Abstract
Objective
Regulatory B cells that inhibit immune responses through interleukin-10 (IL-10) secretion (B10 cells) have been characterized in adults with autoimmune disease. This study examines B10 cells across the entire age range of normal human development, and their changes during pediatric autoimmunity.
Methods
The phenotype and numbers of blood B10 cells were examined in healthy individuals and children with autoimmunity by flow cytometry. B10 cell function was assessed by measuring the effect of B cell-derived IL-10 on CD4+ T cell interferon-gamma (IFN-γ) expression. Serum cytokine levels were measured by enzyme-linked immunosorbent assay (ELISA).
Results
B10 cell frequencies transiently increase during childhood when up to 30% of B cells were competent to produce IL-10, compared to the low frequencies in healthy newborns (3–4%) and adults (7–%). The surface phenotype of B10 cells in children revealed age-dependent variability. B10 cells from children were distinct from proinflammatory cytokine-producing B cells and down-regulated CD4+ T cell IFN-γ production in vitro. Compared to age-matched healthy controls, children with autoimmunity had lower B10 cell frequencies and numbers (decreased by 39% and 48%, respectively), higher IFN-γ and lower interleukin-21 (IL-21) serum levels. IFN-γ inhibited whereas IL-21 promoted B cell IL-10 competence in vitro.
Conclusion
B10 cells, a functionally-defined subset with variable surface phenotype reflective of overall B cell development, transiently expand during childhood. The decreased B10 cell frequencies and numbers in children with autoimmunity may be partially explained by the differential regulation of B10 cell development by IFN-γ and IL-21 and alterations in serum cytokine levels.
Regulatory B cells that inhibit immune responses through the secretion of cytokines have been described in a variety of mouse models of autoimmunity and inflammation (1, 2). A subset of B cells that are competent to express the negative regulatory cytokine IL-10 (B10 cells) is among those best studied. Because phenotypic markers unique to B10 cells have not been identified, these cells are defined by their IL-10 expression following appropriate in vitro stimulation (3, 4). As in mice (5, 6), B10 cells are found at low frequencies in humans (3, 7). Human regulatory B cells have been reported within B cell subsets expressing phenotypic markers associated with transitional B cells (8–12), memory B cells (3, 9, 11, 13), germinal center B cells (14) and plasmablasts (13). Although the functional importance of B10 cells is well described in mice, their role in human autoimmunity remains unclear. In adult humans, B10 cell frequencies are increased or maintained with autoimmunity (3). However, defects in the size and/or functionality of various regulatory B cell compartments have also been reported (8, 10, 11, 15–20).
B10 cell frequencies and numbers are increased in newborn and aged mice (6). Two studies have examined B10 cells in healthy children, one in cryopreserved cord blood samples (3) and one in the context of Wiskott-Aldrich syndrome (21). There are no studies examining B10 cells across the entire age range of normal human development. Common low-grade inflammatory conditions (such as hypertension or obesity) that potentially confound the assessment of immunologic parameters in adults are rare in children (22, 23). Furthermore, the incidence of autoimmunity is lower in children compared to adults (24–30), in contrast with the extensive autoantigen exposure associated with tissue remodeling during normal growth. Additionally, the surface phenotype of blood B cells changes with age during childhood and reflects overall changes in B cell development (31–33). Thereby, studies of B10 cells in the developing human offer unique opportunities to examine this regulatory subset during normal growth and in the context of autoimmunity, and to better define the relationship between B10 cells and surface phenotype-defined developmental B cell subsets.
MATERIALS AND METHODS
Study design
The study protocol was approved by the Duke University Institutional Review Board (IRB) in compliance with the Helsinki Declaration. Participants were recruited from the Research Triangle Area (North Carolina, USA) between February 2012 and July 2015. Following written informed consent, samples were obtained from neonates (umbilical cord samples, n=4), healthy children (n=20), and healthy adults (n=16). An 11-month-old infant was recruited from the University of South Florida under their IRB-approved protocol. Children with autoimmune diseases (n=52) included juvenile idiopathic arthritis (JIA; n=25), juvenile dermatomyositis (JDM; n=13), systemic lupus erythematosus (SLE; n=13) and mixed connective tissue disease (MCTD; n=1). All children with SLE satisfied the American College of Rheumatology criteria (34). 12 of 13 children with JDM had diagnostic muscle biopsy and/or electromyography and satisfied the Bohan and Peter criteria (35). The child with MCTD had myositis, lymphopenia, Raynaud’s phenomenon, periungual telangiectasias, polyarthritis, parotitis, and positive serum autoantibodies (rheumatoid factor, anti-Smith, anti-ribonucleoprotein). All 24 children with JIA satisfied the International League of Associations for Rheumatology criteria (36). Due to sample limitations, there were only 5 white blood cell (WBC) measurements, 14 B10 cell measurements and 11 B10+B10PRO cell measurements in adults, and only 24 B10 cell measurements and 23 B10+B10PRO cell measurements in children with JIA. Exclusion criteria included systemic-onset JIA, intercurrent illness, surgical procedures or vaccination within 4 weeks, and treatment with rituximab, belimumab or cyclophosphamide in the last 12 months. The demographics and study characteristics of healthy children are summarized in Supplementary Table 1, and the clinical data of children with autoimmune diseases are summarized in Supplementary Table 2.
Since JIA, JDM, SLE and MCTD lack a common disease activity assessment tool, physician global assessment as a continuous visual analogue scale score (0–10 cm) was used to assess disease activity and minimize bias due to the use of multiple disease activity assessment tools. 1 of 8 pediatric rheumatologists at the Duke Children’s Health Center scored each participant following their ambulatory clinic visit and prior to any laboratory testing. Scores < 1 were categorized as “inactive disease” and scores ≥1 as “active disease,” respectively.
Cell isolation and culture
Fresh human peripheral blood collected in EDTA (ethylenediaminetetraacetic acid)-coated Vacutainer tubes (BD Biosciences) was processed within 12 hours. WBC counts were measured with a Beckman Coulter automated counter. Serum was obtained after whole blood centrifugation and stored at −80 °C. Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation over a discontinuous gradient (Lymphoprep, Axis-Shield PoC) and quantified manually using a hemocytometer. PBMCs were resuspended in culture medium (RPMI 1640 with 10% fetal calf serum, 200 U/mL penicillin, 200 qµg/mL streptomycin, and 2 mM L-glutamine) and seeded in 48-well flat-bottom tissue culture plates (Genesee Scientific) at a final volume of 1 mL (2×106 PBMCs/mL). Cultures were stimulated with CpG (ODN 2006, 10 qµg/mL; InvivoGen), human recombinant CD40L (1 qµg/mL; Insight Genomics), phorbol esters (PMA, 50 ng/mL; Sigma-Aldrich), ionomycin (1 qµg/mL; Sigma-Aldrich) and Brefeldin A (BFA; BioLegend) as indicated. Alternatively, commercially available magnetic separation-based negative selection kits were utilized to purify B cells (EasySep, StemCell Technologies) and CD4+ T cells (Human CD4+ T Cell Isolation Kit, Miltenyi Biotec). The following human recombinant proteins (100 ng/mL) were used to stimulate B cell IL-10 production in vitro: interleukin-12 (IL-12), IL-21, interleukin-27 (IL-27) and interleukin-35 Fc fusion protein (IL-35 Fc FP) from BioLegend; IFN-γ from PeproTech.
Immunofluorescence staining and flow cytometry analysis
Mononuclear cells were stained using pre-determined optimal concentrations of antibodies for cell surface staining, permeabilized (Cytofix/Cytoperm, BD Biosciences) and stained for intracellular IL-10, IFN-γ, GM-CSF or IL-1β expression as described (3). Fixable cell viability dyes (Fixable Violet Dead Cell Stain, Invitrogen; Zombie Aqua, BioLegend) were used to exclude dead cells. The following anti-human fluorochrome-conjugated monoclonal antibodies (mAbs) were used (clones in parentheses): IgM (MHM-88), IgD (IA6-2), CD3 (OKT3), CD4 (OKT4), CD5 (UCHT2), CD10 (HI10a), CD19 (HIB19), CD20 (2H7), CD21 (Bu32), CD22 (HIB22), CD24 (ML5), CD38 (HIT2), CD44 (IM7), CD48 (BJ40), CD73 (AD2), CD360 (2G1-K12), granulocyte-monocyte colony stimulating factor (GM-CSF) (BVD2-21C11), interleukin-1 beta (IL-1β) (H1b-98) and IL-10 (JES3-9D7) from BioLegend; IgG (G18-145), CD27 (M-T271) and IFN-γ (B27) from BD Pharmingen; interleukin-12 receptor beta 2 subunit (IL-12Rβ2) (305719) from R&D Systems; CD1d (51.1) and CD9 (eBioSN4) from eBiosciences. Surface IgA was visualized using goat polyclonal antibody (Southern Biotech). Immunofluorescence was quantified using a FACSCanto II cytometer (BD Biosciences) and FlowJo (version X) analysis.
