Abstract
The P2X7 receptor (P2X7R) is an ATP-gated ion channel that is a key player in oxidative stress under pathological conditions. The P2X7R is expressed in the retinal pigmented epithelium (RPE) and neural retina. Chronic oxidative stress contributes to the pathogenesis of age-related macular degeneration (AMD). Mice lacking Cu, Zn superoxide dismutase (Sod1) developed chronic oxidative stress as well as AMD-like features, but whether the P2X7R plays a causative role in oxidative stress-induced AMD is unknown. Thus, the main purpose of this study was to test if concurrent knockout (KO) of P2X7R could block AMD-like defects seen in Sod1 KO mice. Using multiple approaches, we demonstrate that Sod1 KO causes AMD-like defects, including positive staining for oxidative stress markers, 3-nitrotyrosine and carboxymethyl lysine, thinning of the RPE and retina, thickening of Bruch’s membrane, presence of basal laminar and linear deposits, RPE barrier disruption and accumulation of microglia/macrophages. Moreover, we find that Sod1 KO mice accumulate more microparticles (MPs) within RPE/choroid tissues. Concurrent KO of the P2X7R protects against AMD-like defects and MP accumulation in Sod1 KO mice. Together, we show for the first time, that deficiency of P2X7R prevents in vivo oxidative stress-induced accumulation of MPs and AMD-like defects. This work could potentially lead to novel therapies for AMD and other oxidative stress-driven diseases.
Keywords: P2X7 receptor, Sod1, AMD, retinal pigmented epithelium (RPE), oxidative stress, microparticle (MP)
1. Introduction
Age-related macular degeneration (AMD) is a blinding and devastating disease among people over the age of 50 worldwide. It is caused by the progressive degeneration of retinal pigmented epithelium (RPE) and photoreceptors in outer retina [1,2]. The prevalence of AMD is expected to increase dramatically as the global population ages [3]. As there is no cure for AMD, better understanding of the initiation and progression of AMD is crucial to finding better and earlier treatment modalities [1].
Numerous in vitro and in vivo studies strongly support a key role for oxidative stress in AMD [4]. Mice lacking Cu, Zn superoxide dismutase (Sod1) induced chronic oxidative stress and developed AMD-like features similar to humans [5], but whether the P2X7 receptor (P2X7R) plays a causative role in oxidative stress-induced AMD is unknown. The P2X7R is an ATP-gated ion channel expressed in RPE and photoreceptor cells [6–9]. It is a key player in oxidative stress under pathological conditions and mediates inflammation in a variety of cell types [10,11]. Previously, we and others found that activation of the P2X7R can cause RPE and photoreceptor apoptosis [6,8,9]. Recently, we demonstrated that oxidative stress induces cultured RPE cells to release microparticles (MPs) that carry drusen components [12]. Cell-derived MPs are small membrane-bound extracellular vesicles shed by activated, stressed, or apoptotic cells through membrane blebbing that range in diameter from 100 to 1000 nm [13]. Interestingly, activation of P2X7R also caused plasma membrane blebbing and release of MPs in non-ocular cells [14–17].
Based on the above findings, we hypothesized that the P2X7R could mediate the release of MPs and AMD-like pathology induced by chronic oxidative stress. Therefore, the main purpose of this study was to test if concurrent knockout (KO) of P2X7R could block AMD-like defects in Sod1 KO mice, a model of chronic oxidative stress and AMD [5]. As in vitro oxidative stress caused RPE to release MPs [12], and P2X7R was involved in release of MPs in non-ocular cells [14–17], we also tested whether in vivo oxidative stress could induce MP release and whether P2X7R deficiency could have protective role in the MP release.
2. Materials and methods
Detailed materials and methods are available in the Supplementary Material.
2.1. Mice
All animal experiments were approved by the institutional animal care and use committee at the University of Michigan. All breeder mice, including P2X7R KO mice (strain B6.129P2-P2rx7tm1Gab/J; stock no. 005576) and Sod1 KO mice (strain B6;129S-Sod1tm1Leb/J; stock no. 002972) were obtained from Jackson Laboratories (Bar Harbor, ME, USA). P2X7R KO and Sod1 KO mice were cross-bred in our facility to generate all needed colonies, including wild type (WT) controls, P2X7R KO, Sod1 KO, and P2X7R/Sod1 double-knockout (DKO) mice. Both male and female offspring aged from 2 to 15 months were used for experiments.
2.2. Genotyping
The mice were genotyped using the PCR primers as described in the Supplementary Material.
2.3. Western Blot Analysis
Western blot analysis was performed as previously described [12].
2.4. Fundus Imaging and SD-OCT
Fundus imaging and spectral domain optical coherence tomography (SD-OCT) were performed as described in the Supplementary Material.
