Abstract
Caged neurotransmitters, in combination with focused light beams, enable precise interrogation of neuronal function, even at the level of single synapses. However, most caged transmitters are, surprisingly, severe antagonists of ionotropic gamma-aminobutyric acid (GABA) receptors. By conjugation of a large, neutral dendrimer to a caged GABA probe we introduce a “cloaking” technology that effectively reduces such antagonism to very low levels. Such cloaked caged compounds will enable the study of the signaling of the inhibitory neurotransmitter GABA in its natural state using two-photon uncaging microscopy for the first time.
Keywords: caged compounds, GABA-A receptors, optical methods, two-photon, biologically inert
Graphical abstract
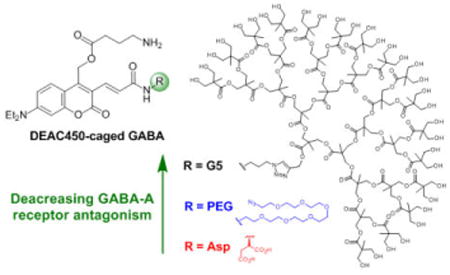
Caged neurotransmitters are known to be quite antagonistic toward the GABA-A receptor. The conjugation of a polyester dendrimer “cloak” to the caged compound DEAC450-GABA significantly decreased its GABA-A receptor antagonism. The chemical probe was inert at concentrations required for effective two-photon photolysis on living cells.
Photochemical probes have revolutionized modern physiological studies. In an era initiated by Galvani, electrodes were the best technology available for real-time stimulation and measurement. However, the photon is more precise and versatile than the electron, so the former is not merely starting to “replace” the latter, but light, in combination with chemistry, is being used to open completely new avenues of physiological discovery[1]. To deliver their potential, the new chemical technologies require novel light sources and so pulsed lasers have been vital for time-resolved chemical biology[2]. Enabled by ultra-fast, pseudo-continuous Ti:sapphire lasers, neurobiology now routinely uses two-photon (2P) microscopy for live cell imaging in complex tissue such as acutely isolated brain slices and living animals. But, as noted by Denk, et al. in their seminal 1990 paper[3], 2P excitation “also provides unprecedented capabilities for three-dimensional, spatially resolved photochemistry, particularly photolytic release of caged effector molecules.” While initial reports of two-photon uncaging used probes[4,5] originally designed for linear actuation, more recently caged compounds designed for 2P excitation have been introduced for Glu, GABA, IP3, calcium, etc[6]. Some of these have even proved useful for independent 2P physiological studies[2]. It is striking that caged Glu probes, in particular, have proved very useful but caged GABA probes much less so. One reason for this must the inherent antagonism of all caged GABA compounds towards GABA-A receptors at concentrations required for effective 2P photolysis in brain tissue[7]. We have developed a new technology, which we call “cloaked caged compounds”, that renders such optical chemical probes biological inert. This technology uses click chemistry to conjugate neutral dendrimers to caged neurotransmitters so as to reduce significantly their antagonism towards GABA-A receptors.
Receptor-specific binding is a key feature of any drug. Very often man-made drugs also activate or interfere with receptors other than their intended target, leading to the so-called “off-target” effects of drugs. Caged compounds are similar to drugs in that they are small, man-made organic molecules, but are dissimilar in that they are designed not to bind to any bioreceptors. In other words, before photolysis they should be biologically inert. Typically, when a photochemical protecting group is covalently attached to a biomolecule to produce the optical probe, the resulting molecule is indeed “caged” in that is totally inactive. However, there was a hint that this was not always the case when it was discovered that the original caged ATP proved to be mildly antagonistic towards skeletal muscle nucleotide binding domains[8].
