Abstract
Despite a growing interest in understanding the cognitive deficits associated with major depressive disorder (MDD), it is largely unknown whether such deficits exist before disorder onset or how they might influence the severity of subsequent illness. The purpose of the present study was to conduct a systematic review and meta-analysis of longitudinal datasets to determine whether cognitive function acts as a predictor of later MDD diagnosis or change in depression symptoms. Eligible studies included longitudinal designs with baseline measures of cognitive functioning, and later unipolar MDD diagnosis or symptom assessment. The systematic review identified 29 publications, representing 34 unique samples, and 121,749 participants, that met the inclusion/exclusion criteria. Quantitative meta-analysis demonstrated that higher cognitive function was associated with decreased levels of subsequent depression (r=−0.088; 95% CI: −0.121, −0.054; p<0.001). However, sensitivity analyses revealed that this association is likely driven by concurrent depression symptoms at the time of cognitive assessment. Our review and meta-analysis indicate that the association between lower cognitive function and later depression is confounded by the presence of contemporaneous depression symptoms at the time of cognitive assessment. Thus, cognitive deficits predicting MDD likely represent deleterious effects of subclinical depression symptoms on performance rather than premorbid risk factors for disorder.
Introduction
A growing literature has found that depression is associated with impaired cognitive functioning (Snyder 2013). For example, broad deficits in neuropsychological functioning have been demonstrated in a number of reviews and meta-analyses on depression Christensen et al. 1997; Rogers et al. 2004; Snyder 2013; Rock et al. 2014), and cognitive deficits have been found to correlate with symptom severity (McDermott & Ebmeier 2009). Theoretical models have been proposed suggesting that cognitive dysfunction could either be a risk factor for later depression or that cognitive function may be impaired as a result of depression (Stern 2003; Barnett et al. 2006). In support of the latter hypothesis, two recent meta-analyses concluded that the deficits in cognitive function persist after remission from depression (Bora et al. 2013; Rock et al. 2014). However, evidence showing that deficits in cognitive function persist after remission from depression, which are primarily derived from cross-sectional studies, does not rule out the possibility that poor cognitive function preceded the onset of depression. The present review and meta-analysis sought to comprehensively summarize the state of the research on cognitive function and its association with future depressive symptoms using data from longitudinal studies.
While the clinical diagnosis of depression is relatively codified according to DSM and ICD criteria, and depression symptoms are readily measured with a number of validated instruments (Lewinsohn et al. 2000; Cuijpers et al. 2004), a similar canonical approach to measuring cognitive function is not present. Thus, it is important to specify a working definition of cognitive function before examining its possible association with depression. Empirical work has reinforced the concept of a single construct of cognitive function through high correlations in performance among disparate cognitive tasks (Spearman 1904). This has led to the derivation of a single factor representing general cognitive function or ability known as “g” (Johnson et al. 2004). The “g” factor is commonly determined by administering a wide range of tasks and using factor analysis to determine the shared variance across these tasks (Ree & Earles 1991). Even in cases when “g” is not calculated as such, there are other customary ways of capturing this general factor by combing across cognitive tasks, e.g., by creating an index of executive functions (Miyake et al., 2000) or measuring the intelligence quotient (Gottfredson 1997).
Although executive functions and intelligence are often considered independently, evidence has suggested that they may best be characterized by an integrated framework based on a common neural network (Barbey et al. 2012), supporting the idea that they reflect a shared general factor of cognitive ability. In light of these findings and the observation that individuals with depression commonly exhibit deficits across a range of cognitive tasks, our review used the broadest measure of cognitive function that was available in a given study. When a higher-order construct was not available, results from individual measures were used. We further restricted analyses to prospective cohort studies with depression assessment before age 65 in order to minimize potential influences of age-related cognitive decline.
We follow our literature review with a quantitative meta-analysis to (1) determine whether cognitive function predicts later depression, (2) evaluate whether differences in the measurement of depression (categorical vs. continuous) influence observed associations, and (3) examine whether sex or age of participants may moderate effects, and (4) whether effects are confounded by depression symptoms at baseline.
Methods
We followed the Meta-Analyses of Observational Studies in Epidemiology (MOOSE) guidelines (Stroup et al. 2000) (Supplemental Table 1) and the literature search strategies suggested by Atkinson et al (Atkinson et al. 2014).
Search Process
Systematic searches were conducted in February 2015, and updated in December 2015 in three electronic databases: PubMed, EMBASE and PsycInfo. Searches were conducted by a research librarian, EM, and overseen by MS. The search syntax was adapted for each database and designed to capture the participants, predictors, comparisons and outcomes described below, according to the PICOS framework (Moher & Liberati 2009). The Boolean operator “OR” was used within categories and the operator “AND” was used between categories. The complete search strategy is described in Supplemental Materials: Search Strategy. Hand searches were conducted of the reference lists of included articles.
Eligibility Criteria
The review included English language studies of empirical studies investigating the longitudinal association of cognitive function (IQ, neuropsychological tests, executive functions) with unipolar depression diagnosis or symptoms. Detailed inclusion/exclusion criteria were as follows:
Participant Characteristics
Eligible studies included children, adolescents and adults under the age of 65. This age range was specified because of the possible impact of cognitive decline in older populations. Articles examining a highly specific population (e.g., with a particular medical diagnosis) were excluded so as to not limit the generalizability of results. Studies that required MDD diagnosis, hospitalization or admission to a treatment center as part of the inclusion criteria at baseline were also excluded from the review so as to permit conclusions about depression onset. This was to ensure that a majority of study participants did not have depression at baseline. However, cohort studies were included even if they did not measure depression at baseline, assuming that these samples would have depression prevalence rates that were comparable to population norms. Subgroup analyses were subsequently performed on the studies that specifically excluded for any history of depression. If there were several papers about one cohort, the paper with larger sample size or better study quality was included.
Predictor Type
Eligible studies included a measure of cognitive, executive or neuropsychological function. The study included a range of cognitive predictors, given that previous studies have found broad deficits in cognitive function in depression (Christensen et al. 1997; Rogers et al. 2004; Snyder 2013; Rock et al. 2014). When more than one cognitive predictor was reported as an outcome in a study, the broadest measure of cognitive functioning was used. If no measure captured broad cognitive functioning, the measure that was most closely aligned with constructs on a standard IQ test (working memory, processing speed, verbal comprehension, or perceptual reasoning) was used in the meta-analysis.
Studies were excluded if cognitive function was assessed via educational achievement, given that these measures can be more easily influenced by learning disabilities than by general cognitive functioning (Siegel 1999). Self-report descriptive measures of cognitive functioning were also excluded in favor of more objective measures. Lastly, since this study sought to investigate general information processing abilities rather than cognitive attributional style, studies with cognitive attribution measures as the sole cognitive measure were excluded.
Comparisons
Search terms used to indicate the possible association between cognitive function and depression included: risk, premorbid, prodromal, onset, predict and association (for a full list, see search terms in the Supplemental Materials). When data were reported from multiple time-points, the analysis accounting for the longest time between assessments was used.
Outcomes
Eligible studies reported depression diagnosis or symptoms determined by investigator or self-report. Presence of comorbidity was permitted given that depression has been found to be highly comorbid with other disorders (Melartin et al. 2002; Kessler et al. 2003). Articles not adequately specifying how depression was assessed or articles that did not uniquely measure depression (ie. only had an index of “mental disorders” or the category of “mood disorders” lumped together (Gale et al. 2010)), were excluded if no unique measure of depression could be obtained from study authors. This is because, although comorbidity was permitted, these could have represented instances of pure anxiety disorders with few or no depressive symptoms or instances of bipolar disorder.
Study Design
Eligible study search terms included: cohort studies, longitudinal, epidemiological, prospective, retrospective, follow-up or case-control studies. Studies not following a longitudinal design were excluded during full-text review. Additionally, studies where cognitive function was assessed only concurrently with or after depression were excluded, given that the purpose of the review was to investigate whether cognitive function predicted later depression.
Studies were included regardless of whether they were reported in peer-review journals in order to account for potentially biased reporting of results. Review articles were included in the search terms to find additional relevant references, but were not included in the meta-analysis. Baseline and protocol studies without follow-up data were excluded.
