Abstract
Introduction
Atrial refractoriness may be an important determinant of atrial fibrillation (AF) risk, but its measurement is not clinically accessible. Because the QT interval predicts incident AF and the atrium and ventricle share repolarizing ion currents, we investigated the association between an individual’s QT interval and atrial effective refractory period (AERP).
Methods
In paroxysmal AF patients presenting for catheter ablation, the QT interval was measured from the surface 12-lead ECG. The AERP was defined as the longest S1-S2 coupling interval without atrial capture using a 600 ms drive cycle length.
Results
In 28 patients, there was a positive correlation between QTC and mean AERP. After multivariate adjustment, a 1 ms increase in QTC predicted a 0.70 ms increase in AERP.
Conclusions
The QTc interval reflects the AERP, suggesting that the QTc interval may be used as a marker of atrial refractoriness relevant to assessing AF risk and mechanism-specific therapeutic strategies.
Keywords: atrial fibrillation, refractory period, QT interval
Introduction
Atrial fibrillation (AF) is a heterogeneous disease. AF results from multiple pathophysiological mechanisms that remain incompletely understood.1, 2 One proposed mechanism involves re-entry determined by the atrial effective refractory period (AERP) and the conduction velocity.3 Shortened refractory periods are thought to be an important component of the multiple wavelet hypothesis for AF,4 with evidence that they contribute to an increased atrial vulnerability for sustained AF.5 A prolonged AERP has also been shown to independently predict the development of AF.6 Despite the possible importance in AF initiation and maintenance, measurement of the atrial refractory period is not readily available in clinical practice.
The QT interval is a non-invasive and readily available electrocardiographic measurement. Both shortened and prolonged QT intervals have been shown to predict incident AF,7, 8 but the exact mechanisms responsible remain unknown. Although the QT interval represents ventricular repolarization, it is possible that these relationships with AF are explained by the fact that, due to individual-level propensities to particular ion channel characteristics spanning the atria and the ventricles,9 the QT interval might be a marker of an individual’s atrial repolarization properties. If true, a readily available measurement might not only unlock an individual’s AF risk, but also provide information regarding the mechanism by which AF might ultimately occur (such as whether due to a shortened refractory period). In addition to a better mechanistic understanding of the relationship between the QT interval and AF, identification of a clinically accessible marker of atrial refractoriness may help to inform therapeutic strategies based on the underlying AF mechanistic subtype and thus improve treatment efficacy. We therefore sought to test the hypothesis that the QT interval is a noninvasive marker of the AERP.
Methods
Study design
This was a sub-study of an ongoing, investigator-initiated, NIH/ NIAAA-supported controlled clinical trial investigating the mechanistic relationship between ethanol and AF (the HOLIDAY [HOw ALcohol InDuces Atrial TachYarrhythmias] Study; ClinicalTrials.gov Identifier: NCT01996943), which is reflected in the inclusion and exclusion criteria.
Consecutive consenting paroxysmal AF patients ages 21-80 undergoing left atrial catheter ablation at the University of California, San Francisco (UCSF) were enrolled between January 2013 and October 2015. All antiarrhythmic medications were held on the morning of the procedure, and dofetilide was held for at least five half-lives. Antiarrhythmic medications were ascertained from the medical record and use was defined as a self-reported dose within 30 days of the procedure. Patients presenting in a rhythm other than sinus, with a depressed left ventricular ejection fraction (<50%), who had received any amiodarone within the past 3 months, or with any history of alcoholism or absolute alcohol abstinence were excluded. Note that the pacing protocol and QT measurements for the current study were all performed prior to implementing the randomization assignment for the parent study, resulting in uniform treatment relevant to the baseline AERP versus QT measurements.
