Abstract
Cancer is a complex disease involving numerous biological processes, which can exist in parallel, can be complementary, or are engaged when needed and as such can replace each other. This redundancy in possibilities cancer cells have, are fundamental to failure of therapy. However, intrinsic features of tumor cells and tumors as a whole provide also opportunities for therapy. Here we discuss the unique and specific makeup and arrangement of cell membranes of tumor cells and how these may help treatment. Interestingly, knowledge on cell membranes and associated structures is present already for decades, while application of membrane modification and manipulation as part of cancer therapy is lagging. Recent developments of scientific tools concerning lipids and lipid metabolism, opened new and previously unknown aspects of tumor cells and indicate possible differences in lipid composition and membrane function of tumor cells compared to healthy cells. This field, coined Lipidomics, demonstrates the importance of lipid components in cell membrane in several illnesses. Important alterations in cancer, and specially in resistant cancer cells compared to normal cells, opened the door to new therapeutic strategies. Moreover, the ability to modulate membrane components and/or properties has become a reality. Here, developments in cancer-related Lipidomics and strategies to interfere specifically with cancer cell membranes and how these affect cancer treatment are discussed. We hypothesize that combination of lipid or membrane targeted strategies with available care to improve chemotherapy, radiotherapy and immunotherapy will bring the much needed change in treatment in the years to come.
Keywords: Cell membrane, Cancer adjuvant, Lipid modulation, Lipidomics
1. Cellular membrane overview
The involvement of lipids in cell structures was first described in 19th century when Charles E. Overton postulate the lipid nature of cellular membranes. Lipids were considered as a cell wall component that maintains the aqueous cytoplasm separately from the extracellular medium. The earliest membrane organization models were postulated 30 years later, ranging from a monolayer to a trilayer of lipids and proteins [1, 2], but the most accepted is the Fluid-mosaic model proposed by Singer and Nicolson in 1972 [3].
The fluid-mosaic model describes a bilayer of lipids in which some proteins are embedded, where lipids are free to rotate, move laterally or exchange between bilayers [4]. Some refinements have been added based on further research, such as the introduction of curvature and pore formation, membrane domains, a higher protein/lipid ratio and lipid interactions with cytoskeleton and surrounding matrix, which limit the freedom of the previous model considerably, but also adds complexity and increases functionality [1, 2, 4-6].
The cellular membrane is a fundamental cell component, not only due to the structural function but also regarding receptors, signaling, enzymatic activity, fusion-fission, endocytosis and transport among others, being responsible for interaction between cell and environment. Thus, research on membranes evolved in how to consider this cellular component, from a simple barrier between aqueous compartments to a more complex and fascinating structure with biological functions and an identity intrinsic to the type of cell or disease. The high lipid compositional complexity, versatility, interactions and distribution are related to concrete functions of the bilayer, determining the characteristics of the membrane or even cell. Lipid related studies revealed to be the key to better understand and comprehend the complexity of cellular mechanisms and related pathologies [2, 7-11]. In that sense, new fields achieved importance such as cellular Lipidomics [12-15] or Membrane Biophysics [16-19]. In spite of solid awareness of lipid distribution, interactions and functionality, there is still a lot to learn in this respect.
1.1. Physical properties
Lipids are composed of a polar head and a relatively long hydrophobic tail. They tend to associate spontaneously in an aqueous medium due to thermodynamic forces, where hydrophobic tails are protected by a layer of hydrophilic heads, resulting in structures like micelles or bilayered sheets which are considered the origin of cell membranes [4, 5].
The presence of hydrophobic and hydrophilic components allows non-covalent interaction with other lipids and proteins, conforming cellular and organelle membranes. Lipid type and distribution within a membrane is not homogeneous. Associations, enrichments and concrete lipid presence determine membrane functionality, showing the important role, often undervalued, of Lipidomics.
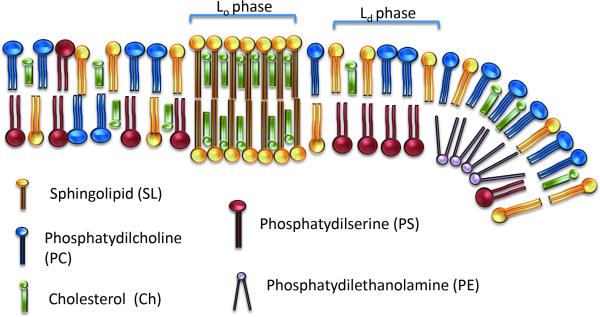
A well-known example is lipid asymmetry between inner and outer membrane leaflets. There is a phospholipid enrichment containing amine or serine moieties in the inner leaflet whereas choline and sphingomyelins (SM) are prevailing on the outside, as is shown in Fig. 1 [1, 12].
Figure 1.
Schematic representation of a cellular membrane depicting a selection of phospholipids as they appear in a bilayer. The liquid-ordered phase (Lo) typically harbors saturated phospholipids and cholesterol and therefore has a relatively rigid nature with a higher density of packing. The liquid-disordered phase is reached at temperatures above the transit temperature (Tm), which is typified by a more loose packing and less rigid nature.
Asymmetry maintenance is an active and energy dependent process which requires the involvement of enzymes such as flippases, scramblases and translocases [1, 12, 20]. The failure to preserve asymmetry is associated with apoptosis and pathological situations [1, 7]. Thus, the exposure of phosphatidylethanolamine (PE) or phosphatidylserine (PS) in the external layer is related to an increase in aggregation and recognition by phagocytic cells, and reacts with molecules like Annexin in apoptotic assays [1, 21]. Signaling lipids like phosphatidylinositol (PI) or phosphatidic acid (PA) are also enriched in the inner leaflet [7, 12]. Due to asymmetry there is a negative inner membrane surface charge that influences hydrolysis of PI mediated by phospholipase C into inositol 1,4,5-triphosphate (IP3) and Diacylglycerol (DAG), known as second messenger molecules [11, 22-24].
Finally, amine and serine moieties of the inner leaflet interact with the cytoskeleton, which forms fences or corrals that highly limits free lipid movement within the membrane and is involved in membrane curvature and in mechanical cell properties [1, 21, 25].
Lateral asymmetry is also widely reported resulting in polarization in some specialized tissues. In general it is assumed that apical areas are enriched in Cholesterol (Ch) and Sphingolipids (SL) contrary to basolateral areas, which present higher amount of phosphatidylcholine (PC) [25, 26]. This distribution is required for barrier formation, transport and sensorial processes of intestinal epithelial cells among other examples [25-28].
On top of that, difference in membrane composition between organelles have been reported, explaining differences in function, strongly related with lipid synthesis. Endoplasmic reticulum (ER) is involved in the synthesis of glycerolipids and Ch, whereas the Golgi complex is where the synthesis of SM and glycosphingolipid takes place [12]. There is trafficking of lipids from these organelles to the membrane, resulting in a gradient of SL and Ch [29, 30]. Thus, secretory organelles are 10-fold enriched in SL and Ch over the Golgi and ER [13]. Mitochondria are typically enriched in PE and Cardiolipin (CL) which has a bacterial origin [29], with low Ch content, whereas the ER presents higher amount of PC and PI [7, 11, 22].
