Abstract
BACKGROUND
The effect of dog exposure on the risk of children developing allergic disease remains controversial. Many analyses have not considered that associations may vary within population subgroups.
OBJECTIVE
Examine whether associations between living with a dog in the first year of life and allergic outcomes vary within subgroups selected a priori (race, gender and delivery mode).
METHODS
Black (n=496) and White (n=196) children enrolled in the WHEALS birth cohort study had a clinical examination at age 2 years to assess eczema and allergen-specific IgE (sIgE) and perform skin prick testing (SPT). Whether the child lived with an indoor dog in the first year of life was assessed through interview, as was doctor diagnosis of asthma at ages 3–6 years.
RESULTS
Living with a dog was associated with decreased odds of having ≥1 positive SPT (OR=0.56, 95%CI 0.34, 0.91) and having eczema (OR=0.34, 95%CI 0.20, 0.60). The association with SPT was stronger in those children born via cesarian-section versus vaginally (OR=0.29, 95%CI 0.12, 0.74 versus OR=0.76, 95%CI 0.43, 1.37, respectively, interaction p=0.087) and in those who were firstborn versus not (OR=0.27, 95%CI 0.11, 0.67 versus OR=0.82, 95%CI 0.45, 1.47, respectively, interaction p=0.044). The association with eczema was stronger in children born vaginally compared with those born via cesarian-section (OR=0.17, 95%CI 0.06, 0.43 versus OR=0.65, 95%CI 0.31, 1.35, respectively, interaction p=0.025) and was stronger in Black versus White children (OR=0.30, 95%CI 0.15, 0.61 versus OR=0.78, 95%CI 0.29, 2.11, respectively, interaction p=0.12). Dog keeping was not significantly inversely associated with having ≥1 elevated sIgE and only approached statistical significance with asthma.
CONCLUSIONS AND CLINICAL RELEVANCE
Results likely vary between studies due to variability of specific exposure-outcome associations in subgroups defined by other factors as well as the relative distributions of those subgroups. Important allergic disorder associations will be missed without subgroup analyses.
Keywords: allergic disease, racial disparities, asthma, atopy, allergic sensitization, dog, pet
INTRODUCTION
The effect of exposure to dogs on the risk of children developing allergic disease remains a controversy. A meta-analysis of 26 publications from 21 birth cohort studies that examined pet exposure and risk of atopic dermatitis (AD) in children reported a “favorable effect” of exposure to dogs and pets on the risk of AD in infants or children.[1] An earlier meta-analysis of birth cohorts of European children concluded that having a dog in the first two years of life was associated with decreased odds of sensitization to at least one aero-allergen.[2] Another recent study from Melbourne reported that dog exposure was protective for food allergy in their cohort of young children.[3] However, a recent study from Finland reported that dog and cat exposure in early life was associated with increased risks of pet allergies.[4]
A question that has received little attention is whether there are any population subgroup-specific differences in observed associations between dog exposure and allergy development. As opposed to adjusting for other risk factors, many reports do not include analyses stratifying by subgroups defined by other risk factors. These subgroup differences, or effect measure modification, could explain, at least in part, some of the differing results (direction and magnitude of association) appearing in the literature. The study of subgroups is not unprecedented in allergic disease research [5], but has predominantly been focused in gene-environment interaction studies, which have highlighted its importance. Much attention has been given to the modifying effects of genes on exposures in studies of allergies and asthma [6–13]. This has been a popular approach but these subgroups analyses have not expanded rapidly to include the study of the modifying effects of lifestyle and environmental factors. By omitting examinations of the potential modifying effects of other non-genetic factors, we may fail to detect important obscured associations or report associations that are only relevant for a majority subgroup of the population. The purpose of this study was to examine whether any associations between dog keeping in the first year of life and various allergic disease related outcomes (eczema, skin prick tests, and allergen-specific sensitization in children at 2 years of age; parentally reported doctor diagnosis of asthma at age 3–6 years) varied within subgroups defined by other important putative risk factors. The analyses examined whether associations varied within subgroups that were selected a priori, including, but not limited to subgroups defined by race, gender and delivery mode.
METHODS
Study Population
The study population for these analyses is comprised of the offspring of the participants in “WHEALS” – a cohort study that enrolled pregnant women who were aged 21–49 years, had reached their second trimester, were from a predefined geographic region, and who were receiving care at one of five Henry Ford Health System (HFHS) obstetrics clinics in urban and suburban Detroit, Michigan. Recruitment began in September 2003 and the last child was born in December 2007. These analyses combine information from the interviews, a clinic visit with a study physician and results from biological and environmental samples. Details of the cohort’s creation have been published.[14] All work was approved by the HFHS Institutional Review Board.