In the case of caged neurotransmitters, the two probes made in 1994, CNB-Glu and CNB-GABA, have been widely used[7]. At the time of invention[9, 10] receptor antagonism was not studied, but a report in 2000 noted that CNB-GABA showed partial antagonism towards GABA-A receptors[11]. In the following year and in contrast to caged GABA, caged glutamate probes were reported to be inert towards APMA receptors. We found that MNI-Glu was inert at concentrations useful for 2P uncaging (up to 12 mM)[12]. Papageorgiou, et al., published similar findings for MNI-Glu in the context of UV uncaging (0.25-1 mM caged compound)[13]. In the same study they found NI-GABA depressed evoked GABA-A currents by 30% but that MNI-Glu was inert towards GABA-A receptors. Thus, we were subsequently surprised to discover that MNI-Glu was not inert towards GABA-A receptors[14,15]. This off-target effect was substantiated by other reports and applies to other caged glutamate probes such CDNI-Glu[16] and RuBi-Glu[16,17]. Much less surprising was that caged GABA probes such as CDNI-GABA[15], RuBi-GABA[18] and PEG-DEAC450-GABA[18] were also found to be quite antagonistic towards GABA-A receptors.
A clue to the potential solution to this “on-target” problem for caged GABA was provided by testing BOC-protected PEG-DEAC450-GABA (i.e. the precursor to the caged compound[18]) when it was found to have substantially reduced GABA-A antagonism (Mike Higley, personal communication). We reasoned that if we could develop a caged GABA probe bearing a substituent that enveloped both ends of the neurotransmitter (hence “cloaked caged compounds”) then we could neutralize most of the detrimental “on-target” side effects of caged GABA probes. Thus, we synthesized a group of 4th and 5th generation (G4 and G5, respectively[19]) 2,2-bis(methylol)propionic acid dendrimer-conjugated molecules to test this hypothesis. We have found that embedding DEAC450-GABA within a G5 dendrimer renders the optical probe biologically inert in a concentration range that is useful for 2P uncaging on neurons in living brain slices.
First, we tested the antagonistic effects of neutral G4 and G5 dendrimers, which were or were not conjugated to a primary alkyl amine (i.e. a GABA surrogate), towards GABA-A receptors (Figure 1, for syntheses see Supplementary Information). Each compound (Figure 1a) was bath applied to a brain slices isolated from adult male and female mice of 3-7 months of age.
Figure 1.
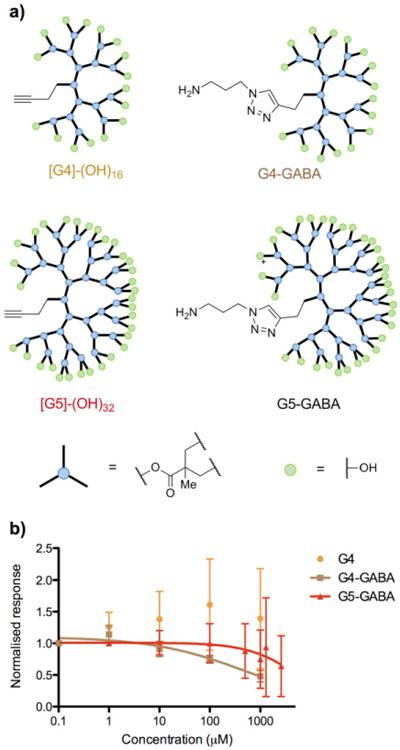
Structures of G4 and G5 dendrimers and pharmacological testing. a) Cartoon representations of G4 and G5 dendrimers with surface hydroxyl functionality; b,) GABA-A antagonism of the dendrimers assessed by dose-dependent blockade of evoked GABAergic inputs onto layer 2/3 pyramidal cells. The IC50 for G4-GABA was 0.52 mM and G5-GABA 5.4 mM. No convergence was found for fitting the G4 dendrimer antagonism, suggesting this compound was not antagonistic. AMPA-R, NMDA-R were blocked during these recordings.
Pyramidal cells in layer 2/3 of the prefrontal cortex were patch-clamped and a stimulation pipette was used to evoke GABAergic responses, as previously described[18] (see Figure S1 for similar recordings of high affinity antagonists). We found that we could detect no interference from the G4 dendrimer at concentrations up to 1 mM (Figure 1b). However the G4-GABA dendrimer showed mild antagonism, with an IC50 = 0.55 +/- 0.28 mM (n = 3). Importantly, increasing the size of the dendrimer to the 5th generation decreased this antagonism dramatically, with our assay providing an estimate for the IC50 of the G5-GABA dendrimer of about 5.4 mM, i.e. effectively no antagonism.
