Abstract
Streptozotocin (STZ)-induced chronic hyperglycemia has a detrimental effect on neurovascular coupling, linked to increased PKC-mediated phosphorylation and PKC isoform expression changes. Here, we sought to determine whether: 1) selective PKC-α/β/γ inhibitor, GF109203X, could reverse the effects of chronic hyperglycemia on cerebrovascular reactivity; 2) pancreatic islet transplantation could prevent the development of cerebrovascular impairment seen in a rat model of Type 1 Diabetes.
We studied the effect of GF109203X in diabetic (DM), non-diabetic (ND), and transplanted (TR) Lewis rats during either sciatic nerve stimulation (SNS) or the topical applications of the large-conductance Ca2+-operated K+ (BKCa) channel opener, NS1619, or the K+ inward rectifier (Kir) channel agonist, KCl. Pial arteriole diameter changes were monitored using a closed cranial window in vivo microscopy technique.
The pial arteriole dilatory response associated with SNS was decreased by ~45%, when comparing DM vs either ND or TR rats. Also, pial arteriolar dilations to topical KCl and NS1619 were largely attenuated in DM rats, but not in ND or TR animals. These responses were completely restored by the acute application of GF109203X to the brain surface. The PKC inhibitor had no effect on vascular responses in normoglycemic and TR animals.
In conclusion, DM-associated chronic impairment of neurovascular coupling may be readily reversed by a PKC-α/β/γ inhibitor or prevented via pancreatic islet transplantation. We believe that specific PCK isoforms (α/β/γ) are mechanistically linked to the neurovascular uncoupling seen with hyperglycemia.
Keywords: neurovascular coupling, diabetes mellitus type 1, potassium channels, PKC, pancreatic islet transplantation, GF109203X
1. Introduction4
Neurovascular coupling (NVC) has been extensively studied over recent decades and many factors appear to participate in this process [4]. Among these, are diffusible paracrine factors released by astrocytes and neurons—e.g., ATP and K+, as well as cyclooxygenase and cytochrome P450 products. Indeed, such redundancy is thought to ensure an adequate O2 and glucose supply to neurons when their energy demands increase over a wide array of conditions.
Type 1 Diabetes Mellitus is a widespread clinical condition [7], that is often associated with encephalopathy (for review see [26]). Indeed, cerebral complications of Type 1 Diabetes Mellitus include microangiopathy, retinopathy, and an increased risk of Alzheimer’s disease [30], stroke, mild cognitive impairment and dementia [2]. Strict glycemic control through intensive insulin therapy can slow, or even stop, the development of such complications [25]. However, intensive insulin therapy is limited by an increased risk for severe hypoglycemic episodes [1]. Islet transplantation can provide glycemic control without exogenous insulin and without the risk of hypoglycemia [6,13,32]. Therefore, in recent years, pancreatic islet transplantation has emerged as a suitable therapeutic option in a selected patient population as a means of curing diabetes and preventing hyperglycemia-associated complications. In experimental animal models with chemically-induced chronic diabetes, evidence indicates a profound impairment in cerebral vasodilatory function [3,23]. We recently reported that neurovascular coupling is negatively affected by uncontrolled chronic hyperglycemia [41]. One possible mechanism responsible for this diabetes-related vascular impairment is chronic activation of protein kinase C (PKC) [29,41]. Findings from in vitro and in vivo models have indicated a vital role for inward rectifier and large conductance, Ca2+-operated K+ channels (Kir and BKCa, respectively) in neurovascular coupling [12,15,27]. According to these models, neuronal activation would lead to calcium increase in the astrocytic endfeet, where activation of BKCa would result in K+ release at the gliovascular interface and activation of Kir channels located on the vascular smooth muscle cells with subsequent hyperpolarization and vasodilation. Interestingly, the literature and our data suggest that Kir and BKCa channels-mediated vasodilations are affected by diabetes [23] and sensitive to inhibition via PKC-mediated phosphorylation [11,21,33,41,45]. Importantly, our work showed that these vascular responses were completely restored by the acute topical application of a general PKC antagonist, calphostin C [41]. On the other hand, the suffusion of a PKC activator, phorbol 12,13-dibutyrate, in ND rats was able to reproduce the vascular reactivity impairments found in a rat model of Type 1 Diabetes Mellitus. Assay of PKC activity in brain samples from DM vs. ND rats revealed a significant gain in activity only in specimens rich in pial vascular plus superficial glia limitans (glio-pial) tissue, but not in bulk cortical gray matter [41]. A subsequent study examining the expression of PKC isoforms in both cerebral cortex and glio-pial tissue, has suggested the specific involvement of PKC-α and, possibly, PKC-βII in the diabetes-related impairment in neurovascular coupling [38]. In the present study, we used a well-established rat in vivo neurovascular coupling model, involving sciatic nerve stimulation-induced pial arteriolar dilation [39,44]. We addressed the core hypothesis that chronic hyperglycemia is accompanied by chronic activation of conventioanl PKC isoforms (especially α, βI, and βII) which, in turn, represses neurovascular coupling efficiency. Additional hypotheses arise from this central premise. First, one can prevent this PKC-linked vascular dysfunction via chronic glycemic normalization through pancreatic islet transplantation. Second, the neurovascular coupling efficiency impairment present in chronically diabetic rats relates to phosphorylation mediated by conventional PKC isoforms. Third, irrespective of the duration of the vascular dysfunction, it is readily reversible using acute PKC blockade via the semi-selective inhibitor of conventional PKC isoforms, GF109203X.