Study Selection
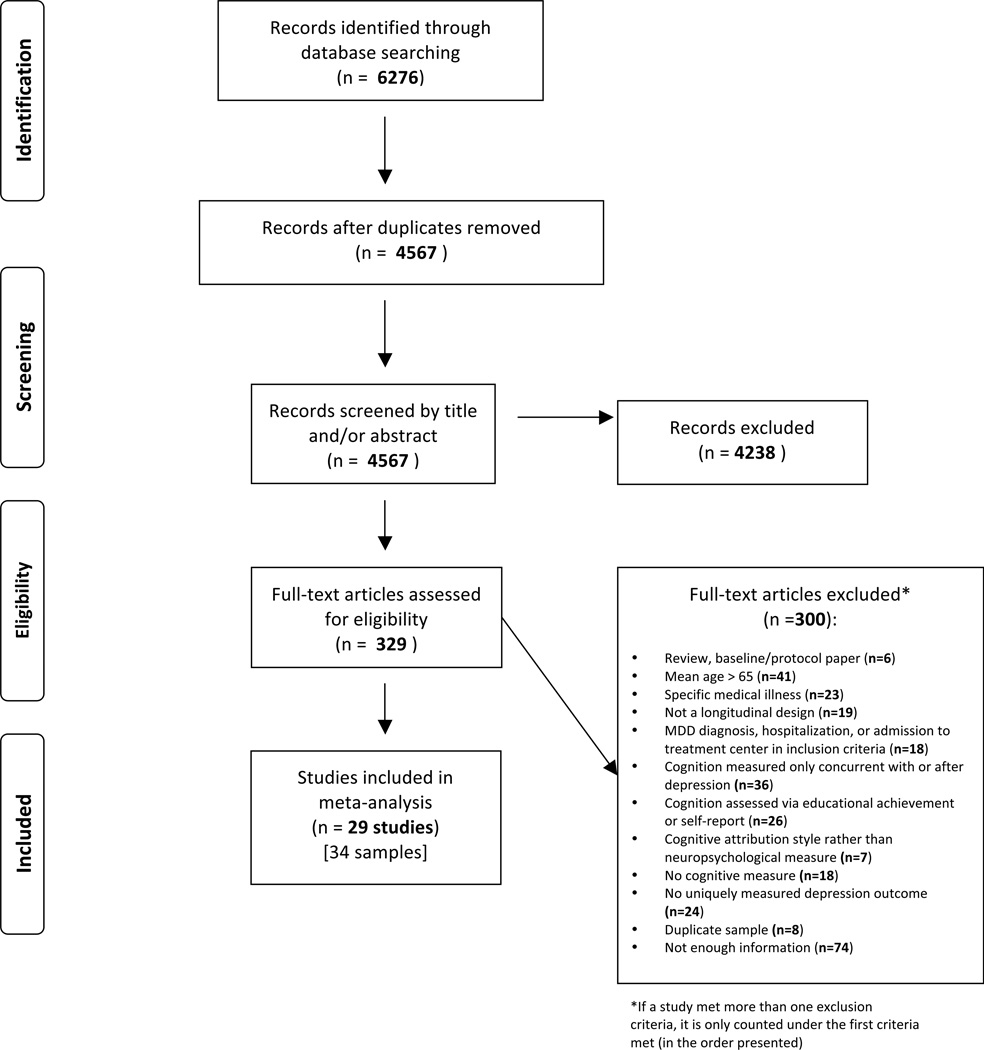
A flow diagram of the process of study selection shows the overall procedures (Figure 1). Records identified from the search processes were combined in EndNote software version X7 and duplicates were removed. Titles and abstracts were reviewed independently by two reviewers (MS & AP) according to the eligibility criteria described above, and marked as either potentially eligible or not eligible. If either author marked an article as potentially eligible, it was included in the full-text review for closer examination by MS and AP, who used an electronic form to indicate if an article should be included or excluded in the final selection. When an article was excluded, the reason for exclusion was selected from a hierarchical list of exclusion criteria presented in Figure 1. Both MS and AP were initially blinded to the other’s decisions. After both MS and AP completed their reviews, discrepancies about whether a study should be included or excluded were resolved via discussion and consensus amongst MS, AP, TM, AH, and TS.
Figure 1. Flow Diagram of the Search Process.
Data Extraction
Study information was extracted independently by MS and AP using an electronic form created for this study. Information was collected and coded based on report characteristics, participant characteristics, study setting, predictors, outcome measures and quality assessment (Cooper 2010). Any discrepancies (approximately 3% of data points) were resolved via consensus.
For studies with categorical depression diagnoses, effect sizes were generally presented as odds ratios. For these studies, the odds ratio plus the upper and lower bound of the confidence interval were recorded. Two of the studies (Zammit et al. 2004; Gale et al. 2008) reported odds ratios greater than one to indicate that lower IQ was associated with greater risk of depression. The other studies reported odds ratios in the reverse, leading to odds ratios less than one for the same direction of the effect. Therefore the odds ratios from the first two studies were recalculated by dividing (1/odds ratio). Another study(Mccord & Ensminger 1997) reported results from chi-squared tests. Additional studies reported mean premorbid IQ scores rather than odds ratios, in which case the means, sample sizes and/or p-values and number of tails of the t-test were utilized to calculate the effect size.
For studies in which depression was measured as a continuous variable, effect sizes were presented as correlation coefficients. The direction of the effect (positive or negative) and the sample size were also extracted to be used in the meta-analysis. Two studies reported only beta weights (Simons et al. 2009; Rawal & Rice 2012) and we were not able to obtain zero-order correlations from the authors. Given that previous reports have suggested that beta weights can be used as an estimate of correlation coefficients in meta-analysis (Peterson & Brown 2005), the beta weights were used instead, and analyses were run both including and excluding these data points.
When insufficient information was presented in the article, authors were contacted requesting further detail. An additional 6 studies were included at this point. One study (Hatch et al. 2007) included effect sizes for the female half of the cohort, but did not provide statistics for the males, but did indicate a null effect. Only women from this study were included in the meta-analyses, but imputing a score of 0 for the males yielded similar overall results.
Follow-up analyses directly compared studies that reported zero-order correlations between IQ at Time 1, Depression at Time 1 and Depression at Time 2 (additional details described in Supplemental Methods).
Statistical Methods
Data were analyzed using Comprehensive Meta-Analysis software (Version 3.0, Biostat Inc, Englewood, NJ, USA). A random-effects model was used for expected heterogeneity across studies. A random-effects model also allows for broader generalization of results to the population at large. Heterogeneity of effects was calculated using the Q statistic, where a significant p-value indicates that true effect varies across studies. The I2 statistic was used to calculate the ratio of the true heterogeneity to total variation, acting as an index of signal-to-noise ratio. When I2 is large, it is has been recommended to consider subgroup analysis or meta-regression to account for the variance (Borenstein et al. 2011).
Subgroup analyses were conducted using a mixed-effects analysis in which a random effects model was used to combine studies within each subgroup, and a fixed effect model was used to combine subgroups and yield the overall effect (the study-to-study variance is not assumed to be the same for all subgroups). Subgroup analyses were conducted comparing type of outcome (continuous vs. categorical), broad vs. specific cognitive measures, and outcomes that were adjusted vs. unadjusted for baseline depression symptoms.
Meta-regression was performed using the following variables on unadjusted analyses: age at baseline, age at follow-up, time between assessments, IQ, percent white, year of cognitive assessment, year of follow-up assessment, percent female, and study quality score.
Study Quality and Publication Bias
Study quality was evaluated independently by MS and AP using a checklist adapted from Luppino & Wit (Luppino et al. 2010). The checklist contained 12 items and studies were rated with a “+” if it met criteria for that item, a “−“ if it did not meet criteria for an item, and a “?” if unclear. “+” were coded as a score of 1 and −/? were coded as a score of 0. Total scores and percentages were calculated. Articles with total scores of 0–3 were considered to be of low quality, scores of 4–7 were considered a medium quality, and scores of 8–12 were considered to be of high quality. Any discrepancies (approximately 3% of data points) were resolved via consensus.
Publication bias was assessed us a funnel plot, which presents study size on the vertical axis (as standard error, in this case) as a function of effect size on the x-axis. When no publication bias exists, the studies should be distributed symmetrically about the combined effect size (Borenstein et al. 2011). If evidence of publication bias was observed, Duval and Tweedie’s Trim and Fill Method was used (Duval & Tweedie 2000), which recalculates effects based on imputing data from studies that are likely to be missing. Lastly, the Fail-Safe N was calculated (Cooper 1979), which indicates the number of missing studies that would need to exist to nullify the observed effect.
