Abstract
Background
Heart transplantation allocation is often restricted from patients with low socioeconomic status (SES) due to concern for worse outcomes. We hypothesized that comorbidities would have a greater impact on risk of severe rejection post-orthotopic heart transplant than would Medicaid insurance and Median Household Income (MHI).
Methods
A retrospective study of 171 patients who underwent orthotopic heart transplant between 7/1999–11/2013 at our facility were followed until 9/2014 for rejection hospitalizations or death. Survival and multivariable analyses with adjustment for age, race, and gender were performed to estimate the risk of severe cellular rejection, ≥2r (hazard ratio [HR], 95% confidence interval [CI]).
Results
Eighteen percent of patients had Medicaid, and 72% of patients had low or medium MHI. Severe rejection occurred in 23% of patients. In the univariable analysis, Medicaid and diabetes were associated with increased risk of rejection while age >60 years, Caucasian race, and male sex were associated with reduced risk [Medicaid 2.32(1.20,4.51), diabetes 2.49(1.09,5.69), age 0.41(0.20,0.84), Caucasian 0.44(0.21,0.93), male 0.49(0.26,0.92)]. Median Household Income had no correlation [MHI 0.79(0.51,1.23)]. In the multivariable adjusted model, Medicaid was not associated with rejection [1.65(0.79,3.41)]; diabetes was strongly associated with risk of severe rejection [3.9(1.59,9.39)], and age >60 years was associated with risk reduction [0.42(0.20,0.82)].
Conclusions
Medicaid insurance and MHI were not associated with increased risk of severe cellular rejection requiring hospitalization post-orthotopic heart transplant in the adjusted model. Rather the presence of diabetes and age ≤60 years were associated with increased risk.
Keywords: Socioeconomic status, Outcomes, Diabetes
Introduction
Hospitalized rejection is associated with worse outcomes post-orthotopic heart transplant.[1–3] Efforts to improve survival have led to further restrictions on patients who are offered advanced therapies.[3–6] Often, underinsured populations are excluded from access to orthotopic heart transplant. In 2003, uninsured patients provided 16.9% of donor organs yet comprised 0.8% of transplant recipients.[6] State safety-net programs and Medicaid allow for some parity in organ allocation. However, some of the existing literature show that underinsurance and low socioeconomic status (SES) are associated with worse outcomes in heart allograft patients particularly from diverse racial/ethnic backgrounds.[2–5,7] We sought to evaluate if comorbidities had a greater impact on initial severe cellular rejection readmission post-orthotopic heart transplant than insurance status and median household income (MHI).
Methods
This study had institutional review board approval. A retrospective evaluation of 171 consecutive patients who underwent orthotopic heart transplant at The Ohio State University Wexner Medical Center from 7/1999 – 11/2013 was performed. Follow-up was through 9/2014 for the outcome of severe cellular rejection requiring hospitalization, classified as International Society for Heart & Lung Transplantation (ISHLT) grade ≥2r on endomyocardial biopsy by center pathologists and cardiologists, or competing risk of death. Insurance status, Medicaid or Non-Medicaid (includes Medicare and private payors) and MHI, which was estimated by the patient’s zip code and corresponding United States 2013 Census data, were assessed. Median Household Income was divided into three even tertiles based upon range of MHI: low MHI was <$38,371, medium MHI was $38,371 – $56,890, and high MHI was >$56,890. Median Household Income was chosen as an estimate of SES given comparable precision to complex neighborhood models for assessment of SES in contemporary studies.[8,9] One patient had no MHI data given the absence of a population in their zip code at the time of the census collection; there were 171 patients with insurance data and 170 patients with MHI data.
The following was assessed during initial heart transplantation hospitalization using the institutional Information Warehouse which is populated from the medical record: demographics (age >60 years [which was chosen given a previous study supporting reduced risk of rejection with age >60 years[2]], race, and sex), presence of common comorbid diseases (atherosclerosis, cerebral vascular accident, diabetes, hypertension, and myocardial infarction), receipt of immunosuppression (tacrolimus, cyclosporine, mycophenolate mofetil, azathioprine, and prednisone), and laboratory values on date of transplant (bilirubin, creatinine, hemoglobin, sodium, and white blood cell count).
Statistical Analysis
Baseline patient characteristics were compared among insurance type and MHI, using chi-square tests for categorical variables and analysis of variance for continuous variables. Cox proportional hazards multivariable analysis with adjustment for age, race, and gender was performed to assess risk of severe rejection (hazard ratio [HR], 95% confidence interval [CI]). Patients were censored at the time of death if death preceded hospitalization as a competing risk. Kaplan-Meier analyses were performed to compare survival times. Statistical analyses were performed using SAS 9.4 (Cary, NC), and the significance level was set at 0.05 for all tests.
