Abstract
The selective phosphorylation of glycogen phosphorylase (GP) by its only known kinase, phosphorylase kinase (PhK), keeps glycogen catabolism tightly regulated. In addition to the obligatory interaction between the catalytic γ subunit of PhK and the phosphorylatable region of GP, previous studies have suggested additional sites of interaction between this kinase and its protein substrate. Using short chemical crosslinkers, we have identified direct interactions of GP with the large regulatory α and β subunits of PhK. These newfound interactions were found to be sensitive to ligands that bind PhK.
Graphical abstract
1. Introduction
The breakdown of glycogen is mediated by phosphorylase kinase (PhK) and glycogen phosphorylase (GP). PhK phosphorylates a single serine on GP, causing activation and subsequent phosphorolysis of glycogen to release glucose-1-phosphate [1]. PhK is also regulated by reversible phosphorylation, and both GP and PhK are also regulated by a variety of allosteric effectors [2, 3]. This complex regulation of PhK and GP is consistent with their large masses. PhK is a hexadecameric complex having four copies of four different subunits (α, β, γ and δ) and a total mass of 1.3 MDa [2]. The γ subunit (44.7 kDa) is catalytic, and the remaining three subunits, α (138.4 kDa), β (125.2 kDa) and δ (16.7 kDa), are regulatory [2], with the δ subunit being a molecule of non-dissociable calmodulin. The substrate GP is a homodimer of 197 kDa that has two distinct faces: a regulatory face, where the phosphorylatable serine and allosteric binding sites reside, and a catalytic face [4].
The recognition of protein substrates by kinases can be complex. The selective phosphorylation of protein substrates depends not only on having appropriate amino acids surrounding the residue to be phosphorylated (primary structure), but also frequently on docking site interactions distinct from the phosphorylation site(s) [5]. After decades of extensive work with peptide substrates, consensus amino acid sequences preferred by most kinases are reasonably well-defined, but docking site interactions between kinases and their substrates are by comparison poorly understood [5-7]. These distinct contact points often play important roles in substrate recognition and phosphorylation [6, 7].
Unlike most other kinases, PhK has only one recognized physiological target, GP. Moreover, GP is known to be phosphorylated only by PhK, making this an unusually specific kinase-substrate pair. Despite this unusual specificity and the importance of these two enzymes, little is known regarding the physical interaction between PhK and GP, and their large sizes complicate traditional binding and structural studies. We know that the N-terminus of GP, which contains its single phosphorylation site, must bind to the active site of γ to be phosphorylated, and the crystal structure of truncated γ with a peptide substrate reveals details of this binding [8]. Importantly, the only comprehensive binding studies on the interactions between PhK and GP found that GP lacking its phosphorylatable N-terminus still bound to PhK with a similar affinity as full-length GP [9], suggesting additional contact site(s). Yeast two-hybrid studies raised the possibility that the regulatory α subunit of PhK may interact with GP [10]. Calmodulin, PhK's δ subunit, has also been shown to bind an N-terminal fragment of GP [11]. Only the β subunit has not been previously implicated in the binding of GP. Here we report the use of short chemical crosslinkers to unambiguously show direct interactions between GP and the two large regulatory subunits of PhK, α and β.
2. Materials and methods
2.1. Enzymes
Non-activated PhK was purified from New Zealand White rabbit psoas muscle as previously described [12], dialyzed into 50 mM HEPES (pH 6.8), 0.2 mM EDTA, and 10% sucrose (w/v), and stored at −80 °C. GP was also isolated from New Zealand White rabbit muscle as described previously [13], and recrystallized with Mg2+ and AMP. After removal of AMP by dialysis into 10 mM HEPES (pH 6.8), GP was stored at −80 °C. The concentrations of PhK and GP were determined spectrally as previously described [12].
2.2. Chemical crosslinking
The crosslinkers 1,5-difluoro-2,4-dinitrobenzene (DFDNB) (CAS No. 327-92-4), succinimidyl iodoacetate (SIA) (CAS No. 39028-27-8), and N-5-azido-2-nitrobenzoyloxysuccinimide (ANB-NOS) (CAS No. 60117-35-3) were from Pierce/ThermoFisher Scientific.
