Abstract
The gonadal hormones testosterone (T) in adult males and estradiol (E2) in adult females have been reported to modulate behavioral effects of Δ9-tetrahydrocannabinol (THC). This study determined whether activational effects of T and E2 are sex-specific, and whether hormones modulate production of the active metabolite 11-hydroxy-THC (11-OH-THC) and the inactive metabolite 11-nor-9-carboxy-THC (THC-COOH). Adult male and female rats were gonadectomized (GDX) and treated with nothing (0), T (10-mm Silastic capsule/100 g body weight), or E2 (1-mm Silastic capsule/rat). Three weeks later, saline or the cytochrome P450 inhibitor proadifen (25 mg/kg; to block THC metabolism and boost THC's effects) was injected i.p.; one h later, vehicle or THC (3 mg/kg females, 5 mg/kg males) was injected i.p., and rats were tested for antinociceptive and motoric effects 15-240 min post-injection. T did not consistently alter THC-induced antinociception in males, but decreased it in females (tail withdrawal test). Conversely, T decreased THC-induced catalepsy in males, but had no effect in females. E2 did not alter THC-induced antinociception in females, but enhanced it in males. The discrepant effects of T and E2 on males’ and females’ behavioral responses to THC suggests that sexual differentiation of THC sensitivity is not simply due to activational effects of hormones, but also occurs via organizational hormone or sex chromosome effects. Analysis of serum showed that proadifen increased THC levels, E2 increased 11-OH-THC in GDX males, and T decreased 11-OH-THC (and to a lesser extent, THC) in GDX females. Thus, hormone modulation of THC's behavioral effects is caused in part by hormone modulation of THC oxidation to its active metabolite. However, the fact that hormone modulation of metabolism did not alter THC sensitivity similarly on all behavioral measures within each sex suggests that other mechanisms also play a role in gonadal hormone modulation of THC sensitivity in adult rats.
Keywords: sex differences, cannabinoids, analgesia, gender, testosterone, estrogen
1. Introduction
Several studies have reported that Δ9-tetrahydrocannabinol (THC) and several other cannabinoid agonists produce greater antinociceptive effects in female compared to male rats (Tseng & Craft, 2001; Romero et al., 2002; Craft et al., 2012). Sex differences in response to cannabinoids may be due to activational effects of gonadal steroid hormones; that is, the different hormone milieu in adult females vs. males may alter their responses to cannabinoids. For example, THC-induced antinociception was greater in female rats tested during late proestrus compared to females tested during estrus and compared to males (Wakley et al., 2011). In rats that were gonadectomized (GDX) as adults, estradiol (E2) treatment had variable effects, either increasing females’ sensitivity to the antinociceptive effects of a single dose of THC (Craft & Leitl, 2008; Wakley et al., 2014), or not significantly affecting antinociceptive potency of THC, whereas progesterone decreased THC's antinociceptive potency (Wakley et al., 2015). Testosterone (T) did not significantly alter GDX males’ antinociceptive sensitivity to THC, but did reduce THC-induced locomotor suppression or catalepsy (Craft & Leitl, 2008; Wakley et al., 2015).
One possible mechanism of gonadal hormone modulation of (and sex differences in) THC sensitivity involves hormone modulation of THC metabolism. Female rats given THC are known to produce more of its major active metabolite, 11-OH-THC, than males do (Narimatsu et al., 1991; Wiley & Burston, 2014), which may contribute to greater and more prolonged THC effects in females compared to males; 11-OH-THC is as potent and efficacious as THC, if not more, in both rats (Ford et al., 1977; Tseng & Craft, 2001) and humans (Lemberger et al., 1972). We previously reported that reducing THC metabolism by pre-treating rats with the cytochrome P450 (CYP) inhibitor proadifen decreased THC-induced antinociception in gonadally intact females but not males, suggesting that greater 11-OH-THC production in females contributes to sex differences in THC-induced antinociception (Tseng et al., 2004). However, both E2 and T can influence hepatic production of the CYP enzymes responsible for metabolism of drugs such as THC. For example, E2 restored CYP2C7 activity in GDX female rats to the level found in gonadally intact females, and increased the level of CYP2C7 activity in male rats, whereas T appeared to be necessary for the normal expression of CYP2C11 in males (Bandiera & Dworschak, 1992).
The present study had two aims. First, we determined whether T and E2 modulated sensitivity to the acute antinociceptive and motoric effects of THC in the same way in adult males and females. If each hormone modulates THC sensitivity similarly in adults of both sexes, this would suggest that the underlying mechanism is the same in both sexes, and that early sexual differentiation (via organizational effects of gonadal hormones or sex chromosome effects) is not necessary for sex-specific responses to THC in adulthood. In contrast, if T and E2 do not modulate THC sensitivity similarly in adult males and females, this would suggest that rats are already sexually differentiated by adulthood in a way that makes them unresponsive or less responsive to the “opposite-sex” hormone, and thus sexual differentiation of THC sensitivity in adult rats must also be due to organizational hormone effects or sex chromosome effects (Becker et al., 2005). A previous study showing that adult gonadectomy did not alter sex differences in THC's antinociceptive potency (Wakley et al., 2015) suggests that mechanisms other than activational hormone effects contribute to sexual differentiation of THC sensitivity in rats.
The second aim of the study was to determine whether hormonal modulation of THC's behavioral effects can be attributed to hormonal modulation of THC metabolism. Half of the rats in each hormone group were pre-treated with the CYP inhibitor proadifen to significantly decrease THC metabolism (Estevez et al., 1974). By increasing THC levels over the 4-hr testing period, we predicted that proadifen would enhance and prolong THC-induced antinociception; however, the proadifen effect would be modulated by gonadal hormones given that they also regulate CYP enzymes responsible for drug metabolism (Bandiera & Dworschak, 1992). Antinociceptive and motoric effects of THC were compared between GDX+0, GDX+T and GDX+E2 rats of each sex pretreated with saline or proadifen, and serum samples were taken immediately after behavioral testing for later quantification of THC, the major active metabolite 11-OH-THC, and the major inactive metabolite THC-COOH.
2. Material and Methods
2.1 Subjects
Male and female Sprague-Dawley rats, 60-102 days old, were used (bred in-house from Harlan stock, Livermore, CA). Rats were housed in same-sex pairs under a 12:12 hr light:dark cycle. Access to rat chow and water was ad libitum except during surgery and testing. Rats were assigned randomly to treatment groups, with the caveat that we avoided assigning same-sex siblings to any treatment group that had 8 or fewer total rats, to ensure genetic variability in each treatment group. All procedures were conducted in accordance with the NIH Guide for Care and Use of Laboratory Animals.