All patients were under general anesthesia, and a strict anesthesia protocol was adhered to in order to avoid drugs that might directly influence electrophysiology properties. Specifically, the following drugs were avoided until after the pacing protocol: fentanyl, rocuronium, succinylcholine, ketamine, scopolamine, and inhaled anesthetics with the exception of nitrous oxide. Allowable agents at induction included propofol bolus, cisatracurium for paralysis, remifentanil for analgesia, and nitrous oxide for amnesia; maintenance pre-transseptal drugs allowed were propofol infusion at 75 mcg/kg/min to 125 mcg/kg/min and remifentanil at 0.1 mcg/kg/min to 0.5 mcg/kg/min. Following the transseptal puncture and during the QT measurement and pacing protocol stage, only propofol was allowed (given sufficient time to assure the absence of any effects due to remifentanil or nitrous oxide).
This study was approved by the UCSF Committee on Human Research and all participants provided informed written consent.
Electrocardiogram (ECG) measurements
The 12-lead surface ECGs were recorded during the electrophysiology study using a computer-based recording system (CardioLab, GE Healthcare, USA). The ECG intervals for all 12 leads were measured from the baseline surface ECG stored in Cardiolab using digital calipers at a paper speed of 50 mm/sec. A sinus beat occurring at the end of the largest number of consecutive beats (with a goal of at least five consecutive sinus beats without any premature atrial or ventricular contractions) was selected for these measurements. The QRS and QT interval were measured both immediately prior to and during atrial pacing from the coronary sinus at a cycle length of 600 ms by a single reader blinded to the associated AERP measurements and the lead with the longest QT interval duration was used. The primary reader repeated all QT measurements in lead II in a blinded fashion, revealing intra-observer agreement of 86%. A second blinded investigator also read all QT measurements in lead II to assess for inter-observer variability, demonstrating 97% agreement. The QT interval was measured from the beginning of the QRS complex to the end of the T wave. If there was a superimposed U wave, the downslope of the T wave was extended and the intersection of this line with the isoelectric baseline was considered to be the end of the QT interval per current consensus guidelines.10 The lead with the longest QT interval duration prior to atrial pacing was used as the primary predictor and corrected by the Framingham formula to be consistent with both consensus guidelines and previous literature demonstrating that the QT interval predicts incident AF.7, 10 The R-R interval of five consecutive sinus beats were measured and the mean of these measurements was used for correction of the QT interval. When five consecutive sinus beats were not available, a minimum of three consecutive beats were used for the calculation.
AERP measurement
The AERP was measured immediately after placement of intra-cardiac catheters and transseptal access and before any ablation or administration of drugs such as isoproterenol. The AERP was defined as the longest S1-S2 coupling interval without atrial capture using a 600 ms drive cycle length and a 2 ms pulse width. The AERP was measured at the proximal bipole of a decapolar catheter at the coronary sinus os, the distal bipole of a decapolar catheter in the coronary sinus (the most lateral aspect of the coronary sinus), the distal bipole of a decapolar or quadripolar catheter in the posterior high right atrium, the distal bipole of a 3.5 mm externally irrigated catheter in the right upper pulmonary vein, and the bipole with the sharpest local signal from a circular duodecapolar catheter in the left upper pulmonary vein. If a pulmonary vein was electrically isolated from a previous catheter ablation, pacing was performed as close as possible to the vein where capture could be achieved. Measurements were performed using a “step-up” method (first capture, to avoid electrical conditioning with the step-down approach) at twice pacing threshold beginning with an atrial extrastimuli of 140 ms and incremental increases of 10 ms; once capture was achieved, an increment down by 5 ms was employed to assure precision within 5 ms.
Covariate ascertainment
Demographics and medical history were determined through patient self-report and verified by chart review.