Results
A flow diagram of the search process is shown in Figure 1. The search identified 2,773 records through PubMed, 2,158 through EMBASE and 1,345 through PsycInfo, for a total of 6,276 records. Duplicate records (1,709) were removed in EndNote, resulting in 4,567 unique records. After title and abstracts were reviewed, 329 records were identified as potentially eligible for the meta-analysis. These 329 records were reviewed in full and 300 articles were excluded hierarchically if they were: a review, baseline or protocol paper (n=6), if the sample had a mean age over 65 at the depression assessment (n=41), if the sample had a specific medical illness (n=23), if the study was not a longitudinal design (n=19), if MDD diagnosis, hospitalization or admission to treatment center was part of the inclusion criteria of the study (n=18), if cognitive function was assessed only concurrently with or after depression (n=36), if cognitive function was assessed by educational achievement or self-report (n=26), if cognitive function was assessed by attribution style rather than neuropsychological measure (n=7), if there was no measure of cognitive function (n=18) or no uniquely measured depression outcome (n=24). Furthermore, if articles reported on the same sample as another article with higher quality or more information, the duplicate articles were excluded (n=8). Lastly, a number of studies appeared to measure both cognitive function and depression, but did not report enough information to be included in the meta-analysis and did not respond to requests for additional information (n=74, approximately 59 unique samples). In total, 29 publication were included in the meta-analysis, comprising 34 samples (one study included two separate birth cohorts and another four reported data separately for men and women).
Study Characteristics
The characteristics of included samples are detailed in Table 1. Sample sizes ranged from 43 to 50,053 with a median of 339.5. The most common types of samples were school samples, (9/34), community samples (8/34), and birth cohorts (7/34). The samples were majority white, with two samples having exclusively black participants. Additionally, 8 samples were men only, 6 samples were women only, and the rest reported combined effect sizes. Mean age of participants at baseline ranged from 3.5 to 59 years with a median of 12 years. Most samples did not report whether comorbid diagnoses were present (23/34).
Table 1.
Characteristics of included studies. Sample size is the number of subjects included in reported analyses of interest. NR = not reported. ~ indicates only a range was reported.
| Study | Name of Cohort | Type of Cohort | Sample Size | Comorbidity | How Depression Assessed |
Mean IQ at Baseline |
Name of Cognitive Measure | Type of Cognitive Measure | Name of Depression Outcome |
Type of Depression Outcome |
Covariates included in Meta-Analysis* |
Percent Female | Race/Ethnicity | Continent | Mean Age at Baseline |
Time Between Assessments (years) |
Year of Initial Assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baer et al. 2013 | Concordia Longitudinal Retirement Project |
Retirees | 333 | NR | Study Assessment |
NR | Montreal Cognitive Assessment | Verbal Comprehension, Perceptual Reasoning, Working Memory |
Center for Epidemiological Studies Depression Scale (CES-D) |
Continuous | Depression symptoms at Time 1 |
NR (both male and female) |
NR | North America | 59.06 | 4 | ~2005 |
| Beaujean et al. 2013 | National Longitudinal Study of Adolescent Health |
Community Sample |
14,322 | NR | Study Assessment |
100.61 | Add Health Picture Vocabulary Test (AHPVT) -- an abridged version of the PPVT-R |
Verbal Comprehension | Created for this study | Continuous | Depression symptoms at Time 1 |
50 | 76.31% White; 16.74% Black; 4.22% Asian; 2.5% American Indian; 11.83% Hispanic; 0.05% Mixed Race |
North America | 15.98 | 7 | 1994 |
| Belsky et al. 2012 | Environmental Risk Longitudinal Twin Study (E-Risk) |
Birth Cohort | 2,123 | conduct disorder, anxiety, psychosis, borderline personality disorder |
Study Assessment |
100 | Wechsler Preschool and Primary Scale of Intelligence (WPPSI) |
General Intelligence, Verbal Comprehension, Perceptual Reasoning |
Children’s Depression Inventory (CDI) |
Continuous | None | 51 | ~90% White | Europe | 5 | 7 | 1999 |
| Betts et al. 2016 | Mater University Study of Pregnancy (MUSP) |
Pre-Birth Cohort | 1,934 | Psychosis, Mania |
Study Assessment |
NR | Peabody Picture Vocabulary Test-Revised (PPVT-R) |
Verbal Comprehension | Composite International Diagnostic Interview (CIDI-Auto) |
Continuous (latent factor) |
None | NR | NR | Australia | 5 | 16 | 1989 |
| Canals et al. 2002 (females) | NR | Community Sample |
99 | NR | Study Assessment |
NR | Academic aptitude test (AAT) | General Intelligence, Verbal Comprehension, Perceptual Reasoning |
Schedules for Clinical Assessment in Neuropsychiatry |
Categorical | None | 100 | NR | Europe | ~11 | 6 | NR |
| Canals et al. 2002 (males) | NR | Community Sample |
100 | NR | Study Assessment |
NR | Academic aptitude test (AAT) | General Intelligence, Verbal Comprehension, Perceptual Reasoning |
Schedules for Clinical Assessment in Neuropsychiatry |
Categorical | None | 0 | NR | Europe | ~12 | 6 | NR |
| Connolly et al. 2014 | Temple University Adolescent Cognition and Emotion (ACE) Project |
Community Sample |
200 | NR | Study Assessment |
NR | Digit Span from Wechsler Intelligence Scale for Children (WISC) |
Working Memory | Children’s Depression Inventory (CDI) |
Continuous | Depression symptoms at Time 1 |
56.5 | 45.2% White; 51.3 % Black |
North America | 12.41 | 1 | NR |
| Der et al. 2009 | US National Longitudinal Survey of Youth 1979 |
Community Sample |
7,458 | Noted a range of health conditions |
Study Assessment |
NR | Armed Services Vocational Aptitude Battery (ASVAB)/Armed Forces Qualification Test (AFQT) |
General Intelligence, Verbal Comprehension |
Center for Epidemiological Studies Depression Scale (CES-D) |
Continuous | Depression symptoms at Time 1 |
52 | 31% Black; 19% Hispanic |
North America | 17.9 | ~22 | 1979 |
| Dubow et al. 2008 (females) | Columbia County Longitudinal Study (CCLS) |
School Children Grade 3 |
215 | NR | Study Assessment |
~104 | California Short-Form Test of Mental Maturity |
General Intellignce | Minnesota Multiphasic Personality Inventory (MMPI) Scale 2 |
Continuous | None | 100 | 90% White; 3% Black; <1%Asian, <1% Hispanic |
North America | 8 | 11 | 1960 |
| Dubow et al. 2008 (males) | Columbia County Longitudinal Study (CCLS) |
School Children Grade 3 |
211 | NR | Study Assessment |
~104 | California Short-Form Test of Mental Maturity |
General Intelligence | Minnesota Multiphasic Personality Inventory (MMPI) Scale 2 |
Continuous | None | 0 | 90% White; 3% Black; <1%Asian, <1% Hispanic |
North America | 8 | 11 | 1960 |
| Evans et al. 2015 | NR | School Children Grades 5–9 |
192 | Some with ADHD |
Study Assessment |
111 | Wechsler Abbreviated Scale of Intellignece (WASI) |
General Intelligence, Verbal Comprehension, Perceptual Reasoning, Working Memory, Set-Shifting |
Children’s Depression Inventory (CDI) |
Continuous | Depression symptoms at Time 1 |
52.1 | 71.4% White; 18.2% Black; 2.6% Asian; 3.6% Hispanic; 4.2% Mixed Race |
North America | 12.36 | 0.3 | ~2000s |
| Franz et al. 2011 | Vietnam Era Twin Study of Aging |
Military Cohort | 1,231 | NR | Study Assessment |
NR | Armed Forces Qualification Test (AFQT Form 7A) |
General Intelligence, Perceptual Reasoning, Working Memory, Processing Speed, Set-Shifting, Inhibition |
Center for Epidemiological Studies Depression Scale (CES-D) |
Continuous | None | 0 | 86% White | North America | 20 | 35 | ~1970 |
| Gale et al. 2008 | Vietnam Experience Study (VES) |
Military Cohort | 3,258 | Mix | Study Assessment |
100 | General technical section of the Army Classification Battery |
General Intelligence, Verbal Comprehension, Arithmetic reasoning |
Diagnostic Interview Schedule (DIS) |