In vitro functional assays
Purified CD4+ T cells (1×106 cells/mL) were cultured for 72 hours with soluble CD3 mAb (clone HIT3a, 1 qµg/mL, BD Pharmingen), CD40L (1 qµg/mL; Insight Genomics) and CpG (ODN 2006, 10 qµg/mL; InvivoGen) in the presence or absence of purified B cells (1×106 cells/mL) (1:1 ratio) in 48-well flat-bottom plates at a final volume of 1 mL. IL-10 receptor (IL-10R)-blocking mAb (5 qµg/mL, clone 3F9, Biolegend) or control rat IgG2a (5 qµg/mL, clone KLH/G2a-1-1, Southern Biotech) was added to the B-T cell cocultures as indicated. During the last 5 hours, cultures were stimulated with PMA, ionomycin and BFA. Cells were then stained with fluorophore-conjugated mAbs against CD3, CD4, CD19 and IFN-γ, followed by flow cytometry analysis as above.
Serum cytokine analysis
Serum IFN-γ, IL-10 and IL-21 concentrations in duplicate samples were quantified by ELISA MAX kits (Biolegend) according to the manufacturer’s protocols with results quantified using a Molecular Devices microplate reader (model Emax). Four-parameter regression analysis was used to fit standard curves and calculate serum sample cytokine values.
Statistical analysis
For all comparisons, two-sided tests were used; alpha was set at 0.05. All differences between groups were tested with the independent sample Mann-Whitney U test (unpaired) unless otherwise indicated. All statistical testing was performed on the IBM SPSS platform (version 22). Graphics were created with GraphPad PRISM (version 7) and IBM SPSS statistics.
RESULTS
The B10 cell compartment transiently expands in childhood
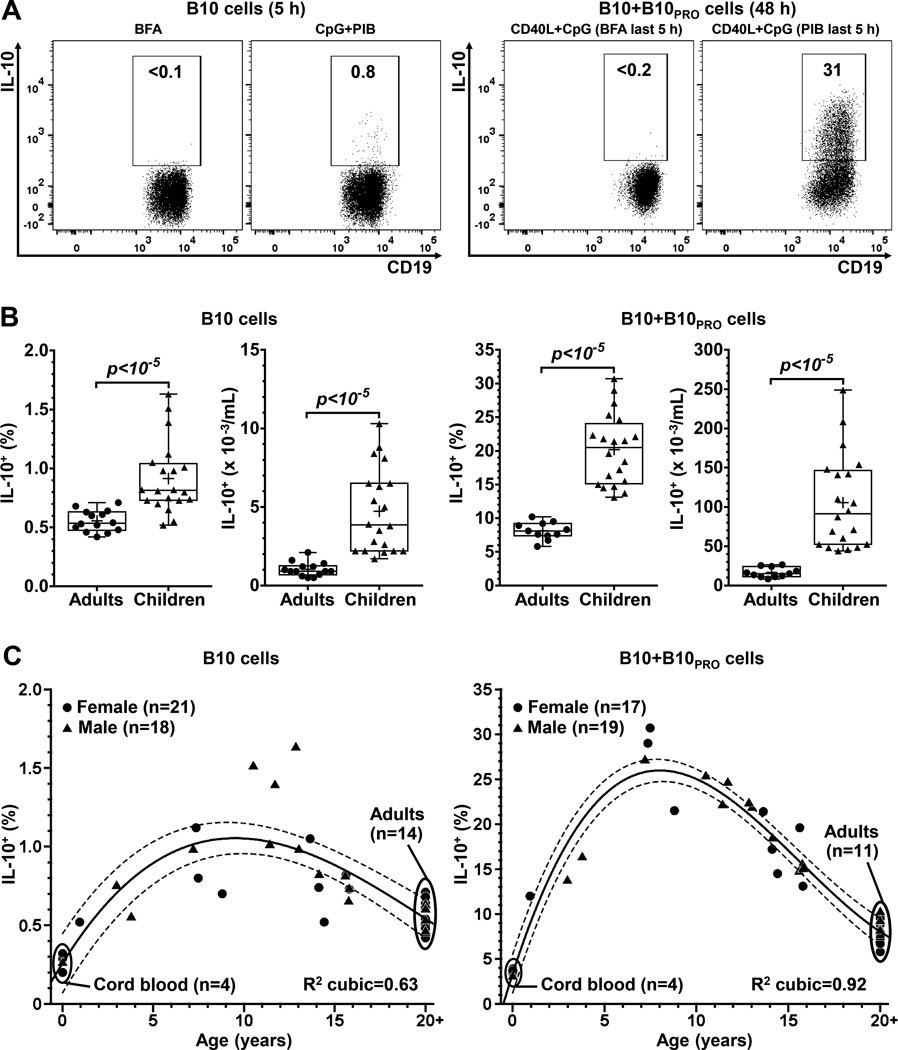
The size of the “B10 cell compartment” (B10 cells and B10 progenitor cells) was assessed (Supplementary Figure 1) as described (3). B10 cells are defined by their capacity to express IL-10 after short-term (5 hours) ex vivo stimulation with PMA, ionomycin and BFA (“PIB”; Figure 1A). B10 progenitor (B10PRO) cells do not express IL-10 following short-term ex vivo stimulation (37) but acquire IL-10-competence following 48 hours of agonistic CD40 stimulation (CD40L) in vitro (Figure 1A); the addition of CpG further enhances the acquisition of IL-10 competence (3). Adding BFA alone during the last 5 hours serves as a negative control for the 0.1–0.2% background staining, similar to the frequencies observed in IL-10-deficient mice (4, 6).
Figure 1.
The B10 cell compartment transiently expands in childhood. A, PBMCs from healthy individuals were cultured with CpG for 5 hours to visualize B10 cells, or with CD40L+CpG for 48 hours to visualize B10+B10PRO cells; BFA or PMA, ionomycin and BFA (“PIB”) added to cultures during the last 5 hours. IL-10+ B cell frequencies were measured by flow cytometry. To position the IL-10+ gate, only BFA (instead of PIB) was added to the cultures during the last 5 hours. Average background staining for B cell IL-10 positivity was 0.1% and 0.2% for B10 and B10+B10PRO cell cultures, respectively; numbers represent the frequencies (% of all CD19+) within the IL-10+ gate. B, Healthy children have increased frequencies and numbers of B10 and B10+B10PRO cells compared to healthy adults. C, The relationship between age and B10 (left panel) or B10+B10PRO (right panel) cell frequencies. Interrupted lines represent the upper and lower limits of the 99% confidence interval of fitted polynomial regression curves, with adults grouped as a single age point (20+). P values were calculated using the independent sample Mann-Whitney U test (unpaired); crosses within boxplots represent means.
Healthy children (n=20, age range: 3–16 years) had significantly increased frequencies and numbers of B10 and B10+B10PRO cells (Figure 1B) when compared to healthy adults (age range: 27–52 years). Mean frequencies of B10 and B10+B10PRO cells in children were increased by 1.7-fold and 2.4-fold, and mean numbers of B10 and B10+B10PRO cells increased by 3.9-fold and 5.7-fold, respectively. Adult B10 (n=14) and B10+B10PRO (n=11) cell measurements were representative of those from >100 healthy adults (3, 7). Children had higher absolute lymphocyte counts (ALC), B cell frequencies and B cell numbers compared to adults (Supplementary Figure 2A). The B10 cell compartment size was similar between males and females (Supplementary Figure 2B). Expansion of the B10+B10PRO cell compartment was transient and peaked in middle childhood (5–11 years); a less prominent relationship with age was observed for B10 cells (Figure 1C). Thus, the B10 cell compartment expands transiently during childhood, with up to 30% of blood B cells becoming competent to produce IL-10 in middle childhood.