Statistical analysis
Normally distributed continuous variables are presented as means ± standard deviation and non-normally distributed continuous variables are presented as medians and interquartile ranges (IQR). The QTC prior to atrial pacing was used as the primary predictor and the QT interval during atrial pacing was used in secondary analyses. Pearson’s correlation coefficient was used to evaluate the correlation between the QTC interval and the mean AERP. The association between QT heterogeneity and AERP heterogeneity was evaluated using linear regression. Measures of heterogeneity for QT and AERP included range, standard deviation, and coefficient of variation. The coefficient of variation was calculated using (standard deviation/mean) *100. The coefficient of variation does not depend on the measurement unit and therefore is a useful comparison of two different measurements. The QT interval, both prior to and during atrial pacing, as a predictor of AERP was examined using mixed-effects linear regression, which accounts for repeated measures within individuals and was adjusted for AERP measurement site alone and in addition to age, sex, diabetes, hypertension, coronary artery disease and history of prior catheter ablation. Confounders were identified a priori based on biological plausibility. Race was not included as a covariate because the study sample was predominantly white.
Assuming a mean QTc of 440 ms and standard deviation of 20 ms, we estimated that 28 patients would provide 80% power to detect a statistically significant correlation with AERP as small as 0.5, justifying use of the selected sample.
Data analysis was completed using Stata 14 (StataCorp, College Station, TX). We considered a two-tailed P value < 0.05 statistically significant.
Results
After exclusion criteria (ventricular pacing, n=2; QRS ≥ 120 ms, n=1), data were available for 28 patients. The study population was predominantly older, white, and male (Table I). Approximately half (46%) of participants had undergone a previous ablation. Patients had discontinued antiarrhythmic medications a median of 3 days prior to the procedure (IQR 2-4 days). The mean corrected QT (QTc) was 432 ± 22 ms and the mean of the AERP from all locations was 283 ± 30 ms. Five individuals (male, n=4) met the definition for a prolonged QTC interval (≥ 450 ms in men and ≥ 460 ms in women).
Table I.
Characteristics of Study Participants
| Characteristic | Cohort n = 28 |
|---|---|
| Mean age (y) | 61 ± 10 |
| Male | 21 (75%) |
| Race | |
| White | 26 (93%) |
| Black | 1 (4%) |
| East Asian | 1 (4%) |
| Hypertension | 7 (25%) |
| Diabetes | 3 (11%) |
| Coronary artery disease | 1 (4%) |
| History of AF ablation | 13 (46%) |
| Antiarrhythmic drug use | 22 (79%) |
| Propafenone | 7 (32%) |
| Flecainide | 10 (45%) |
| Dronedarone | 1 (5%) |
| Dofetilide | 2 (9%) |
| Sotalol | 2 (9%) |
| Mean QT interval (uncorrected), ms | 451 ± 40 |
| Mean QTC, ms | 432 ± 22 |
| Mean AERP, ms | |
| Overall | 283 ± 30 |
| Proximal coronary sinus | 289 ± 36 |
| Distal coronary sinus | 289 ± 50 |
| High right atrium | 310 ± 51 |
| Left pulmonary vein | 257 ± 62 |
| Right pulmonary vein | 265 ± 62 |
AF = atrial fibrillation; AERP = atrial effective refractory period.
QTC interval and AERP
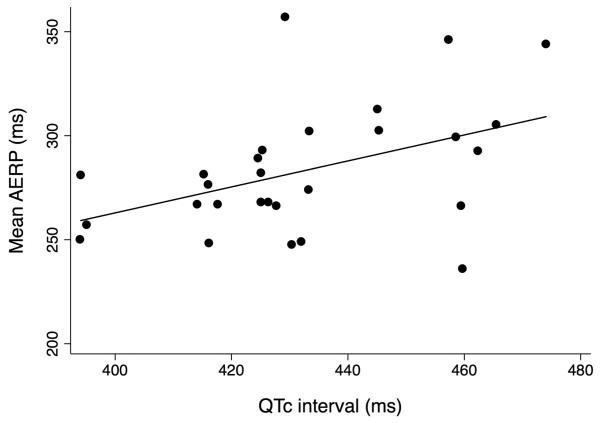
As shown in Figure 1, there was a positive correlation between the QTC interval and mean AERP (Pearson’s correlation coefficient 0.45, p=0.017). In a mixed effects regression model, each 1 ms increase in the QTC interval predicted a 0.63 ms increase in AERP after adjustment for atrial site (95% confidence interval [CI] 0.15-1.10, p=0.01). After additional adjustment for age, sex, diabetes, hypertension, coronary artery disease, and prior ablation, a 1 ms increase in the QTC interval predicted a 0.70 ms increase in AERP (95% CI 0.10-1.3, p=0.02). The relationship between the QTC interval and AERP did not vary by atrial site (interaction P value=0.92). In secondary analyses examining the association between the QT interval during atrial pacing and the AERP, similar results were found. After adjustment for atrial site, each 1 ms increase in the QT interval predicted a 0.60 ms increase in AERP (95% CI 0.31-0.88, p=0.001). After additional adjustment, each 1 ms increase in the QT interval was associated with a 0.59 ms increase in the AERP (95% CI 0.30-0.89, p=0.001).