2. Results
Three sets of Lewis rats were used for this study: 1) euglycemic 4–6 month old non-diabetic controls (ND group, n=11); 2) streptozotocin (STZ)-treated diabetic rats (6 month old, 4 months post-STZ) (DM group, n=6); and 3) STZ-treated diabetic animals, subjected to pancreatic islet transplantation soon after the establishment of the diabetic model, studied 100–110 days after the transplant (TR group, n=7). Blood pressure, body weight, blood glucose, pH, pO2, and pCO2 values in the different groups are reported in Table 1. As expected, body weight was decreased in diabetic rats (DM), whereas blood glucose was increased compared to ND controls and transplanted animals. No other statistically significant differences were found between physiological parameters. The baseline diameters of the arterioles studied was 32.8 ± 3.6 µm for ND, 31.5 ± 2.4 µm for DM, and 34.5 ± 2.7 µm for the TR group (P>0.05).
Table 1.
Vital parameters in normoglycemic control (ND), type 1 diabetic (DM) and transplanted (TR) rats. Mean arterial blood pressure, blood glucose, body weight, arterial blood pH, partial pressure of oxygen (pO2), and partial pressure of carbon dioxide (pCO2) were recorded on the day of experiment.
| Vital parameter | ND | DM | TR |
|---|---|---|---|
| Mean arterial blood pressure (mmHg) |
116±9 | 106±9 | 115±6 |
| Blood glucose (mg/dl) |
134±10 | 559±27* | 171±35 |
| Body weight (g) |
430±18 | 228±19* | 464±23 |
| Blood pH | 7.42± 0.02 |
7.39± 0.02 |
7.41± 0.02 |
| Blood pO2 (mmHg) | 123±20 | 127±15 | 122±15 |
| Blood pCO2 (mmHg) |
42±1 | 39±1 | 40±1 |
, P < 0.05 vs ND and TR
Three types of vasodilatory stimuli were used to test cerebrovascular function in the 3 different groups: sciatic nerve stimulation, NS1619 suffusion and K+ suffusion. In a previous study, we showed that these dilatory responses were impaired in female Sprague-Dawley diabetic rats [41], but no data was available on the Lewis strain used for the present investigation. Therefore, our first endeavor was to compare dilatory responses in the two different rat strains in normo- and hyper-glycemic animals.
2.1. Pial arteriole response to sciatic nerve stimulation in normoglycemic, diabetic (hyperglycemic) and transplanted rats
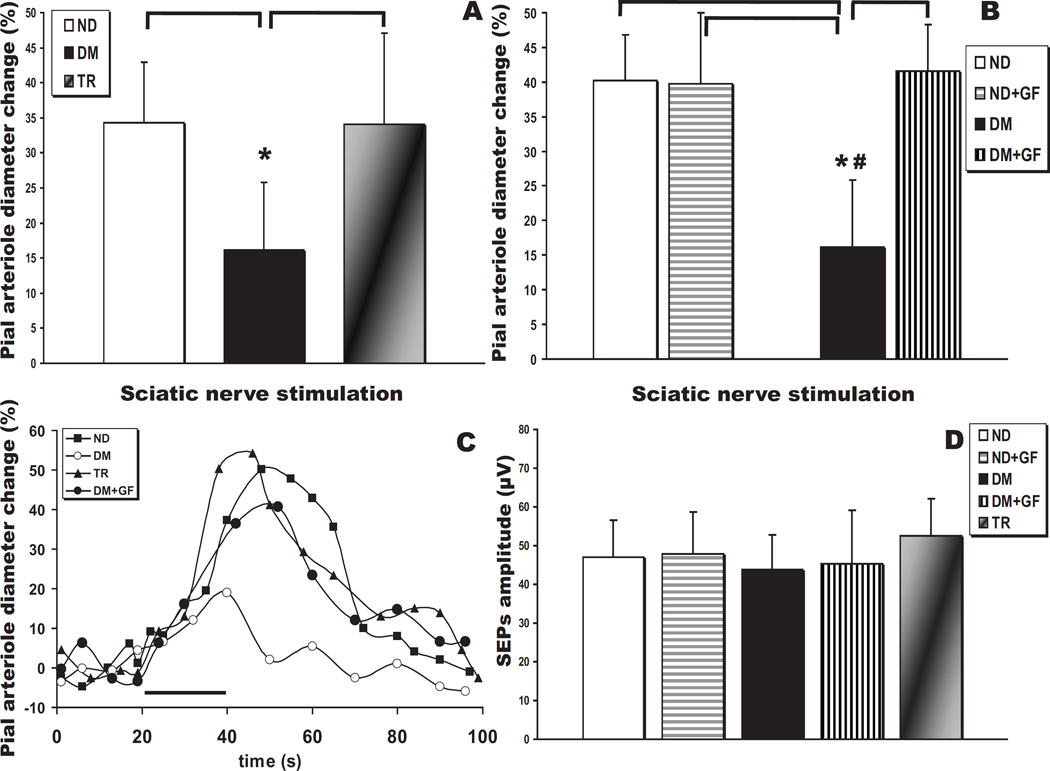
A well established closed cranial window technique was used to monitor the reactivity of pial arterioles overlying the contralateral hindlimb area of the somatosensory cortex (~2 mm caudally to the bregma and 2–3 mm lateral to the sagittal suture). We compared pial arteriole dilations evoked by 20 seconds of sciatic nerve stimulation in ND control (n=11) and DM (n=6) rats. Consistent with previously reported data obtained in Sprague-Dawley rats [41], the average peak response in the Type 1 Diabetes Mellitus group was decreased by 45% (Fig. 1A; P<0.05; representative vascular response curves provided in Fig. 1C). We then evaluated SNS responses in animals subjected to pancreatic islet transplantation. In all animals (n=7) receiving syngeneic islets normoglycemia was restored immediately after transplantation and normal blood glucose levels were maintained up to for 4 months, when the rats were used for the neurovascular reactivity experiments. On the day of the experiment, blood glucose levels in the ND and TR groups were not statistically different (Table 1). Noticeably, the normalization of the blood glucose prevented the depression of the SNS-induced dilation in the transplanted animals as compared to the untreated DM rats (Fig. 1A; P<0.05).