2.2 Apparatus
For the warm water tail withdrawal test, a 2.5-L water bath (Precision Scientifics, Chicago, IL) with the temperature set at 50.0±0.5°C was used. For the paw pressure test, an Analgesy-meter (Ugo-Basile, Varese, Italy) was used. Pressure on the hindpaw, which started at 30 g, increased at a constant rate of 48 g/sec to a maximum of 960 g (20-sec cutoff). Horizontal locomotor activity was measured using a 20 cm × 40 cm × 23 cm Plexiglas cage placed within a photobeam apparatus (Opto-varimex, Columbus Instruments, Columbus, OH): 15 photobeams crossed the width of the cage, 2.5 cm apart and 8 cm above the cage floor. Catalepsy was assessed using a bar test, a 1.5-cm diameter horizontal bar was attached to a ring stand, with the bar set at 12 cm (females) or 15 cm (males) above the surface of the table.
2.3 Surgery
Rats were injected with 3.0 mg/kg chlordiazepoxide and 0.5 mg/kg morphine s.c. Approximately 5 min later, rats were anesthetized with co-administered ketamine (90-100 mg/kg) and xylazine (10 mg/kg) i.p. Ovariectomy in females and orchidectomy in males were conducted as described previously (Stoffel et al., 2003). Immediately after gonadectomy, constant-release Silastic® capsules (0.062 in. i.d./0.125 in. o.d.) were implanted s.c. between the shoulder blades as follows: one 1-mm or 10-mm blank capsule, one 1-mm E2-filled capsule, or one 10-mm T-filled capsule/100 g body weight. We have shown previously that in terms of reproductive behavior and physiology, this T treatment regimen yields GDX males that are similar to gonadally intact males, and the 1-mm E2 treatment yields GDX females that are similar to gonadally intact females in proestrus to estrus (Stoffel et al., 2003). Upon waking from anesthesia, rats were injected with 2.0 mg/kg morphine s.c. as a post-surgical analgesic. After surgery, rats were housed in pairs according to their sex and hormone group, for 21 days before behavioral testing.
2.4 Behavioral Procedure
Rats were pre-tested three times on the tail withdrawal and paw pressure tests the day before drug testing to establish a baseline and to habituate rats to handling. On the drug test day, rats were injected with saline or 25 mg/kg proadifen (SKF525A) i.p.; one h later, 1:1:18 (ethanol:cremaphor:saline) vehicle, or 3 mg/kg (females) or 5 mg/kg (males) THC was injected i.p., and rats were tested for antinociception on the tail withdrawal and paw pressure tests at 15, 30, 60, 90, 120 and 240 min post-injection. Females were given a lower THC dose than males to ensure that any hormone-induced enhancement of THC's effect could be assessed; previous studies indicated that THC is more potent in female than male rats (Tseng & Craft, 2001; Craft et al., 2012), and we wanted. For the tail withdrawal test, the rat was wrapped in a soft cloth with the tail hanging freely; the distal 5 cm of the tail was immersed in the water bath, and latency to withdraw the tail from the water was recorded to the nearest 0.01 sec with a hand-held stopwatch. If no attempt was made to withdraw the tail within 20 sec, the test was terminated to avoid tissue damage. For the paw pressure test, latency to withdraw or attempt to withdraw the paw from the probe was recorded to the nearest 0.1 sec. If no attempt was made to escape when 20 sec (960 g) was reached, the test was terminated to avoid tissue damage. Following the paw pressure test at 30, 60 and 120 and 240 min post-injection, rats were placed into locomotor chambers and the number of photobeam breaks in 5 min was recorded. Following the locomotor test at 30 and 60 min post-injection only (the period of peak THC effect), a catalepsy test was conducted. The rat's forepaws were placed on a raised bar and latency to remove both forepaws or climb onto the bar was recorded to the nearest 0.1 sec with a handheld stopwatch. Rats were taken off the bar after 15 sec if no response was made. Catalepsy testing was limited to two trials during the time of peak THC effect because we have previously found that rats cannot be tested repeatedly on the catalepsy bar, as latencies to respond decrease significantly with repeated testing (Tseng and Craft, 2001).
Following the series of behavioral tests at 240 min post-injection, rats were euthanized and from some rats, trunk blood was collected and centrifuged for 20 min at 2000 rpm; serum was collected and stored at −80°C for later determination of THC and metabolite levels. Additionally, capsules were removed to confirm type and integrity. Some serum samples were lost before analysis, so additional rats were later added to each GDX male and female group; these were not tested in the behavioral procedure but trunk blood was taken at the same time post-THC injection. All protocols were approved by the Washington State University Institutional Animal Care and Use Committee (protocol #4403), and adhered to National Research Council Guidelines (2011).
2.5 Serum cannabinoid analysis
Quantitation of THC and related metabolites in rat blood was accomplished using a liquid chromatography system (Waters Acquity I-Class UPLC, Milford, MA, USA) coupled with a quadrupole time of flight mass spectrometer (QTOF, Waters Xevo G2, Manchester, UK). Each sample was prepared for analysis first by centrifuging at 8000 rpm for 10 min to remove any remaining cells. From the resulting supernatant, 185 uL was spiked with 15 uL of solution containing 200 ppb each of the deuterated standards (THC-d3, OH-THC-d3, and COOH-THC-d3, Cerilliant, Round Rock, TX). Combined with the high resolution and accurate mass of the QTOF platform, these internal standards allow for direct quantitation of each target analyte while minimizing contributions from non-ideal metabolite extraction and instrumental variability. Following the internal standard addition, 400 uL of cold acetonitrile (ACN) was added dropwise to promote protein precipitation while vortexing. Immediately the samples were centrifuged at 4000 g for 10 min at 25°C. 0.6 mL of 1% ammonium hydroxide was added to the sample prior to solid phase extraction (SPE). A mixed mode SPE cartridge (OAXIS Max 1 cc, Waters, Ireland) was used as the primary means of cannabinoid extraction. Each SPE cartridge was conditioned with 1 mL of methanol followed by 1 mL of 1% ammonium hydroxide. Once conditioned, the newly centrifuged sample was loaded onto the SPE cartridge and pulled through the system using a light vacuum (~1-2 psi). Then 0.5 mL of 35% ACN was added and allowed to dry under vacuum for 10 min. Samples were eluted using 1.5 mL of a hexane/ethyl acetate/acetic acid (49:49:2, v/v/v) mixture. The eluent now containing the target analytes was then evaporated under nitrogen at room temperature. Samples were finally reconstituted in 100 uL of a methanol:water solution (80:20, v/v), vortexed, and transferred to an autosampler vial. Analyte separation was achieved using a 50-mm C18 BEH UPLC column (Waters, Milford, MA, USA) held at 40°C. High purity water (Fisher Scientific Co., Fair Lawn, NJ) with 0.1% formic acid (A) and pure acetonitrile (Fisher Scientific Co., Fair Lawn, NJ) with 0.1% formic acid (B) were used as the mobile phases. Initially mobile phase B was increased to 60% from 5% in 0.2 min, followed by an increase to 90% at 3.5 min and held at this level for an additional 0.5 min. At 4 min, mobile B was decreased to its initial condition of 5% within 0.1 min and was held static for 0.9 min to enable column re-equilibration. A total of 10 uL of each prepared sample was injected onto the column with an operational flow rate of 0.3 mL/min.