Categorical | Socioeconomic Status, Ethnicity, Place of service |
0 | 80.8% White; 12.7 % Black |
North America | 20.4 | ~17 | ~1964 |
| Gale et al. 2009 (1958 Cohort) | 1958 National Child Development Survey |
Birth Cohort | 6,369 | NR | Study Assessment |
102.8 | General ability test, devised by the National Foundation for Educational Research in England and Wales |
General Intelligence | Rutter’s Malaise Inventory |
Continuous | None | NR (both male and female) |
NR | Europe | 11 | 22 | 1969 |
| Gale et al. 2009 (1970 Cohort) | 1970 British Cohort Study | Birth Cohort | 6,074 | NR | Study Assessment |
101.8 | British ability Scale | General Intelligence | Rutter’s Malaise Inventory |
Continuous | None | NR (both male and female) |
NR | Europe | 10 | 20 | 1980 |
| Gjerde et al. 1995 (females) | NR | Recruited from a nursery school |
51 | NR | Study Assessment |
118.7 | Wechsler Adult Intelligence Scale (WAIS) |
General Intelligence | General Behavior Inventory (GBI) Depression scale |
Continuous | None | 100 | ~66% White;
~25% Black; ~0.05% Asian |
North America | 18 | 5 | 1983 |
| Gjerde et al. 1995 (males) | NR | Recruited from a nursery school |
45 | NR | Study Assessment |
111.12 | Wechsler Adult Intelligence Scale (WAIS) |
General Intelligence | General Behavior Inventory (GBI) Depression scale |
Continuous | None | 0 | ~66% White;
~25% Black; ~0.05% Asian |
North America | 18 | 5 | 1983 |
| Hatch et al. 2007 | 1946 British Cohort Study | Birth Cohort | 957 | NR | Study Assessment |
NR | National Foundation for Educational Research Test |
General Intelligence, Verbal Comprehension, Perceptual Reasoning |
General Health Questionnaire (GHQ- 28)- severe depression subscale |
Continuous | None | 51 | NR | Europe | 8 | 45 | 1954 |
| Hipwell et al. 2011 | Pittsburgh Girls Study (PGS) |
Community Sample |
195 | NR | Study Assessment |
99.54 | Wechsler Intelligence Scale for Children (WISC) III-R |
Verbal Comprehension | Schedule for Affective Disorders for School- Age Children- Present and Lifetime Version (K-SADS-PL) |
Continuous | Depression symptoms at Time 1 |
100 | 70% Black or multiracial |
North America | 11.54 | 1 | 1998 |
| Horowitz et al. 2003 | NR | Elementary school cohort |
196 | NR | Study Assessment |
104.61 | Wechsler Intelligence Scale for Children (WISC) -R |
General Intelligence, Verbal Comprehension, Perceptual Reasoning |
Schedule for Affective Disorders for School- Age Children- Epidemiological Version (K-SADS-E) |
Categorical | Depression symptoms at Time 1 |
54.2 | 89% White, 14.7% Black, 3.3% Other |
North America | 11.86 | 6 | ~1980 |
| Koenen et al. 2009 | Dunedin Cohort | Birth Cohort | 730 | None | Study Assessment |
NR | Wechsler Intelligence Scale for Children (WISC) |
General Intelligence, Verbal Comprehension, Perceptual Reasoning, Working Memory, Processing Speed |
Diagnostic Interview Assessment (for DSM- IV) |
Categorical | Sex, Socioeconomic Status, Physical Health, childhood maltreatment |
48 | "Primarily White" | New Zealand | 9 | 23 | 1981 |
| McCord & Ensminger 1997 (females) | Woodlawn Cohort | Elementary school cohort |
346 | Some with alcoholism |
Study Assessment |
NR, (13% of sample had scores over 110) |
Unspecified IQ | General Intelligence | Composite International Interview Schedule (according to DSM-III-R) |
Categorical | None | 100 | 100% Black | North America | ~6 | 27 | 1966 |
| McCord & Ensminger 1997 (males) | Woodlawn Cohort | Elementary school cohort |
313 | Some with alcoholism |
Study Assessment |
NR, (11% of sample had scores over 110) |
Unspecified IQ | General Intelligence | Composite International Interview Schedule (according to DSM-III-R) |
Categorical | None | 0 | 100% Black | North America | ~6 | 27 | 1966 |
| Meyer et al. 2004 | NR | Risk Sample | 86 | NR | Study Assessment |
~118 | WISC | General Intelligence, Verbal Comprehension, Perceptual Reasoning |
SCID | Categorical | None | 63 | 90% White, 9% Black, 1% Hispanic |
North America | 11 | 13 | 1989 |
| Papmeyer et al. 2015 | Scottish Bipolar Family Study |
Risk Sample | 111 | NR | Study Assessment |
Controls: 108 Patients: 107 |
National Adult Reading Test (NART) |
Verbal Comprehension | Structured Clinical Interview for DSM Disorders (SCID) symptoms |
Categorical | None | 52 | NR | Europe | 21 | 2 | NR |
| Pine et al. 1997 | NR | Community Sample |
644 | NR | Study Assessment |
100.7 | Unspecified "IQ" | General Intelligence | Based on Diagnostic Interview Schedule for Children |
Continuous | Depression symptoms at Time 1 |
52 | 91% White | North America | 13.8 | 9 | 1983 |
| Quinn & Joormann 2015 | NR | Undergraduate Students |
43 | NR | Study Assessment |
NR | n-back | Working Memory | Beck Depression Inventory II (BDI-II) |
Continuous | Depression symptoms at Time 1 |
63 | 58% White, 30% Hispanic or Latino, 19% Asian, 2% African American, 2% American Indian, 2% Native Hawaiian, 2% Indian |
North America | 19 | 0.14 | NR |
| Rawal & Rice 2012 | Early Prediction of Adolescent Depression study |
Risk Sample | 230 | None | Study Assessment |
97.46 | Wechsler Intelligence Scale for Children (WISC) |
General Intelligence, Verbal Comprehension, Perceptual Reasoning, Working Memory, Processing Speed |
Child and Adolescent Psychiatric Assessment (CAPA) |
Both | Depression symptoms at Time 1, Age, Overgenerality to |
59.6 | NR | Europe | 13.71 | 1 | NR |
| Sharp et al. 2008 | The Child Behaviour Study |
Community Sample |
439 | NR | Study Assessment |
105 | Wechsler Intelligence Scale for Children (WISC) |
General Intelligence, Verbal Comprehension, Perceptual Reasoning |
The Mood and Feelings Questionnaire |
Continuous | Depression symptoms at Time 1 |
52 | 97% White; 0.5% Black; 2.5% Asian |
Europe | 9.4 | 1 | NR |
| Simons et al. 2009 | East Flanders Prospective Twin Survey |
Twin Registry | 444 | NR | Study Assessment |
NR | Principal component from Stroop, Concept Shifting Test, and Letter Digit Substitution Test |
Processing Speed, Set-Shifting, Inhibition |
Structured Clinical Interview for DSM Disorders (SCID) symptoms |
Continuous | Depression symptoms at Time 1 |
100 | NR | Europe | 28 | 2 | NR |
| Slykerman et al. 2015 | Auckland Birthweight Collaborative Study |
Birth Cohort/ Risk Sample |
609 | NR | Study Assessment |
NR | Stanford-Binet Intelligence Scale, 4th Ed |
General Intelligence, Fluid Reasoning, Knowledge, Qunatitative Reasoning, Visual- |
Center for Epidemiological Studies Depression |
Categorical (cut-point) |
None | 49 | NR | New Zealand | 3.5 | 7.5 | ~2000 |
| Sørensen et al. 2012 | Danish Draft-Board Study | Military Cohort | 21,914 | NR | Psychiatric Registry |
Controls: 100 Patients: 96– 98 |
Børge Priens Prøve (BPP) | General Intelligence, Verbal Comprehension, Perceptual Reasoning |
Danish International Classification of diseases (ICD) 8 & 10 |
Categorical | None | 0 | NR | Europe | 19.5 | ~36 | 1968–1989 |
| Vinberg et al. 2013 | NR | High-Risk Twins | 224 | Bipolar
disorder, anxiety, substance abuse, other |
Study Assessment/ Psychiatric Registry |
NR | Cambridge Cognitive Assessment (CAMCOG) |
General Neuropsychological Function, Orientation, Lanugage, Memory, Attention, Abstract Thinking, Visual Perception |
Beck Depression Inventory (BDI) |
Continuous | Depression symptoms at Time 1 |
65 | NR | Europe | 43.9 | 7 | 2003 |
| Zammit et al. 2004 | Swedish Conscripts (1969–1970) |
Military Cohort | 50,053 | None | Hospital Linkage | NR | Unspecified "IQ" | General Intelligence, Verbal Comprehension, Perceptual Reasoning, General Knowledge, Mechanical Knowledge |
International Classification of diseases (ICD) hospital psychiatric admissions |
Categorical | Diagnosis at baseline, disturbed behavior, drug use, raised in a city |
0 | NR | Europe | ~19 | 27 | 1969 |
This column notes covariates included in the meta-analysis. We focused on controlling for depressive symptoms at T1, but some studies reported effect sizes that also included controlling for other variables-- when the zero-order correlations were unavailable, these estimates were used.