Fig. 1.
Neurovascular coupling impairment in 4 month diabetic (T1DM) rats. (A) Sciatic nerve stimulation (SNS)-evoked pial arteriolar responses in type 1 diabetes mellitus rats (DM, n = 6) show a ~45% decrease in the peak diameter change compared to non-diabetic controls (ND, n = 11) or animals subjected to pancreatic islet transplantation (TR, n = 7) (*, P < 0.05). (B) SNS-evoked responses before and after topical suffusion of the classical PKC isoform inhibitor, GF109203X, in ND (n = 4) and DM (n = 6) rats (*, P < 0.05 vs ND and ND + GF109203X; #, P < 0.05 vs DM + GF109203X). (C) Representative time courses of SNS-evoked pial arteriolar diameter changes in non-diabetic control (open circles) and T1DM (closed circles) rats. SNS duration is indicated by the black bar. (D) Summary of the somatosensory evoked potentials (SEPs) P1N1 peak-to-peak amplitudes recorded from ND, ND treated with GF109203X, DM, DM treated with GF109203X, and TR rats. No significant differences were observed.
We then tested the PKC-α/β/γ inhibitor, GF109203X, in both ND (n=4) and DM (n=6) rats to test the hypothesis that PKC-α and/or βII might be responsible for the decreased cerebrovascular function detected in diabetic rats. Interestingly, GF109203X significantly increased the response to SNS in DM rats, producing a dilation not significantly different from controls (Fig.1B). This data is in accordance with the effect of a general PKC inhibitor, Calphostin C, used in a previous study [41]. On the other hand, the SNS response was not affected at all by GF109203X in ND animals (Fig.1B), while Calphostin C substantially decreased that response [41].
The somatosensory evoked potentials (SEPs) recorded during sciatic nerve stimulations, as reflected in the P1N1 amplitudes (Fig. 1D), did not show any significant differences when comparing ND (n = 4), ND+GF109203X (n = 4), DM (n = 6), DM+GF109203X (n = 6) and TR rats (n = 7) (P>0.05). Therefore, the changes in vascular responses to sciatic nerve stimulation in the different groups or treatments were not attributable to alterations in the neuronal activation induced by diabetes and/or drug application.
2.2. BKCa channel function in normoglycemic, diabetic (hyperglycemic), and transplanted rats
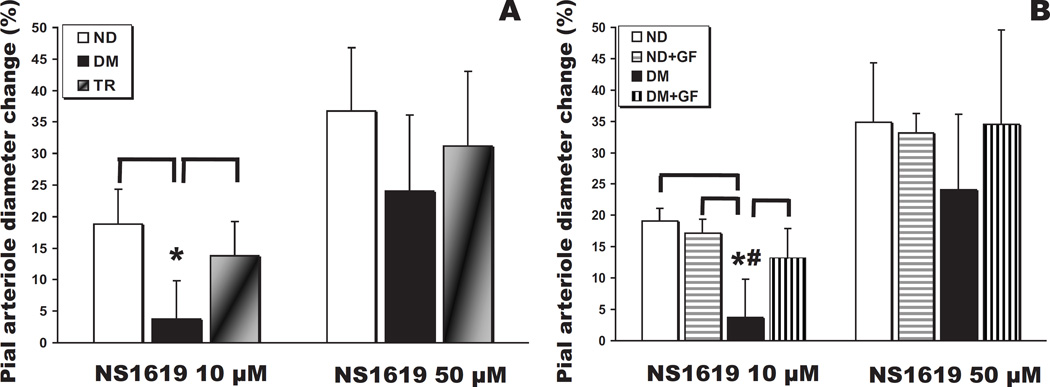
In the diabetic rats of the present study, vasodilations elicited by suffusion of the BKCa channel opener were depressed, similar to the findings reported in a recent paper from our laboratory [41] and others [23]. Thus, NS1619 (10 µM and 50 µM) induced a dose-dependent dilation of pial arterioles in ND and TR animals (Fig. 2A). However, NS1619 failed to induce dilation in DM rats at 10 µM (P<0.05 vs ND and TR), but not at 50 µM (P>0.05). The pial arteriolar dilations to NS1619 were preserved in the TR group (Fig. 2A). The administration of GF109203X was able to reverse the effects of hyperglycemia on NS1619 (10 µM)-mediated dilations (P<0.05 at 10 and P>0.05 at 50 µM, respectively; Fig. 2B). In ND rats, GF109203X had no effect on the responses to NS1619 at both concentrations.
Fig. 2.
(A) Pial arteriolar diameter changes (expressed as a percentage of the baseline diameter) elicited by suffusion of the specific activator of BKCa channels, NS1619(10 µM and 50 µM), in control (ND, n = 11), type 1 diabetes mellitus (DM, n = 6), and transplanted (TR, n = 7) rats (*, P < 0.05). (B) Pial arteriolar responses to NS1619 before and after topical suffusion of the classical PKC isoform inhibitor, GF109203X, in ND (n = 4) and DM (n = 6) rats (*, P < 0.05 vs ND and ND + GF109203X; #, P < 0.05 vs DM + GF109203X).