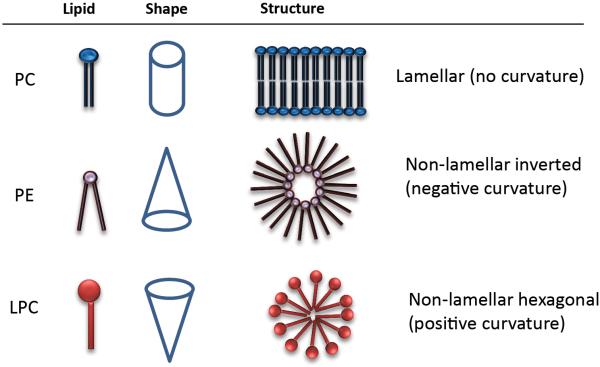
Finally, lipid structure is involved in and affect curvature and distortion of membranes. Phospholipids like PC or SM present a cylindrical shape based on head and tail proportion, and due to their amphiphilicity, spontaneously form a bilayer in an aqueous environment (Fig. 2) [4, 7, 11]. Other lipids such as lysophosphatidylcholine (LPC) and polyphosphoinositides, for instance PIP2, have higher head to tail proportion and have an inverted cone-shape, which causes a membrane positive curvature. On the other hand PA, PE, PS, DAG, ceramides or CL are considered cone-shaped lipids for they present small heads and distort membranes with a negative curvature, as depicted in Fig. 2 [2, 7, 22, 31, 32]. Cone-shaped lipids may adopt non-bilayered, hexagonal and cubic, phases temporarily on which this mesomorphism gives a high versatility to the membrane [4, 18, 23, 33]. These particular lipids influence the curvature of membrane, decreases energy required for fission, fusion, pore formation and vesicle trafficking, whereas also they regulate the activity of several relevant cell-signaling proteins [4, 7, 24, 25]. Fusion, for example, is important in differentiation during embryogenesis and morphogenesis [34] and is involved in the fertilization process, when the membrane of spermatozoids, enriched in LPC, fuse with oocyte [20]. Particular lipids (PE, CL, PA) are recruited in cell or organelle membranes, which together with certain proteins coordinate with the cytoskeleton to carry on fusion and fission [25-27, 32].
Figure 2.
Shape and structure of Phosphatidylcholine (PC), phosphatidylethanolamine (PE) and lyso-phosphatidylcholine (LPC). As is depicted, the makeup of these lipids determine to great extend the geometry of the structures in which they participate.
1.2. Membrane fluidity
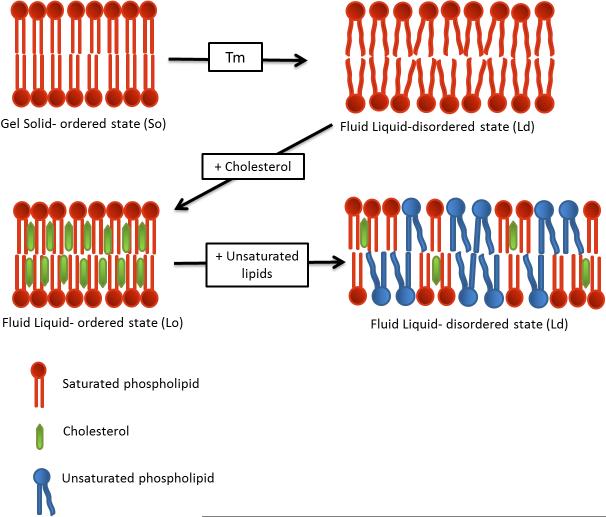
Lipids have different molecular configurations depending on temperature. Lipids change from gel solid-ordered phase (So), with extended hydrophobic tails at the transition temperature (Tm), to a liquid-disordered state (Ld) where the tails are not stretched. The Tm varies depending lipid configuration such as hydrocarbon length, saturation degree, charge, and head group species. At temperatures below Tm, lipids form a bilayer where hydrophobic chains can interact tightly with each other, resulting in a closely packed and rigid membrane. On the contrary, membranes at temperatures above Tm are more fluid and less packed.
At body temperature, cell membrane remains in a Ld state, because of its lipid composition, which means some degree of lateral mobility within is possible [4, 28]. However, apart from lipid phases, membrane fluidity is affected by two main factors: Ch and unsaturated lipids content [17, 30, 31].
Plasma membranes are generally more highly enriched in Ch than other bilayers such as from organelles [12, 31, 33]. When the Ch content is between 8-15%, the bilayer remains in different degrees of Ld phase [18, 35]. However, an enrichment in Ch (to 20-40%) causes an increase in membrane packing (Fig. 3), resulting in a more rigid liquid-ordered bilayer (Lo) [28, 35, 36]. Both membrane states Lo and Ld co-exists in membranes, especially in the outer leaflet [28, 37], as depicted in Fig. 1.
Figure 3.
Different stages of lipid bilayers depending on the composition and ambient temperature. When heating up the membrane changes from a rigid Gel state (So) to a more fluid and less dense Liquid state (Ld) when going through the transition temperature (Tm). Addition of cholesterol stabilizes the effect of temperature by providing denser packing and an increased rigidity. Presence of unsaturated phospholipids results in impaired packing and a higher state of fluidity. The double bounds in these lipids results in bends in the fatty chains causing repulsing and steric hindrance between the lipids.
Saturation status of lipids is as well involved in packing and fluidity. Saturated lipids favor ordered packing of membranes as their straight hydrophobic tails interact with others through van der Waals interactions. Unsaturated lipids have at least one cis double bond which distort the hydrophobic chain which prevents tight packing through steric hindrance. Thus, unsaturated lipids like oleic acid and linoleic acid decrease lipid packing in the membrane and consequently improve fluidity, as shown in Fig. 3 [17, 31, 38-40]. On the contrary, saturated lipids like stearic acid and palmitic acid confer a more rigid and organized membrane [31, 40, 41].
Membrane fluidity is strongly related to permeability [40]. Passive transmembrane transport is slow for most molecules while an increase in rigidity decreases permeability [37, 40]. Comparably, when Ch is removed from a membrane, packaging and rigidity decreases and permeability to water increases [40]. This will be discussed extensively in section 2.
1.3. Membrane domains
Lipid association is very dynamic and can be activated rapidly with little cost of energy. Typically a membrane contains areas which present an enrichment in certain lipids, called domains, which has important consequences for membrane properties, organization and functionalities like cellular polarization and trafficking, binding to external milieu and internal cytoskeleton, transduction of signals, cell growth, migration and entry of viruses, bacteria and nanoparticles [42-45].
There are two main lipid domains, caveolae and lipid rafts [45]. Lipid rafts are nano-sized planar formations (10-200 nm) [1, 5, 43], located in the outer leaflet with an enrichment of SL and Ch [1, 43, 44, 46-49]. These lipid association is dynamic, reversible and quick, where the OH− group of Ch interacts with SL via van der Waals forces and hydrogen bonding [35, 40, 46, 49]. The presence of these lipids is associated with a higher molecular order, Lo, while there is a favorable combination with certain proteins (Fig. 1).
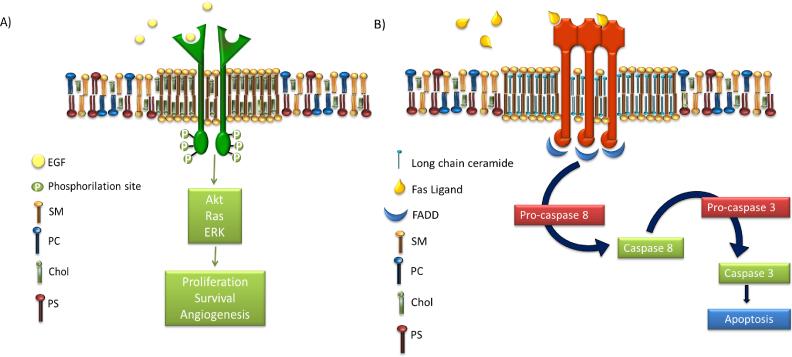
Some authors propose the existence of two different lipid rafts, depicted in Fig. 4: Type 1 enriched in Ch and SL, involved in cell proliferation, and Type 2 enriched in ceramide (Cer), involved in apoptosis [50]. Cer presents a small polar head group and a tendency for self-association caused by intermolecular hydrogen bonding allowing the formation of a relatively stable, ordered and packed state [7, 28, 51]. Even small amounts of Cer are able to displace Ch from Lo domains improving the order [18, 28, 52, 53].
Figure 4.