Emboldened by the success of these model compounds we synthesized the G5-DEAC450-GABA (structure Figure 2, for synthesis see Supplementary Information). We tested the blockade of GABAergic inputs on layer 2/3 pyramidal cells by bath application of the optical probe to brain slices over a wide concentration range (1 – 2300 μM). Our data allowed us to determine that the IC50 was approximately 0.9 mM (the unconjugated G5 dendrimer showed no detectable antagonism, Fig. S2). Unlike high affinity antagonists such as bicuculline and RuBi-GABA (Figure S1), there is considerable biological variability for very low affinity antagonists making precise values difficult to determine. Nevertheless we were very encouraged by the reduction in the IC50 of G5-DEAC450-GABA when compared to our previous DEAC450-caged GABA probes[18], Asp-DEAC450 (IC50 = 0.5 μM) and PEG-DEAC450 (IC50 = 11 μM). Use of bisphosphates to reduce the antagonism of NI-GABA[13] is also effective. The IC50 antagonism of DPNI-GABA is about 0.5 mM[20], but this mononitroindolinyl caged compound, unlike our dinitro CDNI-GABA[15], has not been reported to be 2P active.
Figure 2.
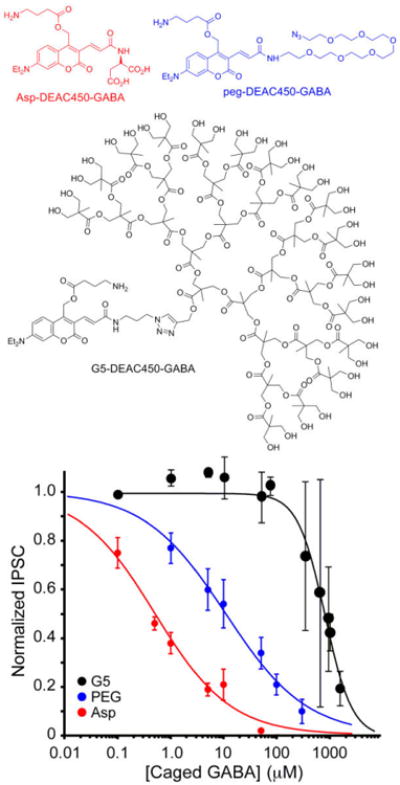
Structure of G5-DEAC450-GABA and pharmacological testing. GABA-A antagonism of G5-DEAC450-GABA was assessed by dose-dependent blockade of evoked GABAergic inputs onto layer 2/3 pyramidal cells by increasing the concentration of caged compound applied to brain slices (Asp- and PEG-DEAC450-GABA are adapted and drawn with permission from[18], copyright 2014 American Chemical Society). AMPA-R and NMDA-R were blocked during these recordings.
CDNI-GABA was, in fact, the first caged GABA probe reported to be effective for 2P optoneurobiology[15], and is starting to be used for biological studies[21,22], therefore we compared the 2P-evoked currents from this probe with G5-DEAC450-GABA. We have found that we can very reliably elicit GABAergic currents from 2P uncaging along he main apical dendrite of CA1 neurons[18]. Thus, we directed a Ti:sapphire laser tuned to either 900 nm or 720 nm to this region (Figure 3a) to make the desired comparison. Uncaging of G5-DEAC450-GABA (bath applied at 0.15-0.20 mM) with increasing power evoked very large currents (Figure 3b shows representative currents using 26-53 mW). Note using PEG-DEAC450-GABA under the same conditions allowed only very modest currents to be evoked (blue line, Figure 3c), due to the high antagonism of the probe. Since the 2P input “dosage” is proportional to (power)[2], [probe], quantum yield (QY) and irradiation time and inversely proportional to wavelength and pulse width[3,23], we normalized the evoked currents for our caged GABA probes for data gathered from many cells (Figure 3c, all cells showed the expected quadratic dependence on power, implying 2P excitation, Fig. S3). The slopes of the two data groups (25 vs 0.8) provides an estimate of the relative effectiveness of CDNI versus G5-DEAC450 for GABA uncaging. Even though the QY was 0.23, we found much more consistent results with G5-DEAC450 than CDNI[15], and we believe this is because of the reduced antagonism of the former towards GABA-A receptors in the concentration ranges used.
