Abstract
The precise role of the prefrontal and posterior parietal cortices in recognition performance remains controversial, with questions about whether these regions contribute to recognition via the availability of mnemonic evidence or via decision biases and retrieval orientation. Here we used an explicit memory cueing paradigm, whereby external cues probabilistically predict upcoming memoranda as old or new, in our case with 75% validity, and these cues affect recognition decision biases in the direction of the cue. The present study applied bilateral transcranial direct current stimulation (tDCS) over prefrontal or posterior parietal cortex, or sham tDCS, to test the causal role of these regions in recognition accuracy or decision biasing. Participants who received tDCS over prefrontal cortex showed increased cue utilization compared to tDCS over posterior parietal cortex and sham tDCS, suggesting that the prefrontal cortex is involved in processes that contribute to decision biases in memory.
Keywords: prefrontal cortex, transcranial direct current stimulation, memory, recognition, decision-making, cueing
1. Introduction
Shifting recognition decision criteria based on contextual cues can be advantageous. Within the memory domain, numerous contextual factors can influence how encountered stimuli are recognized on a day-to-day basis. For example, one may be more likely to decide that an approaching individual is familiar if another reliable source, such as a friend, also thinks that person is familiar. Incorporating external cues can be advantageous because using information above and beyond one’s own memory could increase the likelihood of accurate recognition. Thus, recognition accuracy relies on both memory sensitivity and decision-making processes related to the weighing of mnemonic evidence with contextual cues. Despite its utility, less is known about the neural basis of contextual cue utilization and decision-making in memory tasks, especially when compared to what is known about the neural basis of memory sensitivity.
The explicit memory cueing paradigm is particularly useful for examining mnemonic and decision-making contributions to recognition performance (O’Connor, Han, & Dobbins, 2010). In the explicit memory cueing paradigm participants view a preparatory cue stating “Likely Old” or “Likely New,” and the preparatory cue predicts that an upcoming recognition probe is actually old (studied) or new (unstudied) with a relatively high validity (e.g. 75% true; O’Connor, Han, & Dobbins, 2010). Behaviorally, the explicit preparatory cues bias responding towards the cued recommendation; in other words, toward “old” responses following “Likely Old” cues or toward “new” responses following “Likely New” cues (Jaeger et al., 2012). Functional MRI studies have shown increased BOLD signal in bilateral prefrontal and posterior parietal regions on recognition trials that were preceded by invalid cues compared to valid cues (Jaeger, Konkel, & Dobbins, 2013; O’Connor et al., 2010), suggesting that increased activity in the lateral prefrontal and posterior parietal cortices may also signal when recognition evidence conflicts with the explicitly cued expectation, as is the case with an invalid cue. The different functional roles of the lateral prefrontal and posterior parietal regions in recognition memory is an active area of investigation as these regions have been linked to both accurate memory retrieval (Cansino et al., 2002; Dobbins et al., 2003; Donaldson et al., 2010; Kahn et al., 2004) and decision bias (Aminoff et al., 2015; Miller et al., 2001; Swick and Knight, 1999; Windmann, 2002), suggesting a likely neural substrate for the interplay between retrieval and the decision process. How these regions directly contribute to the combined influence of memory retrieval and cued expectations on the recognition decision process is not known, although the role of the lateral prefrontal cortex in retrieval is largely attributed to executive control processes (Dobbins and Wagner, 2005; Nolde et al., 1998; Wheeler and Buckner, 2003), whereas the role of the posterior parietal cortex in retrieval, although less well understood, is thought to be based on attentional processes (Cabeza et al., 2008; Ciaramelli et al., 2008; Jaeger et al., 2013; Wagner et al., 2005). The present study aims to understand the nature of the roles of the lateral prefrontal or posterior parietal cortices in recognition memory using transcranial Direct Current Stimulation (tDCS) during the explicit memory cueing paradigm.
Directly manipulating intact cortical regions using non-invasive brain stimulation techniques, such as tDCS, can test whether brain regions play a causal role in a cognitive task. Specifically, tDCS provides a good alternative to lesion studies, which can also test for the causal role of brain regions in a cognitive task, because there is no influence of long-term cortical reorganization (Nudo, 2013) and an alternative to neuroimaging techniques because tDCS is causal rather than correlational. During tDCS, weak electrical currents are typically applied through one stimulating electrode, often referred to as the anode, and flow to a return electrode, typically referred to as the cathode, and this is thought to alter the excitability in the underlying cortex (Nitsche and Paulus, 2000; Nitsche et al., 2008, 2005). Specifically, at least in studies of motor cortex, neuronal excitability increased under the positively charge anode (often termed “anodal stimulation”) and attenuated under the negatively charged cathode (often termed “cathodal stimulation”) (e.g. Fregni et al., 2006; Furubayashi et al., 2008; Jeffery et al., 2007; Nitsche and Paulus, 2000). TDCS effects on underlying cortical excitability (Nitsche and Paulus, 2000; Nitsche et al., 2005) have led to measurable changes in behavior following stimulation over lateral prefrontal (Gladwin et al., 2012; Leite et al., 2013; Minati et al., 2012; Nelson et al., 2014; Pope et al., 2015) or posterior parietal cortex (Berryhill & Jones, 2012; Manenti, Brambilla, Petesi, Ferrari, & Cotelli, 2013; Moos, Vossel, Weidner, Sparing, & Fink, 2012; Pergolizzi & Chua, 2016, 2015; Pisoni et al., 2015). Of particular interest here is that tDCS has been useful for investigating the causal role of various neocortical regions in memory processes (Bennabi et al., 2014; Brasil-Neto, 2012; Manenti et al., 2012). For example, administration of bilateral tDCS over lateral parietal cortex during retrieval resulted in increased false recognition using a paradigm known to elicit high rates of false recognition (Pergolizzi & Chua, 2015), and decreased false recognition on an item and source memory paradigm (Pergolizzi and Chua, 2016), consistent with task dependent changes in decision bias.
How the electrodes are placed on the scalp determines where in the brain current flows. Bilateral stimulation can help restrict the delivered current to the cortical area of interest (Neuling et al., 2012), whereas unilateral stimulation requires delivering current through an electrode over a distal region (e.g. the contralateral orbit) that could lead to unintended behavioral effects. Thus, we chose to use bilateral montages to stimulate the lateral prefrontal and posterior parietal cortices, with the stimulating electrode placed on the left hemisphere and the return electrode on the right hemisphere. One interpretation of this montage is that it would increase excitability in the left hemisphere and inhibit the right hemisphere. However, it is worth noting that the idea of anodal-excitation and cathodal-inhibition in tDCS is most consistent in motor studies, whereas, in cognitive studies the anodal-excitation effect is typically found, but the cathodal-inhibition is less common in cognitive studies (for review, see Jacobson et al., 2012b). It has been suggested that the lack of cathodal-inhibition effects may be because many cognitive processes are often bilateral and compensation from the contralateral hemisphere obscures behavioral effects (Jacobson et al., 2012b). Consistent with the compensation idea, tDCS can induce changes through neural networks (Keeser et al., 2011), including cross-hemispheric connectivity such that, for example, unilateral stimulation of the left prefrontal cortex increased coupling with right prefrontal cortex (Stagg et al., 2013). Furthermore, bilateral tDCS resulted in more potent effects on cognitive performance when compared with unilateral tDCS over lateral prefrontal (Fecteau et al., 2007b) and posterior parietal cortex (Heinen et al., 2016). Because we did not have strong hypotheses about cue utilization and laterality (Jaeger et al., 2013), we chose to use a bilateral montage over either lateral prefrontal cortex or posterior parietal cortex to increase our chances of seeing effects on behavior (Fecteau et al., 2007b; Heinen et al., 2016).