2.5. Immunohistochemistry, RPE Flat Mounts and Confocal microscopy
Immunohistochemistry, RPE flat mounts and confocal microscopy were performed as described [5,6,18] with modifications (see Supplementary Material).
2.6. Microparticle Isolation and Quantification by Flow Cytometry
MPs were isolated from RPE/choroid as described in the Supplementary Material. Isolated MPs were quantified as described in our published work [12].
2.7. Transmission Electron Microscopy
Ultrastructure of the RPE/choroid was assessed by transmission electron microscopy (TEM) as previously described [5].
2.8. Statistical Analysis
Data were analyzed using GraphPad Prism version 5 (GraphPad Software, Inc., San Diego, CA). Student's t-test, one-way ANOVA with Sidak’s multiple comparisons test, or two-way ANOVA with Dunnett's multiple comparisons test was used to compare groups. Data are presented as mean ± standard error of the mean (SEM). P values less than 0.05 were considered as statistically significant.
3. Results
3.1. Generation of P2X7R and Sod1 DKO Mice
Sod1 KO mice develop features typical of AMD in humans [5] and are considered a suitable murine model for AMD [2]. To assess the role of the P2X7R in AMD pathology, we generated mice that lack both P2X7R and Sod1 genes. P2X7R KO and Sod1 KO mice were crossed to generate DKO mice and the three other control genotypes of mice used for this study. Representative genotyping results and the absence of full-length P2X7R protein and Sod1 protein in single KO and DKO are shown in Supplementary Figure S1.
3.2. Lack of P2X7R Blocks RPE and Retina Oxidative Stress in Sod1 KO Mice
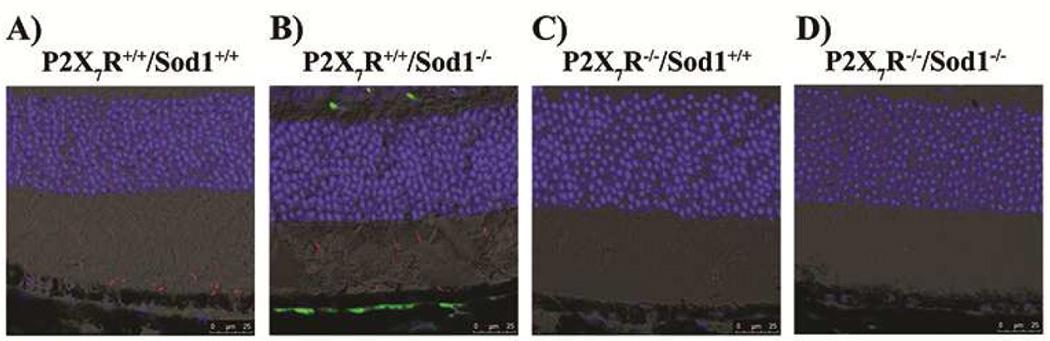
To assess whether P2X7R KO had effects on oxidative stress seen in Sod1 KO mice, we performed confocal microscopy of mouse retina/RPE/choroid cryosections stained for two oxidative stress markers, 3-nitrotyrosine (3-NT) and carboxymethyl lysine (CML). As shown in Figure 1, Sod1 KO mice have stronger 3-NT staining (red) compared with WT mice while P2X7R KO and DKO mice have little 3-NT staining. There was a very strong signal of CML (green) detected in sub-RPE deposits adjacent to the RPE monolayer in the Sod1 KO mice (Fig. 1B), while other genotypes of mice showed little evidence of CML staining in the retina/RPE/choroid sections. The results suggest that P2X7R KO protects the outer retina from oxidative stress induced by Sod1 KO.
Figure 1. Lack of P2X7R Blocks RPE and Retinal Oxidative Stress in Sod1 KO Mice.
(A–D) Representative images of mouse retinal cryosections that were stained for two oxidative stress markers, 3-nitrotyrosine (3-NT, red) and carboxymethyl lysine (CML, green). Nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI; blue). Scale bar, 25 µm. There is accumulation of 3-NT (red) in the photoreceptor outer and inner segments of WT (A) and Sod1 KO (B) mice. Staining for CML (green) is readily detected in sub-RPE deposits adjacent to the RPE monolayer in the Sod1 KO mice (B), while WT (A), P2X7R KO (C) and DKO (D) mice showed little evidence of CML staining.