Schematics of two types of lipid rafts or lipid domains. A) Cholesterol enriched lipid raft with EGFR embedded and part of the downstream cascade. B) Ceramide enriched domain with the Fas receptor embedded and the downstream apoptosis process flow chart. Epidermal growth factor (EGF), sphingomyelin (SM), phosphatidylcholine (PC), cholesterol (Chol), phosphatidylserine (PS) and Fas-Associated protein with Death Domain (FADD).
There are distinct differences between Type 1 and 2 lipid domains. Firstly, Cer enriched lipid rafts are micro or macrodomains in size, as they trend to fuse immediately after formation, whereas Chol/SL lipid rafts are nanodomains with a very short life (10-20 ms) [54]. Moreover, the lateral mobility in Cer domains is slower compared to raft domains containing Ch/SL. This stabilizes embedded proteins resulting in longer protein association [28].
As several important proteins involved in signaling are associated with lipid domains, they are considered hot spots of signaling. The key to explain the affinity of proteins for membrane domains is post-transcriptional protein modifications. These include acylation with fatty acyl moieties like myristoylation of the N-terminal amino group by amide bondage and at least a second fatty acyl substitution on cysteines usually with palmitoyl residues [41, 47, 55]. Some typical examples of proteins related with Type 1 lipid rafts are GPI anchored proteins [5, 44], epidermal growth factor receptor (EGFR) [47, 56], estrogen receptor [57] or insulin-like receptor (IGF-1) [47]. On the other hand, proteins located in Type 2 domains are typically Fas (CD95), TNF-R1 or TRAIL receptor (DR5) [9, 50, 53, 58, 59].
Finally, caveolae are small surface invaginations of 50-100 nm diameter [5, 46, 55] seen in many cell types [43, 55]. These domains are rich in Ch, SL and lipid-anchored proteins. They contain the coat protein caveolin, which polymerization is essential for membrane invagination [8, 43, 55]. Caveolae have basic properties of lipid rafts and are considered to store and down-regulate raft proteins or act as reservoirs for rafts [43].
2. Lipid alterations in cancer
Lipids are regulated in response to pathological, nutritional and pharmacological situations [7]. Although this review is focused on cancer, lipid alteration is also involved in other diseases like cardiopathies, diabetes, atherosclerosis, infectious diseases or neurodegenerative pathologies [14, 43, 60].
Cancer is a disease caused by the loss of the self-control mechanisms of cells due to a wide variety of reasons. Main characteristics of cancer cells are the ability to induce angiogenesis, immortality and resistance to cell death, lack of response to growth suppressor signals and the capability of invasion and metastasis. These altered processes are indeed related with differences in lipid composition in comparison to normal cells [10, 24, 60], although these differences vary regarding type of cancer, stage and sensitivity status [10, 61, 62]. However, there are some common aspects in tumoral cells, mainly related to alterations of fatty acids metabolism or modification of enzymes involved in lipid metabolism [10, 36, 50, 63].
Typically, Ch metabolism is deregulated resulting in higher or lower Ch amount in cancer cells compared to non-tumor cells and therefore variations on membrane fluidity [10, 17, 19]. It has been hypothesized that low Ch cells have more easy deformable membranes and are able to enter blood vessels easier, being highly metastatic [10]. On the other hand, multidrug resistant (MDR) cells present higher amounts of Ch, and a more rigid membrane, which is thus less permeable [10, 64, 65]. In fact, there is a significant increase of Ch and phospholipid levels (PC, PI, PE and others) in MDR cells, and a protein/lipid ratio elevation up to 60% in comparison to sensitive cells [8, 10, 19]. Limitation in permeability decreases drug uptake and explains partially treatment resistance [10, 36, 64]. The increase in Ch content in tumors has been reported to be higher than normal tissues, which can reach up to 50% [66], and is strongly related with an increase of lipid rafts presence [8, 10, 47]. These domains are involved in cell proliferation, differentiation, apoptosis and migration, depending on which proteins are located within. Thus, alterations of lipid rafts domains could be involved in malignant transformations, uncontrolled growth, invasiveness and metastasis [47]. The higher amount of lipid rafts in cancer cells allow the overexpression of growth factor receptors like EGFR, IGF-1 or Sigma receptors [47, 50, 56]. Other proteins like integrins, adherins, receptors CD44 and CD24, involved in tumor progression and invasion, are also located in lipid rafts [42, 47]. The abundance of these proteins in tumor cells are linked with a higher invasive potential and a decreased fluidity of membranes [65]. Besides, there is a higher presence of MDR transporters, like P-glycoprotein (P-gp), which have an important role in MDR development regarding the efflux of chemotherapeutics from the cytoplasm [10, 67].
Cer metabolism has also been described as an effective drug resistance mechanism [9, 19, 68-70]. Cer is present in very small amounts in cell membranes, as intermediates in the metabolism of the more complex SL [51] or as a result of Sphingomyelinase (SMase) activity, which produces Cer from SM [18, 28, 59]. The regulation between Cer and SM is involved in differentiation, proliferation, interplay between proteins and apoptosis through intrinsic and extrinsic pathways [70, 71]. MDR cells maintain low Cer levels by increasing SM synthesis or by preventing SM breakdown [19, 68]. In this situation there are less Type 2 raft domains (Cer enriched) and less apoptosis. Sphingosine kinases regulate a number of process which aid tumor progression such as survival, proliferation and transformation which therefore provide possible targets for therapy [72].
Finally, as the inner leaflet typically consists of negatively charged lipids as PS, in non-transformed cells, acidic pH decreases repulsion between polar groups and surface tension giving a less packaged membrane [19, 73, 74]. A slight increase in alkalinity, as happens in resistant cells, shields the negative charges, attenuates repulsion between polar groups and therefore increases packing. Taken together, the interplay between the alkaline nature of the cytoplasm of MDR cells and the makeup of the cell membrane results in a more dense packing of lipids enhancing rigidity of the membrane resulting in poor chemotherapeutic drug penetration of these cells [19, 73, 74].
3. Membrane modulation strategies in cancer
Traditionally most cancer therapeutics are designed to interact with proteins and nucleic acids. However, taking into account alterations of lipid composition described above and associated functionality in cancer cells, lipid therapy is becoming a very interesting alternative. Modulation of, or interaction with, lipids could change lipid composition, membrane properties or alter these associated functions. Thus, it is reasonable to think that it is possible to treat cancer, or other pathologies, with lipids or molecules that interact with lipids [24].
3.1. Cholesterol depletion
As mentioned above, Ch is the key molecule that keeps raft domains together. Removal of Ch leads to raft and caveolae disruption and dissociation of proteins from these domains, rendering them non-functional [43], resulting in inappropriate cellular signaling events and deregulating cellular functions [16, 47, 50].
There are a wide variety of molecules that decrease Ch levels [50], but only the most representative are listed below. One of them is Filipin, a polyene macrolide that binds Ch and prevents interaction with SL, thereby decreasing stability of membrane rafts [47, 56]. Methyl-beta-cyclodextrin (M CD) has been also widely used as it removes Ch rapidly but not completely [28, 43]. After treatment with these drugs Ch is depleted, rafts are disrupted and as a result EGFR or Estrogen receptors, overexpressed in several cancer types, are decreased [47, 57, 75]. Ch depletion after Emodin treatment has been shown to prevent cancer metastases as well as it decreases CD44 receptor or matrix metalloproteinases levels, suppresses tumor cell migration and impairs metastasis establishment possibilities [47, 76].
Finally, statins lower Ch levels by inhibiting the enzyme 3-hydroxy-methylglutaryl CoA reductase (HMG-CoA reductase) [47, 75]. These drugs, traditionally prescribed to treat cardiovascular diseases, act in early stages of Ch synthesis and are able to decrease lipid raft number and membrane rigidity. Statins cause no effect over healthy cells compared to cancer cells but the main activity of inhibition of HMGCoA has been found in the liver [47, 63]. Although still controversial, a cancer prophylactic effect has been reported in statin users and currently statins are being evaluated in clinical trials in breast cancer [63].