2.3. Kir channel function in normoglycemic, hyperglycemic, and transplanted rats
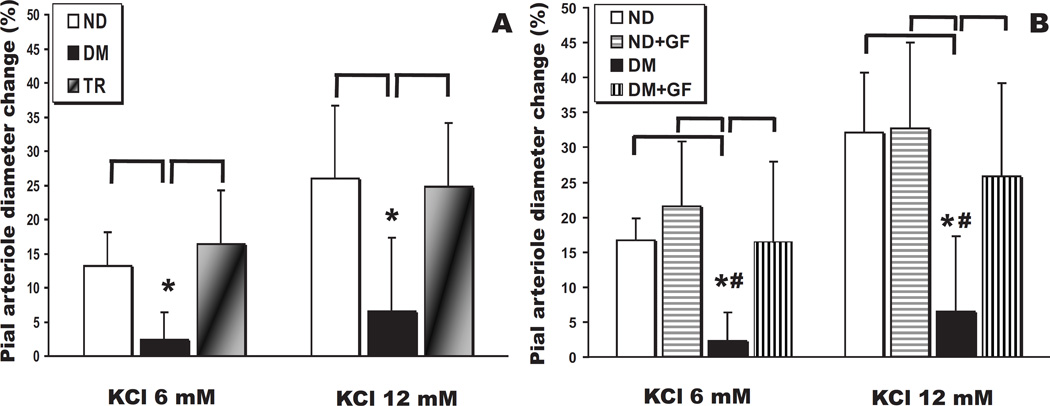
Kir channel-mediated dilation of pial arterioles is shown in Fig. 3. Suffusion with the Kir agonist, K+ (6 and 12 mM), dilated only ND and TR but not DM arterioles (P<0.05 for both concentrations compared to ND and TR groups). Pial arteriolar dilations to K+ were preserved in the TR group (Fig. 3A), demonstrating that glycemic control is able to prevent the loss of reactivity to this dilating agent.
Fig. 3.
(A) Pial arteriolar diameter changes (expressed as a percentage of the baseline diameter) elicited by suffusion of the specific activator of Kir channels, K+ (6 and 12 mM), in control (ND, n = 11), type 1 diabetes mellitus (DM, n = 6), and transplanted (TR, n = 7) rats (*, P < 0.05 for both concentrations). (B) Pial arteriolar responses to K+ before and after topical suffusion of the classical PKC isoform inhibitor, GF109203X, in ND (n = 4) and DM (n = 6) rats (*, P < 0.05 vs ND and ND + GF109203X; #, P < 0.05 vs DM + GF109203X for both concentrations).
On the other hand, similar to pial arteriolar responses to NS1619, the lost K+ responses in diabetic rats were restored to normal levels by the acute PKC inhibition via GF109203X (P<0.05 for both concentrations; Fig. 3B). These findings, therefore, suggest that the normalization of sciatic nerve stimulation responses in diabetic animals in the presence of GF109203X is the result of the restoration of the vascular dilations to NS1619 and K+. Moreover, similarly to NS1619, ND rats showed no significant changes in K+ responses in the presence of PKC inhibitor suffusion (Fig. 3B).
3. Discussion
There are two major findings in the present study: 1) the normalization of blood glucose, via pancreatic islet transplantation in STZ-treated rats, is able to prevent the deleterious effects on cerebrovascular regulation brought on by Type 1 Diabetes Mellitus; 2) the same improvement may be achieved, in chronically diabetic rats, by the topical administration of an inhibitor of conventional PKC isoforms, GF109203X.
Three types of vasodilatory stimuli were used to test cerebrovascular function in the 3 different groups: sciatic nerve stimulation, topically-applied NS1619, and topically-applied K+. Evidence from reports published to date support an important role for BKCa and Kir channels in the communication of a vasodilating signal between astrocytes and arterioles in the process of neurovascular coupling [12,15,27,41,42]. Additional findings point to a repression of BKCa and Kir channel function in diabetic, chronically hyperglycemic rats [23,41]. Present findings confirmed that the K+-channel dysfunction in diabetic rats plays a role in the repression of sciatic nerve stimulation-evoked pial arteriolar dilations. In a previous study, we showed that these dilatory responses were impaired in female Sprague-Dawley diabetic rats [41]. In the present study, we repeated those earlier experiments, but instead using male Lewis rats. We obtained similar results, with the exception that we observed a near-normal response in the diabetic male Lewis rats to the higher dose of NS1619 (50 µM). It has to be noted that NS1619 might have some off target effects, especially toward calcium channels[5]. However, this molcule has been used in a number of publications pertinent to cerebral circulation[16,27,41], making us confident that the results obtained in this paper are valid. We suspect that this difference may be related to the different strain and/or gender of the rats studied. Lewis rats were selected for the present study because they are an inbred strain commonly used in transplantation studies [35]. Indeed, a syngeneic transplantation model was applied in order to eliminate the effect of immune rejection on the transplanted grafts [19]. By doing this, more stable outcome can be obtained to facilitate the further assessment of neurovascular coupling in the rats. In fact, the absence of an immune response toward the transplanted pancreatic islets, likely permitted maintenance of an optimal glycemic control at 4 months following the transplant (Table 1). Importantly, the cerebrovascular reactivities that we measured were never different when comparing transplanted and control animals. This data has strong relevance, since the prevention of chronic hyperglycemia appears to completely prevent the pathophysiologic changes associated with this rat model of uncontrolled type 1 diabetes. However, it yet has to be proven that such changes occur in humans either with unchecked diabetes or with widely fluctuating blood glucose levels. One future study might compare the efficacy of insulin parenteral treatment vs pancreatic islet transplantation in preventing this type of cerebral complication, inasmuch as the glycemic regulation associated with endogenously produced insulin might be superior to exogenous insulin therapeutic regimens.
There is some evidence in the literature of a detrimental effect of acute hyperglycemia on neurovascular coupling[9]. Dorner and coll. measured retinal vein diameter changes in heatlhy volunteers subjected to acute mild hyperglycemia and found that retinal vein dilatory responses to flickering light were depressed in the setting of hyperglycemia. Although, to our knowledge, in the neurovascular coupling literature there is no other mention of venular vasodilation in response to neural activation, this evidence suggests a possible detrimental influence of acute hyperglycemia on neurovascular coupling. Futher studies will need to address this specific issue.