To limit crosslinking, a two-step protocol was used with DFDNB and SIA in the crosslinking of GP with PhK. In step 1, GP was pre-incubated with crosslinker (135 μM DFDNB or 540 μM SIA) for 2-5 min at room temperature (RT). The pre-incubated GP was immediately diluted 10-fold into a solution containing PhK and incubated at 30 °C for 5 min. The final concentrations were: 20 mM HEPES (pH 6.8), 0.1 mM EDTA, 437 μg/mL PhK, 260 μg/mL GP, and 13.5 μM DFDNB or 54 μM SIA (carryover solvent was 0.2% DMSO or ACN). The molar ratio of PhK protomer (αβγδ) to GP monomer was 1:2, while the ratio of DFDNB and SIA to GP monomer was 5:1 and 20:1, respectively. Reaction aliquots were quenched in an equal volume of SDS buffer (125 mM Tris (pH 6.8), 20% glycerol, 5% β-mercaptoethanol, 4% SDS, and trace Coomassie R250) and analyzed on a 4-12% linear SDS-PAGE gel.
ANB-NOS, a photo-activated crosslinker, was treated differently than DFDNB and SIA. GP was incubated with 100 μM ANB-NOS for 5 min at RT in the dark. After removal of unreacted ANB-NOS on a P10 de-salting column, the labeled GP was incubated at RT with PhK under a long-wave UV lamp (366 nm) at a distance of 1 cm for 5 min. The crosslinking reaction was quenched and analyzed as above.
When effectors were included in the DFDNB crosslinking reaction, they were added to the GP/DFDNB solution 15 sec before addition of PhK. The final concentrations of Mg2+, Ca2+, and AMP-PNP in the second step were 4 mM, 330 μM, and 300 μM, respectively. The density of the crosslinked products was determined using ImageJ software. To ensure reproducibility, three different preparations of PhK were used, and all experiments were performed in triplicate.
2.3. Western blotting
Samples for immunodetection were transferred from SDS-PAGE gels to PVDF membranes and blocked with 5% (w/v) nonfat powdered milk in 0.14 M NaCl, 2.7 mM KCl, 6 mM Pi (pH 7.4), 0.1% Tween20, and 0.2% gelatin. The primary antibodies against GP and the α, β, and γ subunits of PhK have been previously characterized and were used as described [14-16]. Colorimetric detection of the immunoreactive bands was performed with AP-conjugated secondary antibodies from Southern Biotechnology.
3. Results and discussion
3.1. Identifying GP-PhK crosslinkers
To limit the amount of crosslinking, a two-step crosslinking approach was used to capture interactions between GP and subunits of PhK. This approach involved pre-labeling GP with a low concentration of crosslinker (step 1) immediately prior to incubation with PhK (step 2). The pre-labeling of GP preferentially captured conjugates between PhK and GP before intramolecular crosslinking within the GP dimer or PhK hexadecamer could dominate. Of seven different crosslinking reagents screened using this two-step method, four (sulfosuccinimidyl 4,4'-azipentanoate; bis(sulfosuccinimidyl)suberate; N-α-maleimidoacet-oxysuccinimide ester; and formaldehyde) failed to capture GP-PhK conjugates, but three (DFDNB, SIA, and ANB-NOS) successfully did so.
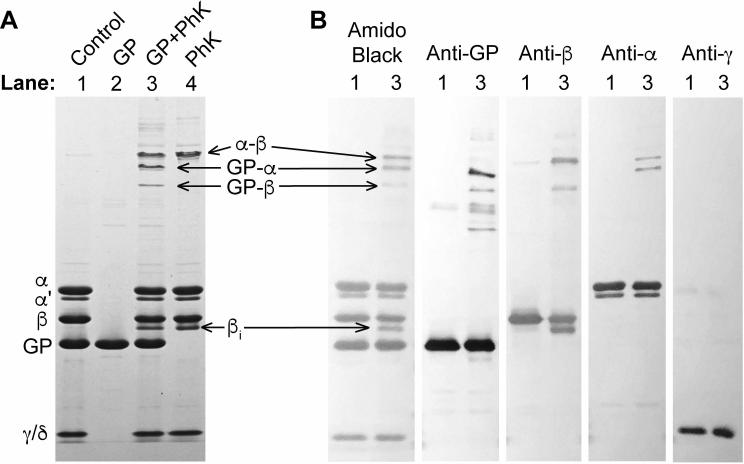
Several major products are formed from DFDNB crosslinking of GP and PhK. The two most intense crosslinked bands are intramolecular PhK products, formed with or without GP present (Fig 1A, lane 4). One is a slower migrating α-β conjugate, and the other is an intra-subunit crosslink of β (Fig 1B). When DFDNB, PhK and GP are present together, two new bands form below the α-β product (Figure 1A, lane 3), the slower being a doublet that cross-reacts with anti-GP and anti-α antibodies, identifying it as a GP-α conjugate. The faster migrating band is a GP-β conjugate. There are additional PhK-GP products above the α-β band; however, these are not well-defined, and their composition is difficult to confidently identify by Western blots due to their proximity to one another. Finally, GP undergoes a small amount of inter-subunit crosslinking to form three, faint bands (Fig 1A, lane 2).