Figure 3.
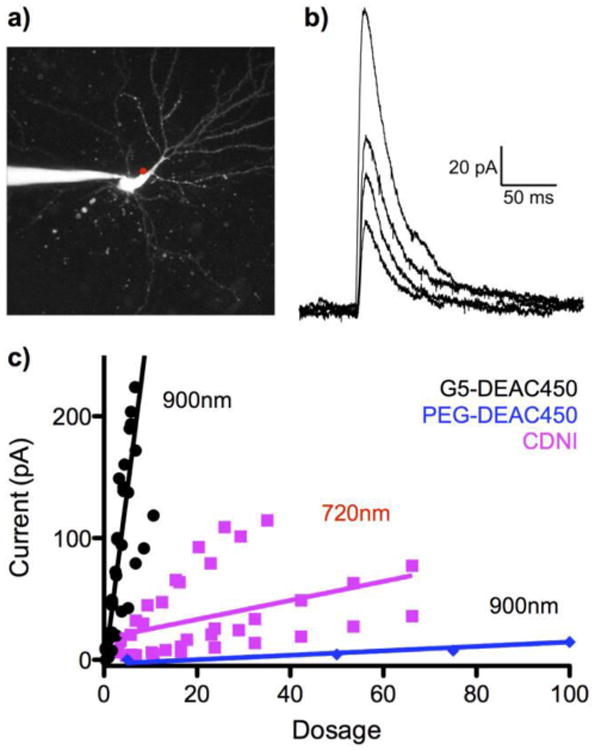
Comparative photolysis of G5-DEAC450-GABA and CDNI-GABA. a) Fluorescent image of a patch-clamped CA1 neuron with the position of uncaging represented by the red dot. The patch pipette can be seen exiting the image frame to the left. b) Representative series of currents evoked by uncaging G5-DEAC450-GABA at 900 nm using increasing power (26, 31, 42, and 53 mW, for cosmetic reasons stray electric noise has been filtered from these traces). c) Summary of power trains for G5-DEAC450 at 900 nm (n = 5, black), CDNI (n = 5, violet) and PEG-DEAC450 (n = 1, blue). “Dosage” = (power)2 × QY × conc. × time/wavelength × pulse width.
Next, we searched for the minimum concentration of CDNI-GABA or G5-DEAC450-GABA that could be used for reliable 2P-evoked GABAergic currents (Figure 4). As we reported previously, CDNI-GABA at 400 μM could be used for 2P uncaging at 720 nm[15] (Figure 4a), but much lower concentrations (< 100 μM) were only useful for 1P uncaging (Figure 4b). In contrast we could use G5-DEAC450-GABA at 66 μM or even 45 μM and still reliably evoke currents by 2P excitation at 900 nm (Figure 4c,d). We also confirmed that G5-DEAC450-GABA was, like previous DEAC450-caged transmitters[16,18], essentially inactive at 720 nm (Fig. S4). These data implied that our “cloaking” strategy could allow highly 2P-active caged neurotransmitters such as DEAC450-GABA to become useful for two-color, two-photon optoneurobiology.
Figure 4.