Figure 1.
QTc interval versus atrial effective refractory period (AERP). The QT interval was measured prior to atrial pacing and corrected by the Framingham formula. Mean AERP was calculated as the mean of the AERP of all atrial sites.
Heterogeneity of QT and AERP
We did not observe any evidence of a relationship between heterogeneity of QTC and heterogeneity of AERP (Table II).
Table II.
Relationship between heterogeneity of QTC interval and AERP
| Measurement | QTC* | AERP† | P value |
|---|---|---|---|
| Range, ms | 30 (19) | 107 ± 59 | 0.53 |
| Standard deviation, ms | 8 (6) | 45 ± 23 | 0.30 |
| Coefficient of variation | 1.9 (1.2) | 16 ± 8.7 | 0.33 |
AERP = atrial effective refractory period.
QTC measurements were non-normally distributed and presented as median (interquartile range).
AERP was normally distributed and presented as mean ± SD.
Discussion
In paroxysmal AF presenting for catheter ablation, the QT interval was independently associated with the AERP both before and after adjustment for demographics, cardiovascular comorbidities, atrial site of AERP measurement, and history of previous ablation. There was no evidence of an association between measurements of QTC heterogeneity and AERP heterogeneity. These findings suggest that the electrocardiographic QTC interval may be utilized as a non-invasive measure of atrial refractoriness, a possible key determinant of AF development.1
Although AF is the most common sustained arrhythmia, there are no known prevention strategies. While there are multiple available therapies, in general, a one-size-fits-all approach is used irrespective of the distinct underlying pathophysiological processes leading to AF and treatment remains limited due to low efficacy and side effects.11, 12 There are likely to be multiple mechanistic subtypes of AF that will need to be better understood in order to design more effective and individualized treatment strategies. However, the understanding of mechanistic subtypes of AF remains in its early stages.11 Subtyping of AF based on clinical features13 and gene expression profiles14 has been proposed. However, identification of AF subtypes based on the ECG would be particularly valuable because of its clinical availability as well as relationship to underlying cardiac electrophysiology.15 Thus, the development of a non-invasive clinical tool to identify distinct mechanisms of AF is an important step in more tailored therapies for AF. In addition, as strategies targeting AF prevention become available, the ECG may serve as an important method to identify individuals who will most benefit from prevention strategies.11, 16
While multiple ECG predictors of AF have been identified, their relationship with AF pathophysiology remains to be fully understood.15 The ERP is a key determinant of reentry, a likely fundamental AF mechanism, at least in some cases.1, 3, 17 While there are multiple frameworks for understanding reentry, such as the leading circle and spiral wave theories,9, 11 as well as single re-entry and multiple re-entry circuits,1 alterations in the ERP remains a key component in each of these models. Potassium channel blockers, a widely used class of anti-arrhythmic drugs, exert their therapeutic effect by increasing the refractory period.9 Additionally, mutations of genes encoding potassium channels in familial AF likely lead to AF by shortening action potential durations and the AERP.18 Additionally, AERP has been shown to increase with age and a prolonged AERP is independently associated with increased development of AF, possibly due to age-related oxidative stress.6 However, despite the critical role of atrial refractoriness in AF initiation and maintenance, its measurement is not readily available. Currently, measurement of the AERP requires an invasive electrophysiology study, but in order to utilize the AERP in determining therapeutic strategies as well as for its potential future use in preventative strategies, a noninvasive measurement of atrial refractoriness is critical.