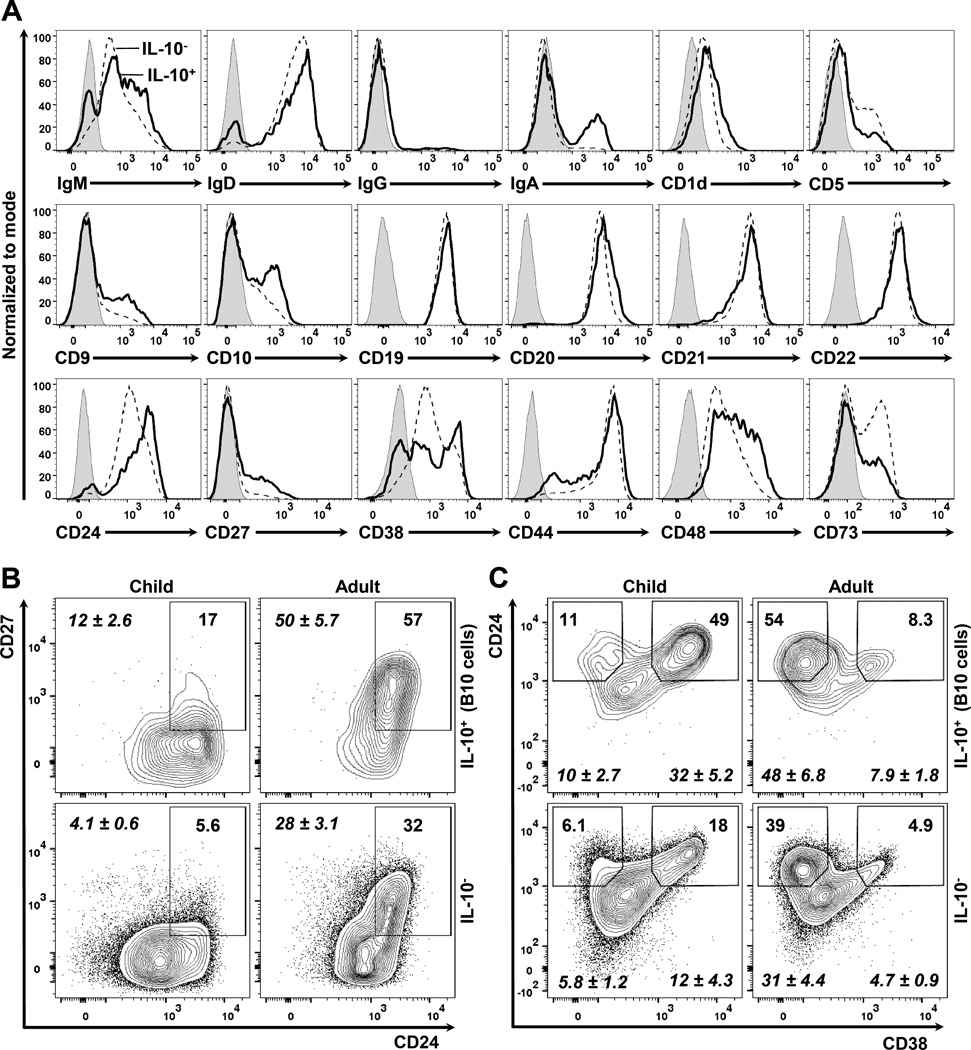
The B10 cell surface phenotype reflects overall B cell development
The surface phenotype of B10 cells in children was variable. B10 cells were enriched within the non-naïve (IgD−), class-switched (IgG+ or IgA+) B cell compartments, indicating their prior exposure to antigen in vivo (Figure 2A). The frequency and density of B10 cell surface IgM, IgD, IgG, IgA, CD19, CD20, CD21, and CD22 expression were similar between subjects, whereas the expression and density of surface markers that dynamically change during B cell development and maturation such as CD5, CD9, CD10, CD24, CD27, CD38, CD44, CD48 and CD73 were more varied between subjects. The 5-hour stimulation with PMA/ionomycin and CpG did not alter the cell surface expression of any of the B cell phenotypic markers presented, although this treatment did induce significant changes in CD360 (IL-21 receptor) and IL-12Rβ2 expression (Supplementary Figure 3). The surface phenotype of B10+B10PRO cells was not examined since it reflects the effects of CD40 and CpG signals during the 48-hour cultures (3).
Figure 2.
The surface phenotype of B10 cells varies during B cell development. PBMCs from healthy children and/or healthy adults were cultured with CpG and stimulated with PIB for 5 hours. The surface phenotype of B10 cells was examined by flow cytometry. Representative data from at least 3 different samples from middle childhood are shown. All flow cytometry plots are gated on CD19+ live lymphocytes. A, Modal histograms of IL-10+ (thick line) and IL-10− (interrupted line) B cells; isotype-matched monoclonal antibody (mAb) staining is shown as a gray shaded area. B–C, Contour plots comparing IL-10+ (B10 cells, top row) with IL-10− (bottom row) B cells from a healthy 7-year-old child (left column) and a healthy adult (right column) in regards to the surface expression of (B) CD24 and CD27 and (C) CD24 and CD38. Numbers represent the cell frequencies (% of total) within each gate, with mean (±SEM) values for children (n=5, age range: 3–11 years) and adults (n=5, age range: 27–52 years) shown in italics.
Regulatory B cells in adults have been “localized” previously to either the memory CD24hiCD27+ or the transitional CD24hiCD38hi B cell compartments (3, 8–13). While most adult B10 cells were CD27+ and ~50% were CD24hiCD27+, only 12% of B10 cells in children were CD24hiCD27+ (Figure 2B, Supplementary Figure 4A). In contrast, 32% of B10 cells in children were CD24hiCD38hi, while only 8% of adult B10 cells were CD24hiCD38hi (Figure 2C, Supplementary Figure 4B). The surface phenotype of B10+B10PRO cells is not presented since it reflects the effects of CD40L and CpG signals during the 48-hour cultures. B10 or B10+B10PRO cell frequencies in children did not correlate with the size of either the memory CD24hiCD27+ or transitional CD24hiCD38hi B cell compartments (Supplementary Figure 5).
Compared to older children, neonates had low B10 and B10+B10PRO cell frequencies (Figure 1C) despite their higher frequencies of CD24hiCD38hi and lower frequencies of CD27+ B cells (31–33). Neonates and adults had similar B10 and B10+B10PRO cell frequencies, despite the dramatically different surface phenotypes of neonatal B cells (high immature/naive and transitional B cell frequencies) compared to high frequencies of memory B cells in adult blood (33). As in adults (3), CD5 was a poor marker for B10 cells in children and CD1d was generally not expressed by blood B cells (Figure 2A). In addition, the low frequencies of B10 and B10PRO cells in cord blood (Figure 1C) contrasts with the abundance of CD5+ B cells in neonates (38). Thus, although there are no cell surface markers specific for B10 cells, these cells were more prevalent within certain surface phenotype-defined B cell subsets depending on the age of the individual.
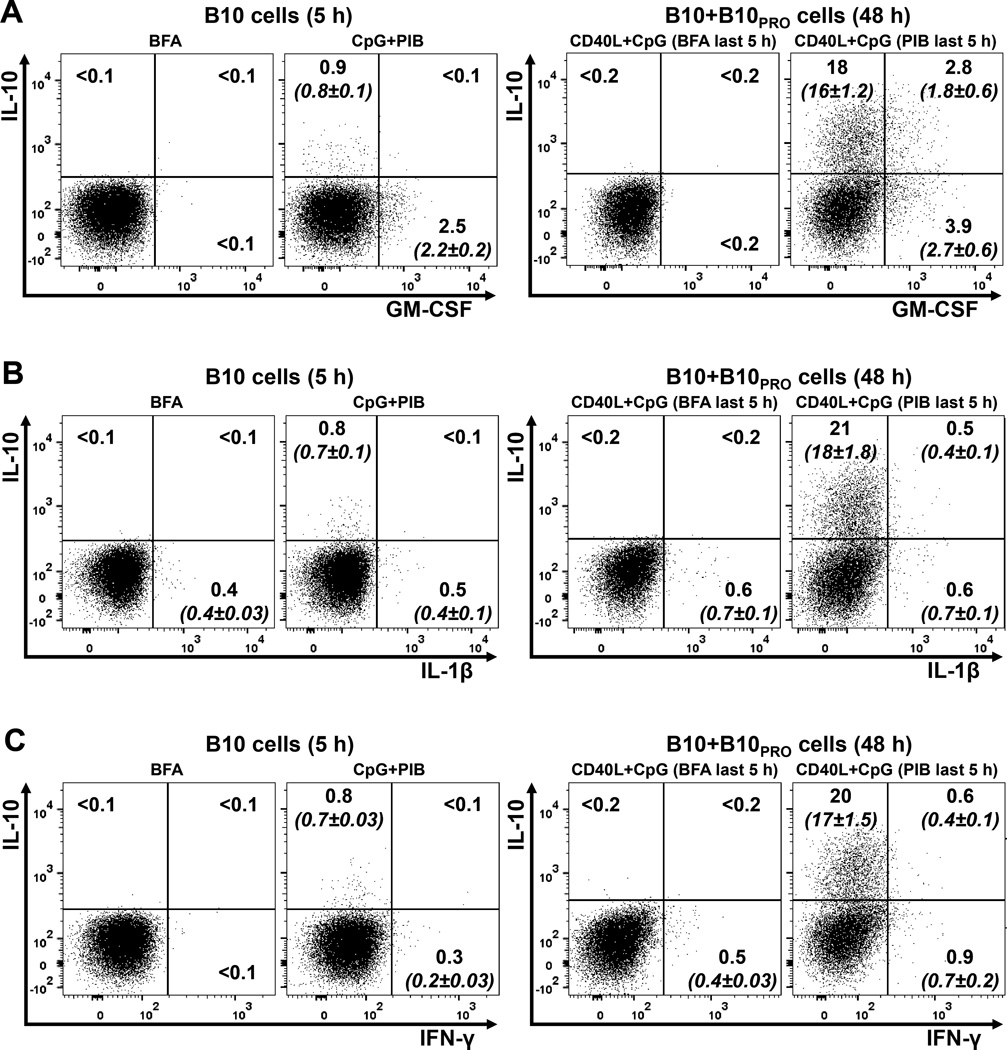
B10 cells do not express GM-CSF, IL-1β, or IFN-γ
Since B10 and B10+B10PRO cells cannot be visualized without PMA/ionomycin stimulation, we examined the possibility that these cells represent activated B cells expressing multiple cytokines in response to non-specific in vitro stimulation. The B10 and B10+B10PRO cell expression profile of three proinflammatory cytokines was examined using purified B cells from three healthy children (Figure 3; Supplementary Table 1). In all three subjects, B10 cells did not express GM-CSF, IL-1β, or IFN-γ, while less than 15% of B10+B10PRO cells expressed GM-CSF and less than 5% expressed IL-1β or IFN-γ. Therefore, the B10 cell compartment in children has a specific cytokine expression profile, characterized by the limited or no expression of proinflammatory cytokines.