3.2. Stabilization of membrane domains
The strategy of Ch depletion has been used to diminish lipid rafts and as a result impair signaling through receptors embedded in this rafts. However, as mention in section 1.3, there are also lipid rafts which harbor pro-apoptotic receptors. Another strategy therefore is to increase Cer levels and use lipid rafts as platforms to induce apoptosis. As a consequence of this approach Ch is displaced from lipid rafts Type 1 by Cer, resulting in recruitment and aggregation of Fas/CD95 death receptors in these rafts, which can be as well pharmacologically modulated [50, 77]. Promotion and stabilization of these pro-apoptotic domains accomplish an amplification of the signal [58, 59]. Fas trimerization recruits Fas-Associated protein with Death Domain (FADD) molecules, which together with the activation of procaspase-8 form the death-inducing signaling complex that activates caspase 3, leading to apoptosis as shown in Fig. 4b [58, 59, 77].
The alkyl-lysophospholipid analogue Edelfosine was the first antitumor drug reported to induce apoptosis in cancer cells through co-clustering of lipid rafts and Fas/CD95 death receptors [59, 77]. This molecule has a modest effect in humans compared to reported side-effects and is scarcely used in humans [78]. Therefore similar compounds like Miltefosine or Perifosine, are being used as antitumor lipid drugs in clinical trials [24, 47, 78]. Upon accumulation in membrane rafts lipid and protein composition in cancer cells are altered promoting recruiting of Fas ligand and apoptosis, with limited effect on normal cells [47, 59, 79].
Another promising compound that induces apoptosis through recruitment of Fas/CD95 into membrane domains is Resveratrol [47, 59]. This polyphenol has been shown to have chemopreventive and antitumor activities [59, 80]. Resveratrol accumulates in lipid rafts previous to its endocytosis [80]. Not surprisingly, good results have been obtained with combined therapy of Resveratrol and death receptor agonists [47].
3.3. Short chain ceramides
Chemotherapeutic treatment require interaction with the membrane to enter a cell, which is one of the main barriers that limits treatment [81, 82]. The outer leaflet of the cell membrane is believed to provide the major barrier to permeation [37, 40]. More rigid and less permeable membranes such as presented by MDR tumor cells challenge interaction with chemotherapeutics and impair entrance [19]. Thus, the capability to modulate membrane fluidity/rigidity emerges as a very useful strategy to improve a variety of treatments.
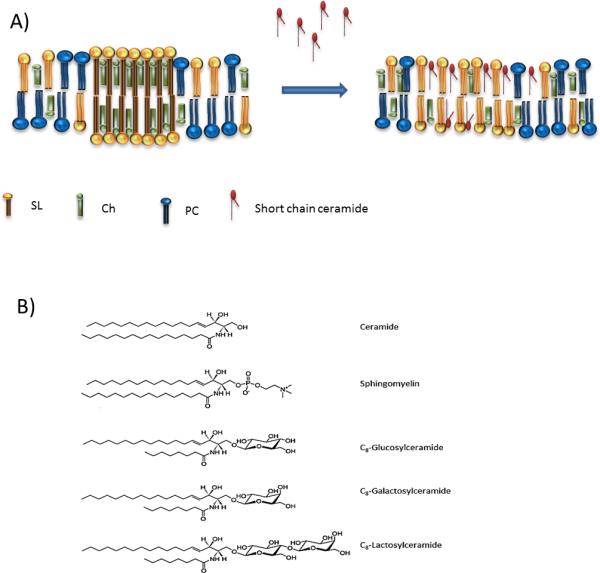
As mentioned, membrane permeability and fluidity depend on Ch content and non-saturated/saturated lipid ratio. However, other factors are important as well. Relatively big head groups of phospholipids can inhibit the formation of tight interactions with the surrounding lipids [28] and changes in the hydrophobic segments of lipids can have an effect in packing tightness [83]. A good example of how lipid structure can influence the fluidity of membrane is provided by members of the Cer family. Cer are closely related to sphingolipids, and several studies show that the Cer effect in membranes vary regarding the length of N-acyl chain [18, 52, 71, 83-85]. Cer molecule with chain lenghts between 4 to 8 carbons is easily incorporated into the external leaflet of cell membranes and, in contrast to long-chained Cer, they are not able to form lipid rafts nor increase rigidity of the membrane [18, 83-85]. This is based on the short length of the hydrophobic chain which is not able to interact tightly with other lipids, as depicted in Fig. 5A. Thus, short chain ceramides (SCC) increase permeability and fluidity of the membrane when already present or when externally added and have been used in cancer treatment for this purpose [52, 81, 83, 85].
Figure 5.
A: Structural depiction of short and long chain ceramides within a membrane. Sphingolipids (SL), cholesterol (Ch), phosphatidylcholine (PC). B: Molecular structures of ceramide, sphingomyelin and three examples of short chain ceramides. The short chain ceramides can be identified by a truncated chain which is significantly shorter compared to other.
The more widely used SCC are N-hexanoyl-sphingomyelin and N-octanoyl-ceramide, composed of a sphingosine backbone where the functional amino group at position C2 is acylated with a fatty acid and the C1 hydroxyl group is linked to a sugar moiety (glucosyl, lactosyl or galactosyl), as shown in Fig. 5 [86]. SCCs, when pre-inserted in membranes, greatly and specifically enhance uptake and action of various amphiphilic anticancer drugs in cancer cells in vitro, without causing membrane leakage, toxicity or other trivial effects by themselves [81, 86, 87]. Cell uptake of doxorubicin, a widely used amphiphilic anthracycline is improved by the addition of SCCs and so resulting in enhanced toxicity to tumor cells [81, 86].
The underlying mechanism has been studied extensively but it is not completely elucidated yet. Studies indicate that it does not involve a specific detergent-like membrane disruption, enhanced endocytosis or decreased ABC transporter mediated efflux (like Pgp), nor involved natural lipid rafts [81]. It is believed that SCCs enhance tumor cell membrane permeability through the potential of glycosphingolipids to form specific permeable microdomains surrounding the drug molecule [82, 87]. These microdomains consist of very small channels or pores constructed of sphingolipids with short open lifetimes (120 ns) and diameters less than 2 nm [81, 82, 88] that allow passage of co-administered drugs [81, 82, 86, 88, 89]. The working hypothesis behind pore formation through sphingolipids is based on the observation that the short acyl chain of SCCs cause imperfect lipid packing and hence create local differences in membrane fluidity and lipophilicity leading to channel formation. The specific geometry of these sphingolipids enable a relatively sharp curvature which is needed for this process.
Application of SCCs is proposed as a novel drug delivery approach to enhance drug bioavailability inside tumor cells in combination with lipid-based nanoscaled drug delivery devices such as liposomes. These liposome carry both pro-active lipid and chemotherapeutic drug ensuring concurrent delivery at the very same spot [82, 89-91]. It is hypothesized that in the vicinity of tumor cells, SCC spontaneously relocate from liposomal to plasma membrane where they self-organize into above mentioned microdomains. Such a spontaneous redistribution, very common for SCC analogs, results in an equilibrium between cells and liposomes [90]. After transfer of SCCs to the membrane it is believed that the encapsulated drug is released due to liposome destabilization and the free drug enters the cell through SCC lined pores without nanoparticle endocytosis [90].
Remarkably, studies indicate that SCCs seem to have a greater impact on tumor cells compared to cells from healthy tissues. Moreover, we (data not shown) and others observed that resistant cells respond better to SCC pre-treatment than native sensitive tumor cells [82, 91]. Typically a cancer cell membrane is more ordered and rigid, especially in MDR cells and SCCs seem to have a more pronounced effect in rigid and liquid ordered membranes with lower permeability [82, 87]. The shorter hydrophobic moiety of SCC, present properties that do not match with the structural order of rigid membranes and as a result there is a decrease in the order of the bilayer [85].