3.3. Lack of P2X7R Prevents Accumulation of Sub-RPE Deposits and Microparticles in Sod1 KO Mice
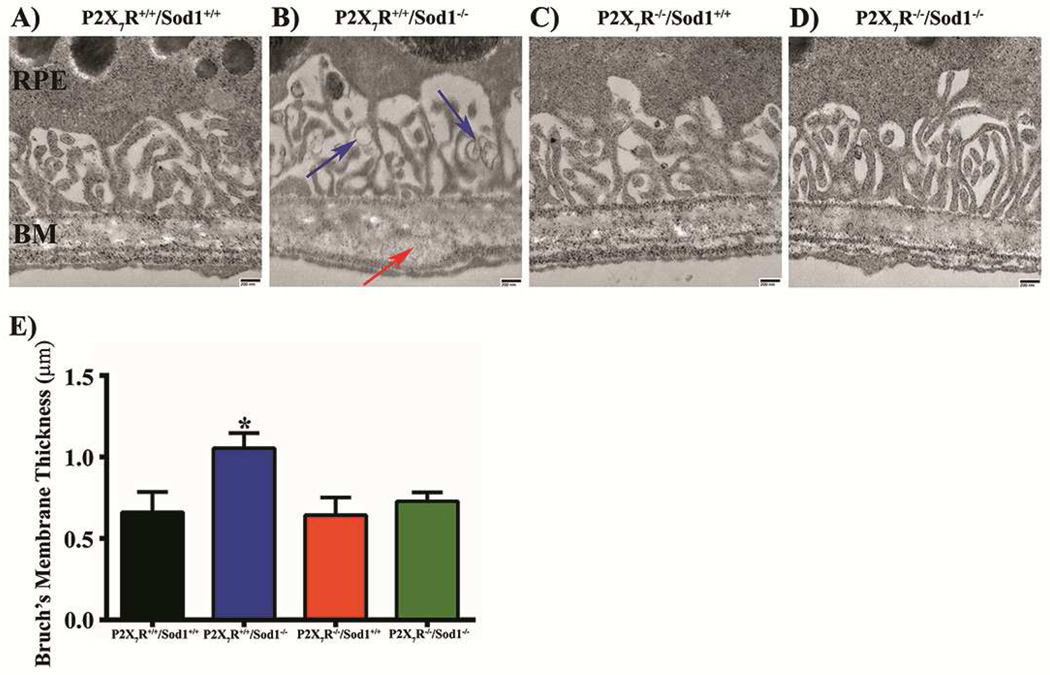
We used TEM to observe the ultrastructure of RPE-Bruch’s membrane (BM) of each genotype of mice aged between 9 and 13 months and to look for basal deposits. Sod1 KO mice showed the BM thickening (Figs. 2B, S2B), basal laminar deposits (BLamD; Fig. S2F), and basal linear deposits (BLinD; Figs. 2B, S2E). Based on the diameter ranged from 100 to 1000 nm in the TEM images, MP-sized vesicles were observed in Sod1 KO mice (Figs. 2B, S2F). These changes were absent in WT (Figs. 2A, S2A), P2X7R KO (Figs. 2C, S2C) and P2X7R/Sod1 DKO (Figs. 2D, S2D) mice. The BM thickness of Sod1 KO mice was significantly increased compared with other three genotypes, and this increase was prevented in the DKO mice (Fig. 2E).
Figure 2. Lack of P2X7R Prevents the Formation of Sub-RPE Deposits, and Thickening of Bruch’s Membrane Observed in Sod1 KO mice.
(A–D) Representative transmission electron microscopy (TEM) images from 10 month old female littermates. RPE, Retinal Pigmented Epithelium; BM, Bruch’s Membrane. Blue arrows: Microparticle-sized objects. Red arrow: basal linear deposits. Scale bar, 200 nm. (E) Quantification of Bruch’s membrane thickness. Data presented as mean ± SEM. n = 2–3. *P < 0.05 compared with all other groups.