2.6 Drugs and Hormones
THC was obtained from the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD), and was prepared in a 1:1:18 ethanol:cremaphor:saline solution. Chlordiazepoxide, morphine sulfate, and proadifen were purchased from Sigma-Aldrich (St. Louis, MO), dissolved in physiological saline and administered in volumes of 1.0 ml/kg. Ketamine and xylazine injectable solutions were purchased from Patterson Veterinary Supply (Devens, MA). Crystalline E2 and T were purchased from Steraloids (Newport, RI); hormone-filled Silastic capsules were constructed in-house (Stoffel et al., 2003).
2.7 Data Analysis
Baseline latencies on the tail withdrawal and paw pressure tests were calculated as the mean of three trials conducted for each rat. Because there were sex differences in baseline nociceptive sensitivity, drug data were converted to percent maximum possible effect (%MPE) before analysis of drug effects; additionally, male and female drug data were analyzed separately because females were given a lower dose of THC than males were. %MPE values for the tail withdrawal and paw pressure tests, and # photobeam breaks after drug were analyzed in each sex via 4-way ANOVA, with factors of hormone (3 levels: 0, T, E2), proadifen (2 levels), THC (2 levels), and time post-injection (4-5 levels, repeated). Catalepsy scores were averaged across the two tests, and these mean values were analyzed in each sex via 3-way ANOVA, with factors of hormone (3 levels), proadifen (2 levels) and THC (2 levels). When rats moved their paws along the bar during a catalepsy test, the test was considered invalid and that score was not included in analyses; in several cases in which both scores were dropped (4 of 113 males, 3 of 112 females), the group mean was used as that rat's score. Serum cannabinoid levels (ng/ml or mg/ml) in THC-treated rats were analyzed in each sex via 2-way ANOVA, with factors of hormone (3 levels) and proadifen (2 levels). Post-hoc comparisons were conducted using Tukey's test, or Dunnett's to compare T- and E2-treated groups to the GDX+0 group. Significance level was p≤0.05 for all statistical tests.
3. Results
3.1 Baselines
Baseline nociceptive latencies were assessed the day before drug testing. Females had shorter nociceptive latencies to respond than males: baseline tail withdrawal latencies averaged 3.19 ± 0.09 sec in females vs. 3.57 ± 0.09 in males (F(1,200)=9.33, p=0.003), and baseline paw pressure latencies averaged 4.48 ± 0.11 in females vs. 4.80 ± 0.12 sec in males (F(1,200)=3.74, p=0.06). There were no hormone group differences in nociceptive baselines, and no differences among rats assigned to different drug treatment groups.
3.2 Gonadal Hormone Modulation of THC-induced Antinociception
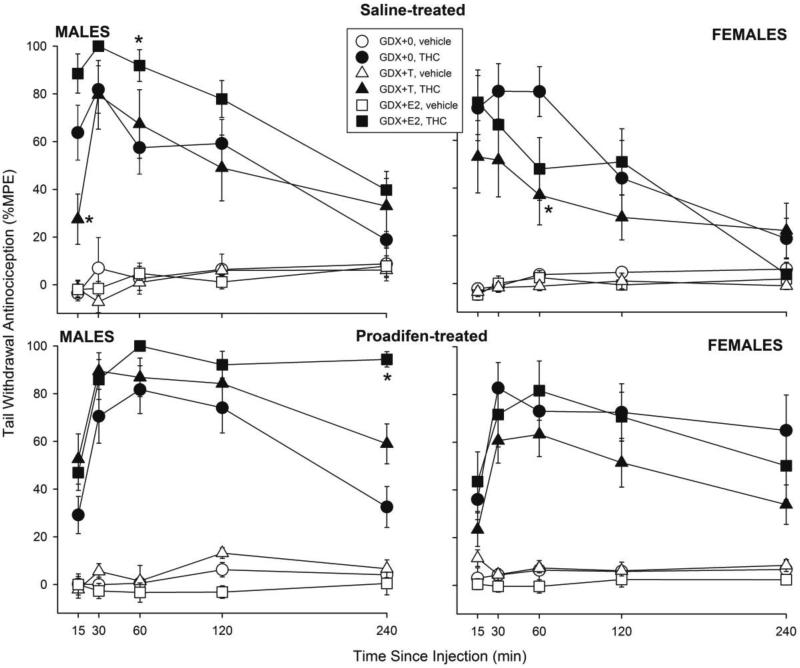
To produce similar levels of antinociception, males were injected with 5 mg/kg THC and females were injected with 3 mg/kg. Figure 1 shows tail withdrawal antinociception in male and female rats in each hormone group that were pretreated with saline (top panels) or proadifen (bottom panels). In GDX males (left panels), E2 increased THC-induced antinociception in both saline- and proadifen-treated groups, with a particularly marked effect at 240 min post-THC injection in proadifen-treated rats; in contrast, T decreased THC-induced tail withdrawal antinociception only at 15 min post-THC injection, in the saline-treated group (Hormone × THC × Proadifen × Time: F(8,400)=3.58, p=0.001; in saline-treated males, Hormone × THC × Time: F(8,196)=2.41, p=0.017); in proadifen-treated males, Hormone × THC × Time: F(8,204)=2.57, p=0.01).
Figure 1.
Gonadal hormone modulation of THC-induced tail withdrawal antinociception in male and female rats. Rats were gonadectomized (GDX) and replaced with no hormone (0), testosterone (T), or estradiol (E2). Three weeks later saline or the cytochrome P450 inhibitor proadifen was injected, and 1 hr later vehicle or THC was administered i.p. (5 mg/kg in males, 3 mg/kg in females, to produce similar levels of antinociception). Each point is the mean ± 1 S.E.M. of 7-12 males or 8-12 females. *significant hormone effect: T- or E2-treated group different from GDX+0 group at same time point, p≤0.05.
In GDX females (Fig. 1 right panels), T decreased THC-induced tail withdrawal antinociception in both saline- and proadifen-treated groups, but this effect was only statistically significant in the saline-treated group (in saline-treated females, Hormone × THC × Time: F(8,204)=1.98, p=0.05; in proadifen-treated females, Hormone × THC: F(2,49)=2.09, p=0.13).