3.4. Cer/SM metabolism modulators
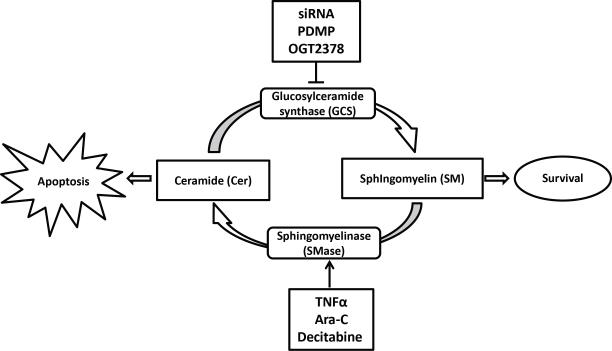
As mentioned, equilibrium between Cer and SM is related with apoptosis or survival [68]. There are several enzymes and routes implied in this regulation, like Glucosylceramide synthase (GCS), an enzyme that converts Cer into SM [68, 69], and SMase, that converts SM to Cer phosphate (Fig. 6) [19, 59]. Some cancer cells present an over expression of GCS or a downregulation of SMase resulting in low levels of Cer and high levels of SM [9, 19, 68]. As an example, SMase downregulation through the SMase gene hypermethylation is observed in 60% of breast tumors [19]. Resistance development to traditional treatments like cisplatin or irradiation are partially explained by the inhibition of SMase, because cells failed to accumulate Cer and therefore do not go into apoptosis [71].
Figure 6.
Schematic representation of the Ceramide/Sphingomyeline cycle and main strategies to modulate it in cancer therapy. Sphingomyelin conversion can be modulated resulting in elevated levels of ceramides which favor apoptosis, while levels of ceramide can be maintained by inhibition of its processing to sphingomyelin.
Thus, modulation through down-regulation or inhibition of GCS results in higher levels of Cer, and thus increased apoptosis and decreased drug resistance [9, 68]. Also the elevated action of SMase reduces available SM to form lipid rafts with the resulting consequences mentioned above [19, 92]. TNFα, etoposide or Cytarabine (Ara-C) are able to activate SMase enzyme, increasing Cer and decreasing SM [9, 71, 92]. SMase activity could also be increased using some epigenetic drugs like decitabine that demethylates the SMase gen [19]. Also the use of siRNA transfection [9, 19, 68], certain analogs of glucosylceramide like PDMP [68, 93] or the imino sugar OGT2378 [9, 68, 94], have demonstrated to inhibit and/or decrease GCS activity and as a result SM [95]. Both strategies decrease Ch lipid rafts and improve Cer-enriched domains in which death receptors are located [19, 59].
3.5. Lipid replacement therapy
Use of lipids in diets and supplements to increase health and prevent illnesses has been followed for centuries [24, 31]. Consumption of mono and polyunsaturated fatty acids (MUFA and PUFA), like linoleic acid [19], oleic acid [24, 96] and marine fish oils [41], is believed to have a protective effect against colon tumorigenesis [39, 47, 97]. They can influence cellular membrane composition through changing plasma properties like fluidity, phase behavior or permeability [39, 41, 47]. Its inclusion in membranes displaces acylated proteins from raft domains and reduces raft formation [39, 41]. The ratio between unsaturated and saturated lipids is believed to be an important factor in cancer development and evolution and as it is influenced by diet this field is gaining interest fast. In fact, progression and incidence of some types of cancer like prostate cancer has been related with high fat diet [56]. However, the main effect of oral consumption is currently thought to be of prophylactic nature in the case of cancer and other diseases [24, 50, 60]. For this reason new analogs are being developed in order to improve the efficacy of lipid-based or lipid-targeted strategies such as derivatives of olive oil (Minerval) [24, 60, 98], of the anesthetic propofol (Propofol-docosahexanoic acid) [24, 98, 99] or lipid analogs of traditional chemotherapeutic drugs [24]. Minerval reduces membrane order and normalizes PE:SM ratio in cancer cells with less effect on healthy tissue. Currently this agent is being evaluated in phase I/II for glioma [78, 98].
Finally, it has been reported that sphingolipids suppress tumor growth by interfering with nutrient absorption. The administration of a synthetic sphingolipid (SH-BC-893), which as well blocks other nutrient pathways, shows favorable tumor response [100].
4. Lipid modulation as adjuvant for cancer therapy
Here we described the crucial importance of lipid distribution within cell membranes and its implication in numerous important functions. The cell membrane undergoes modifications under specific natural conditions such as aging as well as during development of diseases such as cancer. The capability to modulate and manipulate structure, composition and properties of cell membranes has become a very promising reality to treat diseases [98]. Lipid modulation, replacement or supplementation strategies revealed that indeed that cancer therapy may be improved significantly.
Reduction of Ch levels is the most advanced strategy to decrease raft domains with survival and growth receptors, which is highly effective in combination with a wide variety of different therapies, from chemotherapy to radiology or immunotherapy. In fact, combined with chemotherapy like Trastuzumab [47, 101] and tamoxifen [47, 102], EGCG seems to play an important role in decreasing HER expression in multidrug resistance. Statins improve the efficacy response and overall survival combined with first line treatment in hepatocellular carcinoma, acute myeloid leukemia or refractory multiple myeloma [63]. However, sometimes increased Ch results in an advantage. In that way, Yang et al combined an atherosclerosis drug with immunotherapy, showing that TCD8 cells with higher Ch level display increased TCR clustering and underwent a more potent and efficient response [103].
The use of Cer enriched raft domains is also effective in cancer treatment as they are related with apoptosis. A synergistic effect of Cer combined with chemotherapy, docetaxel or paclitaxel, has been shown, which can eliminate the MDR population [104, 105]. From our point of view modulation of membrane fluidity to revert MDR resistance and increase sensitivity to chemotherapeutics is the most promising strategy regarding lipid modulation. SCC analogs demonstrated a specificity for carcinogenic tissues with a more profound effect on tumor cells versus healthy tissues. This class of ceramides not only reverts MDR resistance but even elevates treatment to a better response compared to tumor cells which did not develop MDR, indicating that manipulation of tumor cell membranes has great potential in combination chemotherapy. We and others indeed showed favorable results with a combination of SCC, alone or incorporated in nano-sized doxorubicin and mitoxantrone delivery devices, in cancer models [89-91].
In our opinion, in depth studies of cell membranes and lipid makeup, in other words Lipidomics, of different types of cancer and stages will be used more often in coming years as a prognosis and progression marker, as well as an early predictive tool together with other biomarkers [15, 106]. We believe that the modulation and modification of membrane components will be also used as an adjuvant in cancer and other diseases therapy.
5. Conclusion
The cellular membrane is a uniquely organized and complex component of the cell, responsible of maintaining cellular structure and interaction with the environment. The knowledge of lipid composition and especially the alterations reported in cancer provides a major opportunity to treat and prevent cancer. Understanding these differences and the study on how to apply this knowledge in cancer is part of Lipidomics, a currently underestimated and underappreciated “–omic”.
In conclusion, the cell membrane and possibilities we have to manipulate its composition and function provides a powerful tool in the treatment of cancer either in combination with chemotherapeutics as well as small molecules, which are currently being developed. The cell membrane and its components must be taken into account as key factors in cancer treatment and deserves attention for the development of new therapeutic strategies.
Highlights.
Membrane lipid composition is strongly related with membrane functionality and therefore with individual cell role within tissues.
Lipid alterations are found in cancer cells with variations between stage and cancer type.
Cancer treatment can be improved when combined with lipid modulation strategies.
Deeper evaluation of lipid composition in cancer will be useful as predictive/prognostic tool in the near future.
Acknowledgments
Funding source: This work was supported by the NIH/National Cancer Institute (project n. R01CA181664).