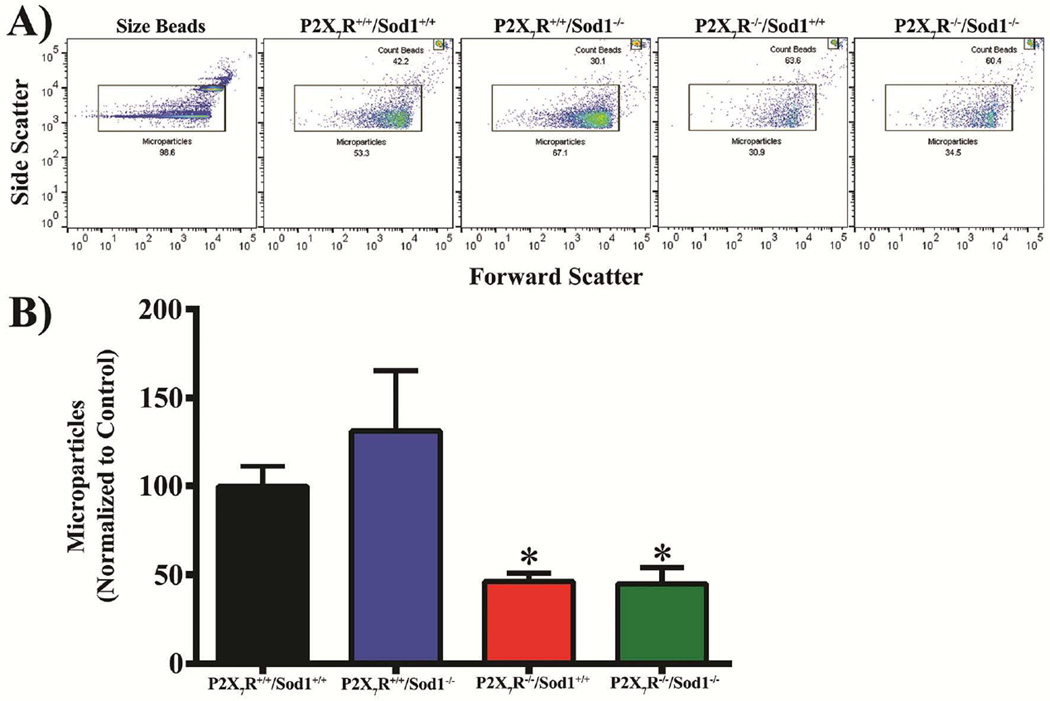
Recently, we reported that in vitro oxidative stress induced RPE cells to release MPs [12]. Others have demonstrated that activation of the P2X7R triggered membrane blebbing and MP release in leukocytes, and this MP shedding was inhibited by P2X7R antagonists [14–17]. To test for a role of P2X7R in the formation of MPs under in vivo oxidative stress due to Sod1 KO, we isolated MPs from RPE/choroid from the four genotypes of mice and analyzed the levels of MPs by flow cytometry. We found that MPs levels were highest in Sod1 KO mice, followed by WT mice; and that P2X7R/Sod1 DKO mice completely blocked MP increase seen in Sod1 KO mice (Fig. 3). Surprisingly, the levels of MPs in the P2X7R/Sod1 DKO mice and P2X7R single KO mice were even lower than those in WT mice (Fig. 3), indicating low-grade oxidative stress may have occurred in WT mice. Thus, it appears that activation of the P2X7R is involved in the release of MPs under in vivo oxidative stress.
Figure 3. Lack of P2X7R Prevents Microparticle Release from the RPE/Choroid Complex in Sod1 KO Mice.
Microparticles (MPs) isolated from RPE/Choroid complex were analyzed by flow cytometry. Beads of a known count were added to all samples in order to quantify MPs per microliter, which were normalized to wild type (P2X7R+/+/Sod1+/+) controls. (A) Forward and side scatter gate was set based on 0.5 and 1 µm beads and maintained across all samples. (B) MPs were quantified by flow cytometry using count beads. MPs levels were highest in Sod1 KO mice, followed by WT mice. P2X7R/Sod1 DKO mice completely blocked MP increase seen in Sod1 KO mice. Data presented as mean ± SEM. n = 4–5. *P < 0.05 compared with Sod1 KO (P2X7R+/+/Sod1−/−) mice.
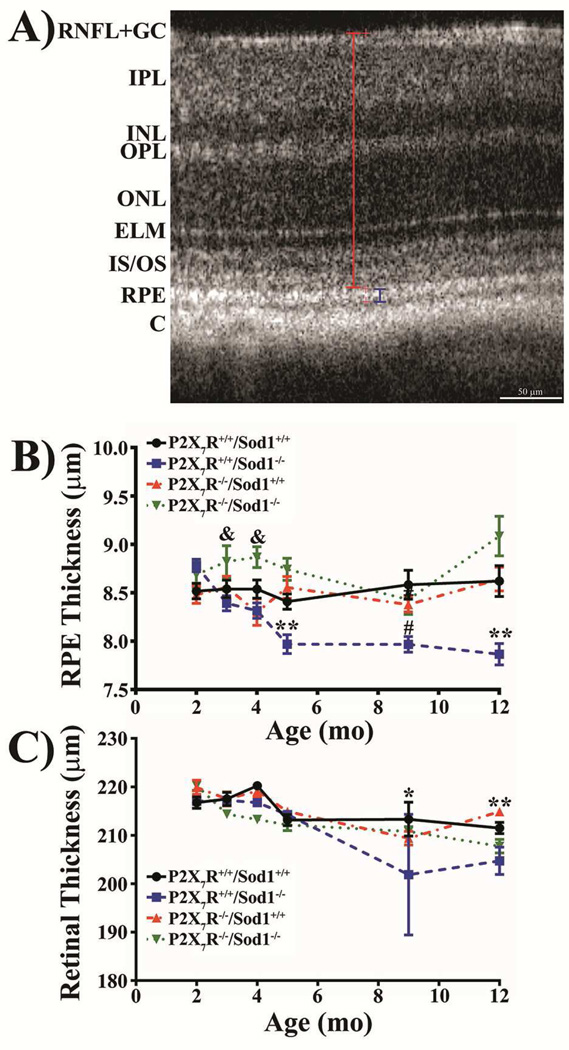
3.4. Lack of P2X7R Improves RPE and Retinal Thinning Seen in Sod1 KO Mice
We used SD-OCT to acquire retinal images from live mice aged between 2 and 12 months. Retinal and RPE thickness were measured as indicated in Figure 4A. Young mice at 2–4 months had similar thickness of RPE (Fig. 4B) and retina (Fig. 4C) among the four genotypes of mice with the exception of the DKO mice that had a significantly thicker RPE at 3 and 4 months of age compared with the Sod1 KO mice. Starting at 5 months of age the Sod1 KO mice exhibited a significantly thinner RPE layer compared with the other three genotypes. The RPE thinning in Sod1 KO mice was maintained through 12 months of age (Fig. 4B). The retina of Sod1 KO mice began thinning at 9 months of age and maintained at 12 months of age, whereas the DKO mice began to develop a thinner retina at 12 months of age (Fig. 4C).
