Abstract
Objective
To describe systemic autoimmune joint diseases following Lyme disease and to compare their clinical features with Lyme arthritis.
Methods
Records of all adult patients referred to our Lyme arthritis clinic over a 13-year period in whom we diagnosed a systemic autoimmune joint disease following Lyme disease were reviewed. For comparison, records of patients enrolled in our Lyme arthritis (LA) cohort over the most recent 2-year period were analyzed. IgG antibodies to Borrelia burgdorferi and to 3 Lyme disease-associated autoantigens were measured.
Results
We identified 30 patients who developed a new-onset systemic autoimmune joint disorder a median of 4 months after Lyme disease, usually erythema migrans (EM). Fifteen had rheumatoid arthritis (RA), 13 had psoriatic arthritis (PsA), and 2 had peripheral spondyloarthropathy (SpA). The 30 patients typically had polyarthritis; and those with PsA/SpA often had previous psoriasis, axial involvement, or enthesitis. In the comparison group of 43 LA patients, monoarticular knee arthritis, without prior EM, was the usual clinical picture. Most systemic autoimmune patients had positive tests for B. burgdorferi IgG antibodies by ELISA, but they had significantly lower titers and lower frequencies of Lyme-associated autoantibodies than LA patients. Prior to our evaluation, the patients often received additional antibiotics for presumed Lyme arthritis without benefit. We prescribed anti-inflammatory therapies, most commonly disease modifying anti-rheumatic drugs (DMARDs), resulting in improvement.
Conclusion
Systemic autoimmune joint diseases, RA, PsA/SpA, may follow Lyme disease. Development of polyarthritis after antibiotic-treated erythema migrans, previous psoriasis, or low-titer B. burgdorferi antibodies are clues to the correct diagnosis.
Keywords: Lyme disease, Lyme arthritis, rheumatoid arthritis, psoriatic arthritis
Lyme arthritis (LA) was identified as a clinical entity in the late 1970’s after an outbreak of monoarticular and oligoarticular arthritis in children in Lyme, Connecticut (1). LA is now known to be a late manifestation of Lyme disease (2) caused by the tick-transmitted spirochete Borrelia burgdorferi (3). Before the cause of the disease was known, about 60% of non-antibiotic-treated patients developed LA within a median duration of 6 months (range, 4 days to 2 years) after the initial erythema migrans (EM) skin lesion (4). These patients experienced intermittent or persistent attacks of swelling and pain primarily in one or a few large joints, especially the knee, over a period of several years (4,5). Small joints, tendons or bursae were occasionally affected, usually in one location at a time. However, while some patients may report a history of a tick bite or EM, arthritis may be the presenting manifestation of the illness, without earlier signs or symptoms of Lyme disease (6).
Serologic testing using a two-test approach of ELISA and Western blot is the mainstay of diagnosis (7). Patients with LA typically have markedly elevated IgG antibody responses to B. burgdorferi with expansion of the response to many spirochetal proteins (8). The majority of LA patients respond to appropriate oral or intravenous (IV) antibiotic treatment, but some patients have persistent proliferative synovitis in previously infected joints, usually a knee, after 2-3 months of such therapy, termed post-infectious, antibiotic-refractory arthritis (9). In these patients, the synovial lesion is similar to that of other forms of chronic inflammatory arthritis, including rheumatoid arthritis or psoriatic arthritis. After oral and IV antibiotic therapy, we treat patients with refractory arthritis with anti-inflammatory therapies, disease modifying anti-rheumatic drugs (DMARDs), or synovectomy (6, 9), analogous to the treatment used for other forms of chronic inflammatory arthritis.
There is strong evidence for an immune-mediated process in patients with antibiotic-refractory LA. This outcome is associated with certain HLA-DR alleles (10), excessive joint inflammation (11), immune dysregulation of the CD4+ T effector / T regulatory cell ratio (12,13), and infection-induced autoimmunity (14). Immune reactivity with spirochetal remnants may also be a factor (2). Using mass spectrometry to identify naturally-presented HLA-DR peptides in synovial tissue (15), we have now identified 4 self proteins, endothelial cell growth factor (ECGF), apolipoproteinB-100 (apoB-100), annexin-A2, and matrix metalloproteinase-10 (MMP-10) that are each targets of T and B cell responses in about 10-35% of patients with LA, particularly in those with antibiotic-refractory arthritis (14-19). Moreover, in patients with antibiotic-refractory LA, the levels of anti-ECGF autoantibodies correlated with obliterative microvascular lesions in synovial tissue, suggesting that these antibodies may have pathologic potential (16). Antibody responses to ECGF were not found in patients with other types of arthritis, and reactivity with MMP-10 and apoB-100 were unusual in other arthritides, but annexin A2 antibody responses occur in several rheumatic diseases (20).
Antibiotic-refractory LA is usually confined to a previously infected joint, most commonly a knee, accompanied by few, if any systemic symptoms (2, 9). However, systemic autoimmune arthritides following Lyme disease are less well described. In the one previous report, 9 of 51 patients presenting with reactive arthritis who lacked a clinical history of Lyme disease were found to have antibody and T-cell responses to B. burgdorferi antigens (21). Although B. burgdorferi antibody titers declined after antibiotic therapy, arthritis rarely resolved. The authors proposed that B. burgdorferi may be among the infectious agents that trigger reactive arthritis.
We report here a cohort of 30 patients who developed new-onset rheumatoid arthritis (RA), psoriatic arthritis or peripheral spondyloarthropathy (PsA/SpA), usually within several months after antibiotic treatment for B. burgdorferi infection. Thus, when inflammatory arthritis develops in the context of Lyme disease, clinicians need to distinguish among three possibilities: patients who have active infection in joints, those who have post-infectious LA, and those who have another form of inflammatory arthritis following Lyme disease.
PATIENTS AND METHODS
Patients
All medical records of adult patients (age greater than 18) seen over a 13-year period (2003-2015) in the Rheumatology Clinic at Massachusetts General Hospital (MGH) for suspected LA in whom we diagnosed a systemic autoimmune joint disease were reviewed. Thirty patients were identified. They were required to have a well-defined systemic autoimmune joint disease occurring within 2 years of definite Lyme disease, the time frame in which LA may develop following EM (4). The autoimmune diseases were classified according to validated criteria (22-24). Patients presenting with systemic autoimmune joint diseases who had a positive serologic test for Lyme disease without prior clinical symptoms of the illness were not included.