Abbreviations
- HMG-CoA reductase
3-hydroxy-methylglutaryl CoA reductase
- Emodin
3-methyl-1,6,8-trihydroxyanthraquinone
- CL
Cardiolipin
- Cer
Ceramide
- Ch
Cholesterol
- DAG
Diacylglycerol
- ER
Endoplasmic reticulum
- EGFR
Epidermal growth factor receptor
- EGCG
Epigallocatechin gallate
- FADD
Fas-Associated protein with Death Domain
- So
Gel solid-ordered state
- GCS
Glucosylceramide synthase
- IP3
Inositol 1,4,5-triphosphate
- IGF-1
Insulin-like receptor
- Ld
Liquid-disordered state
- Lo
Liquid-ordered state
- LPC
Lyso-phosphatidylcholine
- MβCD
Methyl-beta-cyclodextrin
- MUFA
Monosaturated fatty acids
- MDR
Multidrug resistance
- P-gp
P-glycoprotein
- PA
Phosphatidic acid
- PC
Phosphatidylcholine
- PE
Phosphatidylethanolamine
- PI
Phosphatidylinositol
- PS
Phosphatidylserine
- PUFA
Polysaturated fatty acids
- SCC
Short chain ceramides
- SL
Sphingolipids
- SM
Sphingomyelin
- SMase
Sphingomyelinase
- Tm
Transition temperature
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests: none.
References
- 1.Nicolson GL. The Fluid-Mosaic Model of Membrane Structure: still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochim Biophys Acta. 2014;1838(6):1451–66. doi: 10.1016/j.bbamem.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Bagatolli LA, et al. An outlook on organization of lipids in membranes: searching for a realistic connection with the organization of biological membranes. Prog Lipid Res. 2010;49(4):378–89. doi: 10.1016/j.plipres.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175(4023):720–31. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 4.Goni FM. The basic structure and dynamics of cell membranes: an update of the Singer-Nicolson model. Biochim Biophys Acta. 2014;1838(6):1467–76. doi: 10.1016/j.bbamem.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson K, Mouritsen OG, Anderson RG. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9(1):7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- 6.Engelman DM. Membranes are more mosaic than fluid. Nature. 2005;438(7068):578–80. doi: 10.1038/nature04394. [DOI] [PubMed] [Google Scholar]
- 7.Escriba PV, et al. Membranes: a meeting point for lipids, proteins and therapies. J Cell Mol Med. 2008;12(3):829–75. doi: 10.1111/j.1582-4934.2008.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen AW, et al. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84(4):1341–79. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 9.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4(8):604–16. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 10.Hendrich AB, Michalak K. Lipids as a target for drugs modulating multidrug resistance of cancer cells. Curr Drug Targets. 2003;4(1):23–30. doi: 10.2174/1389450033347172. [DOI] [PubMed] [Google Scholar]
- 11.van Meer G, de Kroon AI. Lipid map of the mammalian cell. J Cell Sci. 2011;124(Pt 1):5–8. doi: 10.1242/jcs.071233. [DOI] [PubMed] [Google Scholar]
- 12.Shevchenko A, Simons K. Lipidomics: coming to grips with lipid diversity. Nat Rev Mol Cell Biol. 2010;11(8):593–8. doi: 10.1038/nrm2934. [DOI] [PubMed] [Google Scholar]
- 13.van Meer G. Cellular lipidomics. EMBO J. 2005;24(18):3159–65. doi: 10.1038/sj.emboj.7600798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y, et al. Mass spectrometry based cellular phosphoinositides profiling and phospholipid analysis: a brief review. Exp Mol Med. 2010;42(1):1–11. doi: 10.3858/emm.2010.42.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandu R, Mok HJ, Kim KP. Phospholipids as cancer biomarkers: Mass spectrometry-based analysis. Mass Spectrom Rev. 2016 doi: 10.1002/mas.21510. [DOI] [PubMed] [Google Scholar]
- 16.Almeida PF, Pokorny A, Hinderliter A. Thermodynamics of membrane domains. Biochim Biophys Acta. 2005;1720(1-2):1–13. doi: 10.1016/j.bbamem.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Subczynski WK, Wisniewska A. Physical properties of lipid bilayer membranes: relevance to membrane biological functions. Acta Biochim Pol. 2000;47(3):613–25. [PubMed] [Google Scholar]
- 18.Quinn PJ. Lipid-lipid interactions in bilayer membranes: married couples and casual liaisons. Prog Lipid Res. 2012;51(3):179–98. doi: 10.1016/j.plipres.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Peetla C, Vijayaraghavalu S, Labhasetwar V. Biophysics of cell membrane lipids in cancer drug resistance: Implications for drug transport and drug delivery with nanoparticles. Adv Drug Deliv Rev. 2013;65(13-14):1686–98. doi: 10.1016/j.addr.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flesch FM, Gadella BM. Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochim Biophys Acta. 2000;1469(3):197–235. doi: 10.1016/s0304-4157(00)00018-6. [DOI] [PubMed] [Google Scholar]
- 21.Demchenko AP. The change of cellular membranes on apoptosis: fluorescence detection. Exp Oncol. 2012;34(3):263–8. [PubMed] [Google Scholar]
- 22.Somerharju P, et al. The superlattice model of lateral organization of membranes and its implications on membrane lipid homeostasis. Biochim Biophys Acta. 2009;1788(1):12–23. doi: 10.1016/j.bbamem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Yang L, Ding L, Huang HW. New phases of phospholipids and implications to the membrane fusion problem. Biochemistry. 2003;42(22):6631–5. doi: 10.1021/bi0344836. [DOI] [PubMed] [Google Scholar]
- 24.Escriba PV. Membrane-lipid therapy: a new approach in molecular medicine. Trends Mol Med. 2006;12(1):34–43. doi: 10.1016/j.molmed.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Frohman MA. Role of mitochondrial lipids in guiding fission and fusion. J Mol Med (Berl) 2015;93(3):263–9. doi: 10.1007/s00109-014-1237-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anitei M, Hoflack B. Bridging membrane and cytoskeleton dynamics in the secretory and endocytic pathways. Nat Cell Biol. 2012;14(1):11–9. doi: 10.1038/ncb2409. [DOI] [PubMed] [Google Scholar]
- 27.Chernomordik LV, Kozlov MM. Mechanics of membrane fusion. Nat Struct Mol Biol. 2008;15(7):675–83. doi: 10.1038/nsmb.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.London E. How principles of domain formation in model membranes may explain ambiguities concerning lipid raft formation in cells. Biochimica Et Biophysica Acta-Molecular Cell Research. 2005;1746(3):203–220. doi: 10.1016/j.bbamcr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 29.van Meer G, Sprong H. Membrane lipids and vesicular traffic. Curr Opin Cell Biol. 2004;16(4):373–8. doi: 10.1016/j.ceb.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Hao M, et al. Effects of cholesterol depletion and increased lipid unsaturation on the properties of endocytic membranes. J Biol Chem. 2004;279(14):14171–8. doi: 10.1074/jbc.M309793200. [DOI] [PubMed] [Google Scholar]
- 31.Nicolson GL, Ash ME. Lipid Replacement Therapy: a natural medicine approach to replacing damaged lipids in cellular membranes and organelles and restoring function. Biochim Biophys Acta. 2014;1838(6):1657–79. doi: 10.1016/j.bbamem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Furt F, Moreau P. Importance of lipid metabolism for intracellular and mitochondrial membrane fusion/fission processes. Int J Biochem Cell Biol. 2009;41(10):1828–36. doi: 10.1016/j.biocel.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 33.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9(2):112–24. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bastida-Ruiz D, Van Hoesen K, Cohen M. The Dark Side of Cell Fusion. Int J Mol Sci. 2016;17(5) doi: 10.3390/ijms17050638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quinn PJ, Wolf C. The liquid-ordered phase in membranes. Biochim Biophys Acta. 2009;1788(1):33–46. doi: 10.1016/j.bbamem.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Preetha A, Banerjee R, Huilgol N. Tensiometric profiles and their modulation by cholesterol: implications in cervical cancer. Cancer Invest. 2007;25(3):172–81. doi: 10.1080/07357900701209053. [DOI] [PubMed] [Google Scholar]