Of the 74 papers excluded for having insufficient information (representing 60 unique samples), the median number of subjects was not statistically different between those studies and the studies included in the meta-analysis (n= 288 and n= 339.5, respectively; p=0.19), nor did the average age of study participants at baseline statistically differ (median across studies = 11 vs. 12, respectively; p=0.57). The types of cognitive measures used were also similar across the excluded and included studies, with the most common measure being IQ (28/60 and 20/29), followed by Verbal Comprehension (8/60 and 5/29).
Meta-Analysis
The overall analysis yielded a significant association between cognitive function and subsequent depression outcomes (r=−0.088; 95% CI: −0.121, −0.054; p<0.001; Figure 2). Results were similar when excluding the two studies that used beta-weights (r=−0.071; 95% CI: −0.100, −0.042; p<0.001) or when using episodic memory in place of processing speed in a study reporting both outcomes (r=−0.085; 95% CI: −0.118, −0.051; p<0.001). Follow-up analyses were conducted in the sample of studies reporting educational attainment and self-report outcomes (Supplemental Table 2). Heterogeneity across samples was significant (Q=801.54, p<0.001), suggesting substantial variation across the individual samples. The ratio of the true heterogeneity to total variation was large (I2=95.88%). I2 is on a relative scale and values close to 100 indicate that most of the observed variance is real rather than spurious and suggests that subgroup analyses or meta-regression may help to explain this variability (Borenstein et al. 2011).
Figure 2. Meta-Analysis of the Association Between Cognitive Function and Subsequent Depression.

A forest plot for all studies that investigated associations between cognition and later depression. Results are reported as correlation coefficients denoted by squares, and 95% confidence intervals indicated by lines (effect sizes are converted to correlation coefficients if reported as odds ratios). Meta-analysis results are displayed as the diamond. The overall analysis found a significant effect of cognition on depression (r=−0.088; 95% CI: −0.121, −0.054; p<0.001).
Subgroup Analyses and Meta-Regression
A significant difference was found between categorical and continuous effect sizes (p<0.001). The studies assessing continuous outcomes were found to have a larger effect (r=−0.121; 95% CI: −0.168, −0.073; p<0.001; n=22) than studies assessing categorical outcomes (r=−0.035; 95% CI: −0.054, −0.017; p<0.001; n=12). No differences were observed between studies utilizing broad vs. specific cognitive measures (p=0.57).
Outcomes were also compared adjusting for baseline depression symptoms at the time of cognitive assessment. Nine studies provided enough detail to calculate the partial correlation coefficients adjusting for baseline depression symptoms. Using the partial correlation coefficients led to a null result (r=−0.032; 95% CI: −0.078, 0.014; p=0.169; n=9; Figure 3). The null result does not seem to be due to a lack of power because using the unadjusted correlation coefficients for the same nine studies still led to a significant result (r=−0.120; 95% CI: −0.169, −0.070; p<0.001; n=9; Figure 3). As a follow-up, the difference in effect sizes between the adjusted and unadjusted values for these 9 studies were tested (r=0.082; 95% CI: 0.069, 0.095; p<0.001; n=9), suggesting that the effects are significantly different when directly comparing the values that do and do not control for baseline depression symptoms.
Figure 3. Meta-analysis Comparing Values Adjusted for Baseline Depression Symptoms vs. Unadjusted.
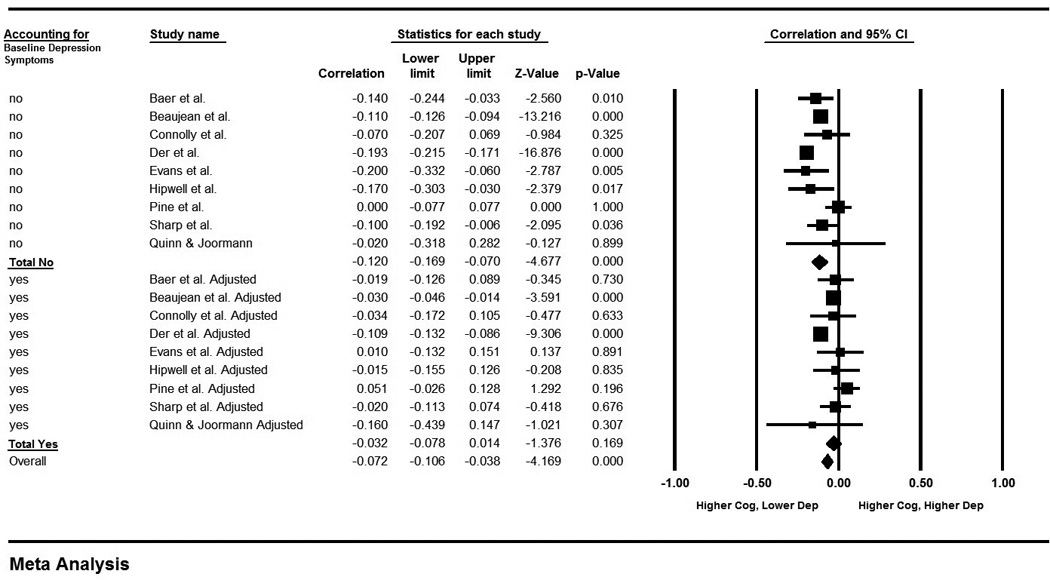
A Forest plot for analysis comparing results from a subset of the same set of studies reported in Figure 2 when using unadjusted values compared to using values that are adjusted for baseline depression. Effects are significant for unadjusted values (r=−0.120; 95% CI: −0.169, −0.070; p<0.001), but not for adjusted values (r=−0.032; 95% CI: −0.078, 0.014; p=0.169). Results are reported as correlation coefficients denoted by squares, and 95% confidence intervals indicated by lines. Meta-analysis results are displayed as diamonds.
Expanding the subgroup analyses to include studies that controlled for baseline depression plus other covariates (Zammit et al. 2004; Simons et al. 2009; Rawal & Rice 2012), and studies that explicitly excluded for a history of depression diagnosis (Gale et al. 2008; Vinberg et al. 2013; Papmeyer et al. 2015), did not change the null result (r=−0.027; 95% CI: −0.058, 0.005; p=0.097; n=15).
None of the meta-regression analyses were significant, indicating that the between-study variance was not attributable to age at baseline, IQ, percent of sample that was female, or percent of the sample that was white age at follow-up, time between assessments, year of cognitive assessment, year of follow-up assessment, or study quality score.
Study Quality and Publication Bias
Multiple samples within a study were combined for the purposes of quality assessment, given that studies within the same publication were found to employ the same methodology. Of the 29 publications reviewed, 12 were rated as high quality and 17 were rated as medium quality. Details are provided in Supplemental Table 1. Comparable results to the overall findings were obtained when just using high quality studies (r=−0.085; 95% CI: −0.141, −0.029; p=0.003).
