Abstract
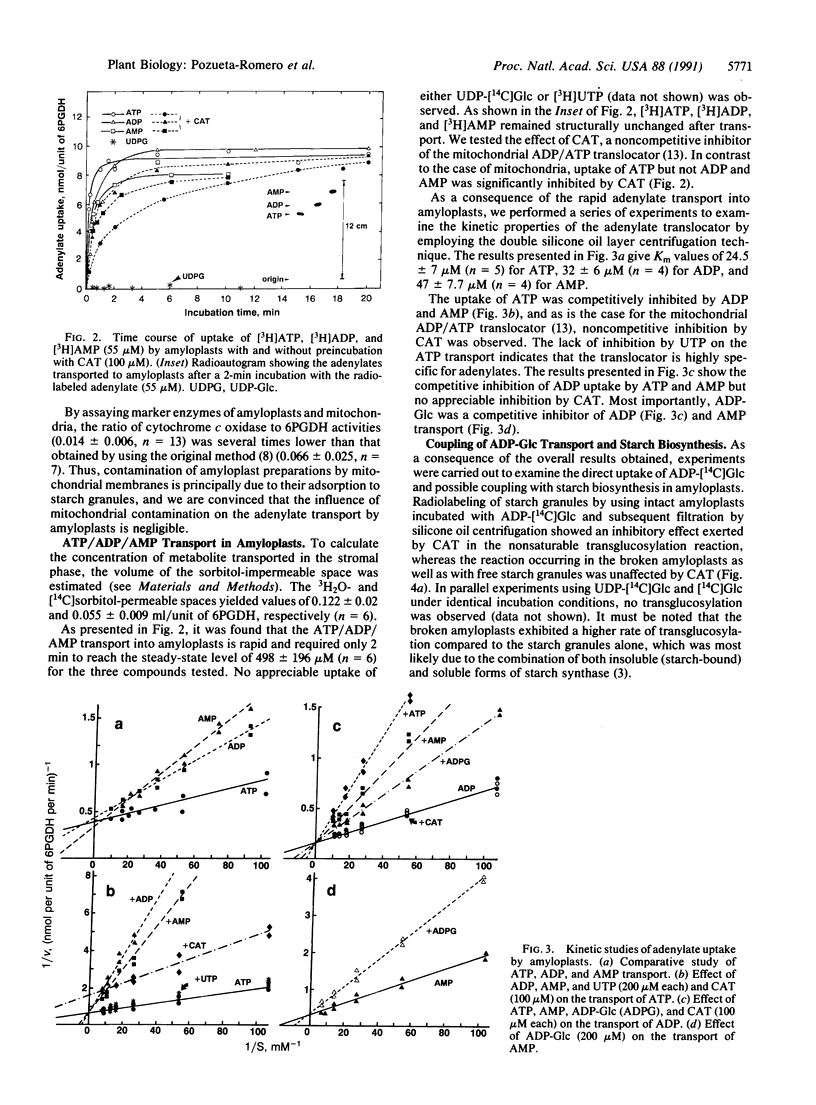
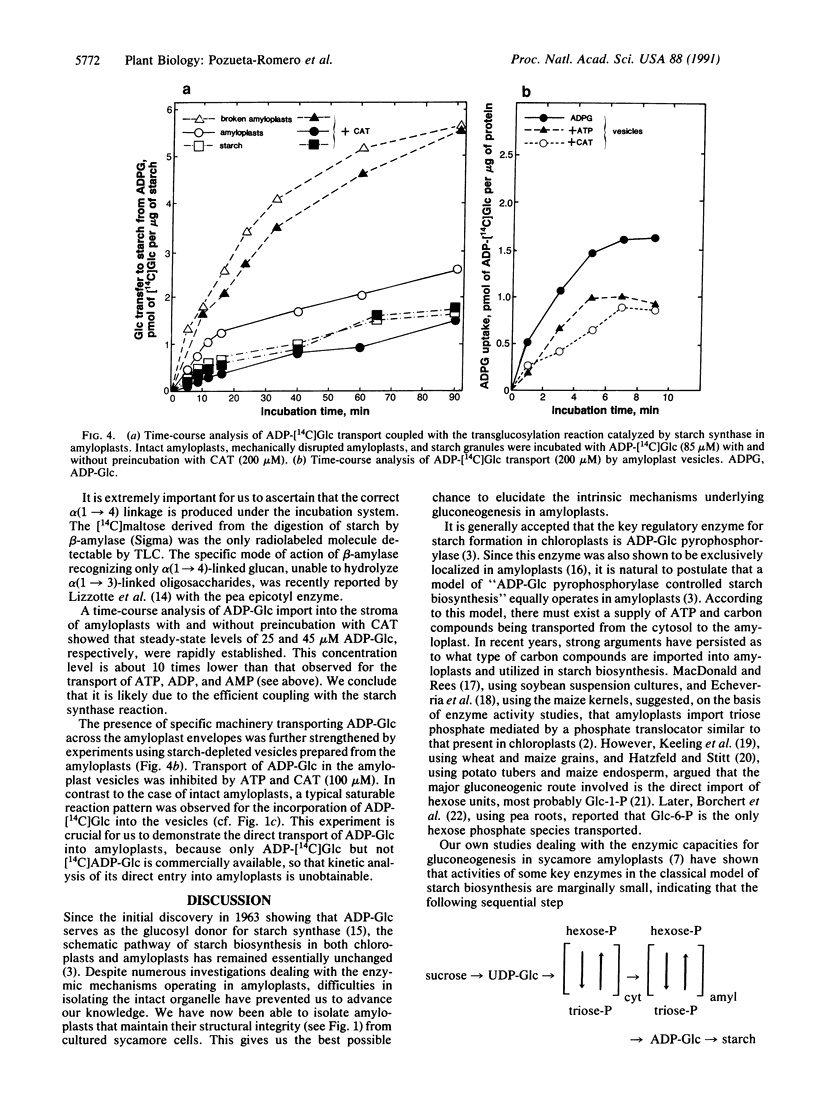
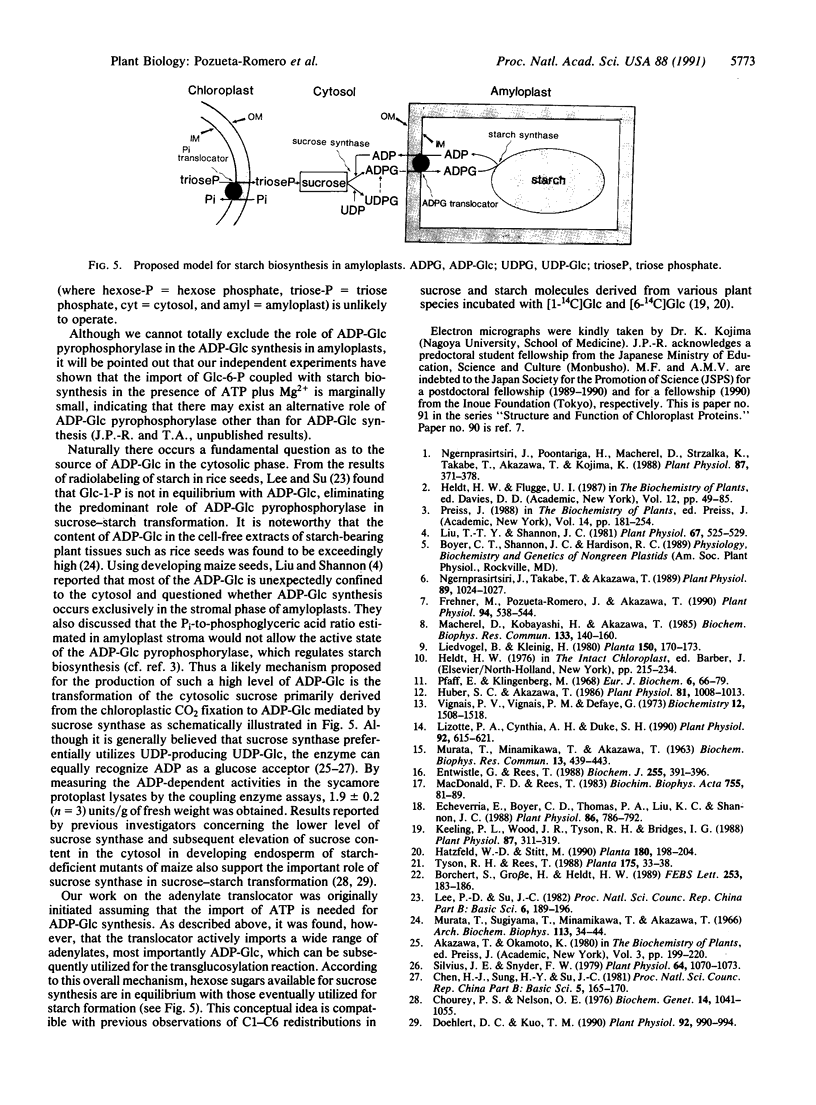
Starch biosynthesis has been studied by using amyloplasts isolated from cultured cells of sycamore trees (Acer pseudoplatanus L.). Highly purified intact amyloplasts, free from mitochondria and starch granules derived from broken amyloplasts, were isolated from a Percoll step gradient. Subsequently, the double silicone oil layer centrifugation technique was used to study adenylate transport in the amyloplasts. An adenylate-specific carrier was found to be active in the uptake of ATP, ADP, AMP, and most importantly, ADPglucose (ADP-Glc). Kinetic analyses showed that the uptake of these adenylates was mutually competitive with each other. In contrast to the mitochondrial adenylate carrier, in amyloplasts only ATP and ADP-Glc uptake were inhibited by carboxyatractyloside. Evidence is presented that the ADP-Glc transported into the amyloplast stroma can be used in starch synthesis catalyzed by starch synthase (ADP-Glc:1,4-alpha-D-glucan 4-alpha-D-glucosyltransferase, EC 2.4.1.21). We propose that starch biosynthesis in amyloplasts is tightly coupled with the direct transport of ADP-Glc synthesized in the cytosol by sucrose synthase (ADP-Glc:D-fructose 2-alpha-D-glucosyltransferase, EC 2.4.1.13)
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chourey P. S., Nelson O. E. The enzymatic deficiency conditioned by the shrunken-1 mutations in maize. Biochem Genet. 1976 Dec;14(11-12):1041–1055. doi: 10.1007/BF00485135. [DOI] [PubMed] [Google Scholar]