Fig. 1.
DFDNB crosslinking of GP and PhK. (A) Coomassie stained 4-12% SDS-PAGE gel with control and crosslinked PhK ± GP. Lane 1 is PhK and GP without DFDNB crosslinking. PhK or GP were crosslinked individually to determine intramolecular crosslinking by DFDNB (lanes 2 and 4). Lane 3 has both GP and PhK present with DFDNB. (B) Amido black staining and Western blot of PhK and GP crosslinked together by DFDNB. Previously characterized antibodies to GP and the α, β, and γ subunits of PhK were used to identify the conjugate species [14-16]. βi, intra-subunit crosslink of β. α’, a naturally occurring splice variant of the α subunit. The lane designations refer to the same samples described in (A). Each section of the Western blot is separated by a lane with a protein ladder of standards.
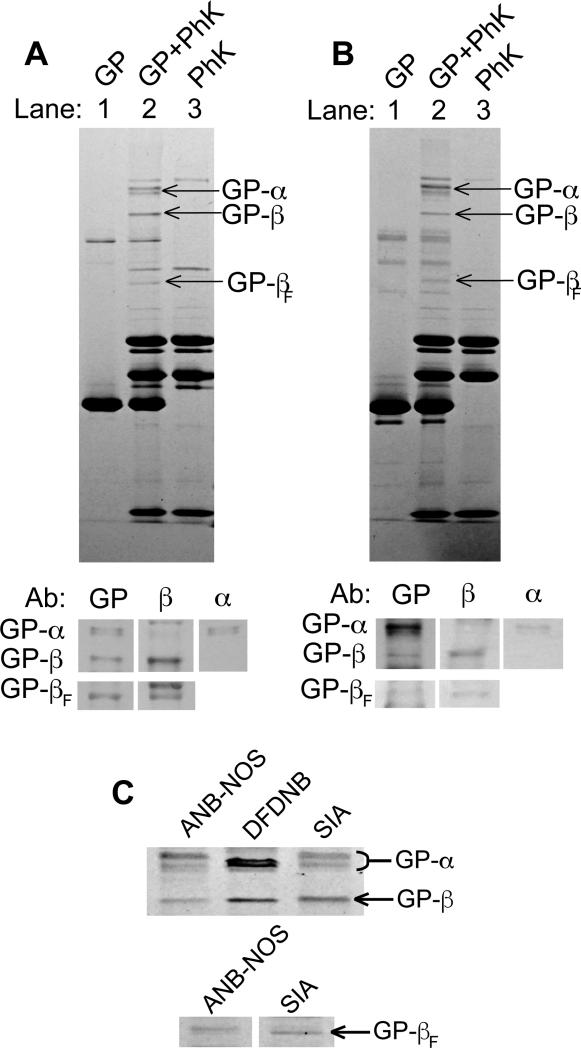
Another crosslinking reagent, SIA, also formed GP-PhK conjugates (Fig 2A). While GP and PhK were both independently crosslinked by SIA (Fig 2A, lanes 1 and 3), when they were present together, three new products formed (lane 2). Western blotting identified the products as GP-α, GP-β, and a second, faster migrating GP-β (GP-βF) conjugates (Fig 2A, bottom panel). The GP-α product is a doublet, like the GP-α formed by DFDNB, suggesting that multiple sites are crosslinked between GP and α.
Fig. 2.
SIA and ANB-NOS crosslinking of GP and PhK. (A) Coomassie-stained 4-12% SDS-PAGE gel and Western blot analysis of SIA crosslinking products. GP and PhK were crosslinked individually to determine intramolecular crosslinking by SIA in lanes 1 and 3, respectively. In lane 2, PhK and GP were crosslinked together with SIA. The major GP-PhK conjugates formed by SIA crosslinking are labeled. The Western blot was carried out as described under Fig. 1 and Materials and Methods. (B) Same as (A), but with ANB-NOS crosslinking. (C) Side-by-side comparison of the co-migrating GP-α and GP-β conjugates formed with all three crosslinkers on Coomassie stained 4-12% SDS-PAGE gel.