For comparison, all 43 adult patients enrolled in our Lyme arthritis cohort study over the most recent 2-year period (2014-2015) were analyzed. All 43 patients met the diagnostic criteria of the Centers for Disease Control and Prevention (CDC) for that disease (25). Patients with arthritis which resolved within 1 month after completion of oral or IV antibiotic therapy were defined as having antibiotic-responsive arthritis, whereas patients with arthritis which persisted longer than 1 month after completion of antibiotic therapy were classified as having post-infectious, antibiotic-refractory arthritis. The study was approved by the MGH Institutional Review Board and written consent was obtained from each subject.
Clinical evaluation
Demographic data were collected and clinical history, including smoking history, history of autoimmune disease in first-degree relatives, personal history of skin psoriasis, and Lyme disease history and antibiotic treatment were recorded. Swollen and tender joint counts were documented. Serum samples were collected from each patient at their initial visit. Rheumatoid factor (RF), anti-citrullinated protein antibodies (ACPA), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) were measured in the MGH Clinical Laboratories.
B. burgdorferi and Lyme-associated autoantibody testing
Serum antibodies to B. burgdorferi were measured in our research laboratory by 2-tier sonicate ELISA and Western blot, as previously described (8, 26). Testing for antibody responses to 3 Lyme disease-associated autoantigens, ECGF, apoB-100, and MMP-10, was also performed, as previously described (14-19). Autoantibody testing was not done for annexin-A2, which is less specific for Lyme disease. For comparison with the LA and post-Lyme systemic autoimmune arthritis groups, serum samples were randomly selected from 43 patients in our new-onset RA cohort (27) and 21 patients with PsA/SpA, none of whom had a history of Lyme disease, and from 72 healthy control subjects. A positive antibody response to each autoantigen was defined as >3 SD above the mean value in healthy subjects.
Statistical analysis
For comparison of multiple groups, one-way analysis of variance (ANOVA) was performed. If significant differences were found by ANOVA, pair-wise comparisons were then made. Non-categorical clinical characteristics and antibody levels were compared by Mann-Whitney rank sum test. Demographic categorical variables were analyzed with Fisher’s exact test. Mean antibody levels between groups were compared by unpaired T test with Welch’s correction. A P value of ≤0.05 was considered statistically significant; all P values are two-tailed.
RESULTS
Clinical characteristics of patients
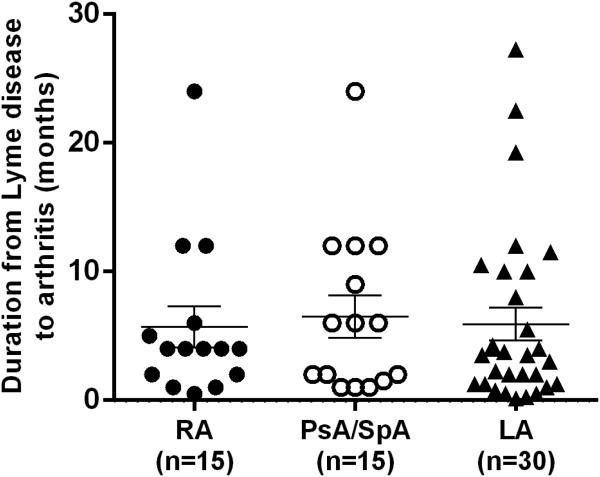
During the 13-year period in which we have been at MGH, 30 patients were referred to us for evaluation of presumed LA in whom we diagnosed a systemic autoimmune joint disorder. Of the 30 patients, 15 had new-onset RA, 13 had new-onset PsA, and 2 had new-onset peripheral SpA. The median duration from the onset of Lyme disease to the start of joint symptoms was 4 months (range, 2 weeks to 2 years) (Figure 1), which is similar to the time frame in which LA may occur after EM (4).
Figure 1.
Duration from Lyme disease to onset of rheumatoid arthritis (RA), psoriatic arthritis or peripheral spondyloarthropathy (PsA/SpA). For comparison, the duration from erythema migrans, the initial skin lesion of the infection, to onset of Lyme arthritis (LA) is shown for historical patients seen in the late 1970s who were not treated with antibiotic therapy (4). Thus, the duration from erythema migrans to the development of arthritis was known.
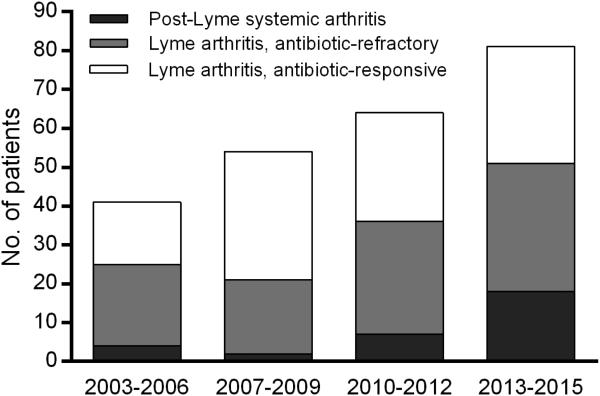
The number of LA patients that we have evaluated has steadily increased over the past 13 years (Figure 2). Moreover, during the most recent 3-year period, the proportion of patients in whom we diagnosed a systemic autoimmune joint disease following Lyme infection increased even more. Of the 30 patients reported here with RA or PsA/SpA following Lyme disease, 18 (60%) were seen during the past 3 years. Overall, during this most recent period, about one-third of the patients referred had LA responsive to antibiotic therapy, another third had antibiotic-refractory LA, and the remaining third had another autoimmune arthritic disease following Lyme disease.
Figure 2.
The number of patients referred for presumptive Lyme arthritis over a 13-year period, according to the year of referral. The patients were stratified into 3 groups according to our diagnoses of post-Lyme systemic autoimmune joint diseases, antibiotic-refractory Lyme arthritis, or antibiotic-responsive Lyme arthritis.