Our study found a positive correlation between the electrocardiographic QTC interval and the AERP. The QTC interval represents ventricular depolarization and repolarization. Shortened and prolonged QT intervals are both associated with AF,7, 8 suggesting that the QTC interval may reflect atrial in addition to ventricular electrophysiology and consistent with previous findings that both shortened and prolonged AERPs are associated with AF development. Despite differences between atrial and ventricular myocytes, there are overlapping potassium channels present in both the atria and ventricles.9 In a canine model, administration of a potassium channel blocker induced polymorphic tachyarrhythmias in the atrium as well as in the ventricle. The induced atrial polymorphic tachycardia degenerated into atrial fibrillation, suggesting that the mechanisms leading to prolonged ventricular repolarization may also be operative in the atrium and mediate the development of atrial fibrillation.19 Additionally, patients with congenital long QT syndrome (LQTS) were found to have prolonged atrial action potential durations and AERPs compared to controls with normal QT intervals. Pacing induced polymorphic atrial tachycardia episodes in patients with LQTS but not in controls, demonstrating that patients with LQTS had altered atrial electrophysiology and characteristics of an atrial “torsades de pointes.”20
The relationship between the QT interval and AERP suggests that an abnormal QT interval predicts AF because it reflects alterations in atrial refractoriness. While the correlation between the QT interval and AERP in our study was not exact, this is unsurprising given the differences in the electrophysiology of the atria and ventricles.9, 21 However, this study suggests that the QTC interval can provide some insight into the underlying atrial electrophysiology. For example, similarly to patients with congenital LQTS,20 individuals with a prolonged QT-associated AF may have a longer AERP, causing increased susceptibility to atrial polymorphic tachyarrhythmias and subsequent AF. In addition to AF related to an atrial torsades, those with a prolonged QT may have AF related to a leaky late sodium current, resulting in a longer action potential duration.22, 23 Therefore, treatment with antiarrhythmic drugs that block sodium, rather than potassium channels, may prove to be more efficacious in these patients. If a long QT does, in fact, represent a diffuse process related to ion channels or age-related injury, it may suggest that those with a longer QT will be less amenable to ablation. In contrast, perhaps those with AF associated with a shorter QT (implying a shorter AERP that may be important to their AF) would respond particularly well to potassium channel blockers. However, future investigations are needed before these findings can be applied to the clinical treatment of atrial fibrillation patients. Additionally, future AF ablation or antiarrhythmic trials may consider QT measurements as predictors of treatment efficacy or toxicity.
We did not find evidence of a relationship between QT heterogeneity and AERP heterogeneity. In addition, the association between the QTC interval and AERP did not vary based on atrial region. This suggests that the QTC interval is a reflection of the overall AERP, rather than region-specific AERP. This is congruent with the notion that the QT interval from different ECG leads serves as multiple views of the same electrophysiology.
There are several limitations to our study. This study was in predominantly white individuals with paroxysmal AF and based on a relatively small sample size in a single medical center. However, given statistically significant findings (consistent with our power calculations), the small size does not negate our results—this would have affected our confidence in excluding an association given negative results and likely affects the precision of our estimates. In addition, a large number of patients had a history of a previous AF ablation which may affect the generalizability of these results to other populations. The unadjusted correlation was relatively weak, calling into question the clinical utility of the QT interval as a marker of the AERP. Indeed, based on the r value of 0.45, it is clear that the majority of the variability of the AERP is not explained by the variability of the QT, demonstrating that likely many other factors influence both independently. However, the strength of the association improved after multivariable adjustment, suggesting that at least the qualitative relationship (a longer QT is generally associated with a longer AERP) may be robust. It is also possible that there were errors in the measurement of the QT interval and AERP. However, as the measurements of the predictors and outcomes were done independently by different individuals, it is unlikely that the predictor was measured differentially based on the outcome and vice versa. Our study was not designed to measure the dynamics of the QTC interval, but rather to study the relationship between the baseline QTC and AERP; therefore, we did not assess changes in the QT interval or QT interval hysteresis as it related to AERP. Antiarrhythmic medications were taken recently prior to the procedure, which may have affected the QT measurements. However, the majority of patients had discontinued antiarrhythmic medications prior to this time. Furthermore, even if both the atria and ventricles were similarly influenced by these drugs, that would support the QT and AERP as correlated markers.