Recent data [43] suggests that resveratrol significantly improved neurovascular coupling capacity in patients with type 2 DM measured by Transcranial Doppler ultrasound. In that study, changes in blood flow velocity (BFV) during a cognitive test battery were used as a surrogate measure of neurovascular coupling. That evidence points toward an impairment of neurovascular coupling in humans in type 2 DM. Another recent study in humans [10] showed changes in hemodynamic response function in early stages of type 2 DM that could be detected as altered functional MRI response profiles. Although that evidence is preliminary and requires further validation, it points toward the direction of possible alterations in neurovascular coupling in humans with type 2 DM, and corroborates our findings in rats. Although the effects of type 2 DM on NVC have not been directly explored in our study, it certainly is a possible future direction of our research.
A key observation in the present study was that the losses in vasodilating function were due to the enhanced activation of one or more of the conventional PKC isoforms. This was based upon the finding that acute administration of a PKC blocker (GF109203X), with selectivity toward conventional PKC isoforms (i.e., α, βI, βII and γ), was accompanied by normalization of pial arteriolar reactivity to sciatic nerve stimulation, NS1619, and K+. Further evidence supporting a role for PKC-α (and perhaps PKC-βII) can be found in a recent paper from our laboratory [38]. Thus, we detected an enhanced protein expression of PKC-α and RACK-1 (which stabilizes the active form of PKC-βII) in the glio-pial tissue fraction. On the other hand, no changes in the PKC-γ isoform were noted.
PKC-α has been linked to the inhibition of ionic channels important for neurovascular coupling, such as inward rectifier K+ channels [28], and, less specifically, of large conductance-Ca2+ operated (BKCa) K+ channels [36]. PKC-α upregulation in diabetes has been documented in brain [31], kidney and heart [20]. Therefore, we speculate that the impairment of neurovascular coupling and cerebrovascular function, occurring in the rat model of Type 1 Diabetes Mellitus, may be mechanistically linked to an increase in PKC-α expression and activity in the pial vessels and/or the astrocytes in contact with them (glia limitans).
The role of PKC-βII in diabetes has been extensively studied, especially in the retina, where it mediates a host of deleterious vascular effects [8,18]. We and others [34] have shown that, PKC-βII protein abundance does not change in the diabetic cerebral cortex or in the glio-pial tissue. Yet, our data on the expression of RACK-1 points to a significant upregulation in the glio-pial tissue, but not in the cerebral cortex, of chronically diabetic rats. This finding could suggest an increased activity of PKC-βII in the diabetic condition, that might have been overlooked by other analyses based on the bulk cerebral cortex sampling. In addition, the role of PKC-βI in diabetes and/or neurovascular coupling appears difficult to disentangle in the literature, probably due to the lack of specific blockers. In many instances, the regulation of PKC-βI and βII seems to follow a similar pattern and have similar pathophysiological implications [8,14]. Therefore, since the pharmacological intervention used in the present study does not allow one to discriminate between the effects of the single isoforms, we cannot, at this time, assign a specific role to PKC-βI in our model.
PKC-γ expression, on the other hand, is decreased in the diabetic glio-pial tissue [38], making it less conceivable that the inhibition caused by GF109203X might exert its effect on cerebrovascular responses through an interaction with this isoform.
In a previous study [41], we found that acute CalC topical exposure in normoglycemic rats was associated with a substantial repression of K+ and sciatic nerve stimulation-induced (but not NS1619-induced) dilations. In the present work, the use of GF109203X, which is selective for the classical PKC isoforms, lacked any effect on the cerebrovascular reactivities to sciatic nerve stimulation, NS1619 and K+. This finding suggests that the complex interactions between hyperglycemia and the different PKC isoforms expression/activity changes produce non-linear effects that are difficult to predict and would warrant further experimentations. Nevertheless, the information conveyed in this study is valuable, because it narrows down the wide array of PKC isoforms that might be involved in the pathophysiology of this diabetic complication and, more importantly, provides evidence for a complete and immediate reversal of a chronic impairment.
Another possible cause of decreased reactivity of the diabetic cerebral vessels, could be the endothelial dysfunction. However, we have demonstrated [44] that, in our model, endothelium does not participate in the response to sciatic nerve stimulation. Another mechanism potentially implicated in the diminished neurovascular coupling response in the diabetic brain could be an impaired neuronal function, as reflected in diminished sciatic nerve stimulation-evoked neuronal responses. This possibility, was ruled out not only by the absence of significant differences in sciatic nerve stimulation-evoked potential amplitudes when comparing diabetic and non-diabetic rats, but also when comparing the electrical responses in the absence and in the presence of GF109203X.
In conclusion, the dysregulation of cerebral perfusion accompanying chronic hyperglycemia, in rat models of type 1 diabetes mellitus, is well-documented. The above pathophysiologic process has been shown to include loss of BKCa and Kir channel-mediated vasodilating functions as well as a significant decrease in the pial arteriolar dilations evoked by somatosensory activation, via sciatic nerve stimulation, in streptozotocin-treated diabetic rats. Present results also suggested that the restoration and maintenance of normoglycemia, via the endogenous production of insulin from transplanted pancreatic islets, are able to prevent the compromise of neurovascular coupling observed in diabetic animals. Moreover, the acute topical administration of an inhibitor of the classical PKC isoforms, GF109203X, was capable of reversing the diabetic cerebrovascular impairments. Both of these neuroprotective mechanisms might have significant clinical implications.