Cue utilization relies on the ability to flexibly bias recognition judgments, which likely relies on top-down control processes governed by the lateral prefrontal cortex (PFC) (Braver, 2012; Miller and Cohen, 2001; Ridderinkhof et al., 2004). Evidence for this comes from patients with left, right, or bilateral prefrontal lesions who showed a more liberal response set on memory tasks compared to controls, even when no other memory impairment was detected (Swick and Knight, 1999; Verfaellie et al., 2004), which was interpreted as impaired strategic processing that allowed setting of an appropriate decision criterion. Functional imaging has also implicated the prefrontal cortex as important for the flexible and appropriate use of decision biases during recognition judgments, with left and right dorsolateral prefrontal activity increasing during blocks when decision bias was shifted trial to trial compared to when decision bias was stable (Miller et al., 2001). Outside the memory domain, prefrontal control processes are also thought to be involved in switching between rules in decision tasks (Braver and Barch, 2002; Cohen and Servan-Schreiber, 1992; Miller and Cohen, 2001), for overriding a bias when contextually inappropriate (e.g., Braver, 2012), and biasing processing of an attended object during a visual attention task (e.g., Desimone & Duncan, 1995). Thus, a role for the PFC in memory bias is consistent with its role in bias in other cognitive domains, and overall the PFC flexibly implements biases based on context to determine the extent of information processing warranted. Related to the explicit memory cueing paradigm used here, explicit cues about the history of an upcoming item (e.g. “Likely Old”) give rise to the need for top-down control from the PFC to flexibly bias decisions based on the cue, and this will lead to liberal responding in the case of “Likely Old” cues or conservative responding in the case of “Likely New” cues. From this perspective, manipulating the PFC using tDCS may enhance bias based on the cue, leading to greater utilization of the cue regardless of whether or not the cue is valid or invalid. Alternatively, given that the PFC is thought to implement biasing in a flexible manner, manipulating the PFC using tDCS may better enable individuals to override biases based on the cue when contextually inappropriate, such as for invalidly cued compared to validly cued trials. Thus, we tested whether or not tDCS over the prefrontal cortex led to greater cue utilization, which would indicate that the prefrontal cortex plays a causal role in biasing recognition judgments.
Alternatively, prefrontal control processes may primarily contribute to retrieval accuracy. FMRI studies have shown greater activity in left lateral PFC for hits relative to correct rejections (Cansino et al., 2002; Donaldson, Wheeler, & Petersen, 2010; Konishi, Wheeler, Donaldson, & Buckner, 2000), and lesions have been shown to lead to reduced accuracy for item judgments after right frontal damage (Curran et al., 1997) and source memory judgments after left and right frontal damage (Janowsky et al., 1989). Given that PFC lesions do not produce amnesia, the PFC is instead thought to guide the selection and evaluation of memories with respect to task goals (Fletcher and Henson, 2001; Kahn et al., 2004). From this perspective, prefrontal control processes select mnemonic evidence and gauge whether the retrieved information is appropriate, and this contributes to overall accuracy. Thus, the current study also examined performance among randomly intermixed uncued trials in the explicit memory cueing paradigm to test whether tDCS over the prefrontal cortex enhances retrieval accuracy.
Similar to the prefrontal cortex, the lateral posterior parietal cortex (PPC) has been implicated in recognition memory, although its contributions are less well understood. Some evidence suggests that the PPC has direct access to mnemonic information (Vilberg and Rugg, 2008; Wagner et al., 2005), likely via connectivity with the medial temporal lobe (Vincent, et al., 2006). This idea is supported by fMRI studies showing increased activity in the left PPC (Guerin and Miller, 2009; Kahn et al., 2004; Leube et al., 2003; Wheeler and Buckner, 2003) and the right PPC (Cansino et al., 2002; Henson et al., 2005; Idaka et al., 2006) for hits compared to correct rejections in item recognition tests, increased activity in the left PPC for correct compared to incorrect source memory (Donaldson, Wheeler, & Petersen, 2010; Hutchinson, Uncapher, & Wagner, 2009; Kahn et al., 2004), and decreased subjective recollection in patients with bilateral PPC lesions (Berryhill et al., 2007; Drowos et al., 2010) with comparatively diminished content when recalling autobiographical memories (Berryhill et al., 2007). This has led to the hypothesis that the PPC performs a role in long-term memory similar to its role in working memory (Berryhill and Olson, 2008; Jones and Berryhill, 2012; Jonides et al., 1998; Owen et al., 2005; Smith and Jonides, 1999, 1998) and maintains retrieved mnemonic information so it is accessible to a decision process. Alternatively, it has been hypothesized that the PPC plays an indirect role in retrieval and exerts its effects via its role in attention or decision-making, which has consequences for memory performance. Indeed, there is evidence that the parietal cortex is not involved in global recognition accuracy because patients with left, right and bilateral PPC lesions show intact basic recognition and source memory abilities (Ally et al., 2008; Davidson et al., 2008; Haramati et al., 2008; Simons et al., 2010). Instead, the memory orienting hypothesis posits that orienting to memory relies on the PPC (Jaeger et al., 2013), whereupon unexpected memory signals shift focus toward novelty or familiarity. Evidence for this comes from an fMRI study using the explicit memory cueing paradigm that showed greater activity in the bilateral PPC for invalidly cued compared to validly cued trials (Jaeger, et al., 2013; O’Connor, et al., 2010). However, there were more subtle differences between the left PPC and the right PPC; with the “Likely New” cue, there was increased activity for invalid compared to valid cueing in the left PPC but not the right PPC, whereas in the right PPC there was only increased activity for invalid compared to valid cueing with the “Likely Old” cue or regardless of whether cues predicted items as old or new (Jaeger et al., 2013). Because old items are presented in the invalid “Likely New” condition, the greater neural response is thought to convey unexpected familiarity and, thus, times when unexpected or contrary mnemonic evidence signals a need to investigate memory further, as in source monitoring. Correspondingly, the left lateralized unexpected familiarity response was shown to overlap with activations from other studies reporting increased activity for source judgments compared to item judgments, consistent with a role in source monitoring (Jaeger et al., 2013). According to the memory orienting hypothesis, the PPC subserves the initial orienting response – the capture and subsequent shift in attention by unexpected recognition signals – which leads to source monitoring attempts to explain the inconsistency (Jaeger, et al., 2013). If the memory orienting hypothesis is correct, tDCS over the PPC should enhance memory orientation, particularly on invalid trials, leading to source monitoring attempts that should improve accuracy on those trials and demonstrate that the parietal cortex has a causal role in attentional and decision-making aspects of memory retrieval.
The present study was designed to investigate the influence of bilateral tDCS (anode left/cathode right) over prefrontal or parietal cortex (Figure 1) on decisional and/or memory retrieval performance during the explicit memory cueing paradigm (Figure 2) compared to sham tDCS. Based on a hypothesized role of the lateral prefrontal cortex in the implementation or overriding of decision bias we tested if tDCS over the prefrontal cortex would result in greater cue utilization, consistent with bias implementation, and/or would result in better performance on invalid trials, consistent with overriding biases, and/or would result in improved recognition accuracy. Based on hypothesized roles of the posterior parietal cortex in retrieval accuracy and/or memory orienting processes, we tested whether tDCS over the posterior parietal cortex would result in enhanced retrieval accuracy, consistent with a direct role in memory retrieval, and/or whether it resulted in enhanced orienting during invalidly cued trials, leading to less errors, consistent with the memory orienting hypothesis of the PPC.