Conclusions
We found that the QTc interval from the surface 12-lead ECG reflects the AERP in paroxysmal AF patients. Because the QTC interval is non-invasive and readily available on the standard 12-lead ECG, this suggests that the QTc interval may be a useful clinical marker of atrial refractoriness. Future investigations are needed to determine if the QTC interval is a clinically useful marker of atrial refractory periods and whether its use can aid in the selection of mechanism-specific therapy in the treatment of individuals with AF.
Acknowledgments
Funding: This work was made possible by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health grant number R01AA022222 and the Joseph Drown Foundation (G.M.M.). This publication was made possible in part by the Clinical and Translational Research Fellowship Program (CTRFP), a program of UCSF’s Clinical and Translational Science Institute (CTSI) that is sponsored in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number TL1 TR000144 and the Doris Duke Charitable Foundation (DDCF), and by R25MD006832 from the National Institute on Minority Health and Health Disparities (K.T.N.). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, UCSF or the DDCF.
Footnotes
Disclosures: Dr. Marcus has received research support from the NIH, PCORI, Medtronic, and Pfizer and is a consultant and equity holder of InCarda. None of the other authors have any relevant disclosures.
References
- 1.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–26. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 2.Andrade J, Khairy P, Dobrev D, Nattel S. The Clinical Profile and Pathophysiology of Atrial Fibrillation: Relationships Among Clinical Features, Epidemiology, and Mechanisms. Circulation Research. 2014;114:1453–1468. doi: 10.1161/CIRCRESAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- 3.Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res. 2014;114:1483–99. doi: 10.1161/CIRCRESAHA.114.302226. [DOI] [PubMed] [Google Scholar]
- 4.Moe GK, Rheinboldt WC, Abildskov JA. A Computer Model of Atrial Fibrillation. Am Heart J. 1964;67:200–20. doi: 10.1016/0002-8703(64)90371-0. [DOI] [PubMed] [Google Scholar]
- 5.Morillo CA, Klein GJ, Jones DL, Guiraudon CM. Chronic rapid atrial pacing. Structural, functional, and electrophysiological characteristics of a new model of sustained atrial fibrillation. Circulation. 1995;91:1588–95. doi: 10.1161/01.cir.91.5.1588. [DOI] [PubMed] [Google Scholar]
- 6.Lee JM, Lee H, Janardhan AH, Park J, Joung B, Pak HN, Lee MH, et al. Prolonged atrial refractoriness predicts the onset of atrial fibrillation: A 12-year follow-up study. Heart Rhythm. 2016;13:1575–80. doi: 10.1016/j.hrthm.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 7.Mandyam MC, Soliman EZ, Alonso A, Dewland TA, Heckbert SR, Vittinghoff E, Cummings SR, et al. The QT interval and risk of incident atrial fibrillation. Heart Rhythm. 2013;10:1562–8. doi: 10.1016/j.hrthm.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen JB, Graff C, Pietersen A, Lind B, Struijk JJ, Olesen MS, Haunso S, et al. J-shaped association between QTc interval duration and the risk of atrial fibrillation: results from the Copenhagen ECG study. J Am Coll Cardiol. 2013;61:2557–64. doi: 10.1016/j.jacc.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt N, Grunnet M, Olesen SP. Cardiac potassium channel subtypes: new roles in repolarization and arrhythmia. Physiol Rev. 2014;94:609–53. doi: 10.1152/physrev.00022.2013. [DOI] [PubMed] [Google Scholar]