Figure 4. Lack of P2X7R Blocks RPE and Retinal Thinning Seen in Sod1 KO Mice.
(A) Representative SD-OCT B-scan image depicting the distances measured. Blue and red brackets depict the region measured for RPE and retinal thickness, respectively. Red crosshairs are location markers used within Bioptigen’s Diver software. RNFL + GC, retinal nerve fiber layer and ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; ELM, external limiting membrane; IS/OS, photoreceptor inner and outer segments; RPE, retinal pigmented epithelium; C, choroid. (B) RPE thickness. **P < 0.01, P2X7R+/+/Sod1−/− compared with all other groups; #P < 0.05, P2X7R+/+/Sod1−/− compared with P2X7R+/+/Sod1+/+; &P < 0.05, P2X7R+/+/Sod1−/− compared with P2X7R−/−/Sod1−/−. (C) Retinal thickness. *P < 0.05, P2X7R+/+/Sod1−/− compared with all other groups; **P < 0.01, P2X7R+/+/Sod1−/− compared with P2X7R+/+/Sod1+/+ and P2X7R−/−/Sod1+/+. Thinning of the RPE layer precedes retinal thinning. The DKO mice protect against RPE and retinal thinning seen in Sod1 KO mice. Data presented as mean ± SEM. n = 4–8.
3.5. Lack of P2X7R Protects against Accumulation of Yellow Spots and Microglia/Macrophages, and RPE Barrier Disruption in Sod1 KO Mice
To test whether concurrent P2X7R KO could affect fundus appearance seen in Sod1 KO mice, we used fundus imaging to examine live mice aged between 6 and 12 months. Yellow spots were present in Sod1 KO mice starting around 7 months (Fig. S3B; red arrows), while WT, P2X7R KO, and P2X7R/Sod1 DKO littermates had limited evidence of these spots in fundus images (Figs. S3A, S3C, S3D). The total amount of spots, quantified by area in pixels, was significantly increased in Sod1 KO mice compared with other three genotypes of mice (Fig. S3E).
As these yellow spots could be due to accumulation of subretinal microglia/macrophages [19], we next asked whether concurrent P2X7R KO could affect RPE barrier integrity and accumulation of microglia/microphages. To this end, RPE flat mounts were double labeled with Phalloidin, for F-actin at RPE cell borders, and with ionized calcium-binding adapter molecule 1 (Iba1), a microglia and macrophage marker [19]. A typical hexagonal pattern of the RPE monolayer was revealed by Phalloidin staining (red) in WT (Fig. S3F), P2X7R KO (Fig. S3H) and P2X7R/Sod1 DKO mice (Fig. S3I), while impaired and interrupted Phalloidin staining was observed in Sod1 KO mice (Fig. S3G). These results suggest that RPE barrier integrity is disrupted in Sod1 KO mice, but maintained in mice that lack both P2X7R and Sod1 genes.
In association with loss of RPE barrier integrity, lack of Sod1 also results in a significant increase in Iba1+ (green) cells (Fig. S3G; white arrows), indicating accumulation of microglia or subretinal macrophages in the RPE layer. The number of microglia or macrophages was significantly attenuated in the DKO mice (Fig. S3J). Thus, it is very likely that the increased yellow spots on fundus images in Sod1 KO mice (Figs. S3B, S3E) are accumulated immune cells, including Iba1+ microglia/macrophages as shown in the RPE flat mounts (Figs. S3G, S3J).
Collectively, our results indicate that the P2X7R mediates accumulation of MPs and AMD-like phenotype in Sod1 KO mice and that targeting the P2X7R can protect against these changes.