Figure 3.
Relationship between IL-10-producing and proinflammatory cytokine-producing B cells. B cells purified from a healthy 12-year-old child (HC17, Supplementary Table 1) were cultured with CpG for 5 hours (B10 cells) or CD40L+CpG for 48 hours (B10+B10PRO cells), with BFA or PIB added to the cultures during the last 5 hours. B cells were stained intracellularly for IL-10 and either GM-CSF (A), IL-1β (B) or IFN-γ (C). The position of all gates was determined using isotype-matched control mAb staining and fluorescence minus one (FMO) controls. These data are representative of those obtained in three separate experiments (n=3; HC3, HC7 and HC17 in Supplementary Table 1). All flow cytometry dot plots were gated on CD19+ live lymphocytes, with numbers representing their frequencies (% of total) within each gate; mean (±SEM; n=3) values are shown within parentheses in italics.
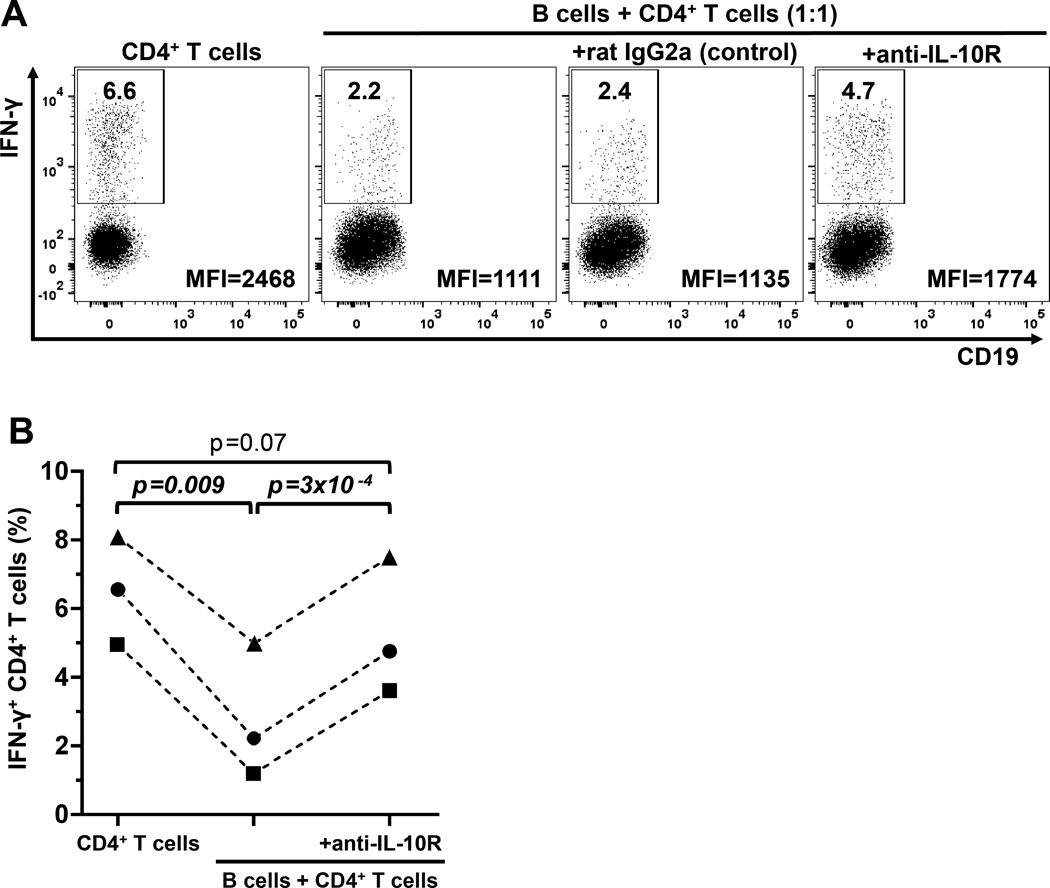
B10 cells negatively regulate CD4+ T cell IFN-γ expression in vitro
The high B10+B10PRO cell frequencies in children provided the unique opportunity to examine the regulatory effects of the entire B10 cell compartment, eliminating the need to fractionate B cell subpopulations based on cell surface marker expression. The regulatory effects of B cell-derived IL-10 on activated CD4+ T cell IFN-γ expression was assessed in three healthy children (Figure 4; Supplementary Table 1). Purified CD4+ T cells stimulated through CD3 were cultured for 72 hours with purified B cells (ratio 1:1) in the presence of CD40L and CpG. The presence of B cells reduced the frequency of CD4+ T cells expressing IFN-γ, while CD4+ T cell IFN-γ expression was normalized by the addition of IL-10 receptor-blocking mAb to the B:T cell cocultures (Figure 4). Thus, B10 cells from children had significant IL-10-dependent negative regulatory effects on CD4+ T cell IFN-γ expression, as occurs in mice (39).
Figure 4.
B cells negatively regulate CD4+ T cell IFN-γ production in vitro via IL-10-dependent mechanisms. A, Purified CD4+ T cells were cultured either alone (1st panel, left to right) or with purified B cells (1:1 ratio) (2nd–4th panels) in the presence of soluble anti-CD3, CD40L and CpG for 72 hours. In the 3rd and 4th panels, the cultures contained either rat IgG2a (control) or IL-10 receptor blocking antibody (anti-IL-10R). PMA, ionomycin and Brefeldin A were added during the final 5 hours of culture. CD4+ T cells are shown as CD19− cells because CD3 and CD4 are down-regulated during the 72-hour stimulation with anti-CD3 mAb and the 5-hour PMA/ionomycin stimulation, respectively. Numbers represent the frequencies (% of all CD19−) within the IFN-γ+ gate; the mean fluorescent intensity (MFI) value for IFN-γ staining is displayed in the right lower corner of each dot plot. B, Cumulative comparisons of IFN-γ+ CD4+ T cell frequencies between the 3 different culture conditions (left to right), CD4+ T cells cultured alone, CD4+ T cells cultured with B cells and CD4+ T cells, cultured with B cells in the presence of anti-IL-10R mAb, respectively, from three separate experiments (n=3; HC14, HC16 and HC20 in Supplementary Table 1). P values were calculated using the paired-sample t-test.