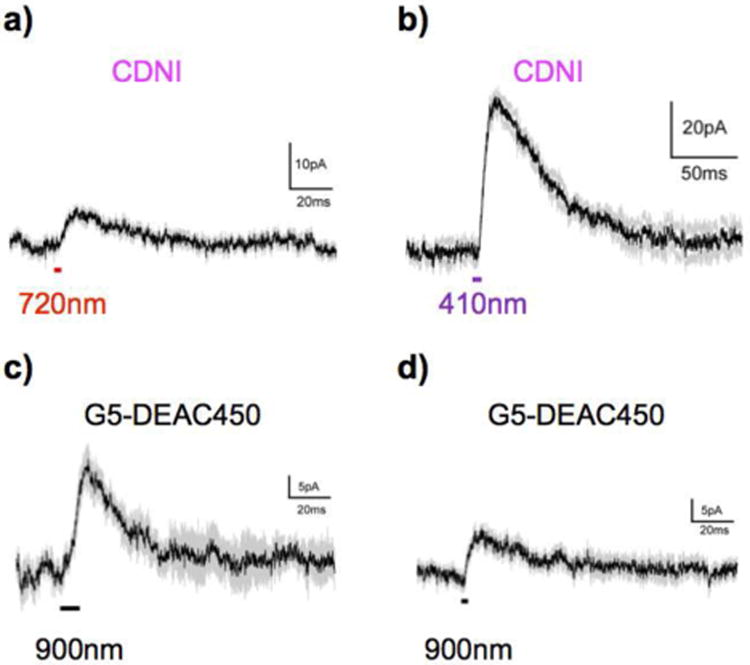
Examples of GABAergic currents evoked by photolysis of low concentrations of G5-DEAC450- and CDNI-GABA. a) CDNI at 720 nm (400 μM, 3 × 1ms, 75 mW, n = 7; b) CDNI at 410 nm (56 μM, 5 ms, 2 mW, n = 5); c) G5-DEAC450 at 900 nm (45 μM, 3 × 3ms, 100 mW, n = 4); d) G5-DEAC450 at 900 nm (66 μM, 3 × 1ms, 50 mW, n = 8). No 2P-evoked currents could be detected with sub-100 μM CDNI-GABA.
In order to test this hypothesis, we paired G5-DEAC450-GABA with CDNI-Glu in a two-color proof-of-principle uncaging experiment. Since our antagonism data suggests the cloaked caged GABA is essentially inert up to about 0.6 mM (Figure 2) we bath applied the probe to brain slices at this concentration. We did this because we knew CDNI-Glu is best used at about 1 mM[21,23-26], and has an IC50 = 0.24 mM for GABA-A receptor blockade[18]. Thus, we patch-clamped a CA1 neuron and monitored voltage changes produced by two-color, 2P uncaging. Irradiation along a dendrite with 720 nm light (10 × 1 ms, 50 mW) fired an action potential (Figure 5). Prior irradiation of the apical dendrite with 900nm (3 × 3 ms, 50 mW) elicited pronounced hyperpolarization, which blocked the action potential, which was restored when only short wavelength irradiation was used (Figure 5).
Figure 5.
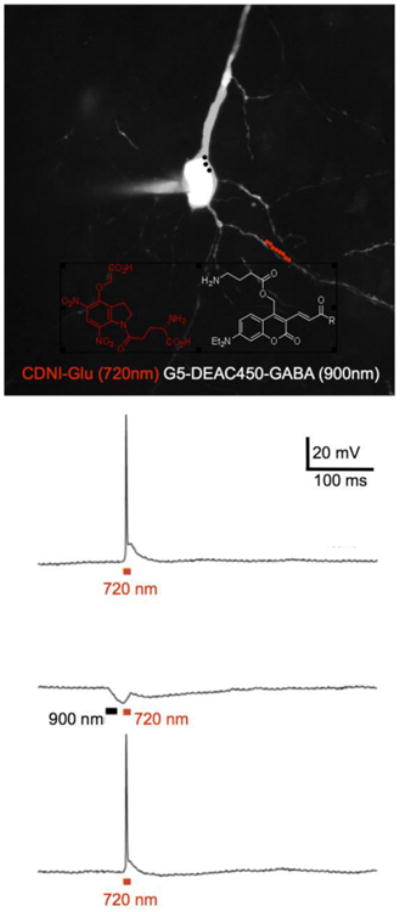
Chromatically orthogonal two-photon uncaging of glutamate and GABA. Example of a fluorescent image of a CA1 pyramidal neuron filled with Alexa594 via a patch pipette taken with excitation at 1070 nm. The red and black dots indicate the experimental protocol for irradiation at 720 nm along a dendrite (red dots) and 900 nm around the soma (black dots). CDNI-Glu and G5-DEAC450-GABA probes were bath applied at 1 mM and 0.6 mM, respectively. Uncaging at 720 nm (10 × 1 ms, 50 mW) fired an action potential (top trace), which could be blocked (middle trace) by prior uncaging at 900 nm (3 × 3ms, 50 mW). Such block was found to be reversible (lower trace).