Figure 1.

Cortical currents modeled using tDCS Explore™. Models depict current flow from tDCS over montage F3/F4 (prefrontal) and CP3/CP4 (parietal). Current flow is depicted with the anode placed on the left hemisphere and the cathode placed on the right hemisphere, as used in the current study. Arrows indicate the direction of current flow. TDCS was administered for twenty minutes at 2mA.
Figure 2.
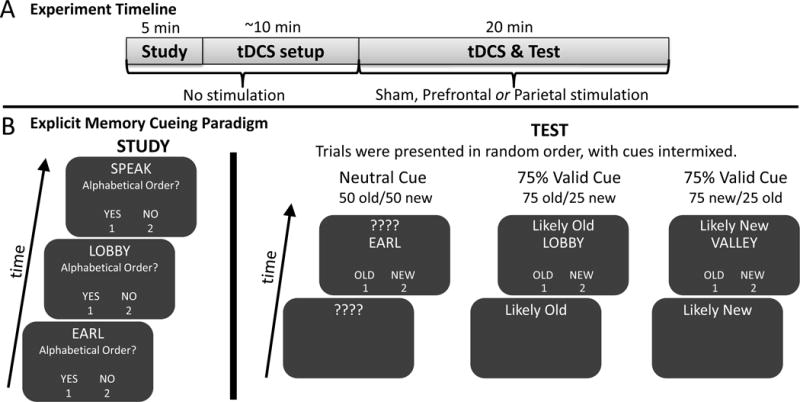
Experiment timeline (A) and schematic of the explicit memory cueing paradigm (B). A) The experiment began with a 5-minute study session, which was followed by tDCS setup. There was not stimulation applied during study or setup. TDCS was administered for 20 minutes during test because were specifically interested in retrieval processes. B) During encoding participants judged whether the first and last letter of the word were in alphabetical order. During retrieval, one of three possible cues preceded the recognition probe, and continued to be presented while participants judged the item as “old” or “new”. Trials were presented in random order, with cues intermixed.
2. Results
2.1 Recognition Memory Performance
We first examined the effects of tDCS on performance using a 3 × 2 × 3 mixed ANOVA with repeated factors of cue type (neutral, Likely Old, Likely New) and response type (hits, correct rejections) and a between-subjects factor of stimulation group (sham, parietal, prefrontal). There was a significant cue type × response type × stimulation group interaction, F(4, 138) = 4.77, MSE = 0.036, p< 0.001, partial η2 = 0.122. Our follow-up analyses showed different effects of stimulation based on cue type. Following neutral cues (Figure 3A), there were there was no stimulation group × response type interaction, F(2, 69) = 1.64, MSE = 0.027, p< 0.849, partial η2 = 0.005, with similar performance between groups for hits and correct rejections. In contrast, there was a marginally significant group × response type interaction in the “Likely Old” cue condition (Figure 3B), F(2, 69) = 3.08, MSE = 0.064, p< 0.052, partial η2 = 0.082, and a significant stimulation group × response type interaction in the “Likely New” cue condition (Figure 3C), F(2, 69) = 3.47, MSE = 0.053, p< 0.04, partial η2 = 0.091, demonstrating that stimulation effected cue utilization. Bonferroni adjusted post-hoc comparisons for stimulation group × response type interaction during “Likely New” cues demonstrated that the prefrontal group showed a greater proportion of correct rejections than the sham group (p<0.035, 95% CI of the difference: [0.005, 0.193]); and numerically fewer hits that the sham group (p<0.075, 95% CI of the difference: [−0.297, 0.010]), suggesting increased use of “Likely New” cues (Figure 3C). However, the parietal group did not differ from the prefrontal group or the sham group, with performance lying between either group.
Figure 3.

Hits and correct rejections for each cue type by stimulation group. There was significant three way interaction. A) Performance during neutral cues was matched between stimulation groups. B) Following the “Likely Old” cue, the prefrontal group shows a numerical trend towards greater cue utilization than the sham and parietal groups although this did not reach significance, whereas, C) following “Likely New” cues, the prefrontal group showed greater cue utilization than the sham, but not the parietal group. Error bars represent SEM.
2.2 Cue utilization scores
Given that there were no significant differences in baseline (i.e., neutral cue) performance, we next examined the effects of tDCS using cue utilization scores calculated for each subject and compared cue utilization between groups. The cue utilization score reflects the degree of cue influence on bias from the individual’s uncued (baseline) bias. In other words, did an individual change their response bias because of the cued recommendation or did they show a general response bias toward “old” or “new” judgments regardless of cue presentation? The former would be indicative of a cue induced decision bias, indicating that response bias shifted based on external information, which we were specifically interested in. A bias toward “old” judgments would be reflected in greater hits and decreased correct rejections and vice versa for a bias toward “new” judgments. Thus, as described in the methods (see Section 4.5), the degree of cue utilization was calculated as the difference between hits and correct rejections as an overall score such that: “Likely Old” cue utilization score = [Likely Old Hit minus Likely Old CR] minus [Uncued Hit minus Uncued CR]; “Likely New” cue utilization = [Likely New CR minus Likely New Hit] minus [Uncued CR minus Uncued Hit]. This score represents the influence of cueing (valid minus invalid) and is adjusted for individual differences in bias at baseline (uncued). This method allows separate analyses for “Likely Old” and “Likely New” cues, which is important because previous research has shown different brain regions associated with unexpected novelty (invalid “Likely Old”) and unexpected familiarity (invalid “Likely New”) (Jaeger et al., 2013).
The ANOVAs for the “Likely Old” and “Likely New” cue utilization score showed a significant effect of tDCS; “Likely Old”: F(2, 69) = 5.220, MSE = 0.076, p< 0.008, partial η2 = 0.131; “Likely New”: F(2, 69) = 3.584, MSE = 0.086, p< 0.033, partial η2 = 0.094. Bonferroni post-hoc comparisons indicated the prefrontal group was significantly more likely to utilize the cues than the sham group following “Likely Old” cues, p<0.010, 95% CI of the difference: [0.0466, 0.4363], and following “Likely New “ cues, p<0.028, 95% CI of the difference: [0.0182, 0.4338]. The prefrontal group was also more likely to utilize the “Likely Old” cue than the parietal group, p<0.048, 95% CI of the difference: [0.001, 0.3907], but not the “Likely New” cue, p<0.760, 95% CI of the difference: [−0.1103, 0.3053]. The parietal group did not differ from the sham group following “Likely Old” cues, p>0.99, 95% CI of the difference: [−0.1492, 0.2405], or following “Likely New” cues, p>0.401, 95% CI of the difference: [−0.0793, 0.3363]. Thus, prefrontal tDCS led to greater cue utilization compared to the sham and parietal groups, particularly for the “Likely Old” cue.
2.3 Overall accuracy
Shifting criteria in response to external cues, if done appropriately, could lead to an increase in overall accuracy because invalid cueing occurs on a minority of trials (25%). Because the prefrontal group relied on cues more, it is possible that this was strategic and led to improved accuracy, or it is possible that they shifted too much and that this would lead to poor performance. Thus, we examined whether or not tDCS over the prefrontal or posterior parietal cortices led to an overall increase in accuracy across cue types. There were no significant differences in overall hit rate [F(2,69) = 1.075, MSE = 0.017, p> 0.347, partial η2 = 0.030; Mean ± SEM; sham: 0.613 ± 0.025; prefrontal: 0.639 ± 0.021; parietal: 0.586 ± 0.03] or correct rejection rate [F(2,69) = 0.812, MSE = 0.010, p> 0.448, partial η2 = 0.023; Mean ± SEM; sham: 0.653 ± 0.024; prefrontal: 0.684 ± 0.019; parietal: 0.691 ± 0.024].