The B10 cell compartment contracts in children with autoimmunity
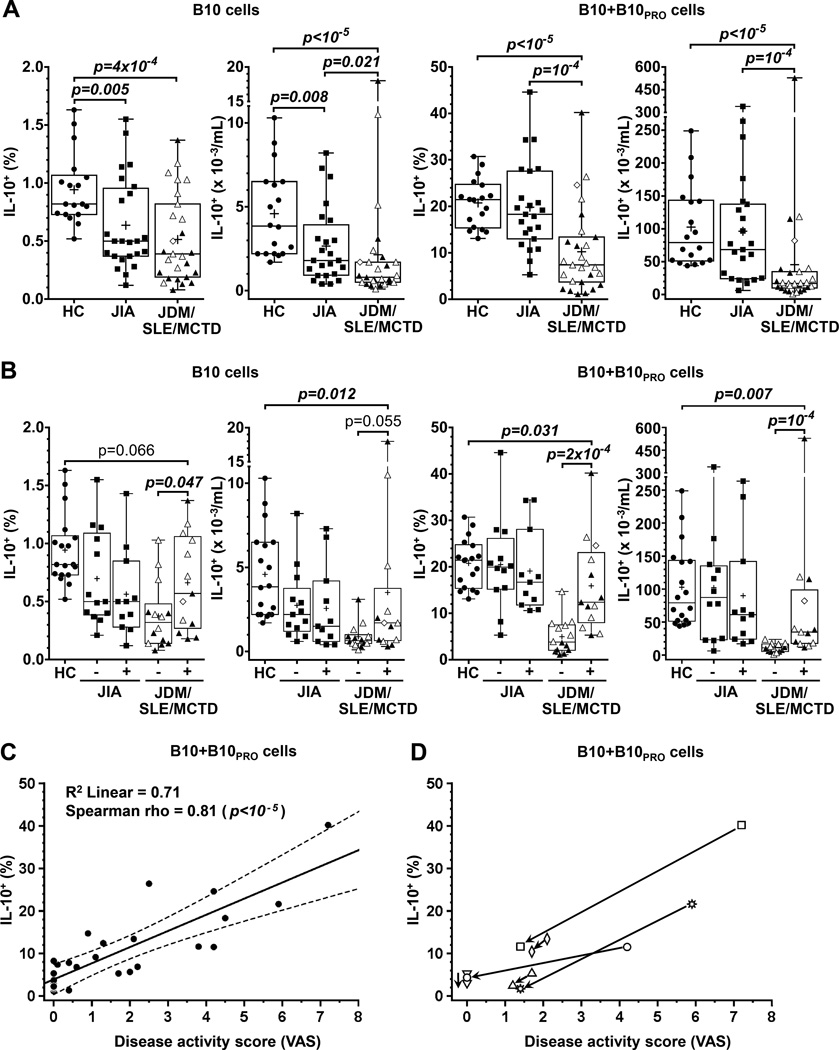
The size of the B10 cell compartment was compared between age-matched healthy children and children with autoimmunity (Supplementary Figure 6A). Most children with autoimmunity had significantly lower B10 cell frequencies and numbers when compared to healthy children (Figure 5A). Children with autoimmunity were further categorized based on data clustering into two groups: 1) children with JIA excluding systemic-onset JIA (“JIA group”), and 2) children with JDM, SLE and MCTD (“JDM/SLE/MCTD group”). B10+B10PRO cell frequencies and numbers were decreased only in the JDM/SLE/MCTD group (Figure 5A). Children with JDM/SLE/MCTD had lower ALC compared to healthy children, but there were no significant differences in B cell frequencies and numbers or WBC counts among disease groups (Supplementary Figure 2C). Thus, the B10 cell compartment was contracted in children with autoimmunity, particularly in the JDM/SLE/MCTD group.
Figure 5.
Children with autoimmunity have a contracted B10 cell compartment compared to age-matched healthy children. A, B10 and B10+B10PRO cell frequencies and numbers are decreased in children with JIA (n=23–24) or JDM/SLE/MCTD (n=27) autoimmunity compared to age-matched healthy children (HC, n=18). Children with JDM, SLE and MCTD are shown as solid triangles, open triangles and open diamonds respectively. B, B10 cell and B10+B10PRO cell frequencies and numbers in children with JIA (n=23) or JDM/SLE/MCTD (n=27) in relation to disease activity (“-” for inactive disease and “+” for active disease); the B10 cell compartment shows a limited expansion with disease activity only in children with JDM/SLE/MCTD. P values were calculated using the independent sample Mann-Whitney U test (unpaired); crosses in the boxplots represent means. C, Correlation of B10+B10PRO cell frequencies with disease activity scores in children with JDM/SLE/MCTD. The interrupted lines represent the upper and lower limits of the 99% confidence interval of the fitted line. D, Longitudinal measurements of B10+B10PRO cell frequencies and disease activity scores in 6 children with JDM/SLE/MCTD (2 with SLE and 4 with JDM). The two time points are connected with a line ending with an arrowhead pointing at the second sampling time point.
The B10 cell compartment expands with active disease in children with JDM/SLE/MCTD
Since JIA, JDM, SLE and MCTD do not share a disease activity measure, physician global assessment was used to assess disease activity following age-matching (Supplementary Figure 6B). Both B10 and B10+B10PRO cell frequencies and numbers increased during active disease in the JDM/SLE/MCTD group but not in the JIA group (Figure 5B). Despite the limited B10+B10PRO cell expansion during active disease in the JDM/SLE/MCTD group, the frequencies and numbers of B10+B10PRO cells remained below those of healthy children (Figure 5B). B10+B10PRO cell frequencies correlated linearly with the VAS scores (Figure 5C). A similar trend was observed when B10+B10PRO cell frequencies were followed longitudinally in 4 children with JDM and 2 with SLE (Figure 5D, Supplementary Table 2). There were no significant differences between disease activity groups in WBC counts, ALC, and B cell frequencies or numbers (Supplementary Figure 2D). Thereby, although the B10 cell compartment is contracted in children with autoimmunity, B10 cells nonetheless retain their ability to expand during active disease in children with JDM and SLE.
Medication effects on the B10 cell compartment
Each group of children with autoimmunity (JIA and JDM/SLE/MCTD) was classified into two “medication subgroups” for each medication based on whether or not the child was on the medication at the time of blood sampling (Supplementary Table 2). No significant differences in B10 or B10+B10PRO cell frequencies between medication subgroups were noted with the exception of mycophenolate mofetil (MMF) in children with SLE. MMF-treated children had significantly higher B10 cell frequencies compared to children not treated with MMF (Supplementary Figure 7A). B10+B10PRO cell frequencies were not significantly higher in MMF-treated children compared to children not treated with MMF (p=0.097, Supplementary Figure 7B). MMF-treated children had lower B cell numbers compared to children not treated with MMF (Supplementary Figure 7C), but there were no significant differences in the age distribution (Supplementary Figure 6C) or WBC counts, ALC and B cell frequencies (Supplementary Figure 7C–D) between MMF-treated children and children not treated with MMF. Therefore, MMF therapy or other treatments did not explain the contracted B10 cell compartment found in children with autoimmunity.
Children with autoimmunity have high IFN-γ and low IL-21 serum levels
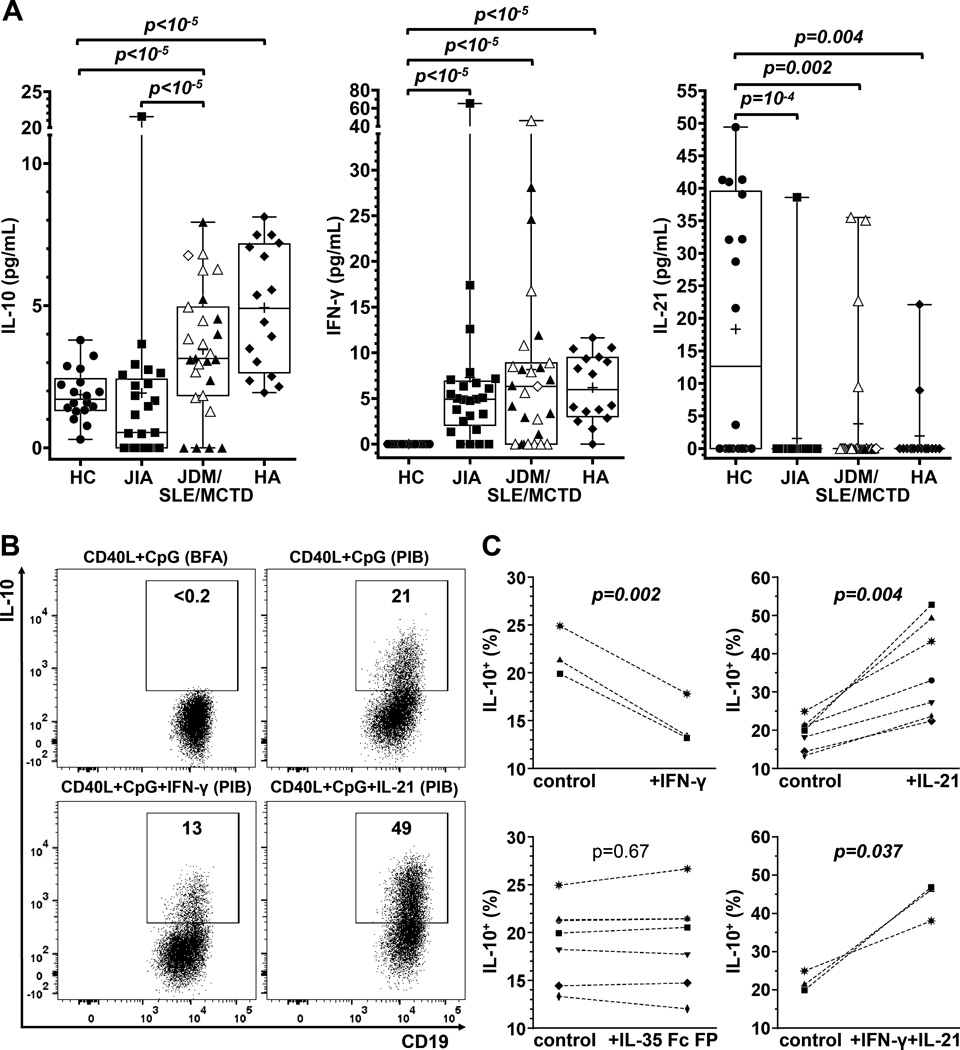
Although inherently low in most subjects, serum IL-10 concentrations were highest in the JDM/SLE/MCTD group and healthy adults (Figure 6A). Higher levels of IL-10 among adults and among children with systemic autoimmunity contrasts with their lower frequencies of blood B10 cells, but multiple cell types produce IL-10 and undoubtedly contribute to the serum IL-10 pool. By contrast, healthy children had significantly lower serum IFN-γ and higher IL-21 levels compared to children with autoimmunity or healthy adults (Figure 6A). However, serum cytokine levels did not correlate with the size of the B10 cell compartment between individuals in any group and regardless of disease activity. Consistent with this, IL-21 promotes whereas IFN-γ inhibits mouse B cell IL-10 expression in vitro (40). Therefore, the differences in serum cytokines could provide a partial explanation for the differences of the B10 cell compartment among our study populations.