Results
Out of a cohort of 171 patients from 1999–2013, the insurance and MHI distribution was the following: Medicaid 18%, Non-Medicaid 82%, Low MHI 22%, Medium MHI 50%, High MHI 27%. Across insurance and MHI groups, there were some significant differences (Table 1). Medicaid patients were on average 14 years younger (p<0.0001), less likely to be Caucasian (p=0.002), and more likely to be a male (p=0.01). Medicaid patients were more likely to be prescribed azathioprine (p=0.04). Otherwise there were no differences in comorbidities, remaining medications, or labs across insurance and MHI groups.
Table 1.
Baseline Characteristics Stratified By Insurance Type and MHI
| Variable | Medicaid % N=31 | Non-Medicaid % N=140 | MHI | ||
|---|---|---|---|---|---|
| Low % N=38 | Medium % N=85 | High % N=47 | |||
| Demographics | |||||
| Age, mean (SD) | 41 (2.2)** | 55 (1.02)** | 52 (1.8) | 54 (1.4) | 52 (2.3) |
| Age Range | 17–64 | 18–70 | 18–70 | 17–69 | 21–70 |
| Race | |||||
| Caucasian | 69** | 90** | 74 | 87 | 91 |
| Sex | |||||
| Male | 55* | 47* | 66 | 79 | 81 |
| Comorbidities | |||||
| Atherosclerosis | 13 | 14 | 18 | 9 | 17 |
| CVA | 9 | 6 | 13 | 5 | 6 |
| Diabetes Mellitus | 66 | 74 | 61 | 76 | 74 |
| Hypertension | 6 | 10 | 5 | 7 | 17 |
| MI | 9 | 29 | 0 | 6 | 4 |
| Medications | |||||
| Immunosuppression Medications | |||||
| All CNI | 69 | 67 | 66 | 68 | 66 |
| Tacrolimus | 41 | 42 | 42 | 40 | 47 |
| Cyclosporine | 35 | 32 | 26 | 36 | 34 |
| Mycophenolate Mofetil | 68 | 72 | 68 | 69 | 68 |
| Azathioprine | 3* | 0* | 3 | 0 | 0 |
| Prednisone | 68 | 72 | 71 | 68 | 66 |
| Labs, mean (SD) | |||||
| Bilirubin mg/dL | 1.19 (0.25) | 1.14 (0.65) | 1.2 (1.27) | 1.2 (0.62) | 1.1 (0.84) |
| Creatinine mg/dL | 1.19 (0.7) | 1.58 (1.4) | 1.68 (1.5) | 1.47 (1.4) | 1.43 (0.8) |
| Hemoglobin g/dL | 10.8 (0.4) | 11.4 (0.5) | 11.2 (1.9) | 11.4 (2.2) | 11.2 (2.0) |
| Sodium mmol/L | 136.1 (0.6) | 136 (0.3) | 136.4(3.8) | 136 (3.3) | 135.8(3.7) |
| WBC K/uL | 11.1 (1.3) | 9.3 (0.4) | 9.9 (6.4) | 9.3 (4.7) | 9.8 (5.5) |
CNI indicates calcineurin inhibitors; CVA, cerebral vascular accident; DL, deciliter; G, gram; K, kilo; L, liter; Mg, milligram; MHI, median household income; MI, myocardial infarction; Mmol, millimol; p, p-value; uL, microliter; WBC, white blood cell count. Bold*, indicates statistical significance with p < 0.05; bold**, p<0.001.
Twenty-three percent had severe rejection requiring hospitalization, and 22% died over the 15-year follow-up. Forty-one percent of patients with Medicaid had severe rejection, and 19% of patients with other insurance had severe rejection (p=0.01) (Table 2, Figure 1). There were significant differences in the ages of patients who had severe rejection (p=0.009). Nineteen percent of male patients compared to 37% female patients had severe rejection (p=0.02). There were differences in medications provided during initial transplant hospitalization for those who developed severe rejection. Patients taking mycophenolate mofetil (p=0.04) and prednisone (p=0.03) compared to patients not taking these medications had higher rates of severe rejection. There were no differences in severe rejection based upon MHI (Figure 2), other comorbidities, nor other medications. Patients taking cyclosporine compared to patients not taking this medication had higher rates of death (p=0.02); otherwise there were no other significant differences between who survived and those who did not (Table 2, Figures 3 and 4).
Table 2.