3. Discussion
We used tDCS to manipulate excitability of the lateral prefrontal and posterior parietal cortices in order to test for the causal role of these regions in recognition accuracy and/or decision biases. During the explicit memory cueing paradigm, the stimulation groups showed no differences in baseline recognition accuracy (hits or correct rejections) following neutral cues, inconsistent with the idea that the lateral prefrontal and/or posterior parietal cortices have a direct role in the availability of mnemonic evidence. In contrast, the prefrontal stimulation group showed increased cue utilization, and corresponding changes in hits and correct rejections, compared to the sham and parietal stimulation groups for the “Likely Old” cue, and compared to the sham group, but not parietal group, for the “Likely New” cue. The increased cue utilization in the prefrontal tDCS group is consistent with its role in top-down control of memory, rather than in memory accuracy per se.
Our results are consistent with neuroimaging results that have linked the prefrontal cortex with bias in recognition tasks rather than memory accuracy. For example, an fMRI study showed that activity in the lateral prefrontal cortex increased during a recognition task that instructed participants to shift between liberal and conservative response biases in one block compared to blocks when solely a liberal or conservative bias was maintained (Miller et al., 2001), suggesting a role of prefrontal cortex toward shifting biases. Similarly, a structural MRI study indicated that frontal lobe volumes best predicted response bias, whereas hippocampal volumes best predicted recognition discriminability (Kramer et al., 2005). Furthermore, event-related potentials at early intervals (300–500 ms post stimulus) over frontal sites were more consistent with response bias, not accuracy (Windmann, 2002). Our results provide complementary support using tDCS, for a role of the prefrontal cortex in bias.
TDCS studies outside of the memory domain have also shown that bilateral tDCS over the prefrontal cortex affected decision processes. In one study, participants were quicker to choose the more frequent alternative in a probabilistic guessing task (Hecht et al., 2010), suggesting stimulation can bias guessing. Other studies have shown less risk taking, reflecting a conservative bias, with bilateral tDCS over prefrontal cortex, using a gambling task (Pripfl et al., 2013) and on a balloon-pumping task (Fecteau et al., 2007a). Taken together, experimental evidence shows that bilateral tDCS alters decision biasing across cognitive domains.
Although our results are clear that the prefrontal group showed increased cue utilization, there are several possible specific mechanisms underlying this effect. One possibility is that greater cue utilization in the prefrontal group may reflect control mechanisms that enhanced maintenance of bias to prevent interference (Braver, 2012). Braver (2012) outlined that cognitive control, particularly in the lateral prefrontal cortex, can be understood as two processes; 1) proactive control – goal relevant information is maintained in anticipation of, and therein to avoid, interference – or 2) reactive control – a correction instantiated only as needed to resolve interference. Greater cue utilization is consistent with proactive control. The mostly valid predictive cues preceded information provided by the recognition probes. Thus, the prefrontal group incorporated the mostly valid cued recommendation to form a bias normally, and maintained it in anticipation of upcoming mnemonic interference. Indeed, lateral PFC control processes have been defined as suppression of interfering memories during retrieval (Aron et al., 2004). Furthermore, proactive control is thought to maintain goal-relevant information (in this case the outcome of a judgment reflected as response bias) through sustained activation of the PFC, whereas reactive control would initiate corrections as needed through transient activations of the PFC (Braver, 2012). Given that tDCS provides a constant current through the entire period, this would be more likely to produce sustained activity consistent with proactive control and providing a mechanistic basis for why the prefrontal group showed greater cue utilization overall.
An alternative possible explanation for increased cue utilization is that tDCS over the prefrontal cortex led to inflexible use of the cue, paralleling behavior seen in patients with lesions. Patients with frontal lesions also show an inability to overcome a bias, essentially inflexibly adhering and perseverating on a previously learned rule when an alternative response may be more appropriate (Braver and Barch, 2002; Cohen and Servan-Schreiber, 1992; Miller and Cohen, 2001). This is especially apparent during bilateral damage. From this perspective, tDCS may have had an interfering effect on PFC function, leading to utilization behavior similar to that seen in individuals with bilateral prefrontal lesions.
Given the numerous findings of differential activity in the PPC using the explicit memory cueing paradigm (Jaeger et al., 2013; O’Connor et al., 2010), it is surprising that there were no significant effects of tDCS over the PPC on performance during cued or uncued trials. During the neutral (uncued) trials, there was no effect of bilateral tDCS on recognition accuracy, inconsistent with the idea that the PPC contributes directly to the availability of mnemonic information. More surprisingly, parietal stimulation did not differ from sham during “Likely Old” or “Likely New” cue utilization scores. Previous work using tDCS with the same stimulation parameters has shown that false alarm rate varied by context, with increased false alarms using a task that oriented towards gist and familiarity (Pergolizzi & Chua, 2015) and decreased false alarms using a task oriented toward recollection (Pergolizzi & Chua, 2016), suggesting that the tDCS parameters used in this study were potent enough to induce changes in the brain. Because previous tDCS studies implicated the parietal cortex in shifting bias during recognition tests (Pergolizzi & Chua, 2016), it is worth noting that although non-significant, during the “Likely New” cues the parietal tDCS group did show a numerical shift in the expected direction. It is possible that the tDCS-induced brain changes may not have been detected at the behavioral level due to the relatively few invalidly cued trials, because memory was relatively weak in this task, or because individual differences may have obscured effects (e.g. Jacobson et al., 2012a; Tseng et al., 2012). In particular, tDCS over the posterior parietal cortex has been shown to effectively alter memory performance in some but not all participants (Jacobson, et al., 2012a), such as those in low but not high performing individuals (Tseng, et al., 2012). Future work is necessary to examine how individual differences interact with the efficacy of tDCS.
It is worth noting that our participants showed relatively low recognition rates, hovering around 60%, which is ~10% above chance and suggests recollection was unlikely. This is important as previous work using bootstrapped Monte Carlo simulations of the explicit memory cueing paradigm showed that the predictive cues only successfully influence bias when one must rely on familiarity, but are ignored if recollection is present (Jaeger et al., 2012). Thus the ability to override a bias may rely on stronger memory signals, such as those elicited by recollection, which may have been absent in our participants, leading to no detectable changes following parietal stimulation. Thus, the relevance of the current findings may be limited to when recollection is absent or low.
We have interpreted our results based on the spatial resolution of tDCS and the montage we used, and have discussed the functional roles of the prefrontal and posterior parietal cortices at a broad level. At a conceptual level, our bilateral tDCS montages were thought to increase left hemispheric and potentially also decrease right hemispheric processing, presumably decreasing interhemispheric competition (Vines et al., 2008), but these montages could also be simultaneously altering competing mechanisms in either hemisphere (Brem et al., 2014). In our previous work, parietal tDCS montages with left anode/right cathode and right anode/left cathode both showed increased false recognition compared to sham tDCS (Pergolizzi & Chua, 2015). Because these montages showed similar results in our previous work, we emphasize the general role of the lateral prefrontal and posterior parietal cortices, and do not make any strong claims about laterality, but cannot rule them out. Nevertheless, these results do provide causal evidence that tDCS over the prefrontal cortex can lead to increased utilization of contextual cues.