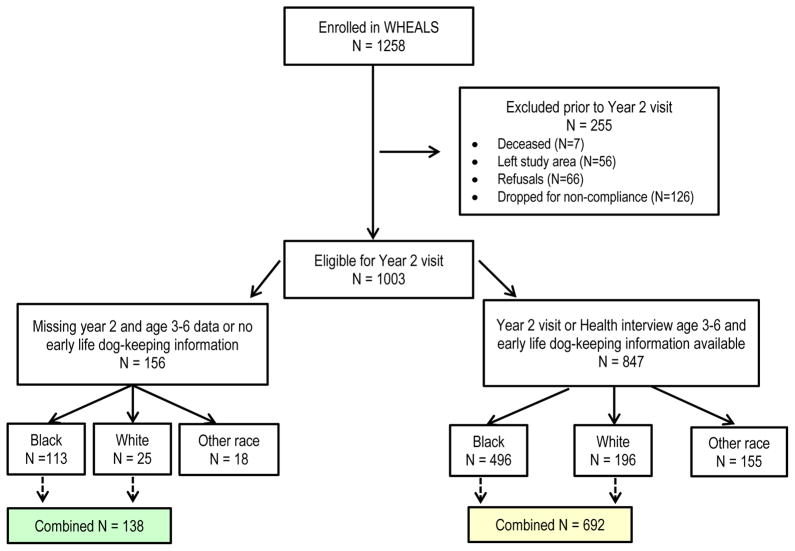
There were 1003 children who were eligible for a 2-year interview and clinic visit. Of these 1003, 830 were either Black (n=609) or White (n=221) and neither Hispanic nor Middle Eastern. The child’s race was based on maternal report for her child. Only those children who are Black or White and neither Hispanic nor Middle Eastern were included in the analyses. The other racial/ethnic subgroups of children were too few in number to analyze as subgroups. Participants who were included and not included in the analyses are compared to assess differences (Table 1). The children included in the present analyses have information on at least one outcome at age 2 years (skin prick testing (SPT), allergen specific IgE (sIgE), evaluation of eczema/atopic dermatitis (AD)) or parental report of doctor diagnosis of asthma at age 3–6 years. Further details on the inclusion and exclusion counts are presented in Figure 1.
Table 1.
Comparison of WHEALS children included or excluded from the current analyses.*
| Child Included in the Analyses | Child Excluded from the Analyses | p value | |
|---|---|---|---|
| N | 692 | 138 | |
| Firstborn | 275 (39.7%) | 43 (31.2%) | 0.058 |
| Ever breastfed the child (n(%) yes) | 508 (76.3%) | 84 (73.7%) | 0.55 |
| Child lived with a smoker | 183 (26.5%) | 45 (32.6%) | 0.14 |
| Mother sensitized to allergens | 390 (58.0%) | 77 (59.2%) | 0.80 |
| Child’s gender | |||
| Male | 348 (50.3%) | 66 (47.8%) | 0.60 |
| Female | 344 (49.7%) | 72 (52.2%) | |
| Race | |||
| Black | 496 (71.7%) | 113 (81.9%) | 0.013 |
| White | 196 (28.3%) | 25 (18.1%) | |
| Delivery type | |||
| Vaginal | 435 (63.0%) | 89 (64.5%) | 0.73 |
| C-section | 256 (37.0%) | 49 (35.5%) | |
There are some missing data. P-values are for a comparison of those included and those excluded.
Figure 1.
The exclusion steps for creation of the analytical data set.
Data Collection
Information on maternal reproductive history was collected via interview during pregnancy at the time of recruitment, as was a maternal blood sample. Additional interviews were conducted with the mothers at 1, 6 and 12-month home visits about the health of their children, breastfeeding and exposure to animals as part of the WHEALS protocol. A telephone interview was conducted at child age 3–6 years to collect information on changes to the child’s health including whether the child had ever been diagnosed with asthma. Dust samples collected from the children’s bedroom floor in the first year of life were analyzed for dog (Can f 1), cat allergen (Fel d 1), cockroach allergen (Bla g 2), dust mite (Der f 1) and endotoxin using previously published methods.[15]
At age 2 years, children were invited for a standardized clinical evaluation by a physician. Using a patient history and physical examination, the physician was then asked to record a response to the following question: “By your clinical evaluation do you believe that this child has or has had atopic dermatitis or eczema?” (hereafter referred to as eczema). The average age at completion of the 2-year clinic visit was 2.2 years (standard deviation (SD) = 0.2 years).
During each interview, a census of dogs kept by the child’s family was performed. Information was also collected about the amount of time the dog usually spent indoors. Indoor dog exposure was defined as having a dog inside the home at least 1 hour per day in the child’s first year of life. The first year of life was selected as we and others have previously shown this to be the time period of exposure associated with subsequent allergy-related outcomes.[16–22]
Skin prick testing was performed by the prick-puncture method on the volar surfaces of the forearms using a Duotip-test® device (Lincoln Diagnostics, Inc., Decatur, IL) for Alternaria, cat, cockroach, dog, Dermatophagoides farinae (Der f), short ragweed, timothy grass, egg, milk and peanut when not contraindicated. A negative saline control and a positive histamine control, 1 mg/ml, were included with all tests. The extracts for skin prick testing were commercial extracts. Wheal and flare responses were measured as the longest diameter 15 minutes after test placement. A positive skin prick test was defined as one producing a wheal with the longest diameter at least 3 mm greater than the negative control.
Levels of allergen-specific IgE (sIgE) to the same allergens were measured in blood samples collected at the clinic visit. sIgE measurement was performed using the Pharmacia UniCAP system (Pharmacia-Upjohn Diagnostic Division, Kalamazoo, Michigan, USA) according to the manufacturer’s standard protocols. Maternal samples were analyzed for a subset of the allergens: Alternaria, cat, cockroach, dog, Dermatophagoides farinae (Der f), short ragweed, timothy grass, and egg. Sensitization was defined as having at least 1 sIgE≥0.35 kU/l.
Statistical Analyses
Characteristics of participants who were included and excluded from the analyses were compared using Chi square and t tests. Percentages (binary outcomes) were used to describe the outcomes. Logistic regression models were used to investigate binary outcomes (at least one positive SPT, at least one positive sIgE, eczema, doctor diagnosis of asthma) to determine whether other factors could explain the observed differences. Odds ratios (OR) and 95% confidence intervals (CI) were calculated based on the models.
