Abstract
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder and the most common form of dementia. Autosomal dominant, familial AD (fAD) is very rare and caused by mutations in amyloid precursor protein (APP), presenilin-1 (PSEN-1), and presenilin-2 (PSEN-2) genes. The pathogenesis of sporadic AD (sAD) is more complex and variants of several genes are associated with an increased lifetime risk of AD. Nuclear and mitochondrial DNA integrity is pivotal during neuronal development, maintenance and function. DNA damage and alterations in cellular DNA repair capacity have been implicated in the aging process and in age-associated neurodegenerative diseases, including AD. These findings are supported by research using animal models of AD and in DNA repair deficient animal models. In recent years, novel mechanisms linking DNA damage to neuronal dysfunction have been identified and have led to the development of noninvasive treatment strategies. Further investigations into the molecular mechanisms connecting DNA damage to AD pathology may help to develop novel treatment strategies for this debilitating disease. Here we provide an overview of the role of genome instability and DNA repair deficiency in AD pathology and discuss research strategies that include genome instability as a component
Keywords: DNA Damage, DNA repair, Alzheimer’s disease
1. Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease affecting nearly 46.8 million people worldwide in 2015. This number is expected to increase to 131.5 million by 2050 due to increasing life expectancy in many populations (Alzheimer’s, 2015; Martin Prince, 2015). Caring for individuals with dementia and the huge costs that this involves represent enormous challenges to societies (Sinha, 2011). The characteristic symptoms of AD are behavioral abnormalities, progressive cognitive and memory impairments, and language impairments (Hardy and Selkoe, 2002; Selkoe, 2002, 2013). Hallmarks of AD pathology include neuronal loss, synaptic dysfunction, senile plaques composed of amyloid β (Aβ) peptides, and neurofibrillary tangles made of hyperphosphorylated tau aggregates (Gotz et al., 2012; Iqbal et al., 2016). Compared to familial AD, sporadic AD is more common and the pathology is multifactorial. Although researchers have not yet established what initiates AD, it is widely considered that the pathogenesis is related to β-amyloid (Aβ) pathology, loss of neuronal function, and uncontrolled neuroinflammation (Selkoe, 1997, 2013). Aβ is produced from APP (Hardy and Higgins, 1992; Selkoe, 1991, 2002). Under normal conditions, APP is processed to produce APPsα, AICD and p3 by α-secretase followed by γ-secretase, in a non-amyloid pathway, but under pathological conditions, APP is cleaved by β-secretase followed by γ-secretase to form Aβ40 and Aβ42, which then aggregate to form Aβ oligomers and fibrils (Haass and Steiner, 2002; Kang et al., 1987; Sinha et al., 1999; Takasugi et al., 2003). Eventually Aβ plaques accumulate in the brain (Hardy and Selkoe, 2002). While there has been much focus on the plaque formation in association with AD, plaques are also seen in normal individuals (Jagust et al., 2010; Klunk et al., 2004; Rodrigue et al., 2012; Rowe et al., 2010; Sperling et al., 2013). The notion that Aβ plaques might not be causative of AD is further supported by the inefficacy of drugs affecting this pathway (Cummings et al., 2014; Godyn et al., 2016; Jia et al., 2014).
Uncontrolled neuroinflammation is a significant pathological feature of normal aging and AD, and it contributes to the cognitive decline (Ryan and Nolan, 2015; Zhang et al., 2015). There are many other factors that contribute to sporadic AD risk, including cardiovascular measures, mitochondrial dysfunction, cholinergic and glutamate transmission defects and DNA damage. There is still no effective treatment for AD (Winslow et al., 2011) and no new drugs have been approved for treatment of AD since 2003 (Godyn et al., 2016). An important relationship exists between DNA damage, repair and aging. Thus we propose further research needs to focus on the relationship between DNA damage, repair and age-related diseases, including AD. The purpose of this article is to review research investigating the role of DNA damage and repair in AD pathogenesis and to provide justification for future AD research and drug development.