3.1 Conclusions
In conclusion, the present experiment suggests that the prefrontal cortex plays a causal role in processes that bias recognition judgments based on external cues. Using tDCS over the prefrontal cortex during the explicit memory cueing paradigm (O’Connor et al., 2010), we showed that the prefrontal group used external cues that predicted items as “old” more than the sham and parietal groups, and also used external cues that predicted items as “new” more than the sham group. The greater cue utilization appears to be related to processes contributing to decision bias rather than memory accuracy, such as top down (proactive) control of bias based on the decision context. Although this experiment did not show significant effects of tDCS over the parietal cortex, several fMRI and lesion studies have implicated the parietal cortex in this task (Dobbins et al., 2012), and future work manipulating recognition accuracy and cue validity would be helpful for resolving the discrepancy between tDCS and fMRI work.
4. Experimental procedure
4.1 Participants
Participants were 81 students at Brooklyn College of the City University of New York (average age 21.4 +/− 4.25 years, 54 female) who participated for course credit or were paid $10 for 1 hour of participation. Eight participants were removed due to poor task compliance as evidenced from primarily hitting one key, and one more was removed due to experimenter error, leaving 72 participants for analyses. All participants had normal or corrected to normal vision, no history of skin conditions, no history of psychiatric or neurological disorders and learned English before age 5. Participants provided written informed consent in accordance with the Human Research Protection Program of the City University of New York.
4.2 Transcranial Direct Current Stimulation (tDCS)
Stimulation was applied via one anode and one cathode rubber electrode each encased in a 35 cm2 saline-soaked sponge pocket. Sponge electrodes were affixed to the scalp using Soterix Medical tDCS EasyStraps™. Direct current was delivered through a battery-driven constant current stimulator with a maximum output of 2mA (1×1 Transcranial Direct Current (tDCS) Low-Intensity Stimulator Model 1224-B, Soterix Medical, USA).
In a between subjects design, participants were randomly assigned to one of three stimulation groups: sham (n=24), prefrontal (n=24), or parietal (n=24). A between subject design was chosen for several reasons, 1) participants are more likely to tell when they are receiving true versus sham stimulation in a within subjects design at 2mA (Wallace et al., 2016), so between subjects is presumably better for blinding, 2) to avoid interference effects from the similarities in word lists and 3) to avoid changes in strategies toward cue utilization based on repeated administration. Sham and parietal groups received the same electrode placement with a bilateral montage (i.e., electrode arrangement) over the lateral parietal cortex with the anode placed over the CP3 and the cathode placed over the CP4 electrode locations of the international 10–20 system for electrode application. This is the montage used in our previous work to successfully elicit changes in memory performance and so was used to maintain consistency for comparison (Pergolizzi and Chua, 2016, 2015). The placement of electrodes was also chosen based on the computational models of tDCS current flow into posterior parietal areas of interest (Figure 1), and because CP3/CP4 was defined as targeting posterior parietal regions in earlier reports using transcranial magnetic stimulation (Mottaghy et al., 2002; Schaal et al., 2015). The prefrontal group received bilateral stimulation over the prefrontal cortex with the anode placed over the F3 and the cathode placed over the F4 electrode locations. Bilateral montages were used because they may produce additive effects on performance compared to unilateral stimulation (Vines et al., 2008), and to avoid distributing stimulation through distal brain regions. The spread of current flow using these montages was modeled using Soterix tDCS Explore software (Kempe et al., 2014), and showed good coverage over lateral prefrontal and posterior parietal cortex (Figure 1).
Stimulation was applied during retrieval. During sham stimulation, 2.0 mA of current was ramped up (30 seconds) and ramped down (30 seconds) before and after a 20-minute period with 0.1 mA of current. During lateral prefrontal and posterior parietal stimulation, 2.0 mA of current was ramped up and delivered continuously for a 20-minute stimulation period and then ramped down.
4.3 Materials
Stimuli were presented on a Dell Optiplex 980 PC connected to a 22″ VGA monitor with presentation and timing controlled via Psychopy v.1.74.02 (Peirce, 2007). Stimuli consisted of 750 words randomly selected from the MRC linguistic database with an average of 5.4 letters (SD = 1.53) and 1.6 syllables (SD = 0.72) and a Kucera-Francis frequency of 28.15 (SD = 26.26). Words were formed into 150 word lists matched on length, number of syllables, frequency, concreteness, familiarity and imageability. Words were presented in white letters on a grey background. Participants were presented with 150 words at study and 300 (150 old, 150 new) words at test. Each test list assigned 50 old (i.e., studied) words and 50 new (i.e., unstudied) words to be presented with the neutral cue, 75 old and 25 new words to be presented with the “Likely Old” cue for 75% validity and 25 old and 75 new words to be presented with the “Likely New” cue for 75% validity. Mapping of word lists to item type (old or new) was counterbalanced across lists. Assignment of cue type (neutral, Likely Old, Likely New) was also varied and validity was counterbalanced across lists (e.g. 75 old words presented with valid “Likely Old” cues became 75 new words presented with valid “Likely New” cues).
4.4 Procedure
Participants completed a single study session with one of the 150 word lists and were instructed to remember the words for a later test. During study, each word was presented one at a time on screen for 2 seconds. In a shallow encoding task, participants indicated whether the first and last letter of each word was in alphabetical order by pressing button ‘1’ for yes and ‘2’ for no (Dobbins et al., 2012; Otten et al., 2001; Selmeczy and Dobbins, 2013). Following the study session, participants were set up to receive tDCS. Once electrodes were in place, tDCS began while participants received instructions for the upcoming recognition test and were given a short practice session. Participants were instructed that they would see the words they studied earlier, as well as “new” words that were unstudied, and would need to judge if they were either “old” (studied) by pressing button ‘1’ or “new” (unstudied) by pressing button ‘2’. Each test word was preceded by a cue that appeared 1s prior to the test word and that indicated the word as “Likely Old”, in which case there was a 75% chance the word was “old”, “Likely New”, in which case there was a 75% chance the word was “new”, or with a series of question marks (????), indicating that there was no external information available about the likelihood that the word was “old” or “new” (i.e., an uncued or neutral condition) (Selmeczy and Dobbins, 2013). A total of 100 words received “Likely Old” cues (75 old, 25 new), 100 words received “Likely New” cues (75 new, 25 old) and 100 words received neutral cues (50 old, 50 new) (Figure 2). Trials were presented in random order, with cues intermixed. All participants were instructed that 75% of the cues were valid. TDCS was administered throughout the duration of the retrieval task.
4.5 Data Analysis
Old/new recognition trials were categorized as hits (H), misses (M), false alarms (FA) and correct rejections (CR) for each cue type (neutral, Likely Old, Likely New). Note in the case of hits and misses and the case of false alarms and correct rejections values are reciprocal, therefore having equal variance. Thus, our primary dependent measures were memory accuracy indexed by hits and correct rejections. We used a 3 × 2 × 3 mixed ANOVA with repeated factors of cue type (neutral, Likely Old, Likely New) and response type (hits, correct rejections) and a between-subjects factor of stimulation group (sham, parietal, prefrontal) to test for the effects of stimulation on performance based on cue type. We followed up significant effects from the ANOVA with Bonferroni adjusted post-hoc comparisons of hits and correct rejections for each cue type.
To further examine whether the influence of the external cues on performance differed by tDCS group, we calculated a cue utilization score that reflects the influence of cueing (valid – invalid) while controlling for individual differences in baseline performance. The cue utilization score was calculated separately for “Likely Old” and “Likely New” trials and reflected the extent to which the presence of the “Likely Old” or “Likely New” cue influenced changes in performance. The “Likely Old” cue utilization score was calculated as: (“Likely Old” Hit – “Likely Old” Correct Rejection) – (Neutral Hit – Neutral Correct Rejection). The “Likely New” cue utilization score was calculated as: (“Likely New” Correct Rejection – “Likely New” Hit) – (Neutral Correct Rejection – Neutral Hit). Scores were compared between groups for the “Likely Old” and “Likely New” cues using one-way ANOVAs. We followed up significant effects from the ANOVA with Bonferroni adjusted post-hoc comparisons.