Inspection of the funnel plot (Figure 4) found no evidence for publication bias to support the hypothesis. In fact, imputation of negative correlations suggested that additional studies favoring the hypothesis may be missing. While the calculated effect size is −0.088 (95% CI: −0.121, −0.054), using Duval and Tweedie’s Trim and Fill Method, the imputed effect size estimate is larger at −0.137 (95% CI: −0.176, −0.099; imputed studies n=11). Lastly, the Fail-Safe N procedure found that 3,583 null studies would need to be located for the combined 2-tailed p-value to exceed 0.05. This suggests that significant results are not likely to be confounded by publication bias and may even underestimate true effect sizes.
Figure 4. Trim and Fill Funnel Plots for All Samples.
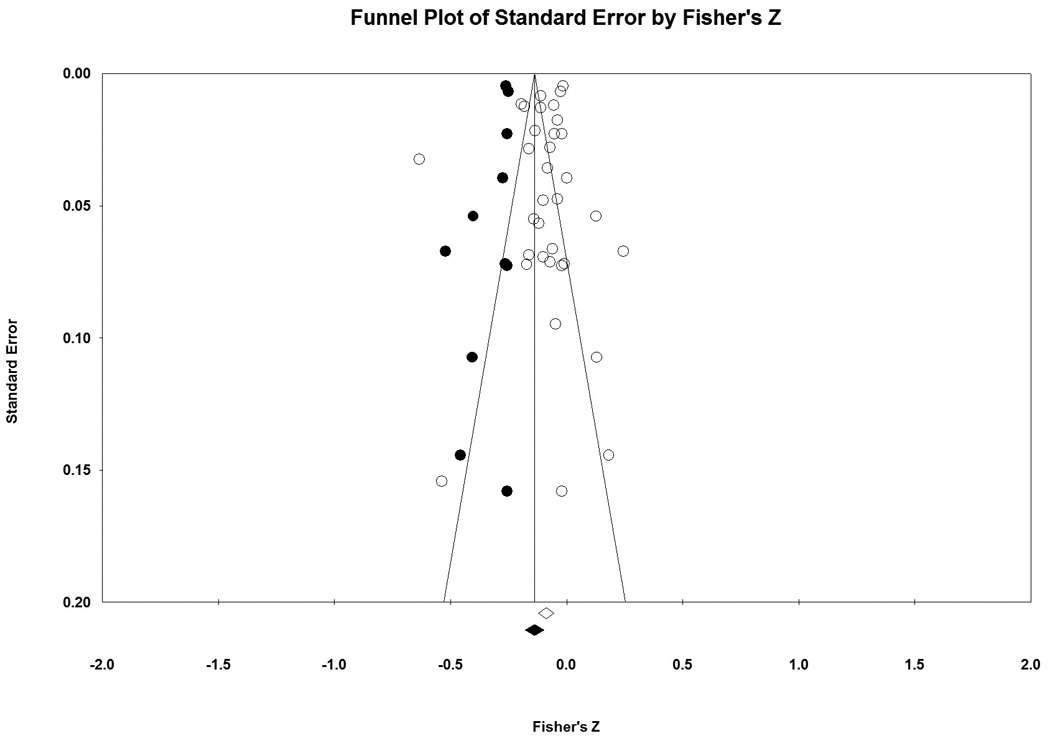
Fisher’s Z, a measure of effect size, is plotted on the x-axis and the standard error is plotted on the y-axis. Larger studies appear toward the top of the graph and smaller studies towards the bottom. In the absence of publication bias, the studies are symmetrically distributed around the mean. Actual studies are shown in open circles and imputed studies would be shown in black circles. No additional studies would be expected in the opposite direction (to the right) of the observed effect, but an additional 11 studies are imputed to the left of the mean effect size. While the calculated effect size is −0.088 (95% CI: −0.121, −0.054), using Duval and Tweedie’s Trim and Fill Method, the imputed effect size estimate is larger at −0.137 (95% CI: −0.176, −0.099; imputed studies n=11).
Discussion
The present systematic review and meta-analysis of 29 longitudinal publications, including 121,749 participants, revealed that after accounting for baseline depression symptoms, variability in cognitive function did not predict subsequent depression. Consistent with prior reports, there was a significant contemporaneous association between higher depression symptoms and lower cognitive function. These patterns have implications for understanding the association between cognitive function and depression and may be important for advancing etiologic and treatment research.
First, our findings reinforce models positing that cognitive function tracks with depression severity (McDermott & Ebmeier 2009). One possible explanation for this contemporaneous association is that depression symptoms interfere with the capacity to complete cognitive assessments, possibly through the general lack of motivation that is one hallmark of the disorder. While this explanation represents an experimental confound for studies focused on assessment of cognitive function, it likewise represents an opportunity for studies to focus more comprehensively on the assessment of both depression and mood disorder symptoms, since performance on cognitive tests may be a sensitive measure of motivational state that could augment self-reported symptoms. Alternatively, the co-occurrence of cognitive deficits and depression symptoms may reflect a shared genetic etiology (Hagenaars et al. 2016) and dysfunction of neural circuits supporting both cognitive and emotional processes (Scult et al. in press). In particular, dysfunction of prefrontal and striatal circuits have been associated with both depression and cognitive function (Keedwell et al. 2005; Aarts et al. 2011).
Second, our findings suggest that prior studies of links between cognitive function and depression that did not assess baseline symptoms may have overestimated the potential protective role of higher cognitive function. While the importance of accounting for subclinical symptoms is not novel, the implementation of such accounting is often neglected in psychiatric epidemiology, given the current categorical, threshold-based diagnostic system. In our meta-analysis, larger effects were observed for continuous as compared to categorical outcomes, which aligns with growing emphasis on dimensional measures of psychopathology (Cuthbert & Kozak 2013).
Third, the associations between cognitive function and either current or later depression were not moderated by data quality or participant age, sex, and race. However, there are a number of additional potential moderators that could not be addressed in the current review and meta-analysis. For example, the number and type of comorbid conditions with depression were often not reported in the included studies, but could represent an important moderator of the observed effects. Modeling the effects of comorbid illness is an important avenue for future research.
One limitation of the current literature is that only a handful of studies reviewed explicitly excluded for depression diagnosis at baseline (Gale et al. 2008; Vinberg et al. 2013; Papmeyer et al. 2015); however, consistent with the finding that controlling for baseline depression symptoms led to a null association between cognition and subsequent depression, there was no effect observed when including these studies in the subgroup analyses, further suggesting that the association between cognitive function and depression is likely contemporaneous. It will be important to follow-up these analyses with individual studies that include rigorous assessment of baseline depression symptoms and other potentially confounding factors to further clarify this relationship.
An open question that remains for future research is whether deficits in cognitive function are likely to predict the development of more sever or recurrent depression later in life. Only one of the included studies (Koenen et al. 2009), specifically investigated recurrent depression and found that lower IQ was associated with greater persistence of disorder.
A final consideration is that while our review and meta-analysis focused on general cognitive function mainly assessed via IQ, there may be specific subtypes of cognitive function that are more predictive of later depression. For example, a lack of cognitive flexibility may more closely match with the ruminative style characteristic of depression, and therefore tasks specifically measuring this component of cognitive function might have some predictive utility. While our review did not find differences between broad and specific measures of cognitive function, there were not enough studies identified to allow for precise consideration of differences between individual cognitive domains.
A limitation of the meta-analysis is that while the present study followed standard guidelines (Stroup et al. 2000; Atkinson et al. 2014), decisions about study selection, data extraction, and quality assessment, include a number of decision points that necessarily involve a level of subjectivity. Reliability was ascertained by using independent raters, however, different inclusion/exclusion criteria or a different approach to the evaluation of those criteria, could result in a different set of studies being included. Ultimately, the quality of the meta-analysis is dependent upon the underlying studies that are analyzed. Furthermore, although studies with insufficient information to be included in the meta-analysis were not found to differ based on basic study data from those studies included in the meta-analysis, nonetheless it is unknown how if at all data from these studies could change the overall results.
Acknowledging these limitations, our present review and meta-analysis found that while an association is evident between cognitive function and later depression, general cognitive function does not appear to be a risk factor for depression, but rather is more likely related to performance decrements associated with concurrent depressive symptoms. Our results suggest that low cognitive function is therefore probably not a causal factor of depression and that clinical practice may benefit more from a focus on how decreased cognitive function in the depressed state is likely to influence treatment outcomes (Gyurak et al. 2015). Our findings have important implications for better understanding depression and for the design of future studies in highlighting the need to control for subthreshold depression symptoms when investigating risk factors for mental illness as well as when assessing cognitive function more generally.