- Doehlert D. C., Kuo T. M. Sugar metabolism in developing kernels of starch-deficient endosperm mutants of maize. Plant Physiol. 1990 Apr;92(4):990–994. doi: 10.1104/pp.92.4.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria E., Boyer C. D., Thomas P. A., Liu K. C., Shannon J. C. Enzyme activities associated with maize kernel amyloplasts. Plant Physiol. 1988 Mar;86(3):786–792. doi: 10.1104/pp.86.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entwistle G., Rees T. A. Enzymic capacities of amyloplasts from wheat (Triticum aestivum) endosperm. Biochem J. 1988 Oct 15;255(2):391–396. doi: 10.1042/bj2550391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frehner M., Pozueta-Romero J., Akazawa T. Enzyme Sets of Glycolysis, Gluconeogenesis, and Oxidative Pentose Phosphate Pathway Are Not Complete in Nongreen Highly Purified Amyloplasts of Sycamore (Acer pseudoplatanus L.) Cell Suspension Cultures. Plant Physiol. 1990 Oct;94(2):538–544. doi: 10.1104/pp.94.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Akazawa T. A novel sucrose synthase pathway for sucrose degradation in cultured sycamore cells. Plant Physiol. 1986 Aug;81(4):1008–1013. doi: 10.1104/pp.81.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling P. L., Wood J. R., Tyson R. H., Bridges I. G. Starch Biosynthesis in Developing Wheat Grain : Evidence against the Direct Involvement of Triose Phosphates in the Metabolic Pathway. Plant Physiol. 1988 Jun;87(2):311–319. doi: 10.1104/pp.87.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T. T., Shannon J. C. Measurement of Metabolites Associated with Nonaqueously Isolated Starch Granules from Immature Zea mays L. Endosperm. Plant Physiol. 1981 Mar;67(3):525–529. doi: 10.1104/pp.67.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizotte P. A., Henson C. A., Duke S. H. Purification and Characterization of Pea Epicotyl beta-Amylase. Plant Physiol. 1990 Mar;92(3):615–621. doi: 10.1104/pp.92.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macherel D., Kobayashi H., Akazawa T., Kawano S., Kuroiwa T. Amyloplast nucleoids in sycamore cells and presence in amyloplast DNA of homologous sequences to chloroplast genes. Biochem Biophys Res Commun. 1985 Nov 27;133(1):140–146. doi: 10.1016/0006-291x(85)91852-2. [DOI] [PubMed] [Google Scholar]