Selected variables were assessed for subgroup differences (effect modification) and confounding in our analyses including: whether the mother was sensitized (sIgE≥0.35 IU/mL) to any of the tested allergens, child’s race (maternal report of Black or White), firstborn status, whether the child was ever breastfed, gender, delivery type, whether the child lived with a smoker and whether the mother had more than a high school degree. We chose all variables that were available in our data set that have traditionally been investigated as risk or preventive factors for allergic outcomes. Since not all children had dust samples for analyses, we included additional models for the subset of children who did have dust samples with individual adjustments for the dust sample results (endotoxin, cat, dust mite and cockroach allergen). Stratum specific estimates with 95% confidence intervals are presented. Those interaction terms with p<0.15 were selected for further discussion in the text as potential effect modifiers. Confounders were defined as variables whose inclusion in the model changed the association between living with an indoor dog in the first year of life and the outcome by more than 20%.
We did not analyze the outcomes of dog sIgE and dog allergen SPT due to the small numbers of children who were positive (n=32 and n=9, respectively). In our study population, as expected, we have higher rates of food sensitization and positive SPTs for food at the age of 2 years than for the aeroallergens.[23] We did not conduct specific time period exposure analyses as the numbers were too small to be able to tease apart the association with a dog strictly in the first year of life as 21 children (17 Black children) only had a dog prenatally and 20 children (17 Black children) had a dog only in the second year. These children were retained in the analyses.
Analyses were repeated using dog allergen (Can f 1) from a home dust sample collected at age 1 month in place of living with a dog and was defined as whether or not any dog allergen was detected in the home (lowest detectable limit=25 ng/mL).[24] We have previously shown that dog allergen levels were highly correlated with the keeping of an indoor dog in this cohort.[24] Some dust samples from homes without dogs had detectable Can f 1.
RESULTS
There were 196 White children and 496 Black children who participated in the clinic visit at age two years. Table 1 indicates that children included in the analyses were more likely to have been White and firstborn. Eighty-two (41.8%) of the White children and 107 (21.6%) of the Black children included in the analyses lived with an indoor dog at some time during their first year of life (Table 2). The Black children were more likely to have had a sensitized mother and a mother who had a high school degree or less education and were more likely to have been born via c-section and to have had: at least 1 positive SPT, at least 1 elevated allergen-specific IgE level, and eczema. (Table 2)
Table 2.
Descriptive information about WHEALS children included in the current analyses.*
| Black Children | White Children | p value | |
|---|---|---|---|
| N | 496 | 196 | |
| Firstborn | 190 (38.3%) | 85 (43.4%) | 0.22 |
| Ever breastfed the child (n(%) yes) | 359 (76.1%) | 149 (76.8%) | 0.84 |
| Child lived with dog in first year | 107 (21.6%) | 82 (41.8%) | <0.001 |
| Mother sensitized to allergens | 295 (61.2%) | 95 (50.0%) | 0.008 |
| Child’s gender | |||
| Male | 248 (50.0%) | 100 (51.0%) | 0.81 |
| Female | 248 (50.0%) | 96 (49.0%) | |
| Delivery type | |||
| Vaginal | 298 (60.2%) | 137 (69.9%) | 0.017 |
| C-section | 197 (39.8%) | 59 (30.1%) | |
| Child lived with a smoker | 190 (38.3%) | 62 (31.6%) | 0.10 |
| Child did not live with a smoker | 306 (61.7%) | 134 (68.4%) | |
| Maternal had high school degree or less | 116 (23.4%) | 23 (11.7%) | 0.001 |
| Maternal had more than high school degree | 380 (76.6%) | 173 (88.3%) | |
| Had at least 1 positive skin prick test | 105 (28.8%) | 25 (16.4%) | 0.003 |
| Had at least 1 elevated allergen-specific IgE | 176 (53.8%) | 45 (39.1%) | 0.007 |
| Ever Eczema | 103 (28.0%) | 19 (12.1%) | <0.001 |
| Ever Asthma diagnosis | 64 (14.4%) | 18 (10.2%) | 0.16 |
There are some missing data.
Living with a dog in the first year of life was associated with decreased odds of having at least 1 positive SPT (OR=0.56, 95%CI 0.34, 0.91) and having eczema (OR=0.34, 95%CI 0.20, 0.60) (Table 3). The association with SPT results was slightly stronger in those children born via c-section versus vaginally (OR=0.29, 95%CI 0.12, 0.74 versus OR=0.76, 95%CI 0.43, 1.37, respectively, interaction p=0.087). The associations with SPT were also significantly different in firstborn children versus those not firstborn (OR=0.27, 95%CI 0.11, 0.67 versus OR=0.82, 95%CI 0.45, 1.47, respectively, interaction p=0.044), and in children who lived with a smoker versus those who did not (OR=0.33, 95%CI 0.14, 0.80 versus OR=0.74, 95%CI 0.41, 1.33, respectively, interaction p=0.14). (Table 3)
Table 3.