ANB-NOS, the third crosslinker that formed GP-PhK conjugates, required a different crosslinking approach than used with DFDNB and SIA. ANB-NOS is a photosensitive crosslinker, containing one functional group that remains inert until activated by UV light. Therefore, GP was labeled in the dark by the amine-selective group on ANB-NOS, and unreacted ANB-NOS was removed by gel filtration prior to incubation of the modified GP with PhK. UV light then activated the second functional group on the crosslinker, allowing it to react with bound PhK. Like SIA, ANB-NOS formed three GP-PhK conjugates (Fig. 2B), which were identified by Western blots as another GP-α doublet, and two GP-β products.
Thus, three different crosslinking reagents apparently form the same GP-PhK conjugates (Fig 2C). In the case of the GP-α doublet and the slower migrating GP-β, these conjugates co-migrate for all three crosslinkers. The faster migrating GP-βF appears with SIA and ANB-NOS, but not DFDNB. The consistent formation of these products suggests that the same interfaces between GP and regions of α and β are being sampled by the different crosslinkers and represent genuine contact points between GP and PhK. Moreover, the short spacer lengths of the crosslinkers (1.5 – 7.7 Å) further argues for direct contact of GP with α and β. Our results are consistent with earlier predictions of direct interactions between GP and the regulatory subunits of PhK based on binding studies. GP lacking its phosphorylatable N-terminus binds to PhK in a competitive ELISA assay, but fails to bind the isolated catalytic subunit [9], suggesting that the regulatory subunits of PhK may be involved in the binding of GP. Our results also agree with a previous yeast two-hybrid study that suggested an interaction between GP and PhK's α subunit [10]. A direct interaction between GP and the β subunit has not been previously suggested. It is feasible that interactions may also occur between GP and PhK's small, regulatory δ subunit [11], but we did not test for the presence of δ in crosslinked conjugates due to the lack of an acceptable antibody for calmodulin.
Our results directly establish for the first time that there are interactions between GP and regulatory subunits of PhK. The location and function of these newfound interactions may be involved in the unusually selective phosphorylation of GP by PhK, because an isolated form of the catalytic γ subunit lacking its calmodulin-binding C-terminus phosphorylates GP less effectively than does γ within the activated PhK complex. Based on at least five published studies, the average kcat for isolated, truncated γ is 56 sec−1 (S.D. ±26) [17-20], whereas the average kcat for activated PhK is 245 sec−1 (S.D. ±119) [20-25]. Although different laboratories and conditions undoubtedly account for some differences in the kcat values, the reported average value for γ within PhK is nevertheless 4.4-fold greater than that of truncated γ. Thus, despite their inhibition of the γ subunit in nonactivated PhK [2], it is possible that the α and β subunits contribute to the catalytic efficiency of γ in the activated complex. The Km values for GP in the above studies are too scattered to allow a meaningful determination of the specificity constants (kcat/Km) for the two forms of γ.
Additional binding sites, distinct from the active site, have been identified in other kinase-substrate pairs. Such sites could enhance the interaction between the substrate and active site through several mechanisms [6]. For example, regions on GP adjacent in 3D structure to its phosphorylation site could bind to α and β and properly position GP's N-terminus at the active site of γ. Alternatively, GP could also bind α and β at a more distant site, anchoring GP to PhK and increasing the frequency of interaction between GP's N-terminus and the active site. Similar docking or recruitment strategies have been seen with other kinases, including MAPK and cyclin-dependent kinases [26, 27]. Another possibility is that GP binding to the regulatory subunits could stabilize a conformation of PhK that promotes phosphorylation through allosteric activation or improved substrate binding, as is seen with PDK1 and MAPK [5, 28].
Prior to this, only PhK's catalytic γ subunit was known with certainty to bind GP; however, we did not detect any crosslinking between GP and γ. Active site interactions of protein kinases with their substrates have been suggested to be transient, facilitating rapid turnover [6, 29]. Thus, it may be that the interaction between GP and γ is too transient for detection by chemical crosslinking compared to the potentially more stable interactions between GP and the regulatory subunits of PhK.