4. Discussion
Here we provide evidence that concurrent knockout of P2X7R and Sod1 blocked chronic oxidative stress, MP accumulation, and AMD-like defects observed in Sod1 KO mice. Knockout of the Sod1 gene alone led to increased oxidative stress in the retina/RPE/choroid, thinning of RPE and retina, BM thickening, accumulation of microglia in the subretinal space, and disruption of the RPE barrier integrity (Figs. 1, 2, 4, Figs. S2, S3). These changes were all attenuated in mice that lack both Sod1 and P2X7R genes (Figs. 1, 2, 4, S2, S3), suggesting that the P2X7R is involved in the oxidative stress-induced damage to the retina/RPE/choroid. Furthermore, the levels of MPs in Sod1 KO RPE/choroid complexes were significantly higher than those in P2X7R/Sod1 DKO and P2X7R single KO ones (Fig. 3).
Oxidative stress plays a key role in the pathogenesis of AMD [4]. Ferrington et al. directly compared immortalized mouse RPE cell lines established from WT, Sod1+/− and Sod2+/− mice, and found that Sod1+/− RPE cells were most susceptible to oxidant-induced cell death [20]. Imamura et al. reported that Sod1 KO mice had oxidative stress and developed sub-RPE deposits, drusen, and RPE degeneration [5]. Consistent with their findings, in this study we observed elevated oxidative damage, as demonstrated by two oxidative stress markers (3-NT and CML) in Sod1 KO mice (Fig. 1), and this increase is associated with thinning of the RPE layer which precedes retinal thinning (Fig. 4). Activation of the P2X7R is involved in the generation of reactive oxygen species (ROS) in microglia [10,21]. Here we show that concurrent knockout of this receptor blocked CML accumulation and resulted in lower 3-NT immunofluorescence induced by Sod1 KO, indicating the P2X7R is also involved in the generation of ROS in retina/RPE/choroid.
In accordance with little oxidative damage of retina/RPE/choroid in DKO mice, a significant prevention of RPE and retina thinning was observed in the DKO mice. However, in mice at 12 months, the retinal thickness in the DKO mice was in-between WT and Sod1 KO mice, indicating that additional factor(s) could play a role in retina thinning at this age.
Thickening of BM is one of the early events in AMD and was found in mouse models of oxidative stress [5,22]. We observed a similar degree of BM thickening in Sod1 KO mice while BM thickness in the DKO mice was similar to that of WT mice (Fig. 2E).
Formation of sub-RPE deposits, BLamD and BLinD, could be due to blebbing/budding from the stressed RPE cells [23,24]. MPs are small membrane-bound vesicles that are released following cell activation and/or apoptosis through a blebbing process [13]. Most recently, we showed that oxidative stress induces loss of membrane complement regulatory proteins (mCRPs) on cultured human RPE cells through release of MPs that carry mCRPs: CD46, CD55 and CD59 [12]. CD46 was reported to be lost from RPE cells at the earliest stage of AMD and present in drusen, and CD59 was detected in the subretinal space [24,25].
Extracellular ATP is an endogenous agonist for P2X7R. RPE cells can release ATP into the extracellular environment in response to various stimuli [26,27]. Extracellular ATP levels were also increased in the vitreous samples of AMD patients with subretinal hemorrhage compared to controls [9]. Released ATP was reported to activate P2X7R in an autocrine or a paracrine manner [28]. Previously, we and others reported that the P2X7R was expressed in human and mouse RPE cells and mediated RPE damage via the Ca2+ pathway, the NLRP3 inflammasome pathway, and/or phagosome-lysosome pathway [6,7,29]. Inhibition or KO of P2X7R also protects against retinal degeneration in another mouse model of AMD induced by Alu RNA [29,30]. Moreover, we show that concurrent KO of the P2X7R completely prevents the accumulation of MPs within the RPE/choroid in Sod1 KO mice (Fig. 3). MP-sized objects were detectable by TEM in the spatial vicinity of BLinD and BLamD in Sod1 KO mice (Figs. 2B, S2F), but not in DKO mice (Figs. 2D, S2D), suggesting a possible relationship between P2X7R-dependent shedding of MPs from the basolateral membrane and the formation of sub-RPE deposits. Given that the P2X7R mediates both membrane blebbing and release of MPs [14–17], our study supports the concept that P2X7R-mediated MP accumulation could be an early event, leading to other AMD-like defects, such as sub-RPE deposits observed by TEM. As MPs isolated from human atherosclerotic plaques can increase monocyte transendothelial migration [31], we speculate that MPs within the RPE/choroid could have a similar function, leading to accumulation of microglia/macrophages in the subretinal space, and thus the yellow spots on the fundus.