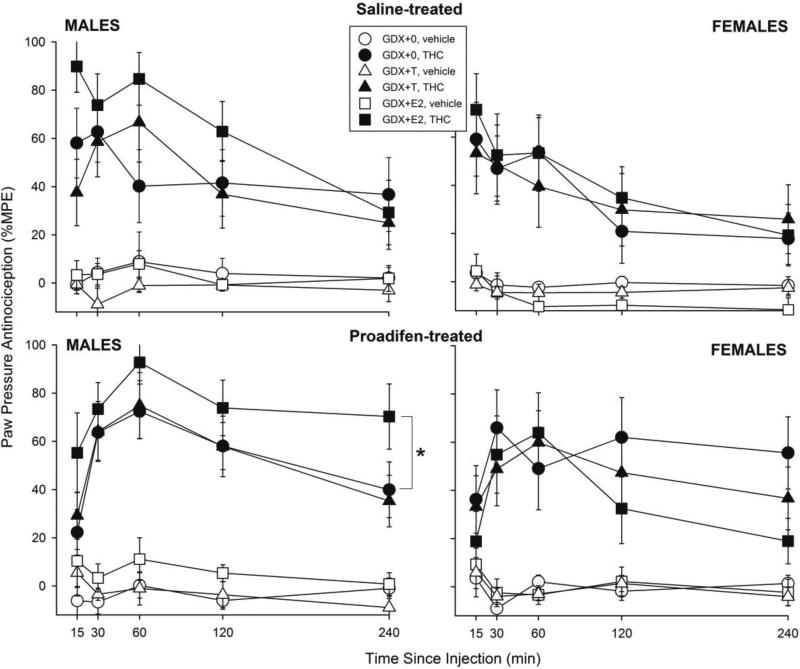
Figure 2 shows paw pressure antinociception in male and female rats in each hormone group that were pretreated with saline (top panels) or proadifen (bottom panels). Similar to the results on the tail withdrawal test, in GDX males (left panels), E2 increased THC's effect on the paw pressure test (Hormone: F(2,100)=6.38, p=0.002). Further analysis revealed that this effect was statistically significant only in the proadifen-treated group (in saline-treated males, Hormone × THC × Time: F(8,196)=1.48, p=0.17; in proadifen-treated males, Hormone: F(2,51)=4.92, p=0.01; 0 vs. E2, p=0.04). In GDX females (Fig. 2 right panels), neither E2 nor T significantly altered THC-induced antinociception on the paw pressure test, in either saline- or proadifen-treated rats.
Figure 2.
Gonadal hormone modulation of THC-induced paw pressue antinociception in male and female rats. Details as described for Fig. 1. *GDX+E2 group significantly different from GDX+0 group, p≤0.05.
3.3 Gonadal Hormone Modulation of THC's Motoric Effects
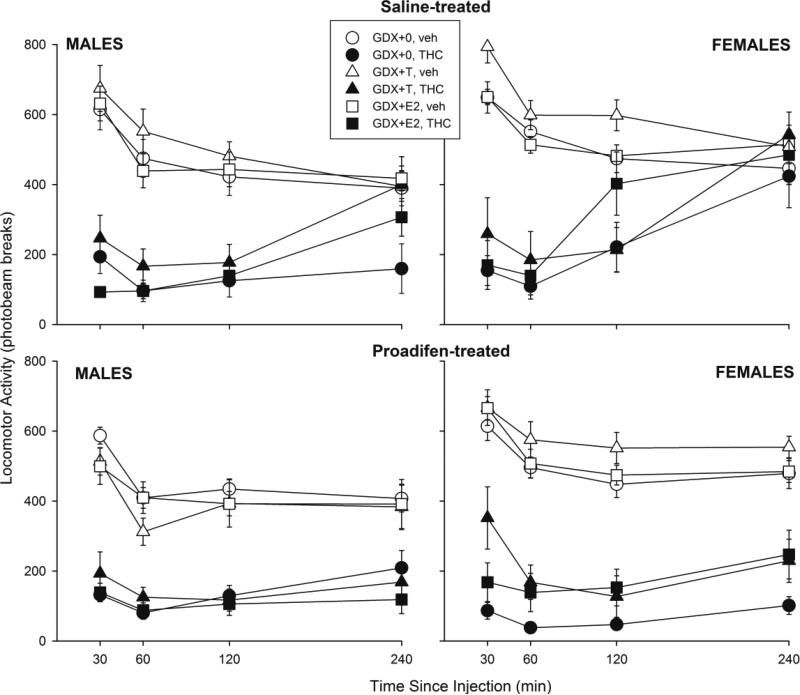
Figure 3 shows locomotor activity in male and female rats in each hormone group that were pretreated with saline (top panels) or proadifen (bottom panels). In GDX males (left panels), THC-induced locomotor suppression appeared to be modulated differently by hormones in saline- vs. proadifen-treated groups (Hormone × THC × Proadifen × Time: F(6,300)=2.98, p=0.008); however, further analyses within the saline-treated groups revealed a non-significant hormone effect (Hormone × THC × Time: F(6,147)=1.90, p=0.08), with T slightly attenuating THC-induced hypolocomotion. In GDX females (Fig. 3 right panels), significant hormone attenuation of THC-induced hypolocomotion was also observed in the overall analysis (Hormone × THC × Time: F(6,300)=2.17, p=0.05). Subsequent analyses revealed a significant hormone effect only in proadifen-treated females (Hormone: F(2,49)=5.01, p=0.01); however, T tended to increase locomotor activity in both vehicle- and THC-treated rats, and the post-hoc test was not significant (0 vs. T, p=0.06).
Figure 3.
Gonadal hormone modulation of THC-induced hypolocomotion in male and female rats. Details as described for Fig. 1.
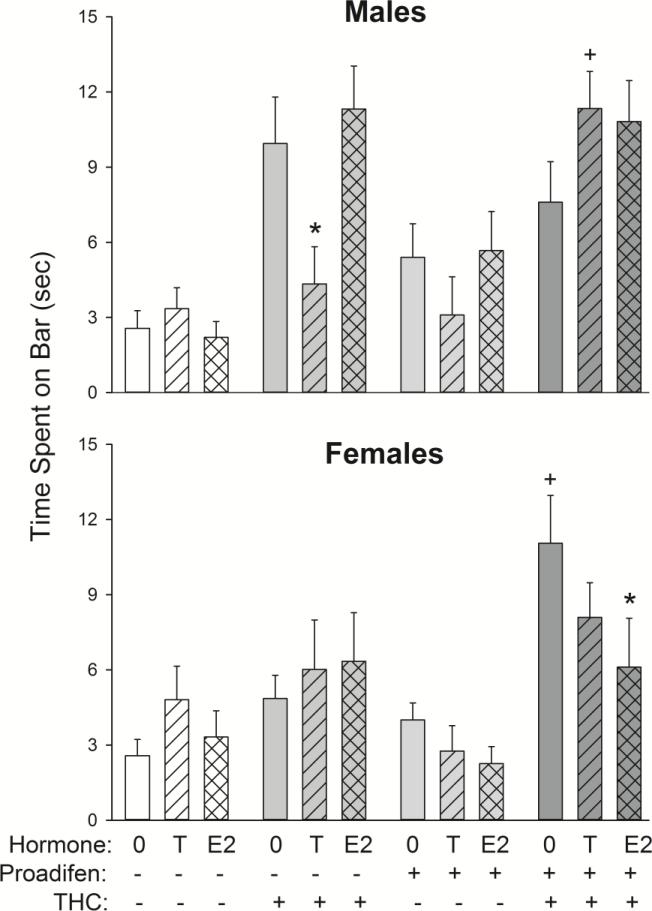
Figure 4 shows catalepsy scores in male and female rats in each hormone group that were pretreated with saline (−) or proadifen (+). In GDX males (top panel), T significantly decreased THC-induced catalepsy in saline- but not proadifen-treated rats (Hormone × THC × Proadifen: F(2,100)=6.82, p=0.002). In GDX females (bottom panel), E2 attenuated catalepsy in proadifen- but not saline-treated rats (Hormone × Proadifen: F(2,100)=3.18, p=0.05).