Since we have shown that DEAC450 uncaging is highly wavelength-selective for 2P excitation at 900 nm[27], our data suggest the cloaked caged neurotransmitter G5-DEAC450-GABA could be very useful for chromatically selective, two-color optoneurobiology. Certainly it is the first example of a caged GABA compound that is highly active to 2P excitation that is also essentially inert towards its target ionotropic receptors. Thus this new caged compound should enable the optical probing of GABA receptors in their native state, in situ for the first time. Further, since G5 dendrimers are easily dissolved in physiological buffer, they can provide an important benefit of solublizing hydrophobic caging chromophores. In particular, there are an emerging set of extended π-electron caging chromophores[28-33] that could appropriate the technology we have developed here, and so become practically useful additions to the chemical tool box available for optoneurobiology.
Supplementary Material
Acknowledgments
GED thanks Mike Higley for helpful discussions.
This work was supported by grants from the NIH (GM053395 and NS069720) to GED
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- 1.Scanziani M, Häusser M. Nature. 2009;461:930. doi: 10.1038/nature08540. [DOI] [PubMed] [Google Scholar]
- 2.Ellis-Davies GCR. ACS Chem Neurosci. 2011;2:185. doi: 10.1021/cn100111a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denk W, Strickler JH, Webb WW. Science. 1990;248:73. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 4.Denk W. Proc Natl Acad Sci U S A. 1994;91:6629. doi: 10.1073/pnas.91.14.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipp P, Niggli E. J Physiol. 1998;508:801. doi: 10.1111/j.1469-7793.1998.801bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brieke C, Rohrbach F, Gottschalk A, Mayer G, Heckel A. Angew Chem Int Edit. 2012;51:8446. doi: 10.1002/anie.201202134. [DOI] [PubMed] [Google Scholar]
- 7.Ellis-Davies GCR. Beilstein J Org Chem. 2013;9:64. doi: 10.3762/bjoc.9.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thirlwell H, Sleep JA, Ferenczi MA. J Muscle Res Cell Motil. 1995;16:131. doi: 10.1007/BF00122531. [DOI] [PubMed] [Google Scholar]
- 9.Wieboldt R, Gee KR, Niu L, Ramesh D, Carpenter BK, Hess GP. Proc Natl Acad Sci U S A. 1994;91:8752. doi: 10.1073/pnas.91.19.8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wieboldt R, Ramesh D, Carpenter BK, Hess GP. Biochemistry. 1994;33:1526. doi: 10.1021/bi00172a032. [DOI] [PubMed] [Google Scholar]
- 11.Molnár P, Nadler JV. Eur J Pharmacol. 2000;391:255. doi: 10.1016/s0014-2999(00)00106-0. [DOI] [PubMed] [Google Scholar]
- 12.Matsuzaki M, Ellis-Davies GCR, Nemoto T, Miyashita Y, Iino M, Kasai H. Nat Neurosci. 2001;4:1086. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canepari M, Nelson L, Papageorgiou G, Corrie J, Ogden D. J Neurosci Meth. 2001;112:29. doi: 10.1016/s0165-0270(01)00451-4. [DOI] [PubMed] [Google Scholar]
- 14.Ellis-Davies GCR, Meucci O, Shimizu S. Society for Neuroscience Annual Conference. 2007;480.6/S14 [Google Scholar]