Covariates Based Upon Severe Rejection or Death
| Severe Rejection % N=40 | p | Death % N= 38 | p | |
|---|---|---|---|---|
| Demographics | ||||
| Medicaid | ||||
| (Yes) | 41 | 0.01 | 18 | 0.6 |
| (No) | 19 | 23 | ||
| MHI | ||||
| (Low) | 26 | 0.51 | 21 | 0.93 |
| (Medium) | 26 | 24 | ||
| (High) | 17 | 21 | ||
| Age | ||||
| Mean (SD) | 48 (13.8) | 0.009 | 54 (13.6) | 0.53 |
| Caucasian | ||||
| (Yes) | 21 | 0.08 | 21 | 0.38 |
| (No) | 38 | 29 | ||
| Male sex | ||||
| (Yes) | 19 | 0.02 | 22 | 0.96 |
| (No) | 37 | 22 | ||
| Atherosclerosis | ||||
| (Yes) | 30 | 0.39 | 26 | 0.63 |
| (No) | 22 | 22 | ||
| CVA | ||||
| (Yes) | 42 | 0.12 | 25 | 0.81 |
| (No) | 22 | 22 | ||
| DM | ||||
| (Yes) | 27 | 0.11 | 20 | 0.29 |
| (No) | 15 | 28 | ||
| Hypertension | ||||
| (Yes) | 6 | 0.09 | 6 | 0.11 |
| (No) | 25 | 24 | ||
| MI | ||||
| (Yes) | 14 | 0.56 | 0 | 0.15 |
| (No) | 24 | 23 | ||
| Immunosuppression Medications | ||||
| Tacrolimus | ||||
| (Yes) | 31 | 0.06 | 18 | 0.26 |
| (No) | 18 | 25 | ||
| Cyclosporine | ||||
| (Yes) | 24 | 0.87 | 33 | 0.02 |
| (No) | 23 | 17 | ||
| Mycophenolate Mofetil | ||||
| (Yes) | 28 | 0.04 | 24 | 0.48 |
| (No) | 13 | 19 | ||
| Azathioprine | ||||
| (Yes) | 24 | 0.58 | 100 | 0.06 |
| (No) | 0 | 22 | ||
| Prednisone | ||||
| (Yes) | 38 | 0.03 | 19 | 0.43 |
| (No) | 13 | 24 | ||
CVA indicates cerebral vascular accident; DM, diabetes mellitus; MHI, median household income; MI; myocardial infarction; p, p-value; SD, standard deviation. Bold, indicates statistical significance with p < 0.05.
Figure 1. Time to Severe Rejection Based On Insurance Type.

This represents unadjusted time to severe rejection based upon having Medicaid or Non-Medicaid health care insurance over 15 years. Patients were censored at the time of death if death preceded hospitalization as a competing risk. The mean time to rejection based upon insurance is also shown.
Figure 2. Time to Severe Rejection Based On MHI.
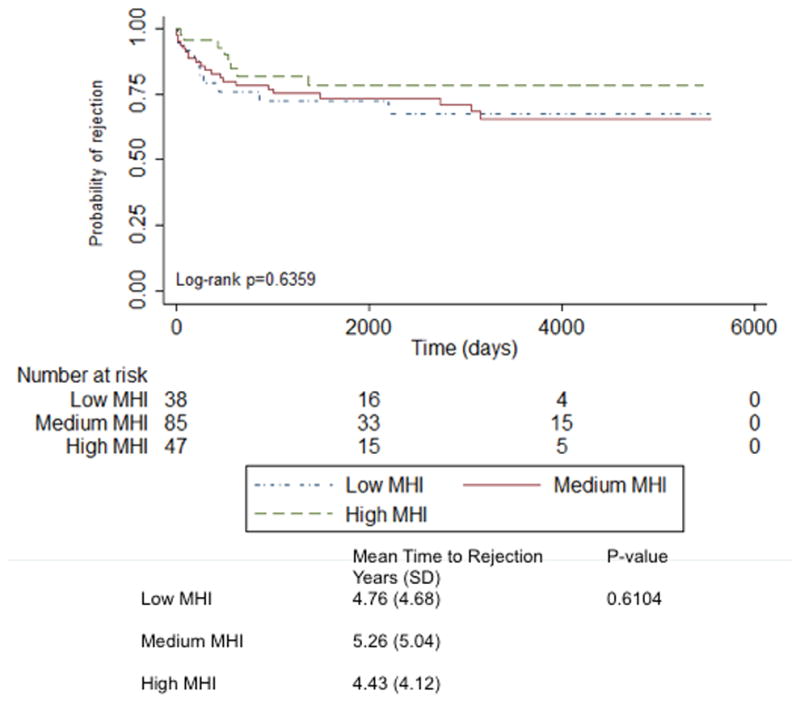
This represents unadjusted time to severe rejection based upon MHI tiers of low, medium, and high over 15 years. MHI indicates median household income. Patients were censored at the time of death if death preceded hospitalization as a competing risk. The mean time to rejection based upon MHI is also shown.