- 37.Krylov AV, et al. Water permeability of asymmetric planar lipid bilayers: leaflets of different composition offer independent and additive resistances to permeation. J Gen Physiol. 2001;118(4):333–40. doi: 10.1085/jgp.118.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wassall SR, et al. Order from disorder, corralling cholesterol with chaotic lipids. The role of polyunsaturated lipids in membrane raft formation. Chem Phys Lipids. 2004;132(1):79–88. doi: 10.1016/j.chemphyslip.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Turk HF, Chapkin RS. Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2013;88(1):43–7. doi: 10.1016/j.plefa.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hill WG, Zeidel ML. Reconstituting the barrier properties of a water-tight epithelial membrane by design of leaflet-specific liposomes. J Biol Chem. 2000;275(39):30176–85. doi: 10.1074/jbc.M003494200. [DOI] [PubMed] [Google Scholar]
- 41.Stulnig TM, et al. Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. J Biol Chem. 2001;276(40):37335–40. doi: 10.1074/jbc.M106193200. [DOI] [PubMed] [Google Scholar]
- 42.Head BP, Patel HH, Insel PA. Interaction of membrane/lipid rafts with the cytoskeleton: impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim Biophys Acta. 2014;1838(2):532–45. doi: 10.1016/j.bbamem.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simons K, Ehehalt R. Cholesterol, lipid rafts, and disease. J Clin Invest. 2002;110(5):597–603. doi: 10.1172/JCI16390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387(6633):569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 45.Lajoie P, Nabi IR. Lipid rafts, caveolae, and their endocytosis. Int Rev Cell Mol Biol. 2010;282:135–63. doi: 10.1016/S1937-6448(10)82003-9. [DOI] [PubMed] [Google Scholar]
- 46.Simons K, Vaz WLC. Model systems, lipid rafts, and cell membranes. Annual Review of Biophysics and Biomolecular Structure. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- 47.Hryniewicz-Jankowska A, et al. Membrane rafts as a novel target in cancer therapy. Biochimica Et Biophysica Acta-Reviews on Cancer. 2014;1845(2):155–165. doi: 10.1016/j.bbcan.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 48.Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol. 2010;11(10):688–99. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- 49.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327(5961):46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 50.Tekpli X, et al. Role for membrane remodeling in cell death: implication for health and disease. Toxicology. 2013;304:141–57. doi: 10.1016/j.tox.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 51.Kolesnick RN, Goni FM, Alonso A. Compartmentalization of ceramide signaling: Physical foundations and biological effects. Journal of Cellular Physiology. 2000;184(3):285–300. doi: 10.1002/1097-4652(200009)184:3<285::AID-JCP2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 52.Goni FM, Alonso A. Effects of ceramide and other simple sphingolipids on membrane lateral structure. Biochim Biophys Acta. 2009;1788(1):169–77. doi: 10.1016/j.bbamem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Megha, et al. Effect of ceramide N-acyl chain and polar headgroup structure on the properties of ordered lipid domains (lipid rafts). Biochim Biophys Acta. 2007;1768(9):2205–12. doi: 10.1016/j.bbamem.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hancock JF. Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol. 2006;7(6):456–62. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parton RG, Richards AA. Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic. 2003;4(11):724–38. doi: 10.1034/j.1600-0854.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 56.Zhuang L, et al. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Research. 2002;62(8):2227–31. [PubMed] [Google Scholar]
- 57.Marquez DC, et al. Estrogen receptors in membrane lipid rafts and signal transduction in breast cancer. Mol Cell Endocrinol. 2006;246(1-2):91–100. doi: 10.1016/j.mce.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 58.Gulbins E, Li PL. Physiological and pathophysiological aspects of ceramide. Am J Physiol Regul Integr Comp Physiol. 2006;290(1):R11–26. doi: 10.1152/ajpregu.00416.2005. [DOI] [PubMed] [Google Scholar]
- 59.Dimanche-Boitrel MT, et al. Role of early plasma membrane events in chemotherapy-induced cell death. Drug Resist Updat. 2005;8(1-2):5–14. doi: 10.1016/j.drup.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 60.Vigh L, et al. The significance of lipid composition for membrane activity: new concepts and ways of assessing function. Prog Lipid Res. 2005;44(5):303–44. doi: 10.1016/j.plipres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Meng X, et al. Cell membrane fatty acid composition differs between normal and malignant cell lines. P R Health Sci J. 2004;23(2):103–6. [PubMed] [Google Scholar]
- 62.Vijayaraghavalu S, et al. Epigenetic modulation of the biophysical properties of drug-resistant cell lipids to restore drug transport and endocytic functions. Mol Pharm. 2012;9(9):2730–42. doi: 10.1021/mp300281t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mullen PJ, et al. The interplay between cell signalling and the mevalonate pathway in cancer. Nat Rev Cancer. 2016 doi: 10.1038/nrc.2016.76. [DOI] [PubMed] [Google Scholar]
- 64.Eytan GD, et al. The role of passive transbilayer drug movement in multidrug resistance and its modulation. J Biol Chem. 1996;271(22):12897–902. doi: 10.1074/jbc.271.22.12897. [DOI] [PubMed] [Google Scholar]
- 65.Niero EL, et al. The multiple facets of drug resistance: one history, different approaches. J Exp Clin Cancer Res. 2014;33:37. doi: 10.1186/1756-9966-33-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Freeman DA, Romero A. Effects of troglitazone on intracellular cholesterol distribution and cholesterol-dependent cell functions in MA-10 Leydig tumor cells. Biochem Pharmacol. 2003;66(2):307–13. doi: 10.1016/s0006-2952(03)00234-x. [DOI] [PubMed] [Google Scholar]
- 67.Sharom FJ. Complex Interplay between the P-Glycoprotein Multidrug Efflux Pump and the Membrane: Its Role in Modulating Protein Function. Front Oncol. 2014;4:41. doi: 10.3389/fonc.2014.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yandim MK, Apohan E, Baran Y. Therapeutic potential of targeting ceramide/glucosylceramide pathway in cancer. Cancer Chemotherapy and Pharmacology. 2013;71(1):13–20. doi: 10.1007/s00280-012-1984-x. [DOI] [PubMed] [Google Scholar]
- 69.Bleicher RJ, Cabot MC. Glucosylceramide synthase and apoptosis. Biochim Biophys Acta. 2002;1585(2-3):172–8. doi: 10.1016/s1388-1981(02)00338-4. [DOI] [PubMed] [Google Scholar]
- 70.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9(2):139–50. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 71.Adada M, Luberto C, Canals D. Inhibitors of the sphingomyelin cycle: Sphingomyelin synthases and sphingomyelinases. Chem Phys Lipids. 2016;197:45–59. doi: 10.1016/j.chemphyslip.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 72.Neubauer HA, et al. An oncogenic role for sphingosine kinase 2. Oncotarget. 2016 doi: 10.18632/oncotarget.11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rauch C. Toward a mechanical control of drug delivery. On the relationship between Lipinski's 2nd rule and cytosolic pH changes in doxorubicin resistance levels in cancer cells: a comparison to published data. Eur Biophys J. 2009;38(7):829–46. doi: 10.1007/s00249-009-0429-x. [DOI] [PubMed] [Google Scholar]
- 74.Petelska AD, Figaszewski ZA. pH Effect of the sphingomyelin membrane interfacial tension. J Membr Biol. 2009;230(1):11–9. doi: 10.1007/s00232-009-9181-5. [DOI] [PubMed] [Google Scholar]