Lastly, we examined overall response accuracy regardless of the cues. In other words, we calculated H, M, CR, FA collapsed across neutral, “Likely Old”, and “Likely New” cues. This reflected overall accuracy and was calculated to verify that overall performance was not maladaptively adjusted regardless of cue influence.
Reaction times were also collected for all responses. There were no significant differences in any of the ANOVAs for reaction time when comparing stimulation groups, and so are not discussed further. For all analyses, comparisons were considered significant at p<0.05, two-tailed, and were analyzed using SPSS 22.0.
Figure 4.
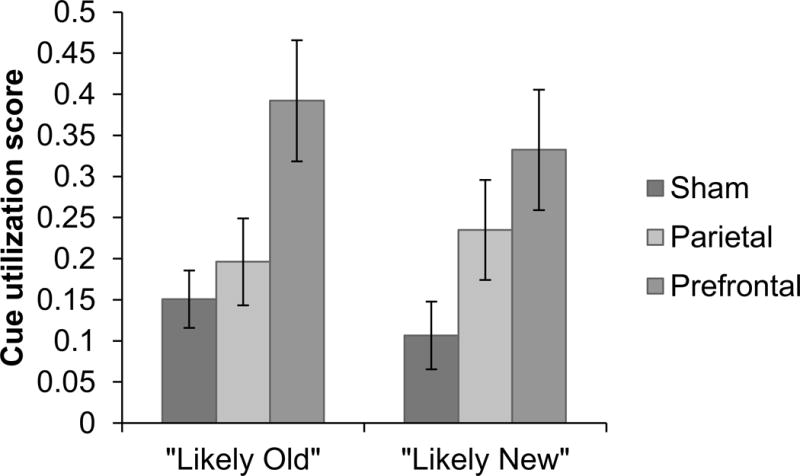
Cue utilization by tDCS group. The prefrontal group showed greater cue utilization, with significantly greater cue utilization scores compared to the sham group for both “Likely Old” and “Likely New” cues, and showed greater cue utilization scores compared to the parietal group for “Likely Old” but not “Likely New” cues. Error bars represent SEM.
Highlights.
We applied tDCS to prefrontal and parietal cortex in an explicit memory cueing task
Cues predicted memoranda as Likely Old or Likely New with 75% validity
The prefrontal group increased use of cues compared to parietal and sham
The prefrontal cortex can bias memory search and recognition decisions
Acknowledgments
The authors wish to thank Rifat Ahmed for help with data collection. EFC was supported by the National Institute of General Medical Sciences and the National Institute on Aging under award number SC2AG046910. DP would like to acknowledge the NIH/NCI Cancer Center Support Grant P30 CA008748 and the NCI award number T32 CA009461 under which she is currently supported. The content is solely responsible of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ally BA, Simons JS, McKeever JD, Peers PV, Budson AE. Parietal contributions to recollection: electrophysiological evidence from aging and patients with parietal lesions. Neuropsychologia. 2008;46:1800–12. doi: 10.1016/j.neuropsychologia.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff EM, Freeman S, Clewett D, Tipper C, Frithsen A, Johnson A, Grafton ST, Miller MB. Maintaining a cautious state of mind during a recognition test: A large-scale fMRI study. Neuropsychologia. 2015;67:132–147. doi: 10.1016/j.neuropsychologia.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Bennabi D, Pedron S, Haffen E, Monnin J, Peterschmitt Y, Van Waes V. Transcranial direct current stimulation for memory enhancement: from clinical research to animal models. Front Syst Neurosci. 2014;8:1–9. doi: 10.3389/fnsys.2014.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Jones KT. tDCS selectively improves working memory in older adults with more education. Neurosci Lett. 2012;521:148–51. doi: 10.1016/j.neulet.2012.05.074. [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Olson IR. The right parietal lobe is critical for visual working memory. Neuropsychologia. 2008;46:1767–74. doi: 10.1016/j.neuropsychologia.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Phuong L, Picasso L, Cabeza R, Olson IR. Parietal Lobe and Episodic Memory: Bilateral Damage Causes Impaired Free Recall of Autobiographical Memory. J Neurosci. 2007;27:14415–14423. doi: 10.1523/JNEUROSCI.4163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil-Neto JP. Learning, memory, and transcranial direct current stimulation. Front Psychiatry. 2012;3:1–4. doi: 10.3389/fpsyt.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn Sci. 2012;16:106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Barch DM. A theory of cognitive control, aging cognition, and neuromodulation. Neurosci Biobehav Rev. 2002;26:809–817. doi: 10.1016/S0149-7634(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Brem AK, Fried PJ, Horvath JC, Robertson EM, Pascual-Leone A. Is neuroenhancement by noninvasive brain stimulation a net zero-sum proposition? Neuroimage. 2014;85:1058–1068. doi: 10.1016/j.neuroimage.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci. 2008;9:613–25. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cansino S, Maquet P, Dolan RJ, Rugg MD. Brain activity underlying encoding and retrieval of source memory. Cereb cortex. 2002;12:1048–56. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Grady CL, Moscovitch M. Top-down and bottom-up attention to memory: a hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia. 2008;46:1828–51. doi: 10.1016/j.neuropsychologia.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Servan-Schreiber D. Context, cortex, and dopamine: A connectionist approach to behavior and biology in schizophrenia. Psychol Rev. 1992;99:45–77. doi: 10.1037/0033-295X.99.1.45. [DOI] [PubMed] [Google Scholar]
- Curran T, Schacter DL, Norman KA, Galluccio L. False recognition after a right frontal lobe infarction: memory for general and specific information. Neuropsychologia. 1997;35:1035–49. doi: 10.1016/S0028-3932(97)00029-8. [DOI] [PubMed] [Google Scholar]
- Davidson PSR, Anaki D, Ciaramelli E, Cohn M, Kim ASN, Murphy KJ, Troyer AK, Moscovitch M, Levine B. Does lateral parietal cortex support episodic memory? Evidence from focal lesion patients. Neuropsychologia. 2008;46:1743–55. doi: 10.1016/j.neuropsychologia.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan JS. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Jaeger A, Studer B, Simons J. Use of explicit memory cues following parietal lobe lesions. Neuropsychologia. 2012;50:2992–3003. doi: 10.1016/j.neuropsychologia.2012.07.037.Use. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Rice HJ, Wagner AD, Schacter DL. Memory orientation and success: separable neurocognitive components underlying episodic recognition. Neuropsychologia. 2003;41:318–33. doi: 10.1016/s0028-3932(02)00164-1. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Wagner AD. Domain-general and domain-sensitive prefrontal mechanisms for recollecting events and detecting novelty. Cereb Cortex. 2005;15:1768–78. doi: 10.1093/cercor/bhi054. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Wheeler ME, Petersen SE. Remember the source: dissociating frontal and parietal contributions to episodic memory. J Cogn Neurosci. 2010;22:377–91. doi: 10.1162/jocn.2009.21242. [DOI] [PubMed] [Google Scholar]
- Drowos DB, Berryhill M, André JM, Olson IR. True memory, false memory, and subjective recollection deficits after focal parietal lobe lesions. Neuropsychology. 2010;24:465–75. doi: 10.1037/a0018902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau S, Knoch D, Fregni F, Sultani N, Boggio P, Pascual-Leone A. Diminishing risk-taking behavior by modulating activity in the prefrontal cortex: a direct current stimulation study. J Neurosci. 2007a;27:12500–12505. doi: 10.1523/JNEUROSCI.3283-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau S, Pascual-Leone A, Zald DH, Liguori P, Theoret H, Boggio PS, Fregni F. Activation of Prefrontal Cortex by Transcranial Direct Current Stimulation Reduces Appetite for Risk during Ambiguous Decision Making. J Neurosci. 2007b;27:6212–6218. doi: 10.1523/JNEUROSCI.0314-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124:849–81. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Santos MC, Lima M, Vieira AL, Rigonatti SP, Silva MTa, Barbosa ER, Nitsche Ma, Pascual-Leone A. Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson’s disease. Mov Disord. 2006;21:1693–1702. doi: 10.1002/mds.21012. [DOI] [PubMed] [Google Scholar]