In Table 1, the clinical and demographic characteristics of the 30 patients with systemic autoimmune arthritides are compared with those of 43 patients with active-infectious or post-infectious, antibiotic-refractory LA. Compared with the LA group, the patients with systemic autoimmune joint disorders, particularly those with RA, were significantly older (median age, 55 versus 44, P=0.03), but similar to early arthritis cohorts (27-29). However, the systemic autoimmune disease group was predominantly male, which is not typical of systemic autoimmune joint diseases, particularly RA (27, 28). There was a trend toward greater tobacco exposure in the systemic autoimmune group, particularly in RA patients for whom smoking is a known risk factor (30), though the majority of patients were non-smokers. Patients with systemic autoimmune arthritides had significantly higher body mass index (BMI) (P<0.0006), particularly for those with PsA/SpA (P<0.0001), consistent with the high frequency of obesity as a comorbid factor in psoriatic arthritis (31). Finally, the systemic autoimmune disease group was significantly more likely to have a family history of autoimmune disease in a first-degree relative than patients with LA (P=0.0004), particularly those with PsA or SpA.
Table 1.
Demographic and Clinical Characteristics Prior to Referral
| Characteristic | Post-Lyme RA (N=15) |
Post-Lyme PsA/SpA (N=15) |
Total Post- Lyme RA, PsA/SpA (N=30) |
Lyme arthritis (N=43) |
|---|---|---|---|---|
| Demographics | ||||
| Age, median (range) | 55 (24-70) P=0.03* |
48 (18-73) | 55 (18-73) P=0.03 |
44 (18-77 |
| Sex, n. male/female | 9/6 | 9/6 | 18/12 | 29/14 |
| Smoking, no. (%) | ||||
| Former | 2 (13) | 3 (20) | 5 (17) | 5 (12) |
| Never | 9 (60) | 11 (73) | 20 (67) | 34 (79) |
| Current | 4 (27) | 1 (7) | 5 (17) | 4 (9) |
| Body Mass Index (BMI), median (range) |
26 (19-38) | 30 (23-45) P<0.0001 |
29 (19-45) P<0.0006 |
24 (18-28) |
|
Family history of
Autoimmunity |
7(47) P=0.03 |
10(67) P=0.0005 |
17(57) P=0.0004 |
7(16) |
| Prior Lyme disease manifestation, no. (%) | ||||
| Early | ||||
| Erythema migrans | 8 (53) P<0.0001 |
9 (60) P<0.0001 |
17 (57) P<0.0001 |
2(5) |
| Early disseminated | 3 (20) | 2 (13) | 5 (17) | 7 (16) |
| Neuroborreliosis | 1 (7) | 1 (7) | 2 (7) | 0 (0) |
| Late | ||||
| Lyme arthritis | 3 (20) | 3 (20) | 6 (20) | 43 (100) |
| Antibiotic treatment** | ||||
| median weeks, (range) | 4 (1.5-16) | 7 (3-16) | 5.5 (1.5-16) | 4 (0-12) |
| Oral, no. (%) | 13 (87) | 12 (80) | 25 (83) | 35 (81) |
| Oral and IV | 2 (13) | 2 (13) | 4 (13) | 8 (18) |
| IV | 0 | 1 (7) | 1 (3) | 0 |
all P values shown are for comparisons with Lyme arthritis group,
at time of initial evaluation
Prior to the onset of joint symptoms, 17 of the 30 patients (57%) with systemic autoimmune joint diseases had EM skin lesions (Table 1). Another 7 patients (23%) had signs or symptoms consistent with early disseminated Lyme disease without EM skin lesions: 5 had flu-like symptoms (such as fever, arthralgias, neck pain) during summer, and 2 others had manifestations of neuroborreliosis (one each with meningitis or facial palsy). The remaining 6 patients had LA followed by onset of systemic autoimmune joint disorders. These 6 patients, 3 with PsA, and 3 with RA, had LA of a hip (n=2) or knee (n=4), associated with expanded IgG responses to 8 or more spirochetal proteins on Western blot. Although the monoarthritis resolved after treatment with antibiotics, or in one case, a synovectomy, involvement in other joints, and skin psoriasis, developed subsequently.
In comparison, among the 43 patients in the LA group, only 2 (5%) had typical EM lesions preceding their arthritis (P<0.0001), and these 2 did not receive antibiotic treatment at the time. An additional 7 patients (16%) recalled flu-like symptoms during summer prior to the onset of arthritis, but did not receive antibiotics at that time. Altogether, only 9 of the 43 patients with LA had signs of early Lyme disease prior to joint involvement compared with 24 of the 30 systemic arthritis patients (P<0.0001).
All 30 patients who subsequently developed systemic autoimmune joint diseases had been treated before referral with appropriate antibiotic regimens for Lyme disease, as recommended by the Infectious Diseases Society of America (IDSA) (32). Generally, this consisted of a 21-day course of oral doxycycline for early Lyme disease, but 13 of the 24 patients (54%) with early Lyme disease received additional courses of oral antibiotic therapy, with a maximum of 4 months of treatment. Five patients had previously received IV antibiotic treatment for ≥1 month for later or more severe manifestations of Lyme disease, including 3 patients with LA and one patient with Lyme meningitis. Among the LA cohort, 35 of the 43 patients (81%) had started or completed a 30-day course of oral doxycycline prior to their referral here, and 8 (19%) had also been treated with a 28-day course of IV ceftriaxone.
Features of Arthritis
Patients with systemic autoimmune joint diseases had a significantly longer duration of arthritis at the time of our evaluation compared with LA patients (median 6.5 months versus 4 months) (Table 2). Moreover, the median duration from onset of arthritis to diagnosis of RA was 12 months, much longer than in most early RA cohorts (26,27). The pattern of joint involvement in the post-Lyme systemic autoimmune group was predominantly one of polyarthritis, which is typical of RA and PsA. Of the 15 patients with RA, 13 (87%) had symmetrical polyarthritis with 5 or more joints involved (median 11 joints, range 5-27), the other 2 patients had 3-to-4 joints affected. Of the 13 patients with PsA, 7 had polyarticular disease (median 9 joints, range 5-35), while 6 had involvement of 2-to-4 joints. In addition, 7 PsA (47%) had unique features of spondyloarthropathy, such as axial involvement, enthesitis, or dactylitis. The 2 patients with non-psoriatic peripheral SpA both had enthesitis and inflammatory oligoarticular arthritis. In comparison, none in the RA group (P=0.006) or LA group (P≤0.0001) had SpA features. Four patients, 2 with RA and 2 with PsA, had radiographic erosions.