The odds ratios (95% confidence intervals) between dog keeping in the first year of life and each early life outcome.*
| At least 1 positive SPT | At least 1 elevated sIgE | Eczema | Doctor Diagnosis of Asthma 3–6 Years | |
|---|---|---|---|---|
| Total number positive/Total number of children assessed | 130/517 | 221/442 | 122/525 | 82/620 |
|
| ||||
| All Children | 0.56 (0.34, 0.91) | 0.87 (0.57, 1.33) | 0.34 (0.20, 0.60) | 0.57 (0.31, 1.03) |
| Black | 0.60 (0.33, 1.09) | 0.91 (0.54, 1.52) | 0.30 (0.15, 0.61) | 0.55 (0.26, 1.16) |
| White | 0.66 (0.26, 1.64) | 1.10 (0.51, 2.35) | 0.78 (0.29, 2.11) | 0.72 (0.26, 2.03) |
| Interaction p | 0.88 | 0.69 | 0.12 | 0.67 |
| Vaginal Delivery | 0.76 (0.43, 1.37) | 0.70 (0.41, 1.19) | 0.17 (0.06, 0.43) | 0.52 (0.25, 1.08) |
| C-section | 0.29 (0.12, 0.74) | 1.23 (0.61, 2.47) | 0.65 (0.31, 1.35) | 0.70 (0.25, 1.95) |
| Interaction p | 0.087 | 0.21 | 0.025 | 0.65 |
| Any Breastfeeding | 0.46 (0.26, 0.81) | 0.78 (0.48, 1.25) | 0.44 (0.24, 0.80) | 0.65 (0.34, 1.24) |
| No Breastfeeding | 0.79 (0.26, 2.35) | 1.03 (0.41, 2.57) | Not estimable (zero cell) | Not estimable (zero cell) |
| Interaction p | 0.40 | 0.59 | --- | --- |
| Maternal Sensitized | 0.58 (0.31, 1.06) | 0.84 (0.48, 1.45) | 0.34 (0.17, 0.68) | 0.43 (0.17, 1.04) |
| No Maternal Sensitization | 0.43 (0.17, 1.09) | 0.69 (0.34, 1.39) | 0.25 (0.09, 0.74) | 0.78 (0.35, 1.74) |
| Interaction p | 0.60 | 0.66 | 0.65 | 0.32 |
| Not Firstborn | 0.82 (0.45, 1.47) | 0.80 (0.47, 1.36) | 0.32 (0.15, 0.65) | 0.68 (0.34, 1.38) |
| Firstborn | 0.27 (0.11, 0.67) | 1.01 (0.51, 1.98) | 0.39 (0.16, 0.94) | 0.39 (0.13, 1.17) |
| Interaction p | 0.044 | 0.60 | 0.72 | 0.41 |
| Male | 0.70 (0.37, 1.34) | 1.05 (0.60, 1.84) | 0.37 (0.18, 0.76) | 0.38 (0.15, 0.93) |
| Female | 0.42 (0.20, 0.89) | 0.69 (0.37, 1.29) | 0.30 (0.12, 0.74) | 0.83 (0.38, 1.82) |
| Interaction p | 0.32 | 0.33 | 0.71 | 0.20 |
| Child lived with a smoker | 0.33 (0.14, 0.80) | 1.31 (0.64, 2.69) | 0.51 (0.21, 1.25) | 0.63 (0.23, 1.76) |
| Child did not live with a smoker | 0.74 (0.41, 1.33) | 0.72 (0.42, 1.20) | 0.28 (0.13, 0.56) | 0.54 (0.26, 1.10) |
| Interaction p | 0.14 | 0.18 | 0.29 | 0.79 |
| Maternal had high school degree or less | 0.39 (0.13, 1.14) | 1.02 (0.40, 2.66) | 0.07 (0.01, 0.51) | 0.35 (0.08, 1.61) |
| Maternal had more than high school degree | 0.61 (0.35, 1.06) | 0.84 (0.53, 1.34) | 0.45 (0.25, 0.81) | 0.63 (0.33, 1.20) |
| Interaction p | 0.45 | 0.72 | 0.077 | 0.48 |
Associations are not adjusted. Adjusted associations appear in the Supplemental Tables.
The association between living with a dog and eczema was significantly stronger in children born vaginally compared with those born via c-section (OR=0.17, 95%CI 0.06, 0.43 versus OR=0.65, 95%CI 0.31, 1.35, respectively, interaction p=0.025) and was stronger in Black versus White children (OR=0.30, 95%CI 0.15, 0.61 versus OR=0.78, 95%CI 0.29, 2.11, respectively, interaction p=0.12), and in children who were born to a mother who had a high school degree or less versus more education (OR=0.07, 95%CI 0.01, 0.51 versus OR=0.45, 95%CI 0.25, 0.81, respectively, interaction p=0.077). (Table 3) Dog keeping was not statistically significantly associated with having at least 1 elevated sIgE (OR=0.87, 95%CI 0.57, 1.33).(Table 3) The inverse association between living with a dog and reporting a doctor diagnosis of asthma at age 3–6 years approached, but did not reach, statistical significance (OR=0.57, 95% CI 0.31, 1.03).(Table 3)
The results were not confounded by any of the examined factors. Tables with adjusted estimates are provided in Supplemental Tables 1–4. The results for associations with detectable dog allergen in the home are presented in Supplemental Table 5. The results using dog allergen largely paralleled those of living with a dog in the first year of life with a few additional subgroup differences emerging. A protective association between having detectable dog allergen in the home and having at least one positive skin prick test was noted for children who had any breastfeeding versus those who had no breastfeeding (OR=0.54, 95%CI 0.33, 0.87 versus OR=1.22, 95%CI 0.51, 2.90, respectively, interaction p=0.10). However, the inverse association between having detectable dog allergen in the home and having eczema was stronger for children who had no breastfeeding versus those who had any breastfeeding (OR=0.33, 95%CI 0.14, 0.79 versus OR=0.70, 95%CI 0.43, 1.14, respectively, interaction p=0.14). The association between having detectable dog allergen and eczema also varied by maternal sensitization status (no maternal sensitization OR=0.24, 95%CI 0.11, 0.51 versus maternal sensitization OR=0.88, 95%CI 0.51, 1.51, respectively, interaction p=0.006). The association between having detectable dog allergen in the home and a doctor diagnosis of asthma varied between male and female children (OR=0.59, 95%CI 0.30, 1.17 versus OR=1.25, 95%CI 0.60, 2.57, respectively, interaction p=0.14).