- Furubayashi T, Terao Y, Arai N, Okabe S, Mochizuki H, Hanajima R, Hamada M, Yugeta A, Inomata-Terada S, Ugawa Y. Short and long duration transcranial direct current stimulation (tDCS) over the human hand motor area. Exp Brain Res. 2008;185:279–286. doi: 10.1007/s00221-007-1149-z. [DOI] [PubMed] [Google Scholar]
- Gladwin TE, den Uyl TE, Fregni FF, Wiers RW. Enhancement of selective attention by tDCS: Interaction with interference in a Sternberg task. Neurosci Lett. 2012;512:33–37. doi: 10.1016/j.neulet.2012.01.056. [DOI] [PubMed] [Google Scholar]
- Guerin SA, Miller MB. Lateralization of the parietal old/new effect: an event-related fMRI study comparing recognition memory for words and faces. Neuroimage. 2009;44:232–42. doi: 10.1016/j.neuroimage.2008.08.035. [DOI] [PubMed] [Google Scholar]
- Haramati S, Soroker N, Dudai Y, Levy DA. The posterior parietal cortex in recognition memory: a neuropsychological study. Neuropsychologia. 2008;46:1756–66. doi: 10.1016/j.neuropsychologia.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Hecht D, Walsh V, Lavidor M. Transcranial direct current stimulation facilitates decision making in a probabilistic guessing task. J Neurosci. 2010;30:4241–5. doi: 10.1523/JNEUROSCI.2924-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen K, Sagliano L, Candini M, Husain M, Cappelletti M, Zokaei N. Cathodal transcranial direct current stimulation over posterior parietal cortex enhances distinct aspects of visual working memory. Neuropsychologia. 2016;87:35–42. doi: 10.1016/j.neuropsychologia.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RNA, Hornberger M, Rugg MD. Further dissociating the processes involved in recognition memory: an FMRI study. J Cogn Neurosci. 2005;17:1058–1073. doi: 10.1162/0898929054475208. [DOI] [PubMed] [Google Scholar]
- Idaka T, Matsumoto A, Nogawa J, Yamamoto Y, Sadato N. Frontoparietal network involved in successful retrieval from episodic memory. Spatial and temporal analyses using fMRI and ERP. Cereb Cortex. 2006;16:1349–60. doi: 10.1093/cercor/bhl040. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Goren N, Lavidor M, Levy DA. Oppositional transcranial direct current stimulation (tDCS) of parietal substrates of attention during encoding modulates episodic memory. Brain Res. 2012a;1439:66–72. doi: 10.1016/j.brainres.2011.12.036. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Koslowsky M, Lavidor M. TDCS polarity effects in motor and cognitive domains: A meta-analytical review. Exp Brain Res. 2012b;216:1–10. doi: 10.1007/s00221-011-2891-9. [DOI] [PubMed] [Google Scholar]
- Jaeger A, Cox JC, Dobbins IG. Recognition confidence under violated and confirmed memory expectations. J Exp Psychol Gen. 2012;141:282–301. doi: 10.1037/a0025687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger A, Konkel A, Dobbins IG. Unexpected novelty and familiarity orienting responses in lateral parietal cortex during recognition judgment. Neuropsychologia. 2013;51:1061–76. doi: 10.1016/j.neuropsychologia.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky JS, Shimamura AP, Squire LR. Source memory impairment in patients with frontal lobe lesions. Neuropsychologia. 1989;27:1043–1056. doi: 10.1016/0028-3932(89)90184-X. [DOI] [PubMed] [Google Scholar]
- Jeffery DT, Norton Ja, Roy FD, Gorassini Ma. Effects of transcranial direct current stimulation on the excitability of the leg motor cortex. Exp Brain Res. 2007;182:281–287. doi: 10.1007/s00221-007-1093-y. [DOI] [PubMed] [Google Scholar]
- Jones K, Berryhill M. Parietal contributions to visual working memory depend on task difficulty. Front Psychiatry. 2012;3:1–11. doi: 10.3389/fpsyt.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Koeppe Ra, Awh E, Reuter-Lorenz Pa, Marshuetz C, Willis CR. The role of parietal cortex in verbal working memory. J Neurosci. 1998;18:5026–34. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I, Davachi L, Wagner AD. Functional-neuroanatomic correlates of recollection: implications for models of recognition memory. J Neurosci. 2004;24:4172–80. doi: 10.1523/JNEUROSCI.0624-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeser D, Meindl T, Bor J, Palm U, Pogarell O, Mulert C, Brunelin J, Moller HJ, Reiser M, Padberg F. Prefrontal Transcranial Direct Current Stimulation Changes Connectivity of Resting-State Networks during fMRI. J Neurosci. 2011;31:15284–15293. doi: 10.1523/JNEUROSCI.0542-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempe R, Huang Y, Parra LC. Simulating pad-electrodes with high-definition arrays in transcranial electric stimulation. J Neural Eng. 2014;11:026003. doi: 10.1088/1741-2560/11/2/026003. [DOI] [PubMed] [Google Scholar]
- Konishi S, Wheeler ME, Donaldson DI, Buckner RL. Neural correlates of episodic retrieval success. Neuroimage. 2000;12:276–86. doi: 10.1006/nimg.2000.0614. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Rosen HJ, Du AT, Schuff N, Hollnagel C, Weiner MW, Miller BL, Delis DC. Dissociations in hippocampal and frontal contributions to episodic memory performance. Neuropsychology. 2005;19:799–805. doi: 10.1037/0894-4105.19.6.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite J, Carvalho S, Fregni F, Boggio PS, Gonçalves ÓF. The Effects of Cross-Hemispheric Dorsolateral Prefrontal Cortex Transcranial Direct Current Stimulation (tDCS) on Task Switching. Brain Stimul. 2013;6:660–667. doi: 10.1016/j.brs.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Leube DT, Erb M, Grodd W, Bartels M, Kircher TTJ. Successful episodic memory retrieval of newly learned faces activates a left fronto-parietal network. Cogn Brain Res. 2003;18:97–101. doi: 10.1016/j.cogbrainres.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Manenti R, Brambilla M, Petesi M, Ferrari C, Cotelli M. Enhancing verbal episodic memory in older and young subjects after non-invasive brain stimulation. Front Aging Neurosci. 2013;5:49. doi: 10.3389/fnagi.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manenti R, Cotelli M, Robertson IH, Miniussi C. Transcranial brain stimulation studies of episodic memory in young adults, elderly adults and individuals with memory dysfunction: A review. Brain Stimul. 2012;5:103–109. doi: 10.1016/j.brs.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller MB, Handy TC, Cutler J, Inati S, Wolford GL. Brain activations associated with shifts in response criterion on a recognition test. Can J Exp Psychol. 2001;55:162–173. doi: 10.1037/h0087363. [DOI] [PubMed] [Google Scholar]
- Minati L, Campanhã C, Critchley HD, Boggio PS. Effects of transcranial direct-current stimulation (tDCS) of the dorsolateral prefrontal cortex (DLPFC) during a mixed-gambling risky decision-making task. Cogn Neurosci. 2012;3:80–88. doi: 10.1080/17588928.2011.628382. [DOI] [PubMed] [Google Scholar]