- 10.Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ, Gorgels A, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119:e241–50. doi: 10.1161/CIRCULATIONAHA.108.191096. [DOI] [PubMed] [Google Scholar]
- 11.Dobrev D, Nattel S. New antiarrhythmic drugs for treatment of atrial fibrillation. Lancet. 2010;375:1212–23. doi: 10.1016/S0140-6736(10)60096-7. [DOI] [PubMed] [Google Scholar]
- 12.Woods CE, Olgin J. Atrial fibrillation therapy now and in the future: drugs, biologicals, and ablation. Circ Res. 2014;114:1532–46. doi: 10.1161/CIRCRESAHA.114.302362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patton KK, Zacks ES, Chang JY, Shea MA, Ruskin JN, Macrae CA, Ellinor PT. Clinical subtypes of lone atrial fibrillation. Pacing Clin Electrophysiol. 2005;28:630–8. doi: 10.1111/j.1540-8159.2005.00161.x. [DOI] [PubMed] [Google Scholar]
- 14.Vanhoutte K, de Asmundis C, Francesconi A, Figysl J, Steurs G, Boussy T, Roos M, et al. Leaving out control groups: an internal contrast analysis of gene expression profiles in atrial fibrillation patients--a systems biology approach to clinical categorization. Bioinformation. 2009;3:275–8. doi: 10.6026/97320630003275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.German DM, Kabir MM, Dewland TA, Henrikson CA, Tereshchenko LG. Atrial Fibrillation Predictors: Importance of the Electrocardiogram. Ann Noninvasive Electrocardiol. 2016;21:20–29. doi: 10.1111/anec.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcus GM. Predicting incident atrial fibrillation: an important step toward primary prevention. Arch Intern Med. 2010;170:1874–5. doi: 10.1001/archinternmed.2010.426. [DOI] [PubMed] [Google Scholar]
- 17.Wakili R, Voigt N, Kaab S, Dobrev D, Nattel S. Recent advances in the molecular pathophysiology of atrial fibrillation. J Clin Invest. 2011;121:2955–68. doi: 10.1172/JCI46315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen YH, Xu SJ, Bendahhou S, Wang XL, Wang Y, Xu WY, Jin HW, et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251–4. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 19.Satoh T, Zipes DP. Cesium-induced atrial tachycardia degenerating into atrial fibrillation in dogs: atrial torsades de pointes? J Cardiovasc Electrophysiol. 1998;9:970–5. doi: 10.1111/j.1540-8167.1998.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 20.Kirchhof P, Eckardt L, Franz MR, Monnig G, Loh P, Wedekind H, Schulze-Bahr E, et al. Prolonged atrial action potential durations and polymorphic atrial tachyarrhythmias in patients with long QT syndrome. J Cardiovasc Electrophysiol. 2003;14:1027–33. doi: 10.1046/j.1540-8167.2003.03165.x. [DOI] [PubMed] [Google Scholar]
- 21.Hatem SN, Coulombe A, Balse E. Specificities of atrial electrophysiology: Clues to a better understanding of cardiac function and the mechanisms of arrhythmias. J Mol Cell Cardiol. 2010;48:90–5. doi: 10.1016/j.yjmcc.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 22.Sossalla S, Kallmeyer B, Wagner S, Mazur M, Maurer U, Toischer K, Schmitto JD, et al. Altered Na(+) currents in atrial fibrillation effects of ranolazine on arrhythmias and contractility in human atrial myocardium. J Am Coll Cardiol. 2010;55:2330–42. doi: 10.1016/j.jacc.2009.12.055. [DOI] [PubMed] [Google Scholar]
- 23.Zygmunt AC, Eddlestone GT, Thomas GP, Nesterenko VV, Antzelevitch C. Larger late sodium conductance in M cells contributes to electrical heterogeneity in canine ventricle. Am J Physiol Heart Circ Physiol. 2001;281:H689–97. doi: 10.1152/ajpheart.2001.281.2.H689. [DOI] [PubMed] [Google Scholar]