- 15.Matsuzaki M, Hayama T, Kasai H, Ellis-Davies GCR. Nat Chem Biol. 2010;6:255. doi: 10.1038/nchembio.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson JP, Kwon HB, Takasaki KT, Chiu CQ, Higley MJ, Sabatini BL, Ellis-Davies GCR. J Am Chem Soc. 2013;135:5954. doi: 10.1021/ja4019379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fino E, Araya R, Peterka DS, Salierno M, Etchenique R, Yuste R. Front Neural Circuits. 2009;3:2. doi: 10.3389/neuro.04.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amatrudo JM, Olson JP, Lur G, Chiu CQ, Higley MJ, Ellis-Davies GCR. ACS Chem Neurosci. 2014;5:64. doi: 10.1021/cn400185r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Astruc D, Boisselier E, Ornelas C. Chem Rev. 2010;110:1857. doi: 10.1021/cr900327d. [DOI] [PubMed] [Google Scholar]
- 20.Trigo FF, Papageorgiou G, Corrie JET, Ogden D. J Neurosci Meth. 2009;181:159. doi: 10.1016/j.jneumeth.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 21.Chiu CQ, Lur G, Morse TM, Carnevale NT, Ellis-Davies GCR, Higley MJ. Science. 2013;340:759. doi: 10.1126/science.1234274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villa KL, Berry KP, Subramanian J, Cha JW, Oh WC, Kwon HB, Kubota Y, So PT, Nedivi E. Neuron. 2016;89:756. doi: 10.1016/j.neuron.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellis-Davies GCR, Matsuzaki M, Paukert M, Kasai H, Bergles DE. J Neurosci. 2007;27:6601. doi: 10.1523/JNEUROSCI.1519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuzaki M, Ellis-Davies GCR, Kanemoto Y, Kasai H. Neural Syst Circuits. 2011;1:2. doi: 10.1186/2042-1001-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayama T, Noguchi J, Watanabe S, Takahashi N, Hayashi-Takagi A, Ellis-Davies GCR, Matsuzaki M, Kasai H. Nat Neurosci. 2013;16:1409. doi: 10.1038/nn.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kantevari S, Matsuzaki M, Kanemoto Y, Kasai H, Ellis-Davies GCR. Nat Methods. 2010;7:123. doi: 10.1038/nmeth.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amatrudo JM, Olson JP, Agarwal HK, Ellis-Davies GCR. Eur J Neurosci. 2015;41:5. doi: 10.1111/ejn.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agarwal HK, Janicek R, Chi SH, Perry JW, Niggli E, Ellis-Davies GCR. J Am Chem Soc. 2016;138:3687–3693. doi: 10.1021/jacs.5b11606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Momotake A, Lindegger N, Niggli E, Barsotti RJ, Ellis-Davies GCR. Nat Methods. 2006;3:35. doi: 10.1038/nmeth821. [DOI] [PubMed] [Google Scholar]
- 30.Picard S, Cueto-Diaz EJ, Genin E, Clermont G, Acher F, Ogden D, Blanchard-Desce M. Chem Commun. 2014;49:10805. doi: 10.1039/c3cc45812a. [DOI] [PubMed] [Google Scholar]
- 31.Cueto Diaz E, Picard S, Klausen M, Hugues V, Pagano P, Genin E, Blanchard-Desce M. Chemistry, Eur J. 2016;22:10848. doi: 10.1002/chem.201601109. [DOI] [PubMed] [Google Scholar]
- 32.Ciuciu AI, Korzycka KA, Lewis WJM, Bennett PM, Anderson HL, Flamigni L. Phys Chem Chem Phys. 2015;17:6554. doi: 10.1039/c4cp05812g. [DOI] [PubMed] [Google Scholar]
- 33.Pawlicki M, Collins HA, Denning RG, Anderson HL. Angew Chem Int Edit. 2009;48:3244. doi: 10.1002/anie.200805257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