DISCUSSION
Effect Modification
In the participating Black and White children of this Detroit metropolitan area cohort the associations between living with a dog in the first year of life and allergic outcomes in early life varied by outcome and subgroups demonstrating effect modification of the associations. Variability of associations within subgroups is not the same as confounding and it is important to note that it is not possible to “adjust away” subgroup differences. Subgroup differences are evidence of effect measure modification meaning summary results representing the combination of the subgroups are misleading. Furthermore, evaluation of effect measure modification must precede evaluation of confounding.
The challenge with evaluating effect measure modification is that a larger sample size is required to more precisely estimate subgroup associations and identify statistically significant relationships. Importantly, effect modification is not that same as biological interaction. Effect measure modification in this work is based on the traditional epidemiological definition of “heterogeneity of effect” rather than biological interaction.[25] Essentially, associations vary, in strength and/or direction, by subgroup and this means that different factors matter differently depending on whether the other factors are present (or absent).
The associations between living with a dog (or having dog allergen in the home) and allergic outcomes vary in subgroups because the relative “importance” of dog keeping likely depends on the other factors that are also associated with the outcome. For example, living with a dog is more strongly associated with decreased odds of having a positive SPT among children born via c-section than children born vaginally. This could be due to a currently proposed underlying biological mechanism explaining the development of sensitization. Children born by c-section have been shown to have different gut microbiota patterns in early life compared to vaginally delivered children. Perhaps these c-sectioned children with a lack of exposure to the mother’s vaginal and gut bacteria are those that benefit from exposure to the microbes associated with dog contact, yielding measures of association that vary by delivery mode and are protective only for c-section babies.
Dog Keeping and Dog Allergen
While largely consistent, there were some differences in stratum-specific results for analyzing reporting living with a dog in the first year of life and having detectable dog allergen in the home. The discrepancies are likely due to the fact that although dog ownership is associated with higher levels of dog allergen in the home, approximately half of homes who report having no dog in the home had detectable levels of dog allergen.[24] The patterns were similar in that living with a dog and having a detectable level of dog allergen in the home were both generally associated with lower odds of the allergic outcomes.
One potential mechanism for how living with a dog could influence risk of allergic outcomes is that dogs could influence the environmental microbiota. Others and we have found that the house dust microbiota is influenced by pets in the home.[28–30] The alteration of the home microbiota could then influence the gut, airway and skin microbiota of the residents. The gut, airway and skin microbiota are related to immune development and regulation of inflammation.[31–40] The barriers to fully assessing the role of the microbiota to explain the observed associations are that the following are unknown: 1) the gut, airway and skin microbiota “signatures” that define “health” as well as the signatures identifying those at the highest risk for developing eczema, sensitization, food allergy or asthma; 2) the mechanisms by which dogs influence their home microbiota; and 3) the ways in which a home environmental microbiota impacts the gut, airway and skin microbiota of the residents.
The dog allergen results could also be related to the microbiota hypothesis. It is possible that dog allergen may be a better proxy for the home microbiota than dog keeping as some homes without dogs still had detectable dog allergen levels in the dust. Studies of how a dog in the home may change the microbiota are needed to better understand the possible mechanisms.
Other Studies
The study of CD14 as a modifier of the effects of endotoxin on allergy-related outcomes is an excellent example for why interaction should be studied.[11, 12] Interactions between the level of endotoxin and CD14 polymorphisms have been reported to relate to total IgE levels [7], lung function [8, 26], eczema [13] and sensitization [13]. The current work extends this successful approach to the investigation of non-genetic factors and the results suggest that it is an approach that needs continued application in allergic disease research.
A previous example of effect modification involving dog exposure comes from analyses of a birth cohort of children (The Cincinnati Childhood Allergy and Air Pollution Study - CCAAPS) with at least one parent with a positive skin prick test. Campo et al. examined the role of endotoxin on the risk for atopy and wheeze at age 1 year and examined whether the association varied by the presence of dogs in the home.(5) They demonstrated that the association between endotoxin and recurrent wheeze changed in direction (to decreased odds) with the presence of dogs in the home.
Given that most prior research had not examined subgroups differences in associations, we were not sure what to expect for these results. We thought that the children may be too young for asthma to either emerge or be well-diagnosed so we were not surprised by the lack of an association. We were also not surprised to see no association with sIgE. Based on our previous work we knew the children were predominantly sensitized with food allergens and the effect of dog may be more important for development of aeroallergen sensitization.[14, 16, 23, 27]
This work supports and extends the earlier work from our Detroit Childhood Allergy Study (CAS), a birth cohort of predominantly White children born in the same region in 1987–1989.(41) In CAS, children who lived with 2 or more dogs or cats in the first year of life had decreased odds of having a positive skin prick test (adjusted OR=0.23, 95% CI 0.09, 0.60) and at least one elevated sIgE (adjusted OR=0.33, 95% CI 0.13, 0.83) at age 6–7 years. Dog exposure was associated with methacholine response in males (2 or more dogs versus non-exposed: 5.1% versus 25.5%, p=0.03) but not in females (2 or more dogs versus non-exposed: 27.0% versus 22.7%, p=0.71). Asthma also was less common in boys who had dog exposure versus boys who did not (2 or more dogs versus non-exposed: 5.1% versus 11.8%, p=0.43) but the difference was not statistically significant, likely due to the small numbers. While the analyses of CAS data considered subgroups defined by child gender, the analyses did not consider some of the other subgroups considered in the present analyses.