Figure 6.
Serum cytokine levels and differential regulation of B cell IL-10 expression by IFN-γ and IL-21. A, Serum IL-10, IFN-γ and IL-21 levels in HC (n=18), age-matched children with JIA (n=25) or JDM/SLE/MCTD (n=27), and healthy adults (n=16). Serum cytokines were measured by ELISA. P values were calculated using the independent sample Mann-Whitney U test (unpaired); crosses in the boxplots represent means. Children with JDM, SLE and MCTD are shown as solid triangles, open triangles and open diamonds respectively. B, Representative effects of IL-21 and IFN-γ on B cell IL-10 competence. Purified B cells from a healthy 12-year-old child were cultured for 48 hours with CD40L+CpG in the presence or absence of IFN-γ or IL-21 and stimulated with either Brefeldin A (BFA) or PMA, ionomycin and BFA (“PIB”) during the last 5 hours. IL-10+ B cell frequencies were measured by immunofluorescence staining with flow cytometry analysis. Numbers represent the frequencies (% of all CD19+) within the IL-10+ gate. C, Cumulative results of IL-10+ B cell frequencies following culture of purified B cells with CD40L+CpG (“control”) alone or with IFN-γ, IL-21 or IL-35 Fc FP. Paired samples (from the same individual) are connected with an interrupted line; P values were calculated using the paired-sample t-test.
IL-21 promotes whereas IFN-γ inhibits human B cell IL-10 competence
In view of the observed differences in serum cytokine levels, along with the reported effects of IFN-γ (40), IL-21 (40), and interleukin-35 (IL-35) family members (41, 42) on B cell IL-10 expression, the effects of these cytokines on B cell IL-10 competence was examined. Purified B cells from healthy children were cultured for 48 hours with CD40L and CpG in the presence or absence of IL-21, IFN-γ, IL-12, IL-27, or IL-35 Fc FP. IL-10 expression was not detectable in any of these cultures without the addition of PIB during the last 5 hours. IL-21 enhanced whereas IFN-γ inhibited B cell IL-10 expression (Figure 6B–C), while IL-35 Fc FP, IL-12 or IL-27 had no effect (Figure 6C; Supplementary Figure 8A–B). When both IL-21 and IFN-γ were added to cultures, the effects of IL-21 on B cell IL-10 expression predominated (Figure 6C; Supplementary Figure 8A). Similar results were obtained with B cells from healthy adults (Supplementary Figure 8C). Thus, B cell IL-10 production was predominantly and differentially regulated by IL-21 and IFN-γ, which may also influence the size of the B10 cell compartment in vivo.
DISCUSSION
The current studies demonstrate that the blood B10 cell compartment is uniquely expanded in children relative to newborns and adults. B10+B10PRO cell frequencies peaked at ~30% in middle childhood (Figure 1C), while the B10 cell compartment normally represents <10% of newborn and adult blood B cells (3). This increase in children did not correlate with the expansion of a specific phenotypically-defined B cell subset (Figure 2, Supplementary Figure 5). Despite differences between the B10 cell compartments of children and adults, B10 cells from children retained their regulatory capacity and down-regulated CD4+ T cell IFN-γ production through IL-10-dependent pathways in vitro (Figure 4). There were also significant reductions in B10 cell and B10+B10PRO cell frequencies and numbers in children with autoimmune disease, but their frequencies nonetheless increased significantly with disease severity in children with JDM and SLE (Figure 5). Dynamic changes in the size of the B10 cell compartment are likely to be functionally important as small increases in B10 cell and B10PRO cell numbers in mice during adoptive transfer experiments can reduce inflammation and autoimmune disease (5, 37, 40, 43), while reducing B10 cell numbers enhances subsequent innate and adaptive immune responses (39, 44, 45). Thus, alterations in the size of the B10 cell compartment in children may differentially affect tolerance regulation and autoimmune disease.
Human regulatory B cells have been reported within a diverse variety of surface phenotype-defined developmental B cell subsets (3, 8–14). While most adult B10 and B10PRO cells express a CD24hiCD27+ memory-like phenotype (3, 7), the surface phenotype of B10 cells in children was variable, and expansion of the B10 cell compartment in children did not correlate with the normal age-related shifts among B cell subsets (Figure 2). B10 cells from children were highly represented within the transitional CD10+CD24hiCD38hiCD44low B cell subset and relatively few pediatric B10 cells expressed simultaneously CD27 and high levels of CD24. A lack of correlation between phenotypically-defined developmental B cell subsets and IL-10-competent B cells is well documented in adults (31–33). The phenotypic variability of B10 cells during normal human development and their lack of correlation with surface phenotype-defined developmental B cell subsets highlights the concept that B10 cells are a unique B cell subset that is functionally programmed to produce IL-10 in response to appropriate B cell antigen receptor (BCR) signals rather than representing a cell lineage or a specific stage of B cell development (2). The transient capacity B10 cells to express IL-10 following appropriate activation in mice is highlighted by their termination of IL-10 production prior to plasmablast and plasma cell differentiation (46). Furthermore, both B10 and B10+B10PRO cells display a specific cytokine-expression profile with limited or no expression of inflammatory cytokines such as GM-CSF (47), IL-1β and IFN-γ (Figure 3). Thus, their capacity to express IL-10 remains the most comprehensive marker for B10 and B10PRO cell identification and function.
Blood B10 cell frequencies increased during childhood, but the most dramatic increase was observed in B10PRO cells (Figure 1C). Although B10 cells are inherently competent to produce IL-10 following PMA/ionomycin stimulation, B10PRO cell IL-10 competence is acquired during prolonged CD40 ligation along with CpG or LPS stimulation in vitro prior to PMA/ionomycin treatment (3). Like B10 cells in adults, B10PRO cells predominantly localize within the blood CD27+ memory B cell subset, but CD40 plus CpG or LPS stimulation does not induce significant numbers of purified CD27-negative B cells to acquire IL-10 competence. Thereby, B10PRO cells are presumed to have encountered the appropriate BCR and transmembrane signals in vivo that initiate IL-10 competence, but they have not acquired the ability to transcribe the il10 gene (2). Consistent with this, BCR crosslinking with anti-IgM antibody in vitro terminates B10PRO cell acquisition of IL-10 competence in humans and mice (2, 3), arguing that non-specific high intensity BCR signals induce a different functional program in B cells. The acquisition of IL-10 competence relates more to the selection of B cells by previous antigen exposure and the activation of specific signaling thresholds that elicit this functional program rather than the simple differentiation of B cells into plasmablasts or plasma cells (2). Moreover, the vast majority of B cells can be activated with CD40 ligand, CpG or LPS, but only a subset of B cells can be induced to express IL-10. Thus, B10PRO cells are likely to represent a precursor pool for the selection of functionally mature B10 cells during antigen-specific cognate interactions with T cells that induce some B10 cells to become B10 effector cells in vivo (40).
Human B10PRO cell maturation into B10 cells was negatively regulated by IFN-γ and positively regulated by IL-21 in vitro (Figure 6) as occurs in mice (40). Remarkably, healthy children had lower serum IFN-γ concentrations as previously documented (48, 49), and higher serum IL-21 when compared to either children with autoimmunity or healthy adults (Figure 6). Thereby, contraction of the B10PRO cell compartment in children with autoimmunity would be expected due to differences in the in vivo cytokine milieu that B cells are exposed to, which would predictably lead to reduced B10 cell numbers. Thus, rather than representing inherent functional defects within the B10 cell compartment, increased serum IFN-γ concentrations during autoimmune disease may reduce the size of the B10PRO cell pool in children. Alterations in the cytokine milieu may similarly account for alterations within regulatory B cell compartments that have been reported to underlie autoimmune disease in adults (8, 10, 18, 19). Consistent with this hypothesis, human IL-21 deficiency results in autoimmune manifestations (50). Given this and the higher levels of IL-21 in healthy children (Figure 6A), our hypothesis is that when stimulated with appropriate antigens plus cognate T cell help, B cells in children are more prone to acquiring IL-10 competence than adult B cells.