3.2. Effectors and GP-PhK crosslinking
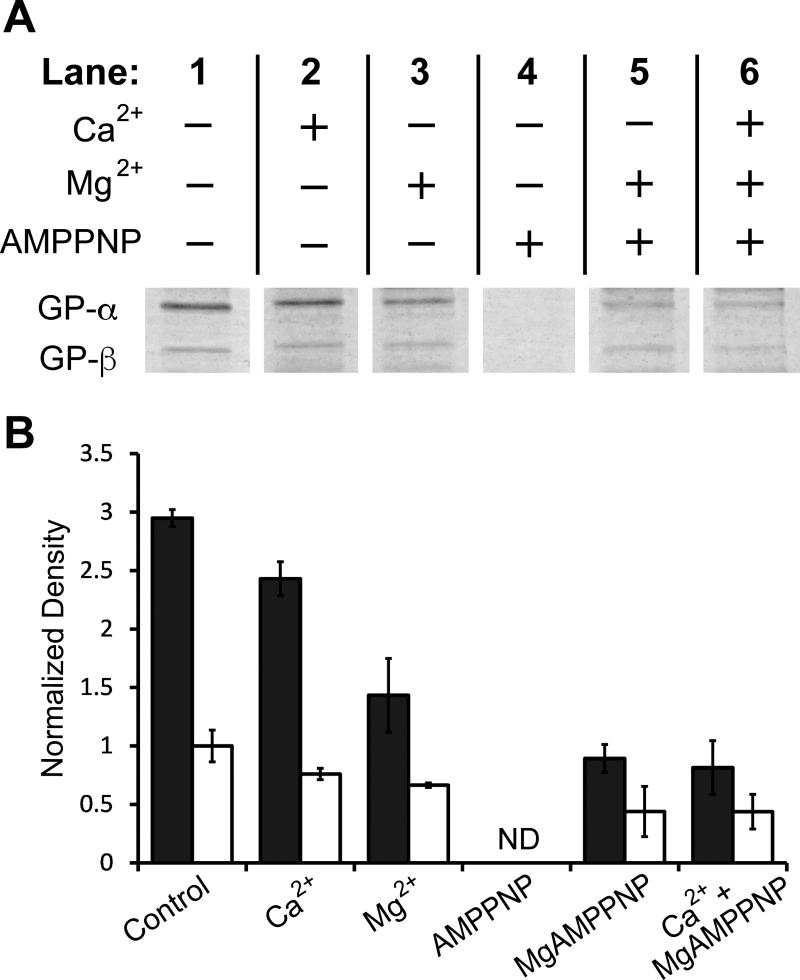
Because in vitro phosphorylation of GP by PhK requires Ca2+ and MgATP (Mg2+ in excess), we determined the effects of these ligands on formation of GP-α and GP-β conjugates during crosslinking with DFDNB. For ATP we substituted the nonhydrolyzable analogue adenylyl-imidodiphosphate (AMP-PNP). The concentrations of AMP-PNP and Ca2+ used were 10-fold above their reported Kd values [30, 31]. Mg2+ was at a concentration only 6.7-fold above its reported Kd value [32], because of the tendency of PhK to aggregate in the presence of high concentrations of this cation [33]. We found that in the absence of divalent cations, AMP-PNP completely blocked formation of the GP-α and GP-β conjugates (Fig 3, compare lanes 4 and 1), whereas inclusion of Mg2+ and Ca2+ with AMP-PNP partially overcame its protection against formation of the GP-PhK conjugates (compare lanes 4 and 6). Control experiments showed that pre-incubation of AMP-PNP and the cations with DFDNB had no effect on its subsequent reactivity (data not shown), indicating that the effect of these ligands on GP-PhK conjugate formation is due to their direct interactions with at least one of the proteins.
Fig. 3.
Influence of PhK effectors on crosslinking by DFDNB. PhK and GP were crosslinked with DFDNB for 5 min in the presence of various effectors after pre-incubation of GP with DFDNB for 2 min. (A) Coomassie-stained GP-α and GP-β conjugates on 4-12% SDS-PAGE gel after crosslinking in the presence of the indicated small molecules. (B) Densities of GP-α (closed bars) and GP-β (open bars) after crosslinking in the absence or presence of effectors and normalized through dividing by the average density of the control GP-β conjugate. Error bars represent the standard deviation of triplicate samples. The lanes in part (A) came from the same experiment and gel. ND, none detected.
Adenine nucleotide affinity labels modify the α, β and γ subunits of PhK [34, 35], and ATP and AMP-PNP bind with similar affinity to γ and an allosteric site [30], most likely on β [34]. At higher concentrations, ATP also binds an allosteric site on GP; however, under our conditions, the potential occupation of the GP allosteric site by AMP-PNP is sub-saturating [36]. Thus, the complete inhibition of GP-α and GP-β formation by AMP-PNP likely results from its binding to the active site or allosteric sites on PhK, inducing conformational changes in α and β that affect either their binding of, or crosslinking with, GP. It must be noted that decreased crosslinking does not necessarily indicate loss of interaction. Crosslinking with a short reagent such as DFDNB samples only a small region of the interface between GP and PhK; so, loss of crosslinking could be due to even the smallest structural rearrangements in the interface.