Figure 4.
Gonadal hormone modulation of THC-induced catalepsy (measured at 30-60 min post-vehicle/THC injection) in male and female rats. Males received 5 mg/kg THC and females received 3 mg/kg THC; catalepsy was assessed at 30-60 min post-injection. Each bar is the mean ± 1 S.E.M. of 7-12 rats. *significant hormone effect: T- or E2-treated group significantly different from GDX+0 group; +significant proadifen effect: proadifen-treated group significantly different from same-sex/hormone, saline-treated group, p≤0.05.
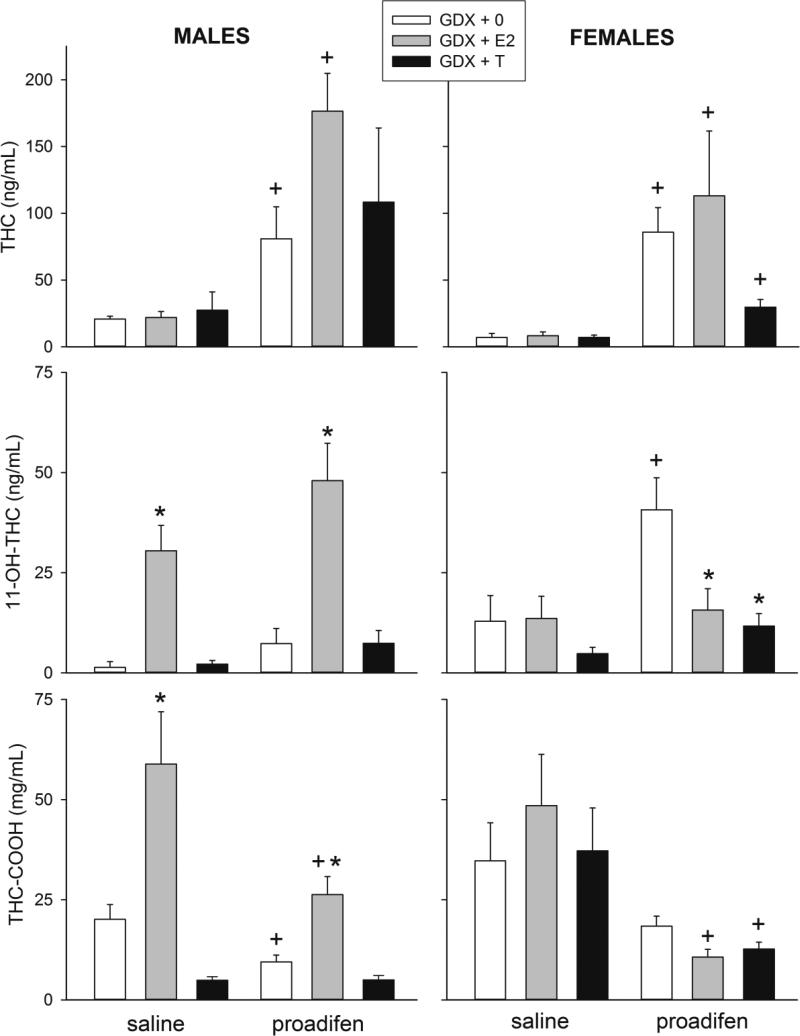
3.4 Serum levels of THC and metabolites
Blood samples were taken after the last behavioral test, at approximately 255 min post-THC injection. Blood samples that were taken from vehicle-treated males and females revealed no detectable THC or metabolites (data not shown). Figure 5 shows serum levels of THC and its major metabolites 11-OH-THC and THC-COOH in males treated with 5 mg/kg THC (left panels) and in females treated with 3 mg/kg THC (right panels). In GDX males, neither E2 nor T significantly altered THC concentrations, although the CYP inhibitor proadifen greatly increased THC levels as expected (Fig. 5, top left panel; Proadifen: F(1,39)=16.56, p<0.001). E2 significantly increased serum levels of the active metabolite 11-OH-THC in both saline- and proadifen-treated GDX males, and proadifen also increased 11-OH-THC (Fig. 5, middle left panel; Hormone: F(2,39)=29.00, p<0.001; Proadifen: F(1,39)=4.73, p=0.04). E2 also increased serum levels of the inactive metabolite THC-COOH in both saline- and proadifen-treated GDX males, and proadifen significantly decreased THC-COOH levels in GDX+0 and GDX+E2 males, but not in GDX+T males (Fig. 5, bottom left panel; Hormone × Proadifen: F(2,39)=3.83, p=0.03).
Figure 5.
Gonadal hormone modulation of serum levels of THC (top panels), its major active metabolite 11-OH-THC (middle panels) and its major inactive metabolite THC-COOH (bottom panels), in THC-treated GDX males (left) and GDX females (right). Males received 5 mg/kg THC and females received 3 mg/kg THC; trunk blood was taken after completion of all behavioral testing, at approximately 255 min post vehicle/THC injection. Each bar is the mean ± 1 S.E.M. of 6-8 samples (rats). *significant hormone effect: T- or E2-treated group significantly different from GDX+0 group; +significant proadifen effect: proadifen-treated group significantly different from same-sex/hormone, saline-treated group, p≤0.05.
Similar to GDX males, in GDX females neither E2 nor T significantly altered serum THC concentrations (Fig. 5, top right panel). However, whereas E2 significantly increased THC metabolite levels in males, E2 did not do so in females (Fig. 5, middle and bottom right panels). As expected, proadifen increased THC levels in all groups of females (Fig. 5, top right panel; Proadifen: F(1,35)=19.92, p<0.001), although this effect was somewhat smaller in GDX+T females than in the other groups (Hormone × Proadifen: F(2,35)=2.63, p=0.09). Regarding 11-OH-THC, again unlike males, proadifen only significantly increased 11-OH-THC in GDX+0 females (Fig. 5, middle right panel; Hormone × Proadifen: F(2,35)=3.45, p=0.04). Proadifen also decreased THC-COOH in GDX females (Fig. 5, bottom right panel; Proadifen: F(1,35)=15.64, p<0.001), but unlike in males, this effect was not hormone-dependent.
4. Discussion
The major questions we sought to answer in this study are: (1) Do the gonadal hormones T and E2 alter THC's behavioral effects in the same way in adult male and female rats? (2) Do T and E2 alter THC metabolism in the same way in adult male and female rats? (3) Can T and E2 effects on behavioral responses to THC be attributed to T and E2 effects on THC metabolism?