Figure 3. Time to Death Based On Insurance Type.

This represents unadjusted time to death based upon having Medicaid or Non-Medicaid health care insurance over 15 years.
Figure 4. Time to Death Based On MHI.
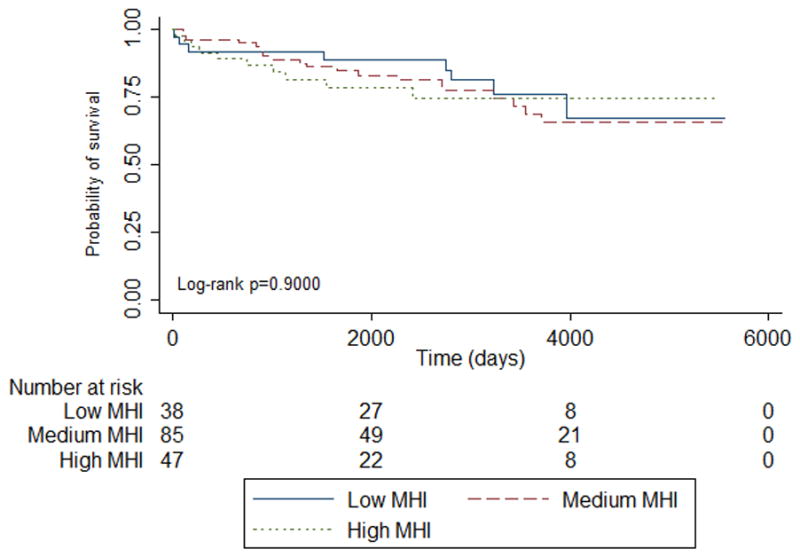
This represents unadjusted time to death based upon MHI tiers of low, medium, and high over 15 years. MHI indicates median household income.
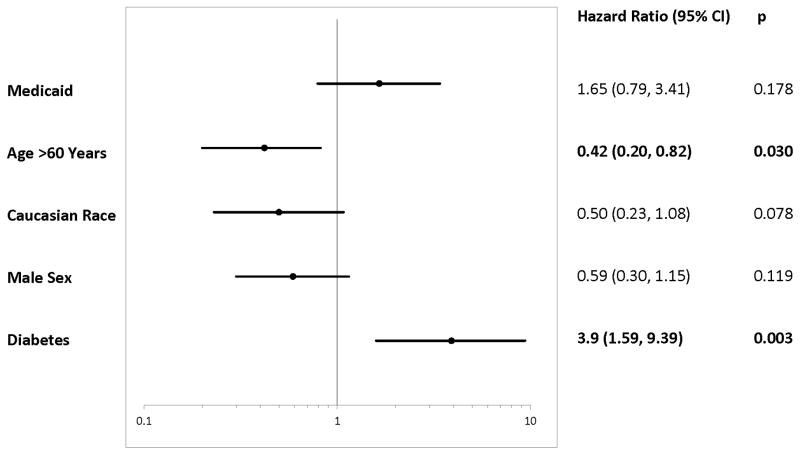
In the univariable analysis, Medicaid, but not MHI, was associated with increased risk of admission for severe rejection [Medicaid 2.32 (1.20, 4.51), MHI 0.79 (0.51, 1.23)], but neither had a significant difference in average time to severe rejection [insurance p= 0.73, MHI p= 0.61] (Figures 1 and 2). Covariates associated with reduced risk included age >60 years, Caucasian race, and male sex [age 0.41 (0.20, 0.84), Caucasian 0.44 (0.21, 0.93), male 0.49 (0.26, 0.92)]. Diabetes predicted increased risk of admission for severe rejection [2.49 (1.09, 5.69)] (Figure 5). Other demographics, comorbidities and medications had no significant association with severe rejection. In the unadjusted multivariable model, Medicaid insurance remained associated with increased risk [2.29 (1.17, 4.50)]. Meanwhile, age >60 years, male sex, and diabetes remained significant [age 0.42 (0.19, 0.91), male 0.49 (0.26, 0.94), diabetes 2.6 (1.14, 5.96)]. However, after adjusting for age, race, and sex in the multivariable model, Medicaid no longer was associated with risk of severe rejection [1.65 (0.79, 3.41)], and male sex was not associated with reduced risk [0.59 (0.30, 1.15)]. The only variables associated with severe rejection in the fully adjusted model were diabetes, increasing risk by nearly four-fold [3.9 (1.59, 9.39)] and age >60 which significantly reduced risk [0.42 (0.20, 0.82)] (Figure 6).