Supplementary Material
Acknowledgments
Financial Support
M.A.S. is supported by an NSF Graduate Research Fellowship. A.R.H. is supported by NIH grants RO1DA033369 & R01AG049789. T.E.M. is supported by R01AG049789.
We would like to thank Harris Cooper PhD at Duke University, who provided invaluable insight into conducting the meta-analysis. We thank all of the authors of studies who provided additional information for this meta-analysis.
Footnotes
Conflicts of Interest
None
References
- Aarts E, van Holstein M, Cools R. Striatal Dopamine and the Interface between Motivation and Cognition. Frontiers in psychology. 2011;2:163. doi: 10.3389/fpsyg.2011.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson KM, Koenka AC, Sanchez CE, Moshontz H, Cooper H. Reporting standards for literature searches and report inclusion criteria: making research syntheses more transparent and easy to replicate. Research Synthesis Methods. 2014 doi: 10.1002/jrsm.1127. [DOI] [PubMed] [Google Scholar]
- Baer LH, Tabri N, Blair M, Bye D, Li KZH, Pushkar D. Longitudinal associations of need for cognition, cognitive activity, and depressive symptomatology with cognitive function in recent retirees. The journals of gerontology. Series B, Psychological sciences and social sciences. 2013;68:655–664. doi: 10.1093/geronb/gbs112. [DOI] [PubMed] [Google Scholar]
- Barbey AK, Colom R, Solomon J, Krueger F, Forbes C, Grafman J. An integrative architecture for general intelligence and executive function revealed by lesion mapping. Brain : a journal of neurology. 2012;135:1154–1164. doi: 10.1093/brain/aws021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett JH, Salmond CH, Jones PB, Sahakian BJ. Cognitive reserve in neuropsychiatry. Psychological medicine. 2006;36:1053–1064. doi: 10.1017/S0033291706007501. [DOI] [PubMed] [Google Scholar]
- Beaujean AA, Parker S, Qiu X. The relationship between cognitive ability and depression: a longitudinal data analysis. Social psychiatry and psychiatric epidemiology. 2013;48:1983–1992. doi: 10.1007/s00127-013-0668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Caspi A, Arseneault L, Bleidorn W, Fonagy P, Goodman M, Houts R, Moffitt TE. Etiological features of borderline personality related characteristics in a birth cohort of 12-year-old children. Development and psychopathology. 2012;24:251–265. doi: 10.1017/S0954579411000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts KS, Williams GM, Najman JM, Alati R. Journal of Psychiatric Research. Vol. 72. Elsevier Ltd; 2016. Predicting spectrums of adult mania, psychosis and depression by prospectively ascertained childhood neurodevelopment; pp. 22–29. [DOI] [PubMed] [Google Scholar]
- Bora E, Harrison BJ, Yücel M, Pantelis C. Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychological medicine. 2013;43:2017–2026. doi: 10.1017/S0033291712002085. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. John Wiley & Sons; 2011. [Google Scholar]
- Canals J, Domènech-Llaberia E, Fernández-Ballart J, Martí-Henneberg C. Predictors of depression at eighteen. A 7-year follow-up study in a Spanish nonclinical population. European child & adolescent psychiatry. 2002;11:226–233. doi: 10.1007/s00787-002-0286-y. [DOI] [PubMed] [Google Scholar]
- Christensen H, Griffiths K, Mackinnon A. A quantitative review of cognitive deficits in depression and Alzheimer-type dementia Selection of Studies. 1997:631–651. [PubMed] [Google Scholar]
- Connolly SL, Wagner Ca, Shapero BG, Pendergast LL, Abramson LY, Alloy LB. Journal of behavior therapy and experimental psychiatry. Vol. 45. Elsevier Ltd; 2014. Rumination prospectively predicts executive functioning impairments in adolescents; pp. 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper HM. Statistically combining independent studies: A meta-analysis of sex differences in conformity research. Journal of Personality and Social Psychology. 1979;37:131–146. [Google Scholar]
- Cooper HM. Research Synthesis and Meta-Analysis: A Step-by-Step Approach. 4th. Washington (DC): U.S: SAGE Publications; 2010. [Google Scholar]
- Cuijpers P, de Graaf R, van Dorsselaer S. Minor depression: risk profiles, functional disability, health care use and risk of developing major depression. Journal of affective disorders. 2004;79:71–79. doi: 10.1016/S0165-0327(02)00348-8. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Kozak MJ. Constructing constructs for psychopathology: the NIMH research domain criteria. Journal of abnormal psychology. 2013;122:928–937. doi: 10.1037/a0034028. [DOI] [PubMed] [Google Scholar]
- Der G, Batty GD, Deary IJ. Intelligence. Vol. 37. Elsevier Inc; 2009. The association between IQ in adolescence and a range of health outcomes at 40 in the 1979 US National Longitudinal Study of Youth; pp. 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubow EF, Boxer P, Huesmann LR. Childhood and adolescent predictors of early and middle adulthood alcohol use and problem drinking: the Columbia County Longitudinal Study. Addiction. 2008;103:36–47. doi: 10.1111/j.1360-0443.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. Trim and Fill: A Simple Funnel-Plot-Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Evans LD, Kouros CD, Samanez-Larkin S, Garber J. Concurrent and Short-Term Prospective Relations Among Neurocognitive Functioning, Coping, and Depressive Symptoms in Youth. Journal of clinical child and adolescent psychology : the official journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division. 2015;53:1–15. doi: 10.1080/15374416.2014.982282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz CE, Lyons MJ, O’Brien R, Panizzon MS, Kim K, Bhat R, Grant MD, Toomey R, Eisen S, Xian H, Kremen WS. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. Vol. 19. American Association for Geriatric Psychiatry; 2011. A 35-year longitudinal assessment of cognition and midlife depression symptoms: the Vietnam Era Twin Study of Aging; pp. 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale CR, Batty GD, Tynelius P, Deary IJ, Rasmussen F. Intelligence in Early Adulthood and Subsequent Hospitalization for Mental Disorders. Epidemiology. 2010;21:70–77. doi: 10.1097/EDE.0b013e3181c17da8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale CR, Deary IJ, Boyle SH, Barefoot J, Mortensen LH, Batty GD. Cognitive ability in early adulthood and risk of 5 specific psychiatric disorders in middle age: the Vietnam experience study. Archives of general psychiatry. 2008;65:1410–1418. doi: 10.1001/archpsyc.65.12.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale CR, Hatch SL, Batty GD, Deary IJ. Intelligence. Vol. 37. Elsevier Inc; 2009. Intelligence in childhood and risk of psychological distress in adulthood: The 1958 National Child Development Survey and the 1970 British Cohort Study. pp. 592–599. [Google Scholar]
- Gjerde PF. Alternative Pathways to Chronic Depressive Symptoms in Young Adults: Gender Differences in Developmental Trajectories. Child Development. 1995;66:1277–1300. [PubMed] [Google Scholar]
- Gottfredson LS. Mainstream science on intelligence: An editorial with 52 signatories, history, and bibliography. Intelligence. 1997;24:13–23. [Google Scholar]
- Guyer AE, Choate VR, Grimm KJ, Pine DS, Keenan K. Journal of the American Academy of Child and Adolescent Psychiatry. Vol. 50. Elsevier Inc; 2011. Emerging depression is associated with face memory deficits in adolescent girls; pp. 180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurak A, Patenaude B, Korgaonkar MS, Grieve SM, Williams LM, Etkin A. Biological psychiatry. Vol. 79. Elsevier; 2015. Frontoparietal Activation During Response Inhibition Predicts Remission to Antidepressants in Patients with Major Depression; pp. 1–8. [DOI] [PubMed] [Google Scholar]