Table 2.
Arthritis Characteristics
| Post-Lyme RA (N=15) |
Post-Lyme PsA/SpA (N=15) |
Total Post- Lyme RA PsA/SpA (N=30) |
Lyme arthritis (N=43) |
|
|---|---|---|---|---|
| Duration of arthritis symptoms, median mos. (range) |
12 (1-48) P=0.009* |
5 (1-36) | 6.5(1-48) P=0.02 |
4 (1-24) |
| Distribution, no. (%) Polyarticular (5+ joints) |
13 (87) P<0.0001 |
9 (60) P<0.0001 |
22 (73) P<0.0001 |
1(2) |
| SpA features (axial disease, enthesitis, dactylitis) |
0 | 7 (47) P<0.0001 |
7 (23) P=0.001 |
0 |
| Skin Psoriasis, no. (%) | ||||
| Prior to LD | 0 | 9 (60) P<0.0001 |
9(30) P=0.006 |
2 (5) |
| After LD | 0 | 4 (27) P=0.01 |
4(13) | 1 (2) |
| ESR mm/h, median (range) |
25 (2-86) | 19 (4-34) | 21 (2-86) | 11 (2-106) |
| CRP, mg/L, median (range) |
14.3 (0.2-118) P=0.07 |
9.3 (2.6-25.8) P=0.05 |
10.5 (0.2-118) P=0.02 |
1.9 (0.1-71.4), |
| RF and/or anti-CCP, no. pos (%) |
6(40) P<0.0001 |
0 | 6(20) P=0.004 |
0 |
All P values shown are for comparison with Lyme arthritis
The LA group almost always had monoarticular or oligoarticular arthritis, with a median of 1 joint affected, most commonly a knee. Only 1 patient had polyarticular involvement with 5 joints involved (bilateral knees, ankles, and one wrist). The majority of patients with antibiotic-refractory arthritis had persistent synovitis in previously involved joints, usually in one knee, but 3 of the 24 patients developed spreading to 1-to-3 other joints during the post-antibiotic period.
Compared with patients in the LA group, the 30 patients with the systemic autoimmune joint diseases usually had higher ESR levels and CRP values (P=0.02), particularly in the PsA/SpA group. Of the 15 RA patients, 6 (40%) had positive results for RF or ACPA, though this is a lower percentage than is usually reported in early RA cohorts (27, 28). As expected, no patient in the PsA/SpA group or LA group had positive results for these antibodies. Of the 13 patients with PsA, 9 (60%) had pre-existing cutaneous psoriasis, but not arthritis, prior to B. burgdorferi infection, whereas 4 (27%) developed both skin psoriasis and arthritis after Lyme disease. Finally, the 2 patients with non-psoriatic peripheral SpA both lacked positive tests for HLA-B27. In the LA group, one of the 43 patients also developed new-onset skin psoriasis following treatment for LA, but to date, she has not developed features of PsA.
B. burgdorferi antibody responses
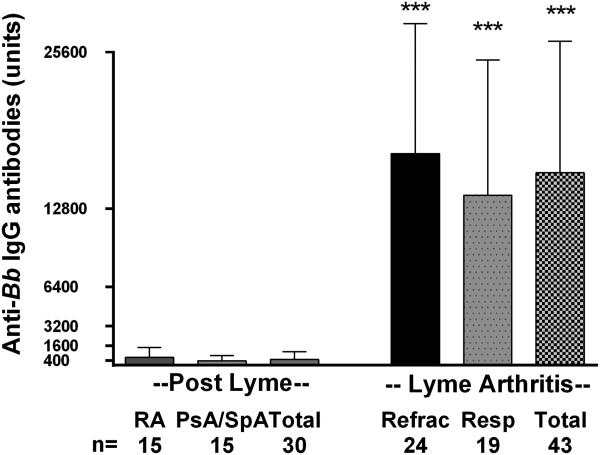
When seen in our clinic, 21 of the 30 patients (70%) with post-Lyme systemic autoimmune joint diseases had equivocal or low-positive IgG antibody responses to B. burgdorferi (a titer of 1:400 of greater) by ELISA, but only 6 patients, 4 of whom had prior LA, had a positive Western blot. In contrast, 42 of the 43 patients with LA, including those in both the antibiotic-responsive and anti-refractory groups, had strongly positive IgG antibody responses by ELISA and Western blot, usually with reactivity with all 10 spirochetal proteins scored using the CDC criteria (Figure 3). The one patient with LA who did not meet CDC criteria had already received several courses of antibiotics, and had met CDC criteria prior to our evaluation. Thus, the magnitude of the antibody responses was significantly lower in patients with post-Lyme systemic autoimmune joint diseases than in those in the Lyme arthritis group (mean titer of 1:486 versus 1:15,733, P<0.0001) (Figure 3). The mean levels of IgM antibodies to B. burgdorferi were the same in both the systemic autoimmune and Lyme arthritis groups, and only 2 patients in each group had a positive IgM Western blot (data not shown). Five post-Lyme systemic autoimmune disease patients, 3 with RA and 2 with PsA, had knee effusions amenable to aspiration, and all had negative PCR results for B. burgdorferi DNA in synovial fluid. In contrast, in the LA group, 12 of 24 patients tested had positive PCR results.
Figure 3.
Anti-Borrelia burgdorferi IgG antibody levels in post-Lyme systemic arthritis, rheumatoid arthritis, psoriatic arthritis or peripheral spondyloarthropathy, or antibiotic-refractory or antibiotic-responsive Lyme arthritis. Mean values and 1 standard deviation are shown. P=<0.0001 for the comparison of IgG antibody levels in the total Lyme arthritis group with each of the other 3 groups. The cut-off value for an equivocal Lyme IgG ELISA test in our laboratory is 400 units, and for a positive test is 800 units.