The meta-analysis of pets and atopic dermatitis (AD) from Pelucchi et al. examined 26 publications from 21 birth cohorts.(1) They concluded that exposure to dogs was “favorable” with respect to the risk of AD. They examined summary estimates across selected subgroups defined by study characteristics (e.g., child age at outcome assessment) and whether the estimated measure of association had been adjusted for certain factors such as family history of allergic disease, parental smoking and education. As mentioned above, adjusting for confounding is not the same as assessing effect measure modification by those factors. In a pooled analysis of 11 European birth cohorts, having a dog in the first two years of life was associated with decreased odds of sensitization to at least one aero-allergen, but was not found to affect asthma risk.[2] In reviewing the published results, it’s important to note that some degree of exposure to dogs is likely needed to develop sensitization to dogs.
The results of our study are difficult to compare to other individual studies as many only enrolled high-risk children with family histories of allergies and few have examined effect modification. In their cohort study of children who had at least one parent with allergic disease, Lodge et al. found no association between having a dog at birth and eczema or allergic sensitization (cat dander, dust mite or rye grass) based on skin prick test results at age 2 years.[42] Among a cohort of children in Wisconsin (COAST study) who had at least one parent with respiratory allergies, those born into a home with a dog were less likely to have atopic dermatitis at age 3 years.[22] In the CCAAPS study, Epstein et al. were unable to determine the association between dog-keeping and eczema in their African-American participants as the rate of dog-keeping was too low, but found dog-keeping was associated with a lower risk of eczema at age 4 years in dog-sensitized children.[17]
In a study (SKARP) that examined the medical records of more than 3000 children in Finland in which 307 had allergy tests only for dog, living with a dog before age 1 year was associated with increased risk of having a positive dog allergy test (dog with or without a cat) and physician diagnosis of dog allergy (dog and cat in home).[(4)] A positive allergy test was defined as having either an elevated sIgE or a positive skin prick test. Not all children had allergy testing and it is possible that only those children whose parents sought care for allergies were tested and care seeking patterns could have varied by pet keeping status. In the HealthNuts cohort of more than 5000 children in Melbourne, children who had no allergic diseases (combinations of eczema, sensitization and food allergy) were most likely to have lived in a household with an indoor dog.[3]
It is important to note that the relative size of important subgroups in our analyses (babies delivered by c-section, firstborns, mothers with low levels of education, Black race), varied compared to these other studies. For, example, our study population was predominantly Black race while other studies had much lower percentages of Black children (e.g., <20% in COAST, ~42% in CCAAPS). In WHEALS, the rate of mothers with a high school degree or less was 25% while that rate was <15% in COAST. As these variables appear to strongly impact the relationships between our exposures and outcomes, their differential distributions in other study populations would consequently account for differences in the results.
Strengths and Limitations
As with any epidemiological study, there were strengths and weaknesses to this work. Households with family members allergic to dogs may have chosen to exclude dogs from their homes. Since most of the dogs in the home were present during the pregnancy as well, we are unable to separate the effects of prenatal and early life exposure. Eczema and atopic dermatitis are separate diagnoses but we used the outcome of physician report of “eczema” or “atopic dermatitis” and did not distinguish between these two separate health conditions – another limitation. Additionally, we did not have detailed information on our potential source population and were not able to assess the representativeness of our analyzed population. Many potential effect modifiers and confounders were considered in the analyses, including race; however, the smaller subgroup sample sizes limit the precision of the estimated associations. Finally, while we examined numerous variables for effect modification, the variables studied did not include paternal atopy or environmental factors such as proximity to highways and exposure to exhaust because these data were not available.
In summary, the evidence presented here supports the continuation of investigating the role that living with a dog may play in allergic disease development. Future studies powered to precisely examine important subgroup associations will likely provide key evidence for understanding the complex relationships between factors and allergic outcomes. Existing studies lacking the sample size to permit precise estimates for subgroup associations still contribute important information on patterns of associations that can influence larger studies addressing effect modification.
Supplementary Material
Acknowledgments
This work was funded by NHLBI, NIAID and the Fund for Henry Ford Hospital.
Footnotes
The work was performed at Henry Ford Hospital.
Conflict of Interest
No author has any financial relationships with a biotechnology and/or pharmaceutical manufacturer that has an interest in the subject matter or materials discussed in the submitted manuscript.