4. Experimental procedures
4.1 Surgical procedures
Adult Lewis male rats (4–6 month old, n=24) were used for cranial window experiments. Experimental procedures were approved by the Animal Care and Use Committee of the University of Illinois at Chicago. As detailed in previously published reports [27,41,44], on the day of the experiment, rats were anesthetized with isoflurane, and mechanically ventilated after orotracheal intubation. The femoral artery and vein on the side not used for the nerve stimulation were cannulated for blood sampling, arterial pressure monitoring, and drug infusions. Rectal temperature was servo-controlled at 37°C. A 10 mm diameter craniotomy was performed over the skull midline. After carefully removing the dura, a glass window outfitted with three ports (artificial cerebrospinal fluid (aCSF) inflow, aCSF outflow, and ICP monitoring) was mounted for pial vessel observation. The space under the cranial window was suffused with aCSF. One hour prior to drug or sciatic nerve stimulation testing, isoflurane was replaced by fentanyl, and anesthesia was maintained with 25 µg/kg/h fentanyl / 70 % N2O / 30 % O2. After negative movement response to tail and paw pinch, paralysis was then induced with d-tubocurarine. Temperature, invasive mean arterial blood pressure, and intracranial pressure were continously monitored and maintained within normal limits. Arterial blood samples were taken at 60-min intervals and analyzed using a blood gas/pH analyzer (Gem3000, Instrumentation Laboratories, Lexington, MA). PaO2, PaCO2, and pH were maintained within physiologic ranges throughout the study.
4.2. Diabetes model and transplantation procedures
Diabetes was induced with a single i.p. streptozotocin (STZ, Sigma, St. Louis, MO) injection (80 mg/kg, freshly dissolved in citrate buffer, pH 4.5) to 2 month old rats (n=13), around 16–18 weeks prior to the experiment. Animals were considered to be diabetic when blood glucose exceeded 350 mg/dL for two consecutive days after STZ. Blood glucose was measured twice a week (Contour glucometer, Bayer, Mishawaka, IN) for the entire period prior to study. Animals in the DM group (n=6) received no insulin.
Syngeneic pancreatic islet transplantation was conducted in the recipient diabetic rats (n=7) 5–7 days after STZ injection using the method described previously [17]. Briefly, donor islets were isolated by conventional collegenase method and cultured at 37°C/5% CO2. On the day of transplantation, 1,000 islets were pooled and prepared for each recipient rat. Under isoflurane anesthesia, a midline incision was made and portal vein was exposed. The islets were injected into the portal vein in 0.3 ml of the same medium through a 25 G needle. After withdrawal of the needle, the injection site was compressed for 2 minutes to minimize blood loss. The incision was closed subsequently and animal was followed for full recovery. Postoperative pain was controlled with scheduled buprenorhine sc injections.
4.3. Experimental protocol
In the typical neurovascular coupling experiment, 1 hour after the anesthesia switch from isoflurane to fentanyl, and regular aCSF suffusion (equilibration period), the rats were subjected to one or two sciatic nerve stimulation episodes followed by NS1619 (10 and 50 µM) or K+ (KCl 6 and 12 mM) suffusions under the cranial window for 5 min at each concentration. In the DM and ND groups, after a recovery period of at least 5 min, we initiated a suffusion of GF109203X (also known as Bisindolylmaleimide I or Gö 6850). GF109203X, at 20 nM, tends to favor the inhibition of PKC-α (IC50 of 8 nM). However, such concentration is thought to inhibit also PKC-βI, -βII and -γ (IC50 of 18, 20, and 21 nM, respectively), but not other PKC isoforms, e.g. δ, ε and ζ (IC50 of 210, 132 and 5800 nM, respectively) [22,37]. Therefore, all the conventional PKC isoforms might be similarly affected by GF109203X. Pial arteriolar diameter changes, relative to baseline, after 40 min suffusion of GF109203X (20 nM) under the cranial window were modest and not significantly different when comparing non-diabetic and diabetic rats (-2 ± 3% and -3 ± 6 %, respectively; P > 0.05). Forty minutes later, a second sciatic nerve stimulation was imposed, followed by re-evaluation of NS1619 and K+-induced dilations.
2.4 Video imaging and data analysis
Intravital video microscopy was used to study pial arteriole diameter changes in response to drugs and/or sciatic nerve stimulation [41]. Images were acquired through a CCD camera (Photometrics, Tucson, AZ) mounted on an upright stereomicroscope (Nikon, Melville, NY). We studied arterioles overlying the hindlimb somatosensory cortex [24]. Starting at 20 s prior to sciatic nerve stimulation and continuing for 60 s post-sciatic nerve stimulation, pial arterioles were monitored and the recordings saved for subsequent analysis. Arteriolar diameter changes were measured off line using Metamorph software (MDS Analytical Technologies, Sunnyvale, CA). The diameter values of 3 different pial arterioles were averaged for each time point. The peak dilation induced by sciatic nerve stimulation was calculated as the percent change relative to the baseline, where baseline was taken as the average diameter value measured over 20 s immediately preceding sciatic nerve stimulation. The dilation induced by NS1619 or K+ was measured after 5 min of perfusion and compared to the baseline diameter.
4.5. Sciatic nerve stimulation and electrophysiologic recordings
The experiments were conducted according to previously published techniques. Briefly, the sciatic nerve on one side was surgically exposed and sectioned. A ring electrode was carefully secured to the nerve ending and connected to an isolated stimulator unit (Stimulator HC, ML155, AD Instruments, Sidney, Australia) controlled via Labchart 7 software (AD Instruments) [40,41]. Electrophysiologic recordings of the somatosensory evoked potentials (SEPs) were obtained through stainless steel screws placed on the border of the cranial window. The signal was amplified, bandpass filtered (0.1–1kHz) and digitized by a PowerLab acquisition board (AD Instruments). Peak-to-peak N1P1 and N2P2 amplitudes were measured and compared before and after administration of GF109203X (and in ND vs DM, and ND vs TR) to evaluate possible effects on SEPs. Values were obtained by averaging 2–4 trains of stimuli per each condition. No significant differences were detected (Fig.1, D).
4.6 Drugs
NS1619 and streptozotocin were obtained from Sigma-Aldrich (St. Louis, MO). GF109203X was purchased from Tocris Bioscience (Bristol, UK).
4.7. Statistical analysis
Statistical comparisons between groups were performed using one-way ANOVA with Tukey post-hoc correction, while the effect of treatments within the same experiment was evaluated via repeated measure one-way ANOVA. Data are expressed as Mean ± sd. Statistical significance was set at P = 0.05; n refers to the number of experimental animals.