Lyme disease-associated autoantibody responses
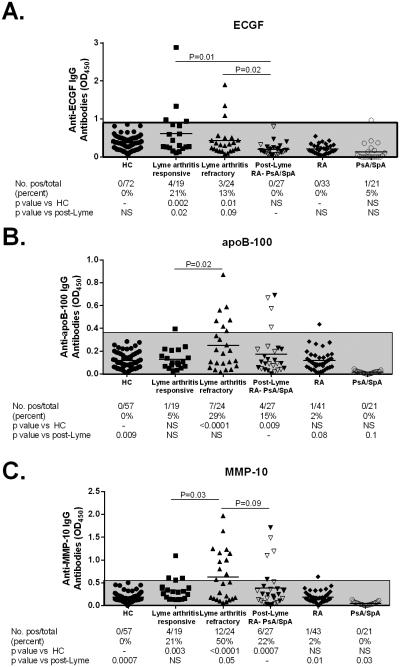
Of the 30 patients with systemic autoimmune joint disorders, 27 had sufficient sera available to test for 3 Lyme disease-associated autoantibodies (ECGF, apoB-100, and MMP-10) (Figure 4). Of the 27 patients, 6 (22%) had positive results for one or more of the Lyme-associated autoantibodies, and 4 (15%) were positive for 2 autoantibodies (apoB-100 and MMP-10). Of the 6 patients, 2 had RA, 3 had PsA, and 1 had SpA. In comparison, 19 of the 43 patients (44%) with LA had one or more of the Lyme-associated autoantibodies (P=0.08). Moreover, the levels of ECGF antibodies, the most specific of the Lyme-associated autoantibodies (14), were significantly increased in the both responsive and refractory LA groups compared with the systemic autoimmune group (P≤0.02). The levels of MMP-10 and apo-B 100 autoantibodies were significantly greater in the refractory versus the responsive LA group (P≤0.03) and MMP-10 levels tended to be greater than in the systemic autoimmune group (P≤0.1). In contrast, autoantibody positivity in the LA group and in the post-Lyme systemic autoimmune group were significantly greater than in healthy controls or in RA or PsA/SpA patients who lacked a history of Lyme disease, only 2 of whom had borderline elevated responses. Of the 3 LA patients with spreading to previously uninvolved joints in the post-infectious period, 2 had positive tests for MMP-10 autoantibodies, and one had reactivity with all 3 autoantibodies.
Figure 4.
Levels of 3 Lyme-disease associated autoantibodies, endothelial cell growth factor (ECGF), apolipoprotein B100 (apoB100) and matrixmetalloproteinase-10 (MMP10), are shown for healthy controls (HC), patients with rheumatoid arthritis (RA) and psoriatic arthritis or spondyloarthropathy (PsA/SpA) who lacked a prior history of Lyme disease, patients with post-Lyme systemic autoimmune joint disorders (RA, PsA/SpA) and patients with Lyme arthritis, grouped by response to antibiotic treatment (responsive and refractory). Bars denote mean values. In the post-Lyme systemic arthritis group, white triangles represent patients with PsA/SpA, while black triangles represent RA patients. The shaded area represents values within 3 standard deviations of the mean value of the healthy control subjects. Statistical comparisons of antibody frequency between groups were performed using Fisher’s exact test.
Treatment and Outcomes
In the systemic autoimmune disease group, we advised treatment with anti-inflammatory agents according to the standard of care. These treatments included steroids (3%), NSAIDS (20%), disease modifying anti-rheumatic drugs (DMARDS) (57%), most commonly methotrexate, but also TNF-inhibitors, or combinations of these agents. All patients treated in this way had control of their arthritis, and none had reactivation of Lyme infection. However, 6 patients (20%) were reluctant to accept the diagnosis of a systemic autoimmune disease, and sought further treatment for Lyme disease elsewhere with additional courses of antibiotic therapy.
Of the 43 LA patients, 11 (26%) had resolution of their arthritis within 1 month of completion of oral antibiotic treatment, and 8 patients (19%) who failed to respond to oral doxycycline had resolution of arthritis within one month of completing IV antibiotic treatment. However, 24 patients (56%) had persistent proliferative synovitis despite treatment with oral and IV antibiotics. Of the 24 patients, 23 (96%) were treated with DMARDs, including hydroxychloroquine, methotrexate or TNF inhibitors. Similar to the systemic autoimmune group, the 24 patients had marked improvement within months, and to date, none had recurrence of Lyme infection and none required a synovectomy.
DISCUSSION
In this study, we report 30 patients with new-onset systemic autoimmune joint disease including RA, PsA, or peripheral SpA, developing a median of 4 months after Lyme disease, particularly after early-stage infection. We have seen increasing numbers of such cases, particularly in the last 3 years, which now constitute about one-third of the patients referred to us for presumed LA. Since the incidence of Lyme disease is increasing dramatically in the United States with an estimated 300,000 new cases annually (33), more rare manifestations of the disease, including post-infectious complications, may be increasing. It is important for clinicians to be aware that a spectrum of infectious and autoimmune joint disorders may occur within the context of Lyme disease: patients may have active B. burgdorferi infection in joints, they may have antibiotic-refractory Lyme synovitis, or they may have another form of autoimmune arthritis following Lyme disease.
The occurrence of RA, PsA, or SpA following soon after Lyme infection may simply be a coincidence, as these autoimmune diseases are relatively common and Lyme disease is epidemic in our geographic area. However, in Olmsted County, Minnesota, from 1955 to 2007, the annual incidence of new RA cases was 0.04% (34). In 2015, we saw 77 new adult patients with any manifestation of Lyme disease of whom 6 had post-Lyme RA or PsA/SpA (8%). However, our study was not population-based, rather we are a referral center for LA, which makes it difficult to compare these percentages.