In regard to the first question, T and E2 did not modulate THC's behavioral effects similarly in adult males and females. For example, T did not consistently alter THC-induced antinociception in males, but decreased it in females (tail withdrawal test). Conversely, T decreased THC-induced catalepsy in males (saline-pretreated group), but not in females. E2 did not alter THC-induced antinociception in females, but significantly enhanced it in males (tail withdrawal and paw pressure tests). The finding that T and E2 modulate behavioral responses to THC in adult rats agrees with previous studies suggesting that gonadal hormone milieu in adults contributes to sexual differentiation of cannabinoid sensitivity in rats (Craft & Leitl, 2008; Wakley et al., 2011; 2014). The finding that T and E2 did not modulate behavioral sensitivity to THC the same way in both males and females indicates that sexual differentiation of THC sensitivity is not simply due to activational effects of gonadal hormones, but also occurs earlier in development, through organizational hormone or sex chromosome effects (Becker et al., 2005). A previous study reporting that adult gonadectomy did not alter sex differences in antinociceptive sensitivity to THC (Wakley et al., 2015) supports this hypothesis.
We are not aware of any previous studies examining T modulation of cannabinoid effects in females and E2 modulation of cannabinoid effects in males. However, T effects that we observed in males and E2 effects in females agree with some of the few previous studies that have examined gonadal hormone influence on behavioral effects of THC. For example, similar to the present study, two previous studies found that in male rats gonadectomized as adults, T decreased THC-induced motoric effects but did not significantly alter THC's antinociceptive effects (Craft & Leitl, 2008; Wakley et al., 2015). Also similar to the present study, two previous studies reported no significant E2 modulation of cannabinoid-induced motor suppression in GDX female rats or mice (Craft & Leitl, 2008; Kalbasi Anaraki et al., 2008). However, in regard to E2 modulation of THC-induced antinociception, two previous studies reported that E2 increased THC's antinociceptive effect in female rats gonadectomized as adults (Craft & Leitl, 2008; Wakley et al., 2014), one reported that E2 decreased the antinociceptive effect of WIN55,212-2 in GDX female mice (Kalbasi Anaraki et al., 2008), and yet another reported no E2 modulation of THC antinociceptive potency in GDX female rats (Wakley et al., 2015). Thus, the lack of E2 modulation of THC effects in GDX females observed in the present study agrees only with the results reported in Wakley et al. (2015). There are several methodological differences among these studies that may contribute to the discrepant results, including species/strain/vendor of rodent, chronicity of E2 administration, and various aspects of THC administration, although the impact of these factors remains to be tested.
In regard to the second question posed in this study, T and E2 also modulated THC metabolism differently in adult male and female GDX rats. At approximately 4 h after THC administration, T tended to decrease serum THC and 11-OH-THC in females but not males, and E2 significantly increased serum levels of active and inactive metabolites of THC in males but not females, compared to same-sex, GDX+0 controls. Again, while the present results demonstrate activational effects of T and E2 on THC metabolism, the discrepant effects of T and E2 between males and females suggest that sex differences in THC metabolism are not simply due to activational effects of hormones. That is, organizational effects of gonadal hormones or sex chromosomes must contribute to the sexually differentiated response to gonadal hormones observed in adult rats (Becker et al., 2005). A previous study reported that neonatal gonadectomy reversed the sex differences in CYP2C7 and CYP2C11 expression normally found in adult rats (Bandiera & Dworschak, 1992), demonstrating that expression of at least two CYP enzymes is sexually differentiated early in life.
Some of the liver enzymes involved in THC metabolism have been identified. In humans, CYP2C9 appears to be the major isozyme involved in 11-hydroxylation of THC (Cho et al., 1999; Watanabe et al., 2007); in rats, CYP2C11 (males only) and CYP2C6 (females and males, with greater production in females) appear to mediate 11-hydroxylation of THC (Narimatsu et al., 1990; 1992). Although other metabolites have been identified, 11-OH-THC is the major active metabolite produced in both rats and humans, and 11-OH-THC is further oxidized (in two steps) to THC-COOH, the major inactive metabolite in rats and humans (Wall et al., 1983; Agurell et al., 1986; Yamamoto et al., 1995; Nadulski et al., 2005). Sex differences in oxidative metabolism of THC have been observed in the rat: at 1 h post-THC injection, gonadally intact females produced primarily 11-OH-THC, whereas males produced 11-OH-THC plus several other (mostly inactive) metabolites (Narimatsu et al., 1991). We have suggested previously that this sex difference in THC metabolism contributes to sex differences in antinociceptive effects of THC – specifically, greater THC effects in females than males – given that proadifen significantly attenuated THC-induced antinociception in gonadally intact female but not male rats (Tseng et al., 2004). Our interpretation of this finding was that, because females make more 11-OH-THC than males do, blocking oxidation with proadifen would be expected to have a greater impact on antinociception in females than in males. The present study demonstrates that proadifen's sexually differentiated effect on THC metabolism is gonadal hormone-dependent.
The sex differences in and gonadal hormone modulation of THC metabolism observed in the present study agree with findings of several previous studies. First, within several hours after THC administration, brain and/or blood levels of the major active metabolite 11-OH-THC have been reported to be greater in gonadally intact female compared to male rats (Tseng et al., 2004; Wiley & Burston, 2014) and in liver microsome preparations from female compared to male rats (Narimatsu et al., 1991). Although we did not statistically compare males and females in the present study because they were not treated with the same dose of THC, GDX+0 females (regardless of proadifen treatment) had higher 11-OH-THC levels than GDX+0 males, despite the fact that females were given 40% less THC than males (see Fig. 5, middle panels). Furthermore, the finding that E2, and to a lesser extent T, modulated THC metabolism corroborates previous studies demonstrating that these gonadal steroid hormones influence hepatic CYP2C enzyme expression in rats (Bandiera et al., 1986; Bandiera & Dworschak, 1992). In the present study, the fact that E2 significantly increased 11-OH-THC levels in GDX males (and E2 increased males’ antinociceptive response to THC) while E2 did not produce these effects in GDX females was unexpected. This result suggests that E2 in adulthood is not required for maintaining the female behavioral and metabolic phenotypes (i.e., females are sexually differentiated before adulthood, in regard to THC sensitivity). In support of this argument, sex differences in response to cannabinoids have been demonstrated in adolescent rats for some behaviors (for review, see Craft et al., 2013), including antinociception (Romero et al., 2002), and adolescent rats also show sexually differentiated 11-OH-THC production, similar to that observed in adults (Wiley & Burston, 2014).