References
- 1.Pelucchi C, Galeone C, Bach JF, La Vecchia C, Chatenoud L. Pet exposure and risk of atopic dermatitis at the pediatric age: a meta-analysis of birth cohort studies. J Allergy Clin Immunol. 2013;132(3):616–22. e7. doi: 10.1016/j.jaci.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Lødrup Carlsen KC, Roll S, Carlsen KH, Mowinckel P, Wijga AH, Brunekreef B, et al. Does pet ownership in infancy lead to asthma or allergy at school age? Pooled analysis of individual participant data from 11 European birth cohorts. PLoS One. 2012;7(8):e43214. doi: 10.1371/journal.pone.0043214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters RL, Allen KJ, Dharmage SC, Lodge CJ, Koplin JJ, Ponsonby AL, et al. Differential factors associated with challenge-proven food allergy phenotypes in a population cohort of infants: a latent class analysis. Clin Exp Allergy. 2015;45(5):953–63. doi: 10.1111/cea.12478. [DOI] [PubMed] [Google Scholar]
- 4.Pyrhönen K, Näyhä S, Läärä E. Dog and cat exposure and respective pet allergy in early childhood. Pediatr Allergy Immunol. 2015;26(3):247–55. doi: 10.1111/pai.12369. [DOI] [PubMed] [Google Scholar]
- 5.Campo P, Kalra HK, Levin L, Reponen T, Olds R, Lummus ZL, et al. Influence of dog ownership and high endotoxin on wheezing and atopy during infancy. J Allergy Clin Immunol. 2006;118(6):1271–8. doi: 10.1016/j.jaci.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koplin JJ, Suaini NH, Vuillermin P, Ellis JA, Panjari M, Ponsonby AL, et al. Polymorphisms affecting vitamin D-binding protein modify the relationship between serum vitamin D (25[OH]D3) and food allergy. J Allergy Clin Immunol. 2016;137(2):500–6. e4. doi: 10.1016/j.jaci.2015.05.051. [DOI] [PubMed] [Google Scholar]
- 7.Williams LK, McPhee RA, Ownby DR, Peterson EL, James M, Zoratti EM, et al. Gene-environment interactions with CD14 C-260T and their relationship to total serum IgE levels in adults. J Allergy Clin Immunol. 2006;118(4):851–7. doi: 10.1016/j.jaci.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Pacheco KA, Rose CS, Silveira LJ, Van Dyke MV, Goelz K, MacPhail K, et al. Gene-environment interactions influence airways function in laboratory animal workers. J Allergy Clin Immunol. 2010;126(2):232–40. doi: 10.1016/j.jaci.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sordillo JE, Kelly R, Bunyavanich S, McGeachie M, Qiu W, Croteau-Chonka DC, et al. Genome-wide expression profiles identify potential targets for gene-environment interactions in asthma severity. J Allergy Clin Immunol. 2015;136(4):885–92. e2. doi: 10.1016/j.jaci.2015.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bønnelykke K, Ober C. Leveraging gene-environment interactions and endotypes for asthma gene discovery. J Allergy Clin Immunol. 2016;137(3):667–79. doi: 10.1016/j.jaci.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lau MY, Dharmage SC, Burgess JA, Lowe AJ, Lodge CJ, Campbell B, et al. CD14 polymorphisms, microbial exposure and allergic diseases: a systematic review of gene-environment interactions. Allergy. 2014;69(11):1440–53. doi: 10.1111/all.12454. [DOI] [PubMed] [Google Scholar]
- 12.Martinez FD. CD14, endotoxin, and asthma risk: actions and interactions. Proc Am Thorac Soc. 2007;4(3):221–5. doi: 10.1513/pats.200702-035AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simpson A, John SL, Jury F, Niven R, Woodcock A, Ollier WE, et al. Endotoxin exposure, CD14, and allergic disease: an interaction between genes and the environment. Am J Respir Crit Care Med. 2006;174(4):386–92. doi: 10.1164/rccm.200509-1380OC. [DOI] [PubMed] [Google Scholar]
- 14.Wegienka G, Havstad S, Joseph CL, Zoratti E, Ownby D, Woodcroft K, et al. Racial disparities in allergic outcomes in African Americans emerge as early as age 2 years. Clin Exp Allergy. 2012;42(6):909–17. doi: 10.1111/j.1365-2222.2011.03946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ownby DR, Peterson EL, Williams LK, Zoratti EM, Wegienka GR, Woodcroft KJ, et al. Variation of dust endotoxin concentrations by location and time within homes of young children. Pediatr Allergy Immunol. 2010;21(3):533–40. doi: 10.1111/j.1399-3038.2009.00918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wegienka G, Johnson CC, Havstad S, Ownby DR, Zoratti EM. Indoor pet exposure and the outcomes of total IgE and sensitization at age 18 years. J Allergy Clin Immunol. 2010;126(2):274–9. 9 e1–5. doi: 10.1016/j.jaci.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epstein TG, Bernstein DI, Levin L, Khurana Hershey GK, Ryan PH, Reponen T, et al. Opposing effects of cat and dog ownership and allergic sensitization on eczema in an atopic birth cohort. J Pediatr. 2011;158(2):265–71. e1–5. doi: 10.1016/j.jpeds.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almqvist C, Egmar AC, Hedlin G, Lundqvist M, Nordvall SL, Pershagen G, et al. Direct and indirect exposure to pets - risk of sensitization and asthma at 4 years in a birth cohort. Clin Exp Allergy. 2003;33(9):1190–7. doi: 10.1046/j.1365-2222.2003.01764.x. [DOI] [PubMed] [Google Scholar]