B10 cells are developmentally and functionally distinct from regulatory T cells, but they work synergistically with regulatory T cells to control autoimmune disease manifestations in mice (39). High frequencies of regulatory T cells from early and middle childhood were recently documented for healthy children in comparison with adolescents and adults (51). Thus, regulatory B10 cells and regulatory T cell expansion during middle childhood may represent a mechanism for promoting tolerance during this unique stage of human development. Thereafter, blood B10 cell frequencies do not change remarkably within adults until they decrease substantially (<0.2%) with old age (7). By contrast, the mouse spleen B10 cell compartment is expanded in neonatal mice, contracts quickly thereafter and remains stable through adulthood, but frequencies expand in aged mice (6). Thereby, immune tolerance mechanisms may be more prominent in children than in adults, especially prior to adolescence.
The B10 cell compartment can expand with autoimmune disease in both adult humans and mice (3, 6), and in individuals prone to developing chronic lymphocytic leukemia (7). In fact, expansion of the B10 cell compartment in some adults with autoimmune disease is similar to the expansion reported herein for children (3). By contrast, however, the B10 cell compartment was significantly contracted in children with autoimmune disease (Figure 5), and the frequencies of B10 cells in children with autoimmune disease resembled those normally observed in healthy adults (Figure 1). Nonetheless, the B10 cell compartment remained dynamic as B10+B10PRO cell frequencies showed a limited increase with disease activity in children with JDM and SLE, but returned to low levels as disease activity decreased (Figure 5). B10 and B10PRO cell frequencies are also significantly reduced in children with Wiskott-Aldrich syndrome relative to healthy children (21). While defects in the size and/or the functional capacity of adult regulatory B cell compartments may contribute to the development of autoimmunity (8, 10, 11, 15–20), the current results suggest the opposite possibility that autoimmunity and inflammation may contribute to the reduced size of the B10 cell compartment. It thereby remains premature to draw conclusions regarding the dynamic function of the B10 cell compartment until there is a better understanding of the factors regulating their numbers and function in vivo during autoimmunity.
The IL-35 family of cytokines has been recently shown to be involved in the development of B cell IL-10 competence (41, 42). However, in our study, the IL-35 family cytokines (IL-12, IL-27, and IL-35 Fc FP) did not promote B cell IL-10 competence (Figure 6, Supplementary Figure 8). It is important to note that our approach for evaluating the involvement of IL-35 in the acquisition of human B cell IL-10 competence is different than the approach previously taken (41) where purified B cells were stimulated for 3 days with PMA and ionomycin prior to immunofluorescence IL-10 staining. The exposure of human B cells to PMA and ionomycin for more than 6 hours results in extensive B cell death (52) and in all our assays only a brief (5 hours) stimulation with PMA and ionomycin was used to maintain cell viability at the time of immunofluorescence IL-10 staining. Furthermore, in both approaches, IL-35 Fc FP was used, a fusion protein that may not have the same properties as native human IL-35. Therefore, the effects of IL-35 on human B cell IL-10 competence in vitro remain to be determined. Nevertheless, the potent effects of IL-21 on human B cell IL-10 competence in vitro open new horizons in the development of autologous B10 cell-based therapies (40).
In conclusion, this first study of B10 cells across the entire age range of developing humans documents that the size of the B10 cell compartment varies during normal human development, and expands transiently in childhood. The peak of the B10 cell compartment expansion occurs during middle childhood, when linear growth rates are lowest (53), suggesting that B10 cells are particularly important in immune tolerance regulation prior to the pubertal growth spurt. The B10 cell compartment responds dynamically to inflammation in both children with JDM/SLE (Figure 5), adults (3, 7) and mice (7, 37), indicating that B10 cell development in vivo may be regulated by antigen exposure and cytokines. Despite the expanded B10 cell compartment in healthy children, serum IL-10 levels remained low in comparison with adults and children with JDM/SLE/MCTD (Figure 6A), indicating that other cells preferentially contribute to serum IL-10 levels and consistent with B10 cells regulating immune responses within local microenvironments rather than through the provision of systemic IL-10 (40). The identification of therapies that restore or augment the B10 cell compartment will clarify these issues and may provide attractive strategies for modulating immune responses and autoimmunity in the future.
Supplementary Material
Acknowledgments
Financial support: This work was supported by grants from the Arthritis Foundation (Clinical to Research Transition Award, project number 5577), the Rheumatology Research Foundation (Scientist Development Award), the Duke Rheumatology T32 training grant and the National Institutes of Health (5R01AI100147).
Footnotes
Conflict of interest disclosure: The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
I.K., J.A.D., and T.F.T. conceived the project, designed, performed or supervised the experiments, analyzed the data, and prepared the manuscript. G.M.V., J.C.P. and J.W.S. contributed to experiments and manuscript preparation.
REFERENCES
- 1.Kalampokis I, Yoshizaki A, Tedder TF. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis research & therapy. 2013;15(Suppl 1):S1. doi: 10.1186/ar3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tedder TF. B10 cells: a functionally defined regulatory B cell subset. J Immunol. 2015;194(4):1395–1401. doi: 10.4049/jimmunol.1401329. [DOI] [PubMed] [Google Scholar]
- 3.Iwata Y, Matsushita T, Horikawa M, DiLillo DJ, Yanaba K, Venturi GM, et al. Characterization of a rare IL-10-competent B cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117(2):530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsushita T, Tedder TF. Identifying regulatory B cells (B10 cells) that produce IL-10. Methods Mol Biol. 2011;677:99–111. doi: 10.1007/978-1-60761-869-0_7. [DOI] [PubMed] [Google Scholar]
- 5.Yanaba K, Bouaziz J-D, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28(5):639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182(12):7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiLillo DJ, Weinberg JB, Yoshizaki A, Horikawa M, Bryant JM, Iwata Y, et al. Chronic lymphocytic leukemia and regulatory B cells share IL-10 competence and immunosuppressive function. Leukemia. 2013;27(1):170–182. doi: 10.1038/leu.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19+CD24hiCD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity. 2010;32(1):129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Bouaziz JD, Calbo S, Maho-Vaillant M, Saussine A, Bagot M, Bensussan A, et al. IL-10 produced by activated human B cells regulates CD4+ T-cell activation in vitro. Eur J Immunol. 2010;40(10):2686–2691. doi: 10.1002/eji.201040673. [DOI] [PubMed] [Google Scholar]
- 10.Flores-Borja F, Bosma A, Ng D, Reddy V, Ehrenstein MR, Isenberg DA, et al. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med. 2013;5(173):173ra23. doi: 10.1126/scitranslmed.3005407. [DOI] [PubMed] [Google Scholar]
- 11.Khoder A, Sarvaria A, Alsuliman A, Chew C, Sekine T, Cooper N, et al. Regulatory B cells are enriched within the IgM memory and transitional subsets in healthy donors but are deficient in chronic GVHD. Blood. 2014;124(13):2034–2045. doi: 10.1182/blood-2014-04-571125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon Q, Pers JO, Cornec D, Le Pottier L, Mageed RA, Hillion S. In-depth characterization of CD24hiCD38hi transitional human B cells reveals different regulatory profiles. J Allergy Clin Immunol. 2015;(15):1352–1354. doi: 10.1016/j.jaci.2015.09.014. S0091-6749. [DOI] [PubMed] [Google Scholar]
- 13.de Masson A, Bouaziz JD, Le Buanec H, Robin M, O'Meara A, Parquet N, et al. CD24hiCD27+ and plasmablast-like regulatory B cells in human chronic graft-versus-host disease. Blood. 2015;125(11):1830–1839. doi: 10.1182/blood-2014-09-599159. [DOI] [PubMed] [Google Scholar]
- 14.Lin W, Cerny D, Chua E, Duan K, Yi JT, Shadan NB, et al. Human regulatory B cells combine phenotypic and genetic hallmarks with a distinct differentiation fate. J Immunol. 2014;193(5):2258–2266. doi: 10.4049/jimmunol.1303214. [DOI] [PubMed] [Google Scholar]
- 15.Quan C, Yu H, Qiao J, Xiao B, Zhao G, Wu Z, et al. Impaired regulatory function and enhanced intrathecal activation of B cells in neuromyelitis optica: distinct from multiple sclerosis. Mult Scler. 2013;19(3):289–298. doi: 10.1177/1352458512454771. [DOI] [PubMed] [Google Scholar]