A ligand-induced structural rearrangement is also apparently the reason that both free Ca2+ and Mg2+ inhibit formation of GP-α and GP-β conjugates by DFDNB (Fig. 3, compare lanes 2/3 and 1), given that both of these cations actually enhance PhK's binding of GP [15]. Free Mg2+ binds to the γ subunit to stimulate PhK's activity [37] and induces several physiochemical changes in the PhK complex [33]. Ca2+ also stimulates PhK's activity [38, 39], but by binding to its regulatory δ subunit, an integral molecule of calmodulin [2]. Further, Ca2+ brings about global conformational changes throughout the PhK complex [40].
The results of this work suggest that multiple conformational changes are likely associated with the interactions between PhK and GP in the phosphorylation-competent complex. That these interactions directly involve regulatory subunits of PhK adds an additional layer of complexity to this already complex, highly regulated system. A deeper understanding of the interaction between PhK and GP and its regulation will help us better understand the strict cellular control of glycogenolysis.
Supplementary Material
Two-step crosslinking successfully captured interactions between two large enzymes.
Regulatory subunits of phosphorylase kinase directly interact with its substrate.
The direct interactions were sensitive to effectors of the kinase.
Acknowledgments
Work supported by NIH grant DK32953. The authors would like to thank Dr. Owen W. Nadeau for helpful discussions.
Abbreviations
- AMP-PNP
adenylyl-imidodiphosphate, a nonhydrolyzable ATP analogue
- ANB-NOS
N-5-azido-2-nitrobenzoyloxysuccinimide
- DFDNB
1,5-difluoro-2,4-dinitrobenzene
- GP
glycogen phosphorylase (nonactivated form)
- PhK
phosphorylase kinase
- RT
room temperature
- SIA
succinimidyl iodoacetate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krebs EG, Kent AB, Fischer EH. The muscle phosphorylase b kinase reaction. J. Biol. Chem. 1958;231:73–83. [PubMed] [Google Scholar]
- 2.Brushia RJ, Walsh DA. Phosphorylase kinase: the complexity of its regulation is reflected in the complexity of its structure. Front. Biosci. 1999;4:D618–641. doi: 10.2741/brushia. [DOI] [PubMed] [Google Scholar]
- 3.Johnson LN. Glycogen phosphorylase: control by phosphorylation and allosteric effectors. FASEB J. 1992;6:2274–2282. doi: 10.1096/fasebj.6.6.1544539. [DOI] [PubMed] [Google Scholar]
- 4.Fletterick RJ, Sprang S, Madsen NB. Analysis of the surface topography of glycogen phosphorylase a: implications for metabolic interconversion and regulatory mechanisms. Can. J. Biochem. 1979;57:789–797. doi: 10.1139/o79-098. [DOI] [PubMed] [Google Scholar]
- 5.Goldsmith EJ, Akella R, Min X, Zhou T, Humphreys JM. Substrate and docking interactions in serine/threonine protein kinases. Chem. Rev. 2007;107:5065–5081. doi: 10.1021/cr068221w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Oliveira PS, Ferraz FA, Pena DA, Pramio DT, Morais FA, Schechtman D. Revisiting protein kinase-substrate interactions: Toward therapeutic development. Sci. Signal. 2016;9:re3. doi: 10.1126/scisignal.aad4016. [DOI] [PubMed] [Google Scholar]
- 7.Remenyi A, Good MC, Lim WA. Docking interactions in protein kinase and phosphatase networks. Curr. Opin. Struct. Biol. 2006;16:676–685. doi: 10.1016/j.sbi.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Lowe ED, Noble ME, Skamnaki VT, Oikonomakos NG, Owen DJ, Johnson LN. The crystal structure of a phosphorylase kinase peptide substrate complex: kinase substrate recognition. EMBO J. 1997;16:6646–6658. doi: 10.1093/emboj/16.22.6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu YH, Carlson GM. Structural features contributing to complex formation between glycogen phosphorylase and phosphorylase kinase. Biochemistry. 1999;38:9562–9569. doi: 10.1021/bi9901836. [DOI] [PubMed] [Google Scholar]