- Moos K, Vossel S, Weidner R, Sparing R, Fink GR. Modulation of top-down control of visual attention by cathodal tDCS over right IPS. J Neurosci. 2012;32:16360–8. doi: 10.1523/JNEUROSCI.6233-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottaghy FM, Döring T, Müller-Gärtner HW, Töpper R, Krause BJ. Bilateral parieto-frontal network for verbal working memory: an interference approach using repetitive transcranial magnetic stimulation (rTMS) Eur J Neurosci. 2002;16:1627–1632. doi: 10.1046/j.1460-9568.2002.02209.x. [DOI] [PubMed] [Google Scholar]
- Nelson JT, McKinley RA, Golob EJ, Warm JS, Parasuraman R. Enhancing vigilance in operators with prefrontal cortex transcranial direct current stimulation (tDCS) Neuroimage. 2014;85:909–917. doi: 10.1016/j.neuroimage.2012.11.061. [DOI] [PubMed] [Google Scholar]
- Neuling T, Wagner S, Wolters CH, Zaehle T, Herrmann CS. Finite-Element Model Predicts Current Density Distribution for Clinical Applications of tDCS and tACS. Front Psychiatry. 2012;3:1–10. doi: 10.3389/fpsyt.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F, Pascual-Leone A. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, Fricke K, Liebetanz D, Lang N, Antal A, Paulus W, Tergau F. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol. 2005;568:291–303. doi: 10.1113/jphysiol.2005.092429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolde SF, Johnson MK, Raye CL. The role of prefrontal cortex during tests of episodic memory. Trends Cogn Sci. 1998;2:399–406. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Nudo RJ. Recovery after brain injury: mechanisms and principles. Front Hum Neurosci. 2013;7:887. doi: 10.3389/fnhum.2013.00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor AR, Han S, Dobbins IG. The Inferior Parietal Lobule and Recognition Memory: Expectancy violation or successful retrieval. J Neurosci. 2010;30:2924–2934. doi: 10.1523/JNEUROSCI.4225-09.2010.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten LJ, Henson RN, Rugg MD. Depth of processing effects on neural correlates of memory encoding: relationship between findings from across- and within-task comparisons. Brain. 2001;124:399–412. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergolizzi D, Chua EF. Transcranial direct current stimulation over the parietal cortex alters bias in item and source memory tasks. Brain Cogn. 2016;108:56–65. doi: 10.1016/j.bandc.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergolizzi D, Chua EF. Transcranial direct current stimulation (tDCS) of the parietal cortex enhances false recognition. Neuropsychologia. 2015;66:88–98. doi: 10.1016/j.neuropsychologia.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Pisoni A, Turi Z, Raithel A, Ambrus GG, Alekseichuk I, Schacht A, Paulus W, Antal A. Separating Recognition Processes of Declarative Memory via Anodal tDCS: Boosting Old Item Recognition by Temporal and New Item Detection by Parietal Stimulation. PLoS One. 2015;10:e0123085. doi: 10.1371/journal.pone.0123085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope PA, Brenton JW, Miall RC. Task-specific facilitation of cognition by anodal transcranial direct current stimulation of the prefrontal cortex. Cereb Cortex. 2015;25:4551–4558. doi: 10.1093/cercor/bhv094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pripfl J, Neumann R, Köhler U, Lamm C. Effects of transcranial direct current stimulation on risky decision making are mediated by “hot” and “cold” decisions, personality, and hemisphere. Eur J Neurosci. 2013;38:3778–3785. doi: 10.1111/ejn.12375. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WPM, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Schaal NK, Williamson VJ, Kelly M, Muggleton NG, Pollok B, Krause V, Banissy MJ. A causal involvement of the left supramarginal gyrus during the retention of musical pitches. Cortex. 2015;64:310–317. doi: 10.1016/j.cortex.2014.11.011. [DOI] [PubMed] [Google Scholar]
- Selmeczy D, Dobbins IG. Metacognitive awareness and adaptive recognition biases. J Exp Psychol Learn Mem Cogn. 2013;39:678–90. doi: 10.1037/a0029469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Peers PV, Mazuz YS, Berryhill ME, Olson IR. Dissociation between memory accuracy and memory confidence following bilateral parietal lesions. Cereb Cortex. 2010;20:479–85. doi: 10.1093/cercor/bhp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and Executive Processes in the Frontal Lobes. Science (80-) 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Neuroimaging analyses of human working memory. Proc Natl Acad Sci U S A. 1998;95:12061–12068. doi: 10.1073/pnas.95.20.12061. doi: VL – 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Lin RL, Mezue M, Segerdahl A, Kong Y, Xie J, Tracey I. Widespread Modulation of Cerebral Perfusion Induced during and after Transcranial Direct Current Stimulation Applied to the Left Dorsolateral Prefrontal Cortex. J Neurosci. 2013;33:11425–11431. doi: 10.1523/JNEUROSCI.3887-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Knight RT. Contributions of prefrontal cortex to recognition memory: electrophysiological and behavioral evidence. Neuropsychology. 1999;13:155–70. doi: 10.1037/0894-4105.13.2.155. [DOI] [PubMed] [Google Scholar]
- Tseng P, Hsu TY, Chang CF, Tzeng OJL, Hung DL, Muggleton NG, Walsh V, Liang WK, Cheng S, Juan CH. Unleashing potential: transcranial direct current stimulation over the right posterior parietal cortex improves change detection in low-performing individuals. J Neurosci. 2012;32:10554–61. doi: 10.1523/JNEUROSCI.0362-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaellie M, Rapcsak SZ, Keane MM, Alexander MP. Elevated false recognition in patients with frontal lobe damage is neither a general nor a unitary phenomenon. Neuropsychology. 2004;18:94–103. doi: 10.1037/0894-4105.18.1.94. [DOI] [PubMed] [Google Scholar]
- Vilberg KL, Rugg M. Memory retrieval and the parietal cortex: a review of evidence from the dual-process perspective. Neuropsychologia. 2008;46:1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vines BW, Cerruti C, Schlaug G. Dual-hemisphere tDCS facilitates greater improvements for healthy subjects’ non-dominant hand compared to uni-hemisphere stimulation. BMC Neurosci. 2008;9:103. doi: 10.1186/1471-2202-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–53. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wallace D, Cooper NR, Paulmann S, Fitzgerald PB, Russo R. Perceived Comfort and Blinding Efficacy in Randomised Sham-Controlled Transcranial Direct Current Stimulation (tDCS) Trials at 2 mA in Young and Older Healthy Adults. PLoS One. 2016;11:e0149703. doi: 10.1371/journal.pone.0149703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional dissociation among components of remembering: control, perceived oldness, and content. J Neurosci. 2003;23:3869–80. doi: 10.1523/JNEUROSCI.23-09-03869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windmann S. Cognitive and Neural Mechanisms of Decision Biases in Recognition Memory. Cereb Cortex. 2002;12:808–817. doi: 10.1093/cercor/12.8.808. [DOI] [PubMed] [Google Scholar]