Highlights.
Neurovascular coupling was impaired in diabetic vs either non-diabetic (ND) or pancreatic islet transplanted-rats (TR).
Pial arteriolar dilations to KCl and NS1619 were suppressed in diabetic rats, but not in ND or TR animals.
These responses were completely restored by the acute application of a PKC inhibitor, GF109203X.
Diabetes-associated chronic impairment of neurovascular coupling may be reversed by a PKC inhibitor.
Diabetes-associated chronic impairment of neurovascular coupling may be prevented via pancreatic islet transplantation.
Acknowledgments
This work was supported by NIH grant 5 RO1 NS0672379-04 to Dale.A. Pelligrino, JDRF postdoctoral fellowship 3-2008-462 to F.V., NIH (ICR Grant 1 U42 RR023245-01) and JDRF grants (#5-2006-398) to J.O.
We would like to thank Dale A. Pelligrino for his guidance and input on this research and dedicate this paper to his memory
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
aCSF, artificial cerebrospinal fluid
BKCa, large-conductance Ca2+-operated K+ channel
DM, diabetic
ND, non-diabetic
NVC, neurovascular coupling
Kir, K+ inward rectifier channel
PKC, protein kinase C
SEPs, somatosensory evoked potentials
STZ, streptozotocin
TR, transplanted rats
References
- 1.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez EO, Beauquis J, Revsin Y, Banzan AM, Roig P, De Nicola AF, Saravia F. Cognitive dysfunction and hippocampal changes in experimental type 1 diabetes. Behav Brain Res. 2009;198:224–230. doi: 10.1016/j.bbr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Arrick DM, Sharpe GM, Sun H, Mayhan WG. Diabetes-induced cerebrovascular dysfunction: role of poly(ADP-ribose) polymerase. Microvasc Res. 2007;73:1–6. doi: 10.1016/j.mvr.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bentzen BH, Olesen SP, Ronn LC, Grunnet M. BK channel activators and their therapeutic perspectives. Front Physiol. 2014;5:389. doi: 10.3389/fphys.2014.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57:3169–3176. doi: 10.2337/db08-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dabelea D, Bell RA, D'Agostino RB, Jr, Imperatore G, Johansen JM, Linder B, Liu LL, Loots B, Marcovina S, Mayer-Davis EJ, Pettitt DJ, Waitzfelder B. Incidence of diabetes in youth in the United States. Jama. 2007;297:2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 8.Das Evcimen N, King GL. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol Res. 2007;55:498–510. doi: 10.1016/j.phrs.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Dorner GT, Garhofer G, Huemer KH, Riva CE, Wolzt M, Schmetterer L. Hyperglycemia affects flicker-induced vasodilation in the retina of healthy subjects. Vision Res. 2003;43:1495–1500. doi: 10.1016/s0042-6989(03)00170-6. [DOI] [PubMed] [Google Scholar]
- 10.Duarte JV, Pereira JM, Quendera B, Raimundo M, Moreno C, Gomes L, Carrilho F, Castelo-Branco M. Early disrupted neurovascular coupling and changed event level hemodynamic response function in type 2 diabetes: an fMRI study. J Cereb Blood Flow Metab. 2015;35:1671–1680. doi: 10.1038/jcbfm.2015.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fakler B, Brandle U, Glowatzki E, Zenner HP, Ruppersberg JP. Kir2.1 inward rectifier K+ channels are regulated independently by protein kinases and ATP hydrolysis. Neuron. 1994;13:1413–1420. doi: 10.1016/0896-6273(94)90426-x. [DOI] [PubMed] [Google Scholar]
- 12.Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- 13.Gangemi A, Salehi P, Hatipoglu B, Martellotto J, Barbaro B, Kuechle JB, Qi M, Wang Y, Pallan P, Owens C, Bui J, West D, Kaplan B, Benedetti E, Oberholzer J. Islet transplantation for brittle type 1 diabetes: the UIC protocol. Am J Transplant. 2008;8:1250–1261. doi: 10.1111/j.1600-6143.2008.02234.x. [DOI] [PubMed] [Google Scholar]
- 14.Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106:1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci U S A. 2010;107:3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holland M, Langton PD, Standen NB, Boyle JP. Effects of the BKCa channel activator, NS1619, on rat cerebral artery smooth muscle. Br J Pharmacol. 1996;117:119–129. doi: 10.1111/j.1476-5381.1996.tb15163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes SJ, Davies SE, Powis SH, Press M. Hyperoxia improves the survival of intraportally transplanted syngeneic pancreatic islets. Transplantation. 2003;75:1954–1959. doi: 10.1097/01.TP.0000066805.39716.23. [DOI] [PubMed] [Google Scholar]
- 18.Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL. Preferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci U S A. 1992;89:11059–11063. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jorns A, Klempnauer J, Tiedge M, Lenzen S. Recovery of pancreatic beta cells in response to long-term normoglycemia after pancreas or islet transplantation in severely streptozotocin diabetic adult rats. Pancreas. 2001;23:186–196. doi: 10.1097/00006676-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Kang N, Alexander G, Park JK, Maasch C, Buchwalow I, Luft FC, Haller H. Differential expression of protein kinase C isoforms in streptozotocin-induced diabetic rats. Kidney Int. 1999;56:1737–1750. doi: 10.1046/j.1523-1755.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- 21.Ko EA, Han J, Jung ID, Park WS. Physiological roles of K+ channels in vascular smooth muscle cells. J Smooth Muscle Res. 2008;44:65–81. doi: 10.1540/jsmr.44.65. [DOI] [PubMed] [Google Scholar]