Microglia disrupt the RPE cell-cell communication by disorganizing tight junctions [32]. We observed similar effects in the Sod1 KO mice which had increased number of microglia/macrophages in the subretinal space and loss of F-actin staining at RPE borders, indicating inflammation and loss of RPE barrier function. All these changes were reversed in DKO mice, suggesting activation of P2X7R or its signaling pathways may mediate the accumulation of microglia/macrophages and RPE barrier disruption seen in the Sod1 KO mice.
The current work used global DKO and single KO mice. Although RPE could be responsible for P2X7R KO-conferred protection against Sod1 KO-induced oxidative stress, MP accumulation, and AMD-like defects based on the fact that the early and many changes observed in this study are involved in the RPE, we don’t know exactly which types of cells are involved. Cell type-specific knockout of both genes would answer this unresolved question.
In conclusion, we have confirmed the previous reports of Sod1 KO resulting in an AMD-like pathology in mice and shown for the first time that genetic deletion of the P2X7R protects Sod1 KO mice from MP release and AMD-like defects. Furthermore, we demonstrate that MPs released under in vivo oxidative stress are spatially associated with sub-RPE deposits. Targeting P2X7R or its signaling pathways could potentially lead to novel therapies for AMD and other oxidative stress-driven diseases.
Supplementary Material
Highlights.
Lack of P2X7 receptor blocks RPE and retina oxidative stress in Sod1 knockout mice.
Lack of P2X7 receptor protects against AMD-like defects in Sod1 knockout mice.
Lack of P2X7 receptor prevents microparticle accumulation in Sod1 knockout mice.
Targeting P2X7 receptor could potentially lead to novel therapies for AMD.
Acknowledgments
The authors thank Austra Liepa for mouse care, breeding and colony management. This work utilized the Core Center for Vision Research funded by P30 EY007003 from the National Eye Institute (NEI), and was supported by the University of Michigan Start-Up Funds. CBR is supported by NEI EY026161.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no competing financial interests.
References
- 1.Bowes Rickman C, Farsiu S, Toth CA, Klingeborn M. Dry age-related macular degeneration: mechanisms, therapeutic targets, and imaging. Invest Ophthalmol Vis Sci. 2013;54 doi: 10.1167/iovs.13-12757. ORSF68–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramkumar HL, Zhang J, Chan CC. Retinal ultrastructure of murine models of dry age-related macular degeneration (AMD) Prog Retin Eye Res. 2010;29:169–190. doi: 10.1016/j.preteyeres.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Lookeren Campagne M, LeCouter J, Yaspan BL, Ye W. Mechanisms of age-related macular degeneration and therapeutic opportunities. J Pathol. 2014;232:151–164. doi: 10.1002/path.4266. [DOI] [PubMed] [Google Scholar]
- 4.Jarrett SG, Boulton ME. Consequences of oxidative stress in age-related macular degeneration. Mol Aspects Med. 2012;33:399–417. doi: 10.1016/j.mam.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imamura Y, Noda S, Hashizume K, et al. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: a model of age-related macular degeneration. Proc Natl Acad Sci U S A. 2006;103:11282–11287. doi: 10.1073/pnas.0602131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang D, Elner SG, Clark AJ, et al. Activation of P2X receptors induces apoptosis in human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2011;52:1522–1530. doi: 10.1167/iovs.10-6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guha S, Baltazar GC, Coffey EE. Lysosomal alkalinization, lipid oxidation, and reduced phagosome clearance triggered by activation of the P2X7 receptor. FASEB J. 2013;27:4500–4509. doi: 10.1096/fj.13-236166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Notomi S, Hisatomi T, Kanemaru T, et al. Critical involvement of extracellular ATP acting on P2RX7 purinergic receptors in photoreceptor cell death. Am J Pathol. 2011;179:2798–2809. doi: 10.1016/j.ajpath.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Notomi S, Hisatomi T, Murakami Y, et al. Dynamic increase in extracellular ATP accelerates photoreceptor cell apoptosis via ligation of P2RX7 in subretinal hemorrhage. PLoS One. 2013;8:e53338. doi: 10.1371/journal.pone.0053338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parvathenani LK, Tertyshnikova S, Greco CR, et al. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer's disease. J Biol Chem. 2003;278:13309–13317. doi: 10.1074/jbc.M209478200. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee S, Das S. P2X7 receptor as a key player in oxidative stress-driven cell fate in nonalcoholic steatohepatitis. Oxid Med Cell Longev. 2015:172493. doi: 10.1155/2015/172493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carver KA, Yang D. N-Acetylcysteine amide protects against oxidative stress-induced microparticle release from human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2016;57:360–371. doi: 10.1167/iovs.15-17117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.György B, Szabó TG, Pásztói M, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verhoef PA, Estacion M, Schilling W, Dubyak GR. P2X7 receptor-dependent blebbing and the activation of Rho-effector kinases, caspases, and IL-1 beta release. J Immunol. 2003;170:5728–5738. doi: 10.4049/jimmunol.170.11.5728. [DOI] [PubMed] [Google Scholar]