The other possibility is that the adjuvant effect of infection may convert the preclinical phase of autoimmune disease to active arthritis. Preclinical phases in which autoantibodies, or in the case of PsA, skin psoriasis, may be present years prior to arthritis (35-38). Numerous infections have been hypothesized to serve as triggers for initiating clinical expression of RA or PsA (39-45). In addition to antigen-specific activation of lymphocytes, microbes can provide the secondary signal necessary for the induction of a pathogenic autoimmune response, referred to as the adjuvant effect of the infection (46). In our patients, the delay of months between the infection and systemic autoimmune disease may reflect the time needed for affinity maturation or epitope spreading of autoimmune responses. Although spirochetal infection itself may be a trigger, perturbations in the microbiome resulting from antibiotic treatment of B. burgdorferi infection could also be a mechanistic factor (47). In addition, it is intriguing that psoriasis may follow EM, the initial lesion of Lyme disease, which may have a direct effect on the skin and its microbiome in addition to effects of antibiotic treatment.
Although other infections may trigger autoimmunity, Lyme disease is uniquely characterized by joint manifestations in its late infectious and post-infectious stages (2, 6). This, along with the presence of B. burgdorferi antibodies which may persist for years after resolution of infection (48), may create diagnostic confusion with delays in diagnosis and the initiation of appropriate anti-inflammatory therapies. However, as described here, infectious or post-infectious LA can usually be distinguished from systemic autoimmune joint diseases by numerous clinical and laboratory features. First, LA is typically characterized by monoarticular or oligoarticular involvement of large joints, and antibiotic-refractory arthritis generally affects one large joint that failed to resolve with antibiotic treatment, most commonly a knee. In contrast, systemic autoimmune joint diseases usually cause polyarthritis or they may also have features of spondyloarthropathy. Second, systemic arthritis patients had significant enrichment of typical risk factors, such as family history of autoimmunity, or obesity. Third, systemic arthritis patients were much more likely to have had EM, which had been treated appropriately with antibiotics. Such therapy would abort the development of subsequent LA. In contrast, in most patients with LA, joint involvement was the presenting manifestation of the disease, and none received antibiotic therapy prior to the development of arthritis. Thus, it is very unlikely that a patient who develops polyarthritis after antibiotic-treated EM has LA.
We also use laboratory tests that help with these distinctions that are not yet available commercially, including antibody titers to B. burgdorferi and Lyme disease-associated autoantibodies. Most importantly, while patients with systemic autoimmune diseases following Lyme infection may have positive antibody tests for B. burgdorferi by ELISA, they usually had only low levels of IgG antibodies to the spirochete, whereas LA patients generally have the highest antibody titers seen in the infection (8). Additionally, of the 4 known Lyme disease-associated autoantibodies, the responses to 3 of them (ECGF, apoB-100, orMMP-10) have reasonable specificity for LA (14, 17, 19). However, since Lyme-associated autoantibodies may develop early in the illness, these tests may still be positive, albeit at lower titer, when patients develop other types of arthritis following Lyme disease, limiting their diagnostic utility in these cases. Yet, the list of known autoantibodies in Lyme disease is presumably incomplete, as only half of patients with antibiotic-refractory Lyme arthritis had one or more of these autoantibody responses. Further, while these autoantibodies have histopathologic correlations in LA, it is not clear whether they have any pathologic role in post-Lyme RA or PsA/SpA. Thus, as knowledge of Lyme-specific autoimmune responses increases, these tests may become more beneficial in differential diagnosis.
Although there are many distinguishing features between post-infectious LA and systemic autoimmune arthritis, there are patients in whom the distinction may be difficult. First, any of the systemic autoimmune diseases may begin with oligoarticular involvement. Six of our patients had monoarthritis of a hip or knee, which might have been the beginning of a systemic autoimmune disease, followed by the development of polyarthritis. However, the expanded antibody response to B. burgdorferi and apparent response of the monoarthritis to antibiotics suggested that this initial event was LA. Moreover, several additional observations raise the question of whether the far end of the spectrum of antibiotic-refractory LA may include the development of symmetrical polyarthritis. First, in a few patients in the LA comparison group, spreading to other joints occurred in the post-antibiotic period. Second, the majority of patients diagnosed here with post-Lyme RA lacked RF or ACPA, and 2 such patients had Lyme disease-associated autoantibodies. Finally, although long-term follow-up is not available for all patients, we are aware of 4 subjects, 2 with RA and 2 with PsA/SpA, who attained drug-free remission, which is atypical in these conditions, but characteristic of LA.
Regardless of whether the occurrence of systemic autoimmune joint disease following Lyme infection is coincidental, induced non-specifically by adjuvant effects of infection, or related to specific Lyme disease-associated autoimmune responses, an important point for the clinician is that post-infectious joint disorders following recommended antibiotic courses for Lyme disease should be treated with DMARDs, rather than additional antibiotics. Some of our patients were reluctant to accept the non-Lyme disease diagnosis and pursued further antibiotic treatment elsewhere. One patient who developed RF- and ACPA-positive RA initially had complete remission of RA on methotrexate, but stopped this medication and sought further treatment for Lyme disease elsewhere with prolonged courses of multiple antibiotics. Three years later, he returned in a wheel chair with radiographic erosions, and deformities and contractures in multiple joints. Given a choice between LA and a chronic illness that may require lifelong immunosuppressive therapy, it is not surprising that patients would find LA a more attractive diagnosis. However, increasing evidence supports that earlier aggressive treatment of inflammatory arthritis is associated with improved radiographic outcomes and chances of sustained remission (49-50). Delays in appropriate DMARD treatment of autoimmune joint disorders by pursuing further antibiotic therapy may lead to poorer clinical outcomes.
ACKNOWLEDGMENTS
The authors thank Gail McHugh for help in the laboratory, Fiona Chen for administrative support, and the patients for their participation in the study.
Dr. Steere receives support from NIH R01 (AI-110175), English, Bonter, Mitchell Foundation, Ounsworth-Fitzgerald Foundation, Littauer Foundation, Lillian B. Davey Foundation, and the Eshe Fund. Dr. Arvikar is supported by a post-doctoral fellowship from the Rheumatology Research Foundation.
Footnotes
COMPETING INTERESTS
The authors declare they have no competing interests
AUTHORS’ CONTRIBUTIONS
SLA and ACS participated in the medical care of the study patients. SLA performed clinical data collection, data interpretation and analysis, and was responsible for the writing of this manuscript. JTC and KBS performed serologic testing and assisted with data interpretation and analysis. AS as senior author, designed and coordinated the study, and contributed to interpretation of the data and the intellectual input in drafting the manuscript. All authors have read and approved this manuscript.