- Hagenaars SP, Harris SE, Davies G, Hill WD, Liewald DCM, Ritchie SJ, Marioni RE, Fawns-Ritchie C, Cullen B, Malik R, METASTROKE Consortium IC for BPG, SpiroMeta Consortium, CHARGE Consortium Pulmonary Group CCA and LG. Worrall BB, Sudlow CLM, Wardlaw JM, Gallacher J, Pell J, McIntosh AM, Smith DJ, Gale CR, Deary IJ. Molecular psychiatry. Nature Publishing Group; 2016. Shared genetic aetiology between cognitive functions and physical and mental health in UK Biobank (N=112 151) and 24 GWAS consortia; p. 031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch SL, Jones PB, Kuh D, Hardy R, Wadsworth MEJ, Richards M. Social science & medicine (1982) Vol. 64. Elsevier Ltd; 2007. Childhood cognitive ability and adult mental health in the British 1946 birth cohort; pp. 2285–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipwell AE, Sapotichne B, Klostermann S, Battista D, Keenan K. Autobiographical memory as a predictor of depression vulnerability in girls. Journal of clinical child and adolescent psychology : the official journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53. 2011;40:254–265. doi: 10.1080/15374416.2011.546037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz JL, Garber J. Journal of the American Academy of Child and Adolescent Psychiatry. Vol. 42. The American Academy of Child and Adolescent Psychiatry; 2003. Relation of intelligence and religiosity to depressive disorders in offspring of depressed and nondepressed mothers; pp. 578–586. [DOI] [PubMed] [Google Scholar]
- Johnson W, Bouchard TJ, Krueger RF, McGue M, Gottesman II. Just one g consistent results from three test batteries. Intelligence. 2004;32:95–107. [Google Scholar]
- Keedwell PA, Andrew C, Williams SCR, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biological Psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Moffitt TE, Roberts AL, Martin LT, Kubzansky L, Harrington H, Poulton R, Caspi A. Childhood IQ and adult mental disorders: a test of the cognitive reserve hypothesis. The American journal of psychiatry. 2009;166:50–57. doi: 10.1176/appi.ajp.2008.08030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Solomon A, Seeley JR, Zeiss A. Clinical implications of ‘subthreshold’ depressive symptoms. Journal of Abnormal Psychology. 2000;109:345–351. [PubMed] [Google Scholar]
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BWJH, Zitman FG. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Archives of general psychiatry. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- Mccord J, Ensminger ME. Multiple risks and comorbidity in an African-American population. Criminal Behaviour and Mental Health. 1997;7:339–352. [Google Scholar]
- McDermott LM, Ebmeier KP. Journal of Affective Disorders. Vol. 119. Elsevier B.V; 2009. A meta-analysis of depression severity and cognitive function; pp. 1–8. [DOI] [PubMed] [Google Scholar]
- Melartin TK, Rytsälä HJ, Leskelä US, Lestelä-Mielonen PS, Sokero TP, Isometsä ET. Current comorbidity of psychiatric disorders among DSM-IV major depressive disorder patients in psychiatric care in the Vantaa Depression Study. The Journal of clinical psychiatry. 2002;63:126–134. [PubMed] [Google Scholar]
- Meyer SE, Carlson Ga, Wiggs Ea, Martinez PE, Ronsaville DS, Klimes–Dougan B, Gold PW, Radke–Yarrow M. A prospective study of the association among impaired executive functioning, childhood attentional problems, and the development of bipolar disorder. Development and Psychopathology. 2004;16:461–476. doi: 10.1017/s095457940404461x. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki aH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex ‘Frontal Lobe’ tasks: a latent variable analysis. Cognitive psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Annals of internal Medicine. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Papmeyer M, Sussmann JE, Hall J, McKirdy J, Peel a, Macdonald a, Lawrie SM, Whalley HC, McIntosh aM. Neurocognition in individuals at high familial risk of mood disorders with or without subsequent onset of depression. Psychological Medicine. 2015:1–11. doi: 10.1017/S0033291715001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RA, Brown SP. On the Use of Beta Coefficients in Meta-Analysis. Journal of Applied Psychology. 2005;90:175–181. doi: 10.1037/0021-9010.90.1.175. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Brook J, Coplan JD. Psychiatric symptoms in adolescence as predictors of obesity in early adulthood: a longitudinal study. American Journal of Public Health. 1997;87:1303–1310. doi: 10.2105/ajph.87.8.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn ME, Joormann J. Control When It Counts: Change in Executive Control Under Stress Predicts Depression Symptoms. Emotion. 2015;Junio 22:1–10. doi: 10.1037/emo0000089. [DOI] [PubMed] [Google Scholar]
- Rawal A, Rice F. Journal of the American Academy of Child and Adolescent Psychiatry. Vol. 51. Elsevier Inc; 2012. Examining overgeneral autobiographical memory as a risk factor for adolescent depression; pp. 518–527. [DOI] [PubMed] [Google Scholar]
- Ree MJ, Earles JA. The stability of g across different methods of estimation. Intelligence. 1991;15:271–278. [Google Scholar]
- Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. Psychological Medicine. 2014;44:2029–2040. doi: 10.1017/S0033291713002535. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Kasai K, Koji M, Fukuda R, Iwanami A, Nakagome K, Fukuda M, Kato N. Executive and prefrontal dysfunction in unipolar depression: a review of neuropsychological and imaging evidence. Neuroscience research. 2004;50:1–11. doi: 10.1016/j.neures.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Scult MA, Knodt AR, Swartz JR, Brigidi BD, Hariri AR. Thinking and Feeling: Individual Differences in Habitual Emotion Regulation and Stress-Related Mood Are Associated With Prefrontal Executive Control. Clinical Psychological Science. doi: 10.1177/2167702616654688. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp C, Petersen N, Goodyer I. Journal of the American Academy of Child and Adolescent Psychiatry. Vol. 47. The American Academy of Child and Adolescent Psychiatry; 2008. Emotional reactivity and the emergence of conduct problems and emotional symptoms in 7- to 11-year-olds: a 1-year follow-up study; pp. 565–573. [DOI] [PubMed] [Google Scholar]
- Siegel LS. Journal of Learning Disabilities. Vol. 32. Boston University; 1999. Issues in the Definition and Diagnosis of Learning Disabilities: A Perspective on Guckenberger v; pp. 304–319. [DOI] [PubMed] [Google Scholar]
- Simons CJP, Jacobs N, Derom C, Thiery E, Jolles J, van Os J, Krabbendam L. Cognition as predictor of current and follow-up depressive symptoms in the general population. Acta psychiatrica Scandinavica. 2009;120:45–52. doi: 10.1111/j.1600-0447.2008.01339.x. [DOI] [PubMed] [Google Scholar]
- Slykerman RF, Thompson J, Waldie K, Murphy R, Wall C, Mitchell EA. Maternal stress during pregnancy is associated with moderate to severe depression in 11-year-old children. Acta Paediatrica. 2015;104:68–74. doi: 10.1111/apa.12787. [DOI] [PubMed] [Google Scholar]
- Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin. 2013;139:81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen HJ, Sæbye D, Urfer-Parnas A, Mortensen EL, Parnas J. Journal of affective disorders. Vol. 136. Elsevier B.V; 2012. Premorbid intelligence and educational level in bipolar and unipolar disorders: a Danish draft board study; pp. 1188–1191. [DOI] [PubMed] [Google Scholar]
- Spearman C. The American Journal of Psychology. Vol. 15. JSTOR; 1904. ‘General Intelligence,’ Objectively Determined and Measured; p. 201. [Google Scholar]
- Stern Y. The Concept of Cognitive Reserve: A Catalyst for Research. Journal of Clinical and Experimental Neuropsychology (Neuropsychology, Development and Cognition: Section A) 2003;25:589–593. doi: 10.1076/jcen.25.5.589.14571. [DOI] [PubMed] [Google Scholar]
- Stroup D, Berlin J, Morton S, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of Observational Studies in Epidemiology: A Proposal for Reporting. JAMA. 2000;283:2008. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Vinberg M, Miskowiak KW, Kessing LV. Impairment of executive function and attention predicts onset of affective disorder in healthy high-risk twins. The Journal of clinical psychiatry. 2013;74:e747–e753. doi: 10.4088/JCP.12m08258. [DOI] [PubMed] [Google Scholar]
- Zammit S, Allebeck P, David AS, Dalman C, Hemmingsson T, Lundberg I, Lewis G. A longitudinal study of premorbid IQ Score and risk of developing schizophrenia, bipolar disorder, severe depression, and other nonaffective psychoses. Archives of general psychiatry. 2004;61:354–360. doi: 10.1001/archpsyc.61.4.354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.