- 15.Baroni M, Pizzirani C, Pinotti M, et al. Stimulation of P2 (P2X7) receptors in human dendritic cells induces the release of tissue factor-bearing microparticles. FASEB J. 2007;21:1926–1933. doi: 10.1096/fj.06-7238com. [DOI] [PubMed] [Google Scholar]
- 16.Constantinescu P, Wang B, Kovacevic K, et al. P2X7 receptor activation induces cell death and microparticle release in murine erythroleukemia cells. Biochim Biophys Acta. 2010;1798:1797–1804. doi: 10.1016/j.bbamem.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Dubyak GR. P2X7 receptor regulation of non-classical secretion from immune effector cells. Cell Microbiol. 2012;14:1697–1706. doi: 10.1111/cmi.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang D, Pan A, Swaminathan A, et al. Expression and localization of the inwardly rectifying potassium channel Kir7.1 in native bovine retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2003;44:3178–3185. doi: 10.1167/iovs.02-1189. [DOI] [PubMed] [Google Scholar]
- 19.Luhmann UF, Carvalho LS, Robbie SJ, et al. Ccl2, Cx3cr1 and Ccl2/Cx3cr1 chemokine deficiencies are not sufficient to cause age-related retinal degeneration. Exp Eye Res. 2013;107:80–87. doi: 10.1016/j.exer.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrington DA, Tran TN, Lew KL, et al. Different death stimuli evoke apoptosis via multiple pathways in retinal pigment epithelial cells. Exp Eye Res. 2006;83:638–650. doi: 10.1016/j.exer.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Bartlett R, Yerbury JJ, Sluyter R. P2X7 receptor activation induces reactive oxygen species formation and cell death in murine EOC13 microglia. Mediators Inflamm. 2013:271813. doi: 10.1155/2013/271813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujihara M, Nagai N, Sussan TE, et al. Chronic cigarette smoke causes oxidative damage and apoptosis to retinal pigmented epithelial cells in mice. PLoS One. 2008;3:e3119. doi: 10.1371/journal.pone.0003119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burns RP, Feeney-Burns L. Clinico-morphologic correlations of drusen of Bruch's membrane. Trans Am Ophthalmol Soc. 1980;78:206–225. [PMC free article] [PubMed] [Google Scholar]
- 24.Ebrahimi KB, Fijalkowski N, Cano M, Handa JT. Decreased membrane complement regulators in the retinal pigmented epithelium contributes to age-related macular degeneration. J Pathol. 2013;229:729–742. doi: 10.1002/path.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogt SD, Curcio CA, Wang L, et al. Retinal pigment epithelial expression of complement regulator CD46 is altered early in the course of geographic atrophy. Exp. Eye Res. 2011;93:413–423. doi: 10.1016/j.exer.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell CH. Release of ATP by a human retinal pigment epithelial cell line: potential for autocrine stimulation through subretinal space. J Physiol. 2001;534:193–202. doi: 10.1111/j.1469-7793.2001.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eldred JA, Sanderson J, Wormstone M, et al. Stress-induced ATP release from and growth modulation of human lens and retinal pigment epithelial cells. Biochem Soc Trans. 2003;31:1213–1215. doi: 10.1042/bst0311213. [DOI] [PubMed] [Google Scholar]
- 28.Piccini A, Carta S, Tassi S, et al. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci U S A. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerur N, Hirano Y, Tarallo V, et al. TLR-independent and P2X7-dependent signaling mediate Alu RNA-induced NLRP3 inflammasome activation in geographic atrophy. Invest Ophthalmol Vis Sci. 2013;54:7395–7401. doi: 10.1167/iovs.13-12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fowler BJ, Gelfand BD, Kim Y, et al. Nucleoside reverse transcriptase inhibitors possess intrinsic anti-inflammatory activity. Science. 2014;346:1000–1003. doi: 10.1126/science.1261754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rautou PE, Leroyer AS, Ramkhelawon B, et al. Microparticles from human atherosclerotic plaques promote endothelial ICAM-1-dependent monocyte adhesion and transendothelial migration. Circ Res. 2011;108:335–343. doi: 10.1161/CIRCRESAHA.110.237420. [DOI] [PubMed] [Google Scholar]
- 32.Ma W, Zhao L, Fontainhas AM, Fariss RN, Wong WT. Microglia in the mouse retina alter the structure and function of retinal pigmented epithelial cells: a potential cellular interaction relevant to AMD. PLoS One. 2009;4:e7945. doi: 10.1371/journal.pone.0007945. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.