- 19.Sandin A, Björkstén B, Bråbäck L. Development of atopy and wheezing symptoms in relation to heredity and early pet keeping in a Swedish birth cohort. Pediatr Allergy Immunol. 2004;15(4):316–22. doi: 10.1111/j.1399-3038.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- 20.Nafstad P, Magnus P, Gaarder PI, Jaakkola JJ. Exposure to pets and atopy-related diseases in the first 4 years of life. Allergy. 2001;56(4):307–12. doi: 10.1034/j.1398-9995.2001.00881.x. [DOI] [PubMed] [Google Scholar]
- 21.Chen CM, Morgenstern V, Bischof W, Herbarth O, Borte M, Behrendt H, et al. Dog ownership and contact during childhood and later allergy development. Eur Respir J. 2008;31(5):963–73. doi: 10.1183/09031936.00092807. [DOI] [PubMed] [Google Scholar]
- 22.Bufford JD, Reardon CL, Li Z, Roberg KA, DaSilva D, Eggleston PA, et al. Effects of dog ownership in early childhood on immune development and atopic diseases. Clin Exp Allergy. 2008;38(10):1635–43. doi: 10.1111/j.1365-2222.2008.03018.x. [DOI] [PubMed] [Google Scholar]
- 23.Wegienka G, Havstad S, Joseph CL, Zoratti E, Ownby D, Johnson CC. Allergic sensitization frequency and wheezing differences in early life between black and white children. Allergy Asthma Proc. 2012;33(6):493–9. doi: 10.2500/aap.2012.33.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicholas C, Wegienka G, Havstad S, Zoratti E, Ownby D, Johnson CC. Dog characteristics and allergen levels in the home. Ann Allergy Asthma Immunol. 2010;105(3):228–33. doi: 10.1016/j.anai.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rothman, Greenland, Lash . Modern Epidemiology. 3. Philadelphia, PA: LIPPINCOTT WILLIAMS & WILKINS; 2008. [Google Scholar]
- 26.Bakolis I, Doekes G, Heinrich J, Zock JP, Heederik D, Kogevinas M, et al. Respiratory health and endotoxin: associations and modification by CD14/-260 genotype. Eur Respir J. 2012;39(3):573–81. doi: 10.1183/09031936.00164410. [DOI] [PubMed] [Google Scholar]
- 27.Wegienka G, Johnson CC, Havstad S, Ownby DR, Nicholas C, Zoratti EM. Lifetime dog and cat exposure and dog- and cat-specific sensitization at age 18 years. Clin Exp Allergy. 2011;41(7):979–86. doi: 10.1111/j.1365-2222.2011.03747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujimura KE, Johnson CC, Ownby DR, Cox MJ, Brodie EL, Havstad SL, et al. Man’s best friend? The effect of pet ownership on house dust microbial communities. J Allergy Clin Immunol. 2010;126(2):410–2. 2 e1–3. doi: 10.1016/j.jaci.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dannemiller KC, Gent JF, Leaderer BP, Peccia J. Influence of housing characteristics on bacterial and fungal communities in homes of asthmatic children. Indoor Air. 2016;26(2):179–92. doi: 10.1111/ina.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barberán A, Dunn RR, Reich BJ, Pacifici K, Laber EB, Menninger HL, et al. The ecology of microscopic life in household dust. Proc Biol Sci. 2015;282(1814) doi: 10.1098/rspb.2015.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson CC, Ownby DR. Allergies and Asthma: Do Atopic Disorders Result from Inadequate Immune Homeostasis arising from Infant Gut Dysbiosis? Expert Rev Clin Immunol. 2016;12(4):379–88. doi: 10.1586/1744666X.2016.1139452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riiser A. The human microbiome, asthma, and allergy. Allergy Asthma Clin Immunol. 2015;11:35. doi: 10.1186/s13223-015-0102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Logan AC, Jacka FN, Prescott SL. Immune-Microbiota Interactions: Dysbiosis as a Global Health Issue. Curr Allergy Asthma Rep. 2016;16(2):13. doi: 10.1007/s11882-015-0590-5. [DOI] [PubMed] [Google Scholar]
- 34.Allen KJ, Koplin JJ. Prospects for Prevention of Food Allergy. J Allergy Clin Immunol Pract. 2016;4(2):215–20. doi: 10.1016/j.jaip.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Williams MR, Gallo RL. The role of the skin microbiome in atopic dermatitis. Curr Allergy Asthma Rep. 2015;15(11):65. doi: 10.1007/s11882-015-0567-4. [DOI] [PubMed] [Google Scholar]
- 36.Prince BT, Mandel MJ, Nadeau K, Singh AM. Gut Microbiome and the Development of Food Allergy and Allergic Disease. Pediatr Clin North Am. 2015;62(6):1479–92. doi: 10.1016/j.pcl.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denner DR, Sangwan N, Becker JB, Hogarth DK, Oldham J, Castillo J, et al. Corticosteroid therapy and airflow obstruction influence the bronchial microbiome, which is distinct from that of bronchoalveolar lavage in asthmatic airways. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brüssow H. Turning the inside out: The microbiology of atopic dermatitis. Environ Microbiol. 2015 doi: 10.1111/1462-2920.13050. [DOI] [PubMed] [Google Scholar]
- 39.Nermes M, Endo A, Aarnio J, Salminen S, Isolauri E. Furry pets modulate gut microbiota composition in infants at risk for allergic disease. J Allergy Clin Immunol. 2015;136(6):1688–90. e1. doi: 10.1016/j.jaci.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 40.Gern JE. Promising candidates for allergy prevention. J Allergy Clin Immunol. 2015;136(1):23–8. doi: 10.1016/j.jaci.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA. 2002;288(8):963–72. doi: 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- 42.Lodge CJ, Allen KJ, Lowe AJ, Hill DJ, Hosking CS, Abramson MJ, et al. Perinatal cat and dog exposure and the risk of asthma and allergy in the urban environment: a systematic review of longitudinal studies. Clin Dev Immunol. 2012;2012:176484. doi: 10.1155/2012/176484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.