- Murata T., Sugiyama T., Minamikawa T., Akazawa T. Enzymic mechanism of starch synthesis in ripening rice grains. 3. Mechanism of the sucrose-starch conversion. Arch Biochem Biophys. 1966 Jan;113(1):34–44. doi: 10.1016/0003-9861(66)90153-6. [DOI] [PubMed] [Google Scholar]
- Ngernprasirtsiri J., Harinasut P., Macherel D., Strzalka K., Takabe T., Akazawa T., Kojima K. Isolation and Characterization of the Amyloplast Envelope-Membrane from Cultured White-Wild Cells of Sycamore (Acer pseudoplatanus L.). Plant Physiol. 1988 Jun;87(2):371–378. doi: 10.1104/pp.87.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngernprasirtsiri J., Takabe T., Akazawa T. Immunochemical Analysis Shows That an ATP/ADP-Translocator Is Associated with the Inner-Envelope Membranes of Amyloplasts from Acer pseudoplatanus L. Plant Physiol. 1989 Apr;89(4):1024–1027. doi: 10.1104/pp.89.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff E., Klingenberg M. Adenine nucleotide translocation of mitochondria. 1. Specificity and control. Eur J Biochem. 1968 Oct 17;6(1):66–79. doi: 10.1111/j.1432-1033.1968.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Silvius J. E., Snyder F. W. Comparative Enzymic Studies of Sucrose Metabolism in the Taproots and Fibrous Roots of Beta vulgaris L. Plant Physiol. 1979 Dec;64(6):1070–1073. doi: 10.1104/pp.64.6.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignais P. V., Vignais P. M., Defaye G. Adenosine diphosphate translocation in mitochondria. Nature of the receptor site for carboxyatractyloside (gummiferin). Biochemistry. 1973 Apr 10;12(8):1508–1519. doi: 10.1021/bi00732a007. [DOI] [PubMed] [Google Scholar]