- 16.Wilde B, Thewissen M, Damoiseaux J, Knippenberg S, Hilhorst M, van Paassen P, et al. Regulatory B cells in ANCA-associated vasculitis. Ann Rheum Dis. 2013;72(8):1416–1419. doi: 10.1136/annrheumdis-2012-202986. [DOI] [PubMed] [Google Scholar]
- 17.Sun F, Ladha SS, Yang L, Liu Q, Shi SX, Su N, et al. Interleukin-10 producing-B cells and their association with responsiveness to rituximab in myasthenia gravis. Muscle Nerve. 2014;49(4):487–494. doi: 10.1002/mus.23951. [DOI] [PubMed] [Google Scholar]
- 18.Vlkova M, Ticha O, Nechvatalova J, Kalina T, Litzman J, Mauri C, et al. Regulatory B cells in CVID patients fail to suppress multifunctional IFN-γ+ TNF-α+ CD4+ T cells differentiation. Clin Immunol. 2015;160(2):292–300. doi: 10.1016/j.clim.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Todd SK, Pepper RJ, Draibe J, Tanna A, Pusey CD, Mauri C, et al. Regulatory B cells are numerically but not functionally deficient in anti-neutrophil cytoplasm antibody-associated vasculitis. Rheumatology. 2014;53(9):1693–1703. doi: 10.1093/rheumatology/keu136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mavropoulos A, Simopoulou T, Varna A, Liaskos C, Katsiari C, Bogdanos DP, et al. Breg cells are numerically decreased and functionally impaired in patients with systemic sclerosis. Arthritis Rheumatol. 2016;68(2):494–504. doi: 10.1002/art.39437. [DOI] [PubMed] [Google Scholar]
- 21.Du HQ, Zhang X, An YF, Ding Y, Zhao XD. Effects of Wiskott-Aldrich Syndrome protein deficiency on IL-10-producing regulatory B cells in humans and mice. Scand J Immunol. 2015;81(6):483–493. doi: 10.1111/sji.12282. [DOI] [PubMed] [Google Scholar]
- 22.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kit BK, Kuklina E, Carroll MD, Ostchega Y, Freedman DS, Ogden CL. Prevalence of and trends in dyslipidemia and blood pressure among US children and adolescents, 1999–2012. JAMA Pediatr. 2015;169(3):272–279. doi: 10.1001/jamapediatrics.2014.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsioni V, Andreoli L, Meini A, Frassi M, Raffetti E, Airo P, et al. The prevalence and incidence of systemic lupus erythematosus in children and adults: a population-based study in a mountain community in northern Italy. Clin Exp Rheumatol. 2015;33(5):681–687. [PubMed] [Google Scholar]
- 25.Kamphuis S, Silverman ED. Prevalence and burden of pediatric-onset systemic lupus erythematosus. Nat Rev Rheumatol. 2010;6(9):538–546. doi: 10.1038/nrrheum.2010.121. [DOI] [PubMed] [Google Scholar]
- 26.Furst DE, Clarke AE, Fernandes AW, Bancroft T, Greth W, Iorga SR. Incidence and prevalence of adult systemic lupus erythematosus in a large US managed-care population. Lupus. 2013;22(1):99–105. doi: 10.1177/0961203312463110. [DOI] [PubMed] [Google Scholar]
- 27.Feldman CH, Hiraki LT, Liu J, Fischer MA, Solomon DH, Alarcon GS, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000–2004. Arthritis Rheum. 2013;65(3):753–763. doi: 10.1002/art.37795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tansley SL, McHugh NJ, Wedderburn LR. Adult and juvenile dermatomyositis: are the distinct clinical features explained by our current understanding of serological subgroups and pathogenic mechanisms? Arthritis Res Ther. 2013;15(2):211. doi: 10.1186/ar4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thierry S, Fautrel B, Lemelle I, Guillemin F. Prevalence and incidence of juvenile idiopathic arthritis: a systematic review. Joint Bone Spine. 2014;81(2):112–117. doi: 10.1016/j.jbspin.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010;62(6):1576–1582. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Gent R, van Tilburg CM, Nibbelke EE, Otto SA, Gaiser JF, Janssens-Korpela PL, et al. Refined characterization and reference values of the pediatric T- and B-cell compartments. Clin Immunol. 2009;133(1):95–107. doi: 10.1016/j.clim.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 32.Piatosa B, Wolska-Kusnierz B, Pac M, Siewiera K, Galkowska E, Bernatowska E. B cell subsets in healthy children: reference values for evaluation of B cell maturation process in peripheral blood. Cytometry B Clin Cytom. 2010;78(6):372–381. doi: 10.1002/cyto.b.20536. [DOI] [PubMed] [Google Scholar]
- 33.Morbach H, Eichhorn EM, Liese JG, Girschick HJ. Reference values for B cell subpopulations from infancy to adulthood. Clin Exp Immunol. 2010;162(2):271–279. doi: 10.1111/j.1365-2249.2010.04206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 35.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292(7):344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 36.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–392. [PubMed] [Google Scholar]
- 37.Matsushita T, Yanaba K, Bouaziz J-D, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118(10):3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gagro A, McCloskey N, Challa A, Holder M, Grafton G, Pound JD, et al. CD5-positive and CD5-negative human B cells converge to an indistinguishable population on signalling through B-cell receptors and CD40. Immunology. 2000;101(2):201–209. doi: 10.1046/j.1365-2567.2000.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling EAE initiation and late-phase immunopathogenesis. J Immunol. 2010;185(4):2240–2252. doi: 10.4049/jimmunol.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshizaki A, Miyagaki T, DiLillo DJ, Matsushita T, Horikawa M, Kountikov EI, et al. Regulatory B cells control T cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012;491(7423):264–268. doi: 10.1038/nature11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang R-X, Yu C-R, Dambuza IM, Mahdi RM, Dolinska M, Sergeey YV, et al. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med. 2014;20(6):633–641. doi: 10.1038/nm.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen P, Roch T, Lampropoulou V, O'Connor RA, Stervbo U, Hilgenberg E, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014;507(7492):366–370. doi: 10.1038/nature12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horikawa M, Minard-Colin V, Matsushita T, Tedder TF. Regulatory B cell production of IL-10 inhibits lymphoma depletion during CD20 immunotherapy in mice. J Clin Invest. 2011;121(11):4268–4280. doi: 10.1172/JCI59266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poe JC, Smith S-H, Haas KM, Yanaba K, Tsubata T, Matsushita T, et al. Amplified B lymphocyte CD40 signaling drives regulatory B10 cell expansion in mice. PLoS One. 2011;6(7):e22464. doi: 10.1371/journal.pone.0022464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horikawa M, Weimer ET, DiLillo DJ, Venturi GM, Spolski R, Leonard WJ, et al. Regulatory B cell (B10 cell) expansion during Listeria infection governs innate and cellular immune responses in mice. J Immunol. 2013;190(3):1158–1168. doi: 10.4049/jimmunol.1201427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maseda D, Smith SH, DiLillo DJ, Bryant JM, Candando KM, Weaver CT, et al. Regulatory B10 cells differentiate into antibody-secreting cells after transient IL-10 production in vivo. J Immunol. 2012;188(3):1036–1048. doi: 10.4049/jimmunol.1102500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li R, Rezk A, Miyazaki Y, Hilgenberg E, Touil H, Shen P, et al. Proinflammatory GM-CSF-producing B cells in multiple sclerosis and B cell depletion therapy. Sci Transl Med. 2015;7(310):310ra166. doi: 10.1126/scitranslmed.aab4176. [DOI] [PubMed] [Google Scholar]
- 48.Sack U, Burkhardt U, Borte M, Schadlich H, Berg K, Emmrich F. Age-dependent levels of select immunological mediators in sera of healthy children. Clin Diagn Lab Immunol. 1998;5(1):28–32. doi: 10.1128/cdli.5.1.28-32.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pranzatelli MR, Tate ED, McGee NR, Colliver JA. Pediatric reference ranges for proinflammatory and anti-inflammatory cytokines in cerebrospinal fluid and serum by multiplexed immunoassay. J Interferon Cytokine Res. 2013;33(9):523–528. doi: 10.1089/jir.2012.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salzer E, Kansu A, Sic H, Majek P, Ikinciogullari A, Dogu FE, et al. Early-onset inflammatory bowel disease and common variable immunodeficiency-like disease caused by IL-21 deficiency. J Allergy Clin Immunol. 2014;133(6):1651–1659. e12. doi: 10.1016/j.jaci.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 51.Holcar M, Goropevsek A, Ihan A, Avcin T. Age-related differences in percentages of regulatory and effector T lymphocytes and their subsets in healthy individuals and characteristic STAT1/STAT5 signalling response in helper T lymphocytes. J Immunol Res. 2015;2015:352934. doi: 10.1155/2015/352934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donjerkovic D, Scott DW. Activation-induced cell death in B lymphocytes. Cell Res. 2000;10(3):179–192. doi: 10.1038/sj.cr.7290047. [DOI] [PubMed] [Google Scholar]
- 53.Abbassi V. Growth and normal puberty. Pediatrics. 1998;102(2 Pt 3):507–511. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.