- 10.Andreeva IE, Rice NA, Carlson GM. The regulatory α subunit of phosphorylase kinase may directly participate in the binding of glycogen phosphorylase. Biochemistry-Moscow+ 2002;67:1197–1202. doi: 10.1023/a:1020927726884. [DOI] [PubMed] [Google Scholar]
- 11.Takrama JF, Graves DJ. Solution conformations of the N-terminal CNBr fragment of glycogen phosphorylase and its interaction with calmodulin. Biochim. Biophys. Acta. 1991;1077:371–378. doi: 10.1016/0167-4838(91)90553-c. [DOI] [PubMed] [Google Scholar]
- 12.King MM, Carlson GM. Synergistic activation by Ca2+ and Mg2+ as the primary cause for hysteresis in the phosphorylase kinase reactions. J. Biol. Chem. 1981;256:11058–11064. [PubMed] [Google Scholar]
- 13.Fischer EH, Krebs EG. The isolation and crystallization of rabbit skeletal muscle phosphorylase b. J. Biol. Chem. 1958;231:65–71. [PubMed] [Google Scholar]
- 14.Wilkinson DA, Marion TN, Tillman DM, Norcum MT, Hainfeld JF, Seyer JM, Carlson GM. An epitope proximal to the carboxyl terminus of the α-subunit is located near the lobe tips of the phosphorylase kinase hexadecamer. J. Mol. Biol. 1994;235:974–982. doi: 10.1006/jmbi.1994.1051. [DOI] [PubMed] [Google Scholar]
- 15.Wilkinson DA, Norcum MT, Fizgerald TJ, Marion TN, Tillman DM, Carlson GM. Proximal regions of the catalytic γ and regulatory β subunits on the interior lobe face of phosphorylase kinase are structurally coupled to each other and with enzyme activation. J. Mol. Biol. 1997;265:319–329. doi: 10.1006/jmbi.1996.0739. [DOI] [PubMed] [Google Scholar]
- 16.Xu YH, Wilkinson DA, Carlson GM. Divalent cations but not other activators enhance phosphorylase kinase's affinity for glycogen phosphorylase. Biochemistry. 1996;35:5014–5021. doi: 10.1021/bi9528107. [DOI] [PubMed] [Google Scholar]
- 17.Chan KF, Graves DJ. Isolation and physicochemical properties of active complexes of rabbit muscle phosphorylase kinase. J. Biol. Chem. 1982;257:5939–5947. [PubMed] [Google Scholar]
- 18.Tabatabai LB, Graves DJ. Kinetic mechanism and specificity of the phosphorylase kinase reaction. J. Biol. Chem. 1978;253:2196–2202. [PubMed] [Google Scholar]
- 19.Newsholme P, Walsh DA. A kinetic re-interpretation of the regulation of rabbit skeletal-muscle phosphorylase kinase activity by Ca2+ and phosphorylation. Biochem. J. 1992;283:845–848. doi: 10.1042/bj2830845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venien-Bryan C, Jonic S, Skamnaki V, Brown N, Bischler N, Oikonomakos NG, Boisset N, Johnson LN. The structure of phosphorylase kinase holoenzyme at 9.9 angstroms resolution and location of the catalytic subunit and the substrate glycogen phosphorylase. Structure. 2009;17:117–127. doi: 10.1016/j.str.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang CY, Yuan CJ, Luo S, Graves DJ. Mutational analyses of the metal ion and substrate binding sites of phosphorylase kinase γ subunit. Biochemistry. 1994;33:5877–5883. doi: 10.1021/bi00185a027. [DOI] [PubMed] [Google Scholar]
- 22.Pete MJ, Liao CX, Bartleson C, Graves DJ. A recombinant form of the catalytic subunit of phosphorylase kinase that is soluble, monomeric, and includes key C-terminal residues. Arch. Biochem. Biophys. 1999;367:104–114. doi: 10.1006/abbi.1999.1256. [DOI] [PubMed] [Google Scholar]
- 23.Skamnaki VT, Owen DJ, Noble ME, Lowe ED, Lowe G, Oikonomakos NG, Johnson LN. Catalytic mechanism of phosphorylase kinase probed by mutational studies. Biochemistry. 1999;38:14718–14730. doi: 10.1021/bi991454f. [DOI] [PubMed] [Google Scholar]