Figure 5. Univariable Analyses of Predictors For Severe Rejection Admission.
Demographics, comorbidities, and medications were used in the univariable analysis. Only Insurance, MHI, and significant covariates are shown. CI, indicates confidence interval; P, p-value; Bold Hazard Ratio, statistical significance with p < 0.05.
Figure 6. Multivariable Adjusted Analyses of Predictors For Severe Rejection Admission.
The model is adjusted for age, race, and gender. CI, indicates confidence interval; P, p-value; Bold Hazard Ratio, statistical significance with p < 0.05.
Discussion
The key findings include: 1) neither Medicaid nor MHI were associated with increased risk of severe rejection post-orthotopic heart transplantation in fully adjusted models; 2) diabetes and age ≤60 years were the only factors associated with increased risk of severe rejection in the fully adjusted model. This suggests that patients with differing forms of health care insurance including safety-net coverage like Medicaid and various MHI backgrounds can have equitable outcomes post-orthotopic heart transplant. However patients with an age ≤60 years and history of diabetes may require additional scrutiny of the immunosuppression regimen, given their heightened risk for severe rejection.
Access to insurance does not equal access to care. Medicaid insurance is the primary form of healthcare coverage for up to 20% of US citizens as of 2015. [10,11] It is a US form of public insurance offered to low income adults, families, pregnant women, children, and disabled citizens.[12] However Medicaid eligibility and coverage policies differ by state.[12] Some state forms of Medicaid are eliminating the option to support advanced therapies like heart transplantation due to concerns for high costs and poor outcomes.[13,14] Several studies have shown that underinsurance and low MHI are associated with worse outcomes post-orthotopic heart transplantation.[2,3] Low socioeconomic status is thought to correlate with: poor follow-up care and reduced adherence to immunosuppression leading to increased risk of allograft rejection.[2,3] Ultimately this trajectory is leading towards greater inequalities in access to care for the poor population.[15]
Our contemporary data suggest that outcomes can be equitable regardless of insurance type and MHI. First, this program employs a multidisciplinary approach to evaluate and manage heart transplantation patients including: assistance with finding financial resources if indicated prior to transplantation (eligibility for Medicaid, Medicare, insurance through a family member, and fund-raising), social support evaluation (inclusion and education of care-giver), formal psychosocial evaluation with Stanford Integrated Psychosocial Assessment for Transplantation scoring and initiation of psychological therapy if indicated, easy access to transplant coordinators pre and post-operatively, and standard multi-medical specialty evaluations. Other centers have also shown that a comprehensive multidisciplinary approach can lead to equity in outcomes across different forms of insurance.[5,16] While utilization of available resources may be higher in lower SES groups, outcomes can still be similar.[5,16]
Second, Medicaid coverage in Ohio is different from other states. Ohio Medicaid ranks in the middle third for eligibility, quality, services offered, and reimbursement compared to the rest of the country.[17,18] The cost of living in Ohio is lower than many states, ranking in the top third of states with the lowest cost of living.[19] Patients with Medicaid in Ohio may have more resources than patients from states that offer lesser Medicaid coverage and have higher costs of living.
Age ≤60 years was linked with a high risk of severe rejection. In our adjusted model, which included age, Medicaid was not associated with risk of rejection. In this study, Medicaid patients were younger than Non-Medicaid patients, which is representative of national profiles given age eligibility requirements for Medicare.[20] Several studies have also shown that younger adults are at higher risk for rejection.[21–23] This has been hypothesized to be largely secondary to immunosenescence, which is a distinct property of aging rather than socioeconomic factors.[21,24] This further highlights the need for fastidious management of immunosuppression regimen in patients ≤60 years regardless of insurance or MHI type.
Diabetes was the only comorbidity associated with risk of severe rejection. While a pre-transplant hemoglobin A1C was not available for this analysis, the institutional protocol followed ISHLT Guidelines by avoiding transplantation in patients with end-organ disease secondary to diabetes and hemoglobin A1C >7.5 at the time of listing.[25] Although the risk of rejection in the setting of pre-transplant diabetes has been mixed in the literature, most studies support the findings of increased risk of rejection with pre-transplant diabetes especially if there is diabetes-related end-organ dysfunction post-transplant.[26–30] In addition, de novo diabetes post-transplant is known to be related to immunosuppression medications and further increases comorbid risks and fatalities.[27,31] This reinforces the need for individual management plans of immunosuppression in the patient with a history of diabetes regardless of insurance type, in addition to good glucose control and management of associated cardiovascular risk factors.[32]
The immunosuppression regimen was associated with outcomes, but the implications are limited in the absence of adherence data. Both mycophenolate mofetil and prednisone are typical medications prescribed upon discharge from heart transplantation hospitalization to prevent rejection, but are also augmented for rejection during initial hospitalization prior to discharge[32]. The increased risk of rejection seen in patients taking mycophenolate mofetil and prednisone may be related to analytical bias. Furthermore, cyclosporine was associated with increased mortality in this study. Cyclosporine usage has been largely replaced by tacrolimus in the heart transplant population due to its known risk of higher mortality, rejection, and comorbidities[33,34].