REFERENCES
- 1.Steere AC, Malawista SE, Snydman DR, Shope RE, Andiman WA, Ross MR, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum. 1977;20:7–17. doi: 10.1002/art.1780200102. [DOI] [PubMed] [Google Scholar]
- 2.Bockenstedt LK, Wormser GP. Review: unraveling Lyme disease. Arthritis Rheumatol. 2014;66:2313–23. doi: 10.1002/art.38756. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steere AC, Grodzicki RL, Kornblatt AN, Craft JE, Barbour AG, Burgdorfer W, et al. The spirochetal etiology of Lyme disease. N Engl J Med. 1983;308:733–40. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- 4.Steere AC, Schoen RT, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med. 1987;107:725–31. doi: 10.7326/0003-4819-107-5-725. [DOI] [PubMed] [Google Scholar]
- 5.Steere AC, Malawista SE, Hardin JA, Ruddy S, Askenase W, Andiman WA. Erythema chronicum migrans and Lyme arthritis. The enlarging clinical spectrum. Ann Intern Med. 1977;86:685–98. doi: 10.7326/0003-4819-86-6-685. [DOI] [PubMed] [Google Scholar]
- 6.Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am. 2015;29:269–80. doi: 10.1016/j.idc.2015.02.004. [review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Recommendations for test performance and interpretation from the Second International Conference on serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep. 1995;44(31):590–1. [PubMed] [Google Scholar]
- 8.Steere AC, McHugh G, Damle N, Sikand VK. Prospective study of serologic tests for Lyme disease. Clin Infect Dis. 2008;47(2):188–95. doi: 10.1086/589242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steere AC, Angelis SM. Therapy for Lyme arthritis: strategies for the treatment of antibiotic-refractory arthritis. Arthritis Rheum. 2006;54:3079–86. doi: 10.1002/art.22131. [DOI] [PubMed] [Google Scholar]
- 10.Steere AC, Klitz W, Drouin EE, Falk BA, Kwok WW, Nepom GT, et al. Antibiotic-refractory Lyme arthritis is associated with HLA-DR molecules that bind a Borrelia burgdorferi peptide. J Exp Med. 2006;203:961–71. doi: 10.1084/jem.20052471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strle K, Shin JJ, Glickstein LJ, Steere AC. Association of a toll-like receptor 1 polymorphism with heightened Th1 inflammatory responses and antibiotic-refractory Lyme arthritis. Arthritis Rheum. 2012;64:1497–507. doi: 10.1002/art.34383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen S, Shin JJ, Strle K, et al. Treg cell numbers and function in patients with antibiotic-refractory or antibiotic-responsive Lyme arthritis. Arthritis Rheum. 2010;62:2127–37. doi: 10.1002/art.27468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vudattu NK, Strle K, Steere AC, Drouin EE. Dysregulation of CD4+CD25(high) T cells in the synovial fluid of patients with antibiotic-refractory Lyme arthritis. Arthritis Rheum. 2013;65:1643–53. doi: 10.1002/art.37910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drouin EE, Seward RJ, Strle K, McHugh G, Li X, Glickstein LJ, et al. A novel human autoantigen, endothelial cell growth factor, is a target of T and B cell responses in patients with Lyme disease. Arthritis Rheum. 2013;65:186–96. doi: 10.1002/art.37732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seward RJ, Drouin EE, Steere AC, Costello CE. Peptides presented by HLA-DR molecules in synovia of patients with rheumatoid arthritis or antibiotic-refractory Lyme arthritis. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M110.002477. M110.002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Londoño D, Cadavid D, Drouin EE, Strle K, McHugh G, Aversa JM, et al. Antibodies to endothelial cell growth factor and obliterative microvascular lesions in the synovium of patients with antibiotic-refractory lyme arthritis. Arthritis Rheumatol. 2014;66:2124–33. doi: 10.1002/art.38618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crowley JT, Drouin EE, Pianta A, Strle K, Wang Q, Costello CE, et al. A highly expressed human protein, Apolipoprotein B-100, serves as an autoantigen in a subgroup of patients with Lyme disease. J Infect Dis. 2015;212:1841–50. doi: 10.1093/infdis/jiv310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pianta A, Drouin EE, Crowley JT, Arvikar S, Strle K, Costello CE, et al. Annexin A2 is a target of autoimmune T and B cell responses associated with synovial fibroblast proliferation in patients with antibiotic-refractory Lyme arthritis. Clin Immunol. 2015;160:336–41. doi: 10.1016/j.clim.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowley JT, Strle K, Drouin EE, Pianta A, Arvikar SL, Wang Q, et al. Matrix metalloproteinase-10 is a target of T and B cell responses that correlate with synovial pathology in patients with antibiotic-refractory Lyme arthritis. J Autoimmun. 2016;69:24–37. doi: 10.1016/j.jaut.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cañas F, Simonin L, Couturaud F, Renaudineau Y. Annexin A2 autoantibodies in thrombosis and autoimmune disease. Thrombosis Res. 2015;135:226–30. doi: 10.1016/j.thromres.2014.11.034. [review] [DOI] [PubMed] [Google Scholar]
- 21.Weyand CM, Goronzy JJ. Immune responses to Borrelia burgdorferi in patients with reactive arthritis. Arthritis Rheum. 1989;32:1057–64. doi: 10.1002/anr.1780320902. [DOI] [PubMed] [Google Scholar]
- 22.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, III, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 23.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. CASPAR Study Group. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54:2665–73. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 24.Rudwaleit M, van der Heijde D, Landewé R, Akkoc N, Brandt J, Chou CT, et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011;70:25–31. doi: 10.1136/ard.2010.133645. [DOI] [PubMed] [Google Scholar]
