1997. [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004a;23(Suppl 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004b;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Frodl T, Reinhold E, Koutsouleris N, Reiser M, Meisenzahl EM. Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. Journal of Psychiatric Research. 2010;44:799–807. doi: 10.1016/j.jpsychires.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Gershon A, Sudheimer K, Tirouvanziam R, Williams LM, O'Hara R. The long-term impact of early adversity on late-life psychiatric disorders. Current Psychiatry Reports. 2013;15:352. doi: 10.1007/s11920-013-0352-9. [DOI] [PubMed] [Google Scholar]
- Harkness KL, Bruce AE, Lumley MN. The role of childhood abuse and neglect in the sensitization to stressful life events in adolescent depression. Journal of Abnormal Psychology. 2006;115:730–741. doi: 10.1037/0021-843X.115.4.730. [DOI] [PubMed] [Google Scholar]
- Herpfer I, Hezel H, Reichardt W, Clark K, Geiger J, Gross CM, Heyer A, Neagu V, Bhatia H, Atas HC, Fiebich BL, Bischofberger J, Haas CA, Lieb K, Normann C. Early life stress differentially modulates distinct forms of brain plasticity in young and adult mice. PLoS One. 2012;7:e46004. doi: 10.1371/journal.pone.0046004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubczyk A, Klimkiewicz A, Krasowska A, Kopera M, Slawinska-Ceran A, Brower KJ, Wojnar M. History of sexual abuse and suicide attempts in alcohol-dependent patients. Child Abuse and Neglect. 2014;38:1560–1568. doi: 10.1016/j.chiabu.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplow JB, Widom CS. Age of onset of child maltreatment predicts long-term mental health outcomes. Journal of Abnormal Psychology. 2007;116:176–187. doi: 10.1037/0021-843X.116.1.176. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Magee WJ. Childhood family violence and adult recurrent depression. Journal of Health and Social Behavior. 1994;35:13–27. [PubMed] [Google Scholar]
- Krishnadas R, Palaniyappan L, Lang J, McLean J, Cavanagh J. Psychoticism and salience network morphology. Personality and Individual Differences. 2014;57:37–42. [Google Scholar]
- Luebbe AM, Bell DJ. Positive and negative family emotional climate differentially predict youth anxiety and depression via distinct affective pathways. Journal of Abnormal Child Psychology. 2014;42:897–911. doi: 10.1007/s10802-013-9838-5. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ, Katz M, Schatzberg AF. Developmental cascades linking stress inoculation, arousal regulation, and resilience. Frontiers in Behavioral Neuroscience. 2009;3:32. doi: 10.3389/neuro.08.032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Molecular Psychiatry. 2011;16:252–264. doi: 10.1038/mp.2010.80. [DOI] [PubMed] [Google Scholar]
- Martins CM, Von Werne Baes C, Tofoli SM, Juruena MF. Emotional abuse in childhood is a differential factor for the development of depression in adults. The Journal of Nervous and Mental Disease. 2014;202:774–782. doi: 10.1097/NMD.0000000000000202. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Early life influences on life-long patterns of behavior and health. Mental Retardation and Developmental Disabilities Research Reviews. 2003;9:149–154. doi: 10.1002/mrdd.10074. [DOI] [PubMed] [Google Scholar]
- Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, Mulsant B, Reynolds CF. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Research. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Navalta CP, Polcari A, Webster DM, Boghossian A, Teicher MH. Effects of childhood sexual abuse on neuropsychological and cognitive function in college women. The Journal of Neuropsychiatry and Clinical Neurosciences. 2006;18:45–53. doi: 10.1176/jnp.18.1.45. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Justus KR, Schatzberg AF, Lyons DM. Mild early life stress enhances prefrontal-dependent response inhibition in monkeys. Biological Psychiatry. 2005;57:848–855. doi: 10.1016/j.biopsych.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Parks ED, Balon R. Autobiographical memory for childhood events: patterns of recall in psychiatric patients with a history of alleged trauma. Psychiatry. 1995;58:199–208. doi: 10.1080/00332747.1995.11024726. [DOI] [PubMed] [Google Scholar]
- Polak AR, Witteveen AB, Reitsma JB, Olff M. The role of executive function in posttraumatic stress disorder: A systematic review. Journal of Affective Disorders. 2012;141:11–21. doi: 10.1016/j.jad.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Sanders B, Becker-Lausen E. The measurement of psychological maltreatment: early data on the Child Abuse and Trauma Scale. Child Abuse and Neglect. 1995;19:315–323. doi: 10.1016/s0145-2134(94)00131-6. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker RW, Dunbar GC. The Mini-International Neuropsychiatric Inventory (M.I.N.I.): the development and validation of a structured diagnostic interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;(Suppl 20):22–33. [PubMed] [Google Scholar]
- Sheline YI, Pieper CF, Barch DM, Welsh-Bohmer K, McKinstry RC, MacFall JR, D'Angelo G, Garcia KS, Gersing K, Wilkins C, Taylor W, Steffens DC, Krishnan RR, Doraiswamy PM. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Archives of General Psychiatry. 2010;67:277–285. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. The Journal of Neuroscience. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychological Bulletin. 2013;139:81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E563–E572. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, Millner A, Galvan A, Davidson MC, Eigsti IM, Thomas KM, Freed PJ, Booma ES, Gunnar MR, Altemus M, Aronson J, Casey BJ. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udo D, Anja S, Victoria B, Peter Z, Thomas L, Dominik G, Katharina D, Christa H, Patricia O, Jochen B, Christian L, Christian P, Carsten K, Volker A, Walter H, Thomas S, Harald K. Limbic Scars: Long-Term Consequences of Childhood Maltreatment Revealed by Functional and Structural Magnetic Resonance Imaging. Biological Psychiatry. 2012;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- van Harmelen AL, van Tol MJ, van der Wee NJ, Veltman DJ, Aleman A, Spinhoven P, van Buchem MA, Zitman FG, Penninx BW, Elzinga BM. Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biological Psychiatry. 2010;68:832–838. doi: 10.1016/j.biopsych.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Vietze P, Falsey S, Sandler H, O'Connor S, Altemier WA. Transactional approach to prediction of child maltreatment. Infant Mental Health Journal. 1980;1:248–261. [Google Scholar]
- Wise LA, Zierler S, Krieger N, Harlow BL. Adult onset of major depressive disorder in relation to early life violent victimisation: a case-control study. Lancet. 2001;358:881–887. doi: 10.1016/S0140-6736(01)06072-X. [DOI] [PubMed] [Google Scholar]
- Wu G, Feder A, Cohen H, Kim JJ, Calderon S, Charney DS, Mathe AA. Understanding resilience. Frontiers in Behavioral Neuroscience. 2013;7:10. doi: 10.3389/fnbeh.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.