- 75.Irwin ME, et al. Lipid raft localization of EGFR alters the response of cancer cells to the EGFR tyrosine kinase inhibitor gefitinib. J Cell Physiol. 2011;226(9):2316–28. doi: 10.1002/jcp.22570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang Q, et al. Emodin inhibits tumor cell adhesion through disruption of the membrane lipid Raft-associated integrin signaling pathway. Cancer Res. 2006;66(11):5807–15. doi: 10.1158/0008-5472.CAN-06-0077. [DOI] [PubMed] [Google Scholar]
- 77.Gajate C, Mollinedo F. Lipid rafts and Fas/CD95 signaling in cancer chemotherapy. Recent Pat Anticancer Drug Discov. 2011;6(3):274–83. doi: 10.2174/157489211796957766. [DOI] [PubMed] [Google Scholar]
- 78.Alves AC, et al. Biophysics in cancer: The relevance of drug-membrane interaction studies. Biochim Biophys Acta. 2016;1858(9):2231–44. doi: 10.1016/j.bbamem.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 79.Mollinedo F, et al. ET-18-OCH3 (edelfosine): a selective antitumour lipid targeting apoptosis through intracellular activation of Fas/CD95 death receptor. Curr Med Chem. 2004;11(24):3163–84. doi: 10.2174/0929867043363703. [DOI] [PubMed] [Google Scholar]
- 80.Delmas D, et al. Importance of lipid microdomains, rafts, in absorption, delivery, and biological effects of resveratrol. Ann N Y Acad Sci. 2013;1290:90–7. doi: 10.1111/nyas.12177. [DOI] [PubMed] [Google Scholar]
- 81.Veldman RJ,ZS, van Blitterswijk WJ, Verheij M. N-hexanoyl-sphingomyelin potentiates in vitro doxorubicin cytotoxicity by enhancing its cellular influx. Br J Cancer. 2004;90(4):917–925. doi: 10.1038/sj.bjc.6601581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Hell AJ,MM, van Blitterswijk WJ, Gueth DM, Braumuller TM, Pedrosa LRC, Song JY, Marrink SJ, Koning GA, Jonkers J, Verheij M. Defined lipid analogues induce transient channels to facilitate drug-membrane traversal and circumvent cancer therapy resistance. Sci Rep. 2013;3:1949. doi: 10.1038/srep01949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Novotny J, et al. Short-chain ceramides decrease skin barrier properties. Skin Pharmacol Physiol. 2009;22(1):22–30. doi: 10.1159/000183923. [DOI] [PubMed] [Google Scholar]
- 84.Grosch S, Schiffmann S, Geisslinger G. Chain length-specific properties of ceramides. Prog Lipid Res. 2012;51(1):50–62. doi: 10.1016/j.plipres.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 85.Chiantia S, Kahya N, Schwille P. Raft domain reorganization driven by short- and long-chain ceramide: a combined AFM and FCS study. Langmuir. 2007;23(14):7659–65. doi: 10.1021/la7010919. [DOI] [PubMed] [Google Scholar]
- 86.van Lummel M, et al. Enriching lipid nanovesicles with short-chain glucosylceramide improves doxorubicin delivery and efficacy in solid tumors. FASEB J. 2011;25(1):280–9. doi: 10.1096/fj.10-163709. [DOI] [PubMed] [Google Scholar]
- 87.van Hell AJ, et al. Membrane organization determines barrier properties of endothelial cells and short-chain sphingolipid-facilitated doxorubicin influx. Biochim Biophys Acta. 2014;1841(9):1301–7. doi: 10.1016/j.bbalip.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 88.Siskind LJ,FS, Bui M, Colombini M. Sphingosine Forms Channels in Membranes That Differ Greatly from Those Formed by Ceramide. Journal of Bioenergetics and Biomembranes. 2005;37(4):227–236. doi: 10.1007/s10863-005-6632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Lummel M, v.B.W., Vink SR, Veldman RJ, van der Valk MA, Schipper D, Dicheva BM, Eggermont AMM, ten Hagen TLM, Verheij M, Koning GA. Enriching lipid nanovesicles with short-chain glucosylceramide improves doxorubicin delivery and efficacy in solid tumors. FASEB J. 2009;25(1):280–289. doi: 10.1096/fj.10-163709. [DOI] [PubMed] [Google Scholar]
- 90.Veldman RJ,ZS, Koning GA, van Hell Al, Zerp S, Vink SR, Storm G, Verheij M, van Blitterswijk WJ. Coformulated N-Octanoyl-glucosylceramide Improves Cellular Delivery and Cytotoxicity of Liposomal Doxorubicin. 2005:704–710. doi: 10.1124/jpet.105.087486. [DOI] [PubMed] [Google Scholar]
- 91.Pedrosa LR, et al. Improving intracellular doxorubicin delivery through nanoliposomes equipped with selective tumor cell membrane permeabilizing short-chain sphingolipids. Pharm Res. 2013;30(7):1883–95. doi: 10.1007/s11095-013-1031-6. [DOI] [PubMed] [Google Scholar]
- 92.Megha, London E. Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): implications for lipid raft structure and function. J Biol Chem. 2004;279(11):9997–10004. doi: 10.1074/jbc.M309992200. [DOI] [PubMed] [Google Scholar]
- 93.Kok JW, Sietsma H. Sphingolipid metabolism enzymes as targets for anticancer therapy. Curr Drug Targets. 2004;5(4):375–82. doi: 10.2174/1389450043345452. [DOI] [PubMed] [Google Scholar]
- 94.Kraveka JM, et al. Involvement of dihydroceramide desaturase in cell cycle progression in human neuroblastoma cells. J Biol Chem. 2007;282(23):16718–28. doi: 10.1074/jbc.M700647200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Siddiqui A, et al. Mixed backbone antisense glucosylceramide synthase oligonucleotide (MBO-asGCS) loaded solid lipid nanoparticles: in vitro characterization and reversal of multidrug resistance in NCI/ADR-RES cells. Int J Pharm. 2010;400(1-2):251–9. doi: 10.1016/j.ijpharm.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Escrich E, Moral R, Solanas M. Olive oil, an essential component of the Mediterranean diet, and breast cancer. Public Health Nutrition. 2011;14(12A):2323–2332. doi: 10.1017/S1368980011002588. [DOI] [PubMed] [Google Scholar]
- 97.Davidson LA, et al. Chemopreventive n-3 polyunsaturated fatty acids reprogram genetic signatures during colon cancer initiation and progression in the rat. Cancer Research. 2004;64(18):6797–804. doi: 10.1158/0008-5472.CAN-04-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Escriba PV, et al. Membrane lipid therapy: Modulation of the cell membrane composition and structure as a molecular base for drug discovery and new disease treatment. Prog Lipid Res. 2015;59:38–53. doi: 10.1016/j.plipres.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 99.Siddiqui RA, et al. Anticancer properties of propofol-docosahexaenoate and propofoleicosapentaenoate on breast cancer cells. Breast Cancer Res. 2005;7(5):R645–54. doi: 10.1186/bcr1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim SM, et al. Targeting cancer metabolism by simultaneously disrupting parallel nutrient access pathways. J Clin Invest. 2016 doi: 10.1172/JCI87148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eddy SF, Kane SE, Sonenshein GE. Trastuzumab-resistant HER2-driven breast cancer cells are sensitive to epigallocatechin-3 gallate. Cancer Res. 2007;67(19):9018–23. doi: 10.1158/0008-5472.CAN-07-1691. [DOI] [PubMed] [Google Scholar]
- 102.Farabegoli F, et al. (−)-Epigallocatechin-3-gallate downregulates Pg-P and BCRP in a tamoxifen resistant MCF-7 cell line. Phytomedicine. 2010;17(5):356–62. doi: 10.1016/j.phymed.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 103.Yang W, et al. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature. 2016;531(7596):651–5. doi: 10.1038/nature17412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Feng LX, et al. Synergistic enhancement of cancer therapy using a combination of ceramide and docetaxel. Int J Mol Sci. 2014;15(3):4201–20. doi: 10.3390/ijms15034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Vlerken LE, et al. Modulation of intracellular ceramide using polymeric nanoparticles to overcome multidrug resistance in cancer. Cancer Res. 2007;67(10):4843–50. doi: 10.1158/0008-5472.CAN-06-1648. [DOI] [PubMed] [Google Scholar]
- 106.Yan G, et al. Lipidome in colorectal cancer. Oncotarget. 2016 doi: 10.18632/oncotarget.7960. [DOI] [PMC free article] [PubMed] [Google Scholar]