- 24.Biorn AC, Bartleson C, Graves DJ. Site-directed mutants of glycogen phosphorylase are altered in their interaction with phosphorylase kinase. Biochemistry. 2000;39:15887–15894. doi: 10.1021/bi001755l. [DOI] [PubMed] [Google Scholar]
- 25.Skamnaki VT, Oikonomakos NG. Kinetic characterization of the double mutant R148A/E182S of glycogen phosphorylase kinase catalytic subunit: the role of the activation loop. J. Protein Chem. 2000;19:499–505. doi: 10.1023/a:1026553532289. [DOI] [PubMed] [Google Scholar]
- 26.Dimitri CA, Dowdle W, MacKeigan JP, Blenis J, Murphy LO. Spatially separate docking sites on ERK2 regulate distinct signaling events in vivo. Curr. Biol. 2005;15:1319–1324. doi: 10.1016/j.cub.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 27.Harper JW, Adams PD. Cyclin-dependent kinases. Chem. Rev. 2001;101:2511–2526. doi: 10.1021/cr0001030. [DOI] [PubMed] [Google Scholar]
- 28.Komander D, Kular G, Deak M, Alessi DR, van Aalten DM. Role of T-loop phosphorylation in PDK1 activation, stability, and substrate binding. J. Biol. Chem. 2005;280:18797–18802. doi: 10.1074/jbc.M500977200. [DOI] [PubMed] [Google Scholar]
- 29.Adams JA. Kinetic and catalytic mechanisms of protein kinases. Chem. Rev. 2001;101:2271–2290. doi: 10.1021/cr000230w. [DOI] [PubMed] [Google Scholar]
- 30.Cheng A, Fitzgerald TJ, Bhatnagar D, Roskoski R, Jr., Carlson GM. Allosteric nucleotide specificity of phosphorylase kinase: correlation of binding, conformational transitions, and activation. Utilization of lin-benzo-ADP to measure the binding of other nucleoside diphosphates, including the phosphorothioates of ADP. J. Biol. Chem. 1988;263:5534–5542. [PubMed] [Google Scholar]
- 31.Cohen P. The role of calcium ions, calmodulin and troponin in the regulation of phosphorylase kinase from rabbit skeletal muscle. Eur. J. Biochem. 1980;111:563–574. doi: 10.1111/j.1432-1033.1980.tb04972.x. [DOI] [PubMed] [Google Scholar]
- 32.Clerch LB, Huijing F. The role of magnesium in muscle phosphorylase kinase activity. Biochim. Biophys. Acta. 1972;268:654–662. doi: 10.1016/0005-2744(72)90269-0. [DOI] [PubMed] [Google Scholar]
- 33.Liu W, Nadeau OW, Sage J, Carlson GM. Physicochemical changes in phosphorylase kinase induced by its cationic activator Mg2+ Protein Sci. 2013;22:444–454. doi: 10.1002/pro.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King MM, Carlson GM. Affinity labeling of rabbit skeletal muscle phosphorylase kinase by 5'-(p-fluorosulfonylbenzoyl) adenosine. FEBS Lett. 1982;140:131–134. doi: 10.1016/0014-5793(82)80537-1. [DOI] [PubMed] [Google Scholar]
- 35.Guliaeva NV, Vul'fson PL, Severin ES. [Inhibition of the phosphorylase kinase activity by ATP analogs and their binding to the enzyme subunits] Biochemistry-USSR. 1978;43:373–382. [PubMed] [Google Scholar]
- 36.Walcott S, Lehman SL. Enzyme kinetics of muscle glycogen phosphorylase b. Biochemistry. 2007;46:11957–11968. doi: 10.1021/bi7005527. [DOI] [PubMed] [Google Scholar]
- 37.Chelala CA, Torres HN. Activation of muscle phosphorylase b kinase by Mg++ Biochem. Biophys. Res. Commun. 1968;32:704–709. doi: 10.1016/0006-291x(68)90296-9. [DOI] [PubMed] [Google Scholar]
- 38.Ozawa E, Hosoi K, Ebashi S. Reversible stimulation of muscle phosphorylase b kinase by low concentrations of calcium ions. J. Biochem.-Tokyo. 1967;61:531–533. doi: 10.1093/oxfordjournals.jbchem.a128582. [DOI] [PubMed] [Google Scholar]
- 39.Brostrom CO, Hunkeler FL, Krebs EG. The regulation of skeletal muscle phosphorylase kinase by Ca2+ J. Biol. Chem. 1971;246:1961–1967. [PubMed] [Google Scholar]
- 40.Nadeau OW, Carlson GM, Gogol EP. A Ca(2+)-dependent global conformational change in the 3D structure of phosphorylase kinase obtained from electron microscopy. Structure. 2002;10:23–32. doi: 10.1016/s0969-2126(01)00678-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.