- 25.Wharton M, Chorba TL, Vogt RL, Morse DL, Buehler JW. Case definitions for public health surveillance. MMWR Recomm Rep. 1990;39:1–43. [PubMed] [Google Scholar]
- 26.Dressler F, Whalen JA, Reinhardt BN, Steere AC. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 27.Arvikar SL, Collier DS, Fisher MC, Unizony S, Cohen GL, McHugh G, Kawai T, Strle K, Steere AC. Clinical correlations with Porphyromonas gingivalis antibody responses in patients with early rheumatoid arthritis. Arthritis Res Ther. 2013;5:R109. doi: 10.1186/ar4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuriya B, Xiong J, Boire G, Haraoui B, Hitchon C, Pope J, et al. CATCH Investigators. Earlier time to remission predicts sustained clinical remission in early rheumatoid arthritis--results from the Canadian Early Arthritis Cohort (CATCH). J Rheumatol. 2014;41:2161–6. doi: 10.3899/jrheum.140137. [DOI] [PubMed] [Google Scholar]
- 29.Khraishi M, MacDonald D, Rampakakis E, Vaillancourt J, Sampalis JS. Prevalence of patient-reported comorbidities in early and established psoriatic arthritis cohorts. Clin Rheumatol. 2011;30:877–85. doi: 10.1007/s10067-011-1692-7. [DOI] [PubMed] [Google Scholar]
- 30.Klareskog L, Stolt P, Lundberg K, Källberg H, Bengtsson C, Grunewald J. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-Restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 31.Labitigan M, Bahče-Altuntas A, Kremer JM, Reed G, Greenberg JD, Jordan N, et al. Higher rates and clustering of abnormal lipids, obesity, and diabetes mellitus in psoriatic arthritis compared with rheumatoid arthritis. Arthritis Care Res. 2014;66:600–7. doi: 10.1002/acr.22185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 33.Nelson CA, Saha S, Kugeler KJ, Delorey MJ, Shankar MB, Hinckley AF. Incidence of clinician-diagnosed Lyme disease, United States, 2005-2010. Emerg Infect Dis. 2015;21:1625–31. doi: 10.3201/eid2109.150417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955-2007. Arthritis Rheum. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.del Puente A, Knowler WC, Pettitt DJ, Bennett PH. The incidence of rheumatoid arthritis is predicted by rheumatoid factor titer in a longitudinal population study. Arthritis Rheum. 1988;31:1239–1244. doi: 10.1002/art.1780311004. [DOI] [PubMed] [Google Scholar]
- 36.Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–386. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 37.Mankia K, Emery P. Preclinical rheumatoid arthritis: progress toward prevention. Arthritis Rheum. 2016;68:779–788. doi: 10.1002/art.39603. [review] [DOI] [PubMed] [Google Scholar]
- 38.Gladman DD, Shuckett R, Russell ML, Thorne JC, Schacheter RK. Psoriatic arthritis (PSA)—an analysis of 220 patients. Q J Med. 1987;62:127–41. 2010;62:1576-1582. doi: 10.1002/art.27425. [PubMed] [Google Scholar]
- 39.Ollier WE, Harrison B, Symmons D. What is the natural history of rheumatoid arthritis? Best Pract Res Clin Rheumatol. 2001;15:27–48. doi: 10.1053/berh.2000.0124. [DOI] [PubMed] [Google Scholar]
- 40.Rosenstein ED, Greenwald RA, Kushner LJ, Weissmann G. Hypothesis: the humoral immune response to oral bacteria provides a stimulus for the development of rheumatoid arthritis. Inflammation. 2004;28:311–8. doi: 10.1007/s10753-004-6641-z. [Review] [DOI] [PubMed] [Google Scholar]
- 41.Johnson TM, Duvic M, Rapini RP, Rios A. AIDS exacerbates psoriasis. N Engl J Med. 1985;313:1415. [PubMed] [Google Scholar]
- 42.Telfer NR, Chalmers RJ, Whale K, Colman G. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128:39–42. [PubMed] [Google Scholar]
- 43.Seetharam KA, Sridevi K. Chikungunya infection: a new trigger for psoriasis. J Dermatol. 2011;38:1033–1034. doi: 10.1111/j.1346-8138.2011.01200.x. [DOI] [PubMed] [Google Scholar]
- 44.Pattison E, Harrison BJ, Griffiths CE, Silman AJ, Bruce IN. Environmental risk factors for the development of psoriatic arthritis: results from a case-control study. Ann Rheum Dis. 2008;67:672–6. doi: 10.1136/ard.2007.073932. [DOI] [PubMed] [Google Scholar]
- 45.Horton DB, Scott FI, Haynes K, Putt ME, Rose CD, Lewis JD, et al. Antibiotic Exposure, Infection, and the Development of Pediatric Psoriasis: A Nested Case-Control Study. JAMA Dermatol. 2015;11:1–9. doi: 10.1001/jamadermatol.2015.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rose NR. The adjuvant effect in infection and autoimmunity. Clin Rev Allergy Immunol. 2008;34:279–82. doi: 10.1007/s12016-007-8049-7. [DOI] [PubMed] [Google Scholar]
- 47.Scher JU, Littman DR, Abramson SB. Review: Microbiome in Inflammatory Arthritis and Human Rheumatic Diseases. Arthritis Rheumatol. 2016;68:35–45. doi: 10.1002/art.39259. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalish RA, McHugh G, Granquist J, Shea B, Ruthazer R, Steere AC. Persistence of immunoglobulin M or immunoglobulin G antibody responses to Borrelia burgdorferi 10-20 years after active Lyme disease. Clin Infect Dis. 2001;33:780–5. doi: 10.1086/322669. [DOI] [PubMed] [Google Scholar]
- 49.Kuriya B, Xiong J, Boire G, Haraoui B, Hitchon C, Pope J. Earlier time to remission predicts sustained clinical remission in early rheumatoid arthritis--results from the Canadian Early Arthritis Cohort (CATCH) J Rheumatol. 2014;41:2161–6. doi: 10.3899/jrheum.140137. [DOI] [PubMed] [Google Scholar]
- 50.van Nies JA, Tsonaka R, Gaujoux-Viala C, Fautrel B, van der Helm-van Mil AH. Evaluating relationships between symptom duration and persistence of rheumatoid arthritis: does a window of opportunity exist? Results on the Leiden early arthritis clinic and ESPOIR cohorts. Ann Rheum Dis. 2015;74:806–12. doi: 10.1136/annrheumdis-2014-206047. [DOI] [PubMed] [Google Scholar]