In regard to the third question, T and E2 modulation of rats’ behavioral responses to THC could reasonably be argued to be due to T and E2 modulation of THC metabolism – in some cases. For example, E2 significantly increased 11-OH-THC production in males, which, given 11-OH-THC's equal or greater potency compared to THC (Ford et al., 1977; Sanders et al., 1979; Browne & Weissman, 1981; Tseng & Craft, 2001), should increase THC's behavioral effects. Greater THC-induced antinociception was indeed observed in GDX+E2 males compared to GDX+0 males (see Figs. 1-2). E2 enhancement of THC-induced antinociception was particularly pronounced at 240 min post-injection in the proadifen-treated male rats, which showed the highest serum THC and 11-OH-THC levels of any group (see Fig. 5). In contrast, E2 did not alter THC's motoric effects (see Figs. 3-4). On the locomotor test, activity was quite low in THC-treated GDX+0 males, so the failure to observe E2 enhancement of THC's sedative effects may have been confounded by a floor effect. Although it should have been possible to observe E2 enhancement of THC-induced catalepsy in males, this behavioral test was conducted at 30-60 min post-THC injection, and we did not measure serum cannabinoid levels at these earlier time points; thus, we cannot draw firm conclusions regarding the relationship between E2 modulation of THC effect on that behavior and E2 modulation of THC metabolism. In summary, E2 enhancement of THC-induced antinociception in GDX males may be due to E2 enhancement of 11-hydroxylation of THC (perhaps by altering the expression of CYP2C isozymes: Bandiera & Dworschak, 1992), but the present study does not provide evidence to support the argument that E2-induced increases in 11-OH-THC production also increase adult males’ sensitivity to the motoric effects of THC.
T modulation of THC's behavioral effects may also be attributable to T modulation of THC metabolism, in some cases. T reduced GDX females’ sensitivity to THC-induced antinociception on the tail withdrawal test, and T also tended to decrease serum THC and 11-OH-THC in GDX females. However, T did not alter females’ responses to THC on the paw pressure test or on tests of motor function. The lack of concordance of T effects across all tests suggests that other pharmacokinetic factors besides THC metabolism (e.g., THC access to anatomical loci mediating particular behavioral effects), and/or pharmacodynamic factors (e.g., T modulation of CB1 receptor expression: Niu et al., 2012) also modulate THC effect.
5. Conclusion
The present study demonstrates that sexual differentiation of behavioral responses to THC are not simply due to activational effects of gonadal hormones. T and E2 can modulate adult rats’ sensitivity to some behavioral effects of THC and to THC metabolism, but T and E2 do not have the same effects in males and females, indicating that sexual differentiation begins to occur earlier in development. In regard to the clinical relevance of this study, it is not yet known whether gonadal hormones modulate cannabinoid effects or metabolism in humans. In regard to sex differences in cannabinoid analgesia, one study reported that oral nabilone was anti-hyperalgesic against experimental heat pain in women but not men (Redmond et al., 2008), whereas another study reported no sex differences in the analgesic effects of smoked dronabinol or marijuana against cold pressor pain, in daily marijuana users (Cooper et al., 2013). Sex differences have been reported for several other effects of cannabinoids in humans (for review, see Craft et al., 2013), and sex differences in THC pharmacokinetics have also been reported in humans (Nadulski et al., 2005). We are not aware of any studies examining, for example, the impact of hormone contraceptives on sensitivity to any cannabinoid drug. However, given that gonadal hormones are known to regulate the expression of some CYP enzymes (Gandhi et al., 2004; Zhang et al., 2007) as well as cannabinoid receptors (Notarnicola et al., 2008) in humans, it is likely that gonadal hormones can alter cannabinoid sensitivity in humans.
Highlights.
Testosterone and estradiol did not similarly modulate THC-induced antinociception in adult male and female rats.
Testosterone and estradiol did not similarly modulate THC metabolism in adult male and female rats.
Within sex, hormone modulation of THC behavioral effect may be attributed to hormone modulation of THC metabolism.
Sexual differentiation of THC sensitivity is not simply due to activational effects of hormones.
Acknowledgments
The authors thank Kelly Hewitt for excellent technical assistance. This work was supported by funds provided for cannabinoid research by the State of Washington Initiative Measure 502, and by the National Institutes of Health (grant DA01644).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agurell S, Halldin M, Lindgren J-E, Ohlsson A, Widman M, Gillespie H, Hollister L. Pharmacokinetics and metabolism of Δ1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol Rev. 1986;38:21–42. [PubMed] [Google Scholar]
- Bandiera S, Dworschak C. Effects of testosterone and estrogen on hepatic levels of cytochromes P450 2C7 and P450 2C11 in the rat. Arch Biochem Biophysics. 1992;296:286–95. doi: 10.1016/0003-9861(92)90574-g. [DOI] [PubMed] [Google Scholar]
- Bandiera S, Ryan DE, Levin W, Thomas PE. Age- and sex-related expression of cytochromes P450f and P450g in rat liver. Arch Biochem Biophys. 1986;248:658–76. doi: 10.1016/0003-9861(86)90521-7. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–73. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Browne RG, Weissman A. Discriminative stimulus properties of Δ9-tetrahydrocannabinol: Mechanistic studies. J Clin Pharmacol. 1981;21:227S–234S. doi: 10.1002/j.1552-4604.1981.tb02599.x. [DOI] [PubMed] [Google Scholar]
- Cho AK, Narimatsu S, Kumagai Y. Metabolism of drugs of abuse by cytochromes P450. Addiction Biol. 1999;4:283–301. doi: 10.1080/13556219971498. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Comer SD, Haney M. Comparison of the analgesic effects of dronabinol and smoked marijuana in daily marijuana smokers. Neuropsychopharmacology. 2013;38:1984–92. doi: 10.1038/npp.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft R, Leitl M. Gonadal hormone modulation of the behavioral effects of Delta(9)-tetrahydrocannabinol in male and female rats. Eur J Pharmacol. 2008;578:37–42. doi: 10.1016/j.ejphar.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Craft RM, Kandasamy R, Davis SM. Sex differences in anti-allodynic, anti-hyperalgesic and anti-edema effects of Δ(9)-tetrahydrocannabinol in the rat. Pain. 2013;154:1709–17. doi: 10.1016/j.pain.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Craft RM, Marusich JA, Wiley JL. Sex differences in cannabinoid pharmacology: a reflection of differences in the endocannabinoid system? Life Sci. 2013;92:476–81. doi: 10.1016/j.lfs.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Wakley AA, Laggart JD. Sex differences in CB1 vs. CB2 receptor-selective antagonism of antinociception produced by delta-9-THC and CP55,490 in the rat. J Pharmacol Exp Ther. 2012;340:787–800. doi: 10.1124/jpet.111.188540. [DOI] [PubMed] [Google Scholar]