There are limitations in representing a single center’s experience, which could underestimate the role of insurance type and MHI in post-orthotopic heart transplant outcomes. It is acknowledged that MHI is a crude proxy for SES given that values are obtained via zip code rather than direct patient confirmation; yet this is an accepted form of assessing risks associated with MHI for a given population.[8,9,35–37] Furthermore, a single variable measure, such as MHI, is representative of diverse populations since an index measure, as proposed by Diez-Roux et al., must be validated in each population via factor analysis.[38] Outcomes were based on insurance type and MHI at heart transplantation; thus any possible post-transplantation switchover was not included in this analysis. Adherence to the medication regimen and immunosuppression drug levels was not tracked, but the discharge regimen is still useful to assess risk of admission for severe rejection in this adult population with typically high adherence rates.[39,40] Operative risk factors (pre-operative hospitalization or inotropes, recipient organ sex-mismatch, ischemic time), body mass index, smoking history, and recipient education level were not included given limitations to the retrospective dataset. However at the time of listing, transplant patients cannot be active smokers and generally must have a body mass index of 35 or less.[25] Considering that this analysis was performed in a moderate-sized heart transplantation program (n=10.5–47 transplants/year)[41] spanning approximately 15 years with similar demographics to the average program, the results are meaningful.
Conclusions
In this single center transplant population, Medicaid insurance and MHI were not associated with increased risk of severe cellular rejection requiring hospitalization post-orthotopic heart transplant. The presence of diabetes and age ≤60 years conferred increased risk. Further investigation is needed to improve transplant management of patients who are young and diabetic. Additional study across transplant programs with comprehensive multidisciplinary teams would be warranted to validate these results.
Acknowledgments
Funding Sources: Dr. Breathett received support from a T32 training grant (5T32 HL116276-02) from the National Institute of Health. Dr. Smith received support from the Robert Wood Foundation/Harold Amos Medical Faculty Development Program (72427).
The authors thank the Department of Internal Medicine and Division of Cardiovascular Medicine at The Ohio State University Wexner Medical Center for their support in proceeding with this study.
Footnotes
Author Contributions: Drs. Breathett and Smith conceived and designed the project. Drs. Breathett, Willis, and Smith were responsible for data analysis and data collection. Dr. Breathett was responsible for writing the article, and Drs. Willis, Foraker, and Smith critically reviewed the article and provided additional revisions
Disclosures: The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parra AV, Rodrigues V, Cancella S, Cordeiro JA, Bestetti RB. Impact of socioeconomic status on outcome of a Brazilian heart transplant recipients cohort. Int J Cardiol. 2008;125:142–3. doi: 10.1016/j.ijcard.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 2.Allen JG, Weiss ES, Arnaoutakis GJ, Russell SD, Baumgartner WA, Shah AS, et al. Insurance and education predict long-term survival after orthotopic heart transplantation in the United States. J Heart Lung Transplant. 2012;31:52–60. doi: 10.1016/j.healun.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Singh TP, Givertz MM, Semigran M, DeNofrio D, Costantino F, Gauvreau K. Socioeconomic Position, Ethnicity, and Outcomes in Heart Transplant Recipients. Am J Cardiol. 2010;105:1024–9. doi: 10.1016/j.amjcard.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Morris AA, Cole RT, Veledar E, Bellam N, Laskar SR, Smith AL, et al. Influence of Race/Ethnic Differences in Pre-Transplantation Panel Reactive Antibody on Outcomes in Heart Transplant Recipients. J Am Coll Cardiol. 2013;62:2308–15. doi: 10.1016/j.jacc.2013.06.054. [DOI] [PubMed] [Google Scholar]