- 22.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- 23.Mayhan WG, Mayhan JF, Sun H, Patel KP. In vivo properties of potassium channels in cerebral blood vessels during diabetes mellitus. Microcirculation. 2004;11:605–613. doi: 10.1080/10739680490503410. [DOI] [PubMed] [Google Scholar]
- 24.Meno JR, Nguyen TS, Jensen EM, Alexander West G, Groysman L, Kung DK, Ngai AC, Britz GW, Winn HR. Effect of caffeine on cerebral blood flow response to somatosensory stimulation. J Cereb Blood Flow Metab. 2005;25:775–784. doi: 10.1038/sj.jcbfm.9600075. [DOI] [PubMed] [Google Scholar]
- 25.Nathan DM, Lachin J, Cleary P, Orchard T, Brillon DJ, Backlund JY, O'Leary DH, Genuth S. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003;348:2294–2303. doi: 10.1056/NEJMoa022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Northam EA, Rankins D, Cameron FJ. Therapy insight: the impact of type 1 diabetes on brain development and function. Nat Clin Pract Neurol. 2006;2:78–86. doi: 10.1038/ncpneuro0097. [DOI] [PubMed] [Google Scholar]
- 27.Paisansathan C, Xu H, Vetri F, Hernandez M, Pelligrino DA. Interactions between adenosine and K+ channel-related pathways in the coupling of somatosensory activation and pial arteriolar dilation. Am J Physiol Heart Circ Physiol. 2010;299:H2009–H2017. doi: 10.1152/ajpheart.00702.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park WS, Kim N, Youm JB, Warda M, Ko JH, Kim SJ, Earm YE, Han J. Angiotensin II inhibits inward rectifier K+ channels in rabbit coronary arterial smooth muscle cells through protein kinase Calpha. Biochem Biophys Res Commun. 2006;341:728–735. doi: 10.1016/j.bbrc.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 29.Pelligrino DA, Koenig HM, Wang Q, Albrecht RF. Protein kinase C suppresses receptor-mediated pial arteriolar relaxation in the diabetic rat. Neuroreport. 1994;5:417–420. doi: 10.1097/00001756-199401120-00011. [DOI] [PubMed] [Google Scholar]
- 30.Profenno LA, Porsteinsson AP, Faraone SV. Meta-Analysis of Alzheimer's Disease Risk with Obesity, Diabetes, and Related Disorders. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Ramakrishnan R, Sheeladevi R, Suthanthirarajan N. PKC-alpha mediated alterations of indoleamine contents in diabetic rat brain. Brain Res Bull. 2004;64:189–194. doi: 10.1016/j.brainresbull.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 33.Schubert R, Nelson MT. Protein kinases: tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci. 2001;22:505–512. doi: 10.1016/s0165-6147(00)01775-2. [DOI] [PubMed] [Google Scholar]
- 34.Shiba T, Inoguchi T, Sportsman JR, Heath WF, Bursell S, King GL. Correlation of diacylglycerol level and protein kinase C activity in rat retina to retinal circulation. Am J Physiol. 1993;265:E783–E793. doi: 10.1152/ajpendo.1993.265.5.E783. [DOI] [PubMed] [Google Scholar]
- 35.Stagner JI, Rilo HL, White KK. The pancreas as an islet transplantation site. Confirmation in a syngeneic rodent and canine autotransplant model. Jop. 2007;8:628–636. [PubMed] [Google Scholar]
- 36.Tian L, Philp JA, Shipston MJ. Glucocorticoid block of protein kinase C signalling in mouse pituitary corticotroph AtT20 D16:16 cells. J Physiol. 1999;516(Pt 3):757–768. doi: 10.1111/j.1469-7793.1999.0757u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- 38.Vetri F, Chavez R, Xu HL, Paisansathan C, Pelligrino DA. Complex modulation of the expression of PKC isoforms in the rat brain during chronic type 1 diabetes mellitus. Brain Res. 2012;1490:202–209. doi: 10.1016/j.brainres.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vetri F, Menicucci D, Lapi D, Gemignani A, Colantuoni A. Pial arteriolar vasomotion changes during cortical activation in rats. Neuroimage. 2007;38:25–33. doi: 10.1016/j.neuroimage.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 40.Vetri F, Xu H, Mao L, Paisansathan C, Pelligrino DA. ATP hydrolysis pathways and their contributions to pial arteriolar dilation in rats. Am J Physiol Heart Circ Physiol. 2011;301:H1369–H1377. doi: 10.1152/ajpheart.00556.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vetri F, Xu H, Paisansathan C, Pelligrino DA. Impairment of neurovascular coupling in type 1 diabetes mellitus in rats is linked to PKC modulation of BK(Ca) and Kir channels. Am J Physiol Heart Circ Physiol. 2012;302:H1274–H1284. doi: 10.1152/ajpheart.01067.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vetri F, Xu HL, Mao L, Pelligrino DA. Society for Neuroscience meeting. Washington, DC: 2008. Diabetes-related changes in neurovascular coupling in rats are gender-specific. [Google Scholar]
- 43.Wong RH, Raederstorff D, Howe PR. Acute Resveratrol Consumption Improves Neurovascular Coupling Capacity in Adults with Type 2 Diabetes Mellitus. Nutrients. 2016;8 doi: 10.3390/nu8070425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu HL, Mao L, Ye S, Paisansathan C, Vetri F, Pelligrino DA. Astrocytes are a key conduit for upstream signaling of vasodilation during cerebral cortical neuronal activation in vivo. Am J Physiol Heart Circ Physiol. 2008;294:H622–H632. doi: 10.1152/ajpheart.00530.2007. [DOI] [PubMed] [Google Scholar]
- 45.Zhou XB, Wulfsen I, Utku E, Sausbier U, Sausbier M, Wieland T, Ruth P, Korth M. Dual role of protein kinase C on BK channel regulation. Proc Natl Acad Sci U S A. 2010;107:8005–8010. doi: 10.1073/pnas.0912029107. [DOI] [PMC free article] [PubMed] [Google Scholar]