- Estevez VS, Englert LF, Ho BT. Effects of SKF-525-A on the metabolism of (-)-Δ9-tetrahydrocannabinol in the rat brain and liver. Res Comm Chem Pathol Pharmacol. 1974;8:389–92. [PubMed] [Google Scholar]
- Ford RD, Balster RL, Dewey WL, Beckner JS. Δ9-THC and 11-OH-Δ9-THC: Behavioral effects and relationship to plasma and brain levels. Life Sci. 1977;20:1993–2004. doi: 10.1016/0024-3205(77)90178-3. [DOI] [PubMed] [Google Scholar]
- Ghandi M, Aweeka F, Greenblatt RM, Blaschke TF. Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol. 2004;44:499–523. doi: 10.1146/annurev.pharmtox.44.101802.121453. [DOI] [PubMed] [Google Scholar]
- Kalbasi Anaraki D, Sianati S, Sadeghi M, Ghasemi M, Paydar MJ, Mehr SE, Dehpour AR. Modulation by female sex hormones of the cannabinoid-induced catalepsy and analgesia in ovariectomized mice. Eur J Pharmacol. 2008;586:189–96. doi: 10.1016/j.ejphar.2008.02.055. [DOI] [PubMed] [Google Scholar]
- Kato R, Yamazoe Y. Sex-specific cytochrome P450 as a cause of sex- and species-related differences in drug toxicity. Toxicol Lett. 1992;64/65:661–67. doi: 10.1016/0378-4274(92)90245-f. [DOI] [PubMed] [Google Scholar]
- Lemberger L, Crabtree RE, Rowe HM. 11-Hydroxy-Δ9-tetrahydrocannabinol: Pharmacology, disposition and metabolism of a major metabolite of marihuana in man. Science. 1972;177:62–3. doi: 10.1126/science.177.4043.62. [DOI] [PubMed] [Google Scholar]
- Nadulski T, Pragst F, Weinberg G, Roser P, Schnelle M, Fronk E-M, Stadelmann AM. Randomized, double-blind, placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of Δ9-tetrahydrocannabinol (THC) after oral application of THC versus standardized cannabis extract. Ther Drug Monit. 2005;27:799–809. doi: 10.1097/01.ftd.0000177223.19294.5c. [DOI] [PubMed] [Google Scholar]
- Narimatsu S, Watanabe K, Matsunaga T, Yamamoto I, Imaoka S, Funae Y, Yoshimura H. Cytochrome P-450 isozymes in metabolic activation of Δ9-tetrahydrocannabinol by rat liver microsomes. Drug Metab Disp. 1990;18:943–48. [PubMed] [Google Scholar]
- Narimatsu S, Watanabe K, Matsunaga T, Yamamoto I, Imaoka S, Funae Y, Yoshimura H. Cytochrome P-450 isozymes involved in the oxidative metabolism of Δ9-tetrahydrocannabinol by liver microsomes of adult female rats. Drug Metab Disp. 1992;20:79–82. [PubMed] [Google Scholar]
- Narimatsu S, Watanabe K, Yamamoto I, Yoshimura H. Sex differences in the oxidative metabolism of Δ9-tetrahydrocannabinol in the rat. Biochem Pharmacol. 1991;41:1187–94. doi: 10.1016/0006-2952(91)90657-q. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide for the care and use of laboratory animals. 8th ed. The National Academies Press; Washington DC: 2011. [Google Scholar]
- Niu KY, Zhang Y, Ro JY. Effects of gonadal hormones on the peripheral cannabinoid receptor 1 (CB1R) system under a myositis condition in rats. Pain. 2012;153:2283–91. doi: 10.1016/j.pain.2012.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notarnicola M, Messa C, Orlando A, Bifulco M, Laezza C, Gazzerro P, Caruso MG. Estrogenic induction of cannabinoid CB1 receptor in human colon cancer cell lines. Scand J Gastroenterol. 2008;43:66–72. doi: 10.1080/00365520701559011. [DOI] [PubMed] [Google Scholar]
- Redmond WJ, Goffaux P, Potvin S, Marchand S. Analgesic and antihyperalgesic effects of nabilone on experimental heat pain. Curr Med Res Opin. 2008;24:1017–24. doi: 10.1185/030079908x280635. [DOI] [PubMed] [Google Scholar]
- Romero E, Fernández B, Sagredo O, Gomez N, Urigüen L, Guaza C, de Miguel R, Ramos J, Viveros M. Antinociceptive, behavioural and neuroendocrine effects of CP 55,940 in young rats. Brain Res Dev Brain Res. 2002;136:85–92. doi: 10.1016/s0165-3806(02)00306-1. [DOI] [PubMed] [Google Scholar]
- Sanders J, Jackson DM, Starmer GA. Interactions among the cannabinoids in the antagonism of the abdominal constriction response in the mouse. Psychpharmacology. 1979;61:281–5. doi: 10.1007/BF00432273. [DOI] [PubMed] [Google Scholar]
- Stoffel EC, Ulibarri CM, Craft RM. Gonadal steroid hormone modulation of nociception, morphine antinociception and reproductive indices in male and female rats. Pain. 2003;103:285–302. doi: 10.1016/s0304-3959(02)00457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng A, Craft R. Sex differences in antinociceptive and motoric effects of cannabinoids. Eur J Pharmacol. 2001;430:41–7. doi: 10.1016/s0014-2999(01)01267-5. [DOI] [PubMed] [Google Scholar]
- Tseng A, Harding J, Craft R. Pharmacokinetic factors in sex differences in Delta(9)-tetrahydrocannabinol-induced behavioral effects in rats. Behav Brain Res. 2004;154:77–83. doi: 10.1016/j.bbr.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Wakley AA, Craft RM. Antinociception and sedation following intracerebroventricular administration of Δ9-tetrahydrocannabinol in female vs. male rats. Behav Brain Res. 2011;216:200–6. doi: 10.1016/j.bbr.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Wakley AA, McBride AA, Vaughn LK, Craft RM. Cyclic ovarian hormone modulation of supraspinal Δ9-tetrahydrocannabinol-induced antinociception and cannabinoid receptor binding in the female rat. Pharmacol Biochem Behav. 2014;124C:269–77. doi: 10.1016/j.pbb.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Wakley AA, Wiley JL, Craft RM. Gonadal hormones do not alter the development of antinociceptive tolerance to delta-9-tetrahydrocannabinol in adult rats. Pharmacol Biochem Behav. 2015;133:111–21. doi: 10.1016/j.pbb.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall ME, Sadler BM, Brine D, Taylor H, Perez-Reyes M. Metabolism, disposition, and kinetics of delta-9-tetrahydrocannabinol in men and women. Clin Pharmacol Ther. 1983;34:352–63. doi: 10.1038/clpt.1983.179. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Yamaori S, Funahashi T, Kimura T, Yamamoto I. Cytochrome P450 enzymes involved in the metabolism of tetrahydrocannabinols and cannabinol by human hepatic microsomes. Life Sci. 2007;80:1415–19. doi: 10.1016/j.lfs.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Burston JJ. Sex differences in Δ9-tetrahydrocannabinol metabolism and in vivo pharmacology following acute and repeated dosing in adolescent rats. Neurosci Lett. 2014;576:51–55. doi: 10.1016/j.neulet.2014.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto I, Watanabe K, Narimatsu S, Yoshimura H. Recent advances in the metabolism of cannabinoids. Int J Biochem. 1995;27:741–6. doi: 10.1016/1357-2725(95)00043-o. [DOI] [PubMed] [Google Scholar]
- Zhang H, Cui D, Wang B, Han Y-H, Balimane P, Yang Z, Sinz M, Rodrigues AD. Pharmacokinetic drug interactions involving 17α-ethinylestradiol. Clin Pharmacokinet. 2007;46:133–57. doi: 10.2165/00003088-200746020-00003. [DOI] [PubMed] [Google Scholar]