- 5.Pamboukian SV, Costanzo MR, Meyer P, Bartlett L, Mcleod M, Heroux A. Influence of race in heart failure and cardiac transplantation: Mortality differences are eliminated by specialized, comprehensive care. J Card Fail. 2003;9:80–6. doi: 10.1054/jcaf.2003.11. [DOI] [PubMed] [Google Scholar]
- 6.Herring AA, Woolhandler S, Himmelstein DU. Insurance Status of U.S. Organ Donors and Transplant Recipients: The Uninsured Give, but Rarely Receive. Int J Health Serv. 2008;38:641–52. doi: 10.2190/HS.38.4.d. [DOI] [PubMed] [Google Scholar]
- 7.Allen JG, Weiss ES, Arnaoutakis GJ, Russell SD, Baumgartner WA, Conte JV, et al. The Impact of Race on Survival After Heart Transplantation: An Analysis of More Than 20,000 Patients. Ann Thorac Surg. 2010;89:1956–64. doi: 10.1016/j.athoracsur.2010.02.093. [DOI] [PubMed] [Google Scholar]
- 8.Foraker RE, Patel MD, Whitsel EA, Suchindran CM, Heiss G, Rose KM. Neighborhood Socioeconomic Disparities and 1-year Case Fatality after Incident Myocardial Infarction: The Atherosclerosis Risk in Communities (ARIC) Community Surveillance (1992–2002) Am Heart J. 2013;165:102–7. doi: 10.1016/j.ahj.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith SA, Hasan AK, Binkley PF, Foraker RE. The impact of insurance and socioeconomic status on outcomes for patients with left ventricular assist devices. J Surg Res. 2014;191:302–8. doi: 10.1016/j.jss.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medicaid Enrollment Surges Across the U.S. US News World Rep; n.d. [accessed August 21, 2015]. http://www.usnews.com/news/articles/2015/02/24/medicaid-enrollment-surges-across-the-us. [Google Scholar]
- 11. [accessed August 21, 2015];Total Monthly Medicaid and CHIP Enrollment. n.d http://kff.org/health-reform/state-indicator/total-monthly-medicaid-and-chip-enrollment/
- 12.(DCD) DCD. Who is eligible for Medicaid? HHS.gov; 2015. [accessed May 10, 2016]. http://www.hhs.gov/answers/medicare-and-medicaid/who-is-eligible-for-medicaid/index.html. [Google Scholar]
- 13.organdonor.gov. [accessed July 30, 2015];Organ Transplantation: The Process. 2014 http://www.organdonor.gov/about/transplantationprocess.html.
- 14.Allen JE, Unit ANM. Arizona Medicaid Cuts Transplants. ABC News; 2010. [accessed July 30, 2015]. http://abcnews.go.com/Health/Health_Care/medicaid-cuts-make-organ-transplants-unaffordable/story?id=12177059. [Google Scholar]
- 15.Deaton A. The Great Escape: Health, Wealth, and the Origins of Inequality. Princeton University Press; 2015. Reprint edition. [Google Scholar]
- 16.Dew MA, Goycoolea JM, Harris RC, Lee A, Zomak R, Dunbar-Jacob J, et al. An internet-based intervention to improve psychosocial outcomes in heart transplant recipients and family caregivers: development and evaluation. J Heart Lung Transplant. 2004;23:745–58. doi: 10.1016/j.healun.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Arellano AR, de Wolfe S. [accessed May 4, 2016];Unsettling Scores, A Ranking of State Medicaid Programs. 2007 http://www.citizen.org/hrg1807#national_results.
- 18.Weissman JS, Zaslavsky AM, Wolf RE, Ayanian JZ. State Medicaid Coverage and Access to Care for Low-income Adults. J Health Care Poor Underserved. 2008;19:307–19. doi: 10.1353/hpu.2008.0021. [DOI] [PubMed] [Google Scholar]
- 19.Cost of Living Data Series 2015 Annual Average. Missouri Economic Research and Information Center; n.d. [Google Scholar]
- 20.Medicare C. Baltimore MS 7500 SB, Usa M: 2014. [accessed August 21, 2015]. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/CMS-Statistics-Reference-Booklet/2014.html. [Google Scholar]
- 21.Renlund DG, Gilbert EM, O’Connell JB, Gay WA, Jones KW, Burton NA, et al. Age-associated decline in cardiac allograft rejection. Am J Med. 1987;83:391–8. doi: 10.1016/0002-9343(87)90746-7. [DOI] [PubMed] [Google Scholar]
- 22.George JF, Pamboukian SV, Tallaj JA, Naftel DC, Myers SL, Foushee MT, et al. Balancing rejection and infection with respect to age, race, and gender: Clues acquired from 17 years of cardiac transplantation data. J Heart Lung Transplant. 2010;29:966–72. doi: 10.1016/j.healun.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Kobashigawa JA, Kirklin JK, Naftel DC, Bourge RC, Ventura HO, Mohanty PK, et al. Pretransplantation risk factors for acute rejection after heart transplantation: a multiinstitutional study. The Transplant Cardiologists Research Database Group. J Heart Lung Transplant Off Publ Int Soc Heart Transplant. 1993;12:355–66. [PubMed] [Google Scholar]
- 24.Bradley BA. Rejection and recipient age. Transpl Immunol. 2002;10:125–32. doi: 10.1016/s0966-3274(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 25.Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J Heart Lung Transplant Off Publ Int Soc Heart Transplant. 2016;35:1–23. doi: 10.1016/j.healun.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 26.Rhenman MJBR. Diabetes and heart transplantation. J Heart Transplant. 2006;7:356–8. [PubMed] [Google Scholar]
- 27.Moro JA, Martínez-Dolz L, Almenar L, Martínez-Ortiz L, Chamorro C, García C, et al. Impact of Diabetes Mellitus on Heart Transplant Recipients. Rev Esp Cardiol. 2006;59:1033–7. doi: 10.1157/13093980. [DOI] [PubMed] [Google Scholar]
- 28.Saraiva J, Sola E, Prieto D, Antunes MJ. Diabetes as an outcome predictor after heart transplantation. Interact Cardiovasc Thorac Surg. 2011;13:499–504. doi: 10.1510/icvts.2010.256321. [DOI] [PubMed] [Google Scholar]
- 29.Morgan JA, John R, Weinberg AD, Colletti NJ, Mancini DM, Edwards NM. Heart transplantation in diabetic recipients: a decade review of 161 patients at Columbia Presbyterian. J Thorac Cardiovasc Surg. 2004;127:1486–92. doi: 10.1016/j.jtcvs.2003.11.063. [DOI] [PubMed] [Google Scholar]
- 30.Russo MJ, Chen JM, Hong KN, Stewart AS, Ascheim DD, Argenziano M, et al. Survival After Heart Transplantation Is Not Diminished Among Recipients With Uncomplicated Diabetes Mellitus An Analysis of the United Network of Organ Sharing Database. Circulation. 2006;114:2280–7. doi: 10.1161/CIRCULATIONAHA.106.615708. [DOI] [PubMed] [Google Scholar]
- 31.Marchetti P. New-onset diabetes after transplantation. J Heart Lung Transplant Off Publ Int Soc Heart Transplant. 2004;23:S194–201. doi: 10.1016/j.healun.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Costanzo MR, Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29:914–56. doi: 10.1016/j.healun.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 33.Grimm M, Rinaldi M, Yonan NA, Arpesella G, Arizón Del Prado JM, Pulpón LA, et al. Superior prevention of acute rejection by tacrolimus vs. cyclosporine in heart transplant recipients--a large European trial. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2006;6:1387–97. doi: 10.1111/j.1600-6143.2006.01300.x. [DOI] [PubMed] [Google Scholar]
- 34.Penninga L, Møller CH, Gustafsson F, Steinbrüchel DA, Gluud C. Tacrolimus versus cyclosporine as primary immunosuppression after heart transplantation: systematic review with meta-analyses and trial sequential analyses of randomised trials. Eur J Clin Pharmacol. 2010;66:1177–87. doi: 10.1007/s00228-010-0902-6. [DOI] [PubMed] [Google Scholar]
- 35.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–10. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foraker RE, Rose KM, Kucharska-Newton AM, Ni H, Suchindran CM, Whitsel EA. Variation in rates of fatal coronary heart disease by neighborhood socioeconomic status: the atherosclerosis risk in communities surveillance (1992–2002) Ann Epidemiol. 2011;21:580–8. doi: 10.1016/j.annepidem.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagstaff A, Watanabe N. What difference does the choice of SES make in health inequality measurement? Health Econ. 2003;12:885–90. doi: 10.1002/hec.805. [DOI] [PubMed] [Google Scholar]
- 38.Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 39.Kl G, AJCW-W Patient compliance at one year and two years after heart transplantation. J Heart Lung Transplant Off Publ Int Soc Heart Transplant. 1998;17:383–94. [PubMed] [Google Scholar]
- 40.Blanca Martínez Pérez A, López Suárez A, Rodríguez Rodríguez J, Sobrino Márquez JM, Lage Gallé E. Medication adherence in patients who undergo cardiac transplantation. Transplant Proc. 2013;45:3662–4. doi: 10.1016/j.transproceed.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 41.Russo MJ, Iribarne A, Easterwood R, Ibrahimiye AN, Davies R, Hong KN, et al. Post–Heart Transplant Survival Is Inferior at Low-Volume Centers Across All Risk Strata. Circulation. 2010;122:S85–91. doi: 10.1161/CIRCULATIONAHA.109.926659. [DOI] [PubMed] [Google Scholar]