2. DNA damage in AD
Increased DNA damage in AD and age-related decline in DNA repair may exacerbate AD progression (Lovell et al., 1999; Lovell and Markesbery, 2007a; Weissman et al., 2009; Weissman et al., 2007; Yang et al., 2008). Several types of DNA damage are associated with neurodegeneration, including bulky adducts, abasic sites, DNA single-strand breaks (SSBs), DNA double-strand breaks (DSBs), base mismatches, insertions and deletions (Caldecott, 2008; Jeppesen et al., 2011; Martin, 2008; McKinnon, 2009; Obulesu and Rao, 2010). Accumulation of genomic DNA damage can be caused by increased rates of damage formation, e.g. epithelial cells of the respiratory tract exposed to air pollution and cigarette smoke, and reduced cellular DNA repair capacity such as those caused by rare hereditary defects as well as age-related alterations. Cells have multiple mechanisms to repair DNA lesions (Figure 1). The major DNA repair pathways are: base excision repair (BER), nucleotide excision repair (NER), mismatch repair (MMR) and double strand break repair (DSBR) and direct reversal (Jeppesen et al., 2011). These are distinct but overlapping DNA repair pathways that repair the different types of lesions in DNA (Figure 1). For example, NER repairs bulky adducts or photoproducts generated by UV-light while oxidative damage, abasic site and DNA SSBs are repaired by BER. MMR repairs base mismatches as well as insertions or deletions. DNA DSBs can be repaired by two different pathways, homologous recombination (HR) or non-homologous end joining (NHEJ).
Figure 1. Pathways of DNA repair.
Genetic or environmental factors cause several types of DNA damage, including bulky adducts, abasic sites, DNA SSBs, DSBs, base alkylation, methylation, base mismatches, insertions and deletions. These DNA lesions may contribute to neuronal loss and AD progression. The major DNA repair pathways are base excision repair (BER), nucleotide excision repair (NER), mismatch repair (MMR), double strand break repair (DSBR) and direct reversal. DSBR includes homologous recombination (HR) and non-homologous end joining (NHEJ).
2.1 Oxidative damage in AD
Oxidative DNA lesions, which are derived from reactive oxygen species (ROS), can result in DNA mutations and promote cancer, aging and inflammation (Oka et al., 2008; Thanan et al., 2015). The most significant DNA lesion affecting the progression of AD is likely oxidative DNA damage (Canugovi et al., 2013; Madabhushi et al., 2014). The brain has a high rate of energy production and consumption. Increased DNA oxidation was reported in post mortem brains of humans with AD (Bradley-Whitman et al., 2014; Butterfield et al., 2001; Finkel and Holbrook, 2000; Gabbita et al., 1998; Lovell et al., 1999; Lovell and Markesbery, 2007a; Markesbery and Lovell, 2006; Wang et al., 2005), mild cognitive impairment (MCI) (Lovell and Markesbery, 2007b; Wang et al., 2006) and preclinical AD (Lovell et al., 2011). It was also observed in many studies that DNA repair is dysregulated in AD (Bucholtz and Demuth, 2013; Canugovi et al., 2014; Forestier et al., 2012; Leandro et al., 2013; Pinto et al., 2013; Weissman et al., 2007; Wu et al., 2014). These findings suggest that oxidative DNA damage and less than optimal DNA repair may play a potential role in the pathogenesis of AD.
Attack by ROS on DNA results in a multitude of oxidized base adducts, one of which is 8-hydroxyguanine (8-OHG). 8-OHG is commonly used as a marker for oxidative stress to DNA, and it was increased in mitochondrial and nuclear DNA in the cortex of AD patients (Mecocci et al., 1994). In addition, the levels of several oxidative adducts in nuclear DNA and mitochondrial DNA (mtDNA) were quantified in samples from the superior and middle temporal gyri, inferior parietal lobule, and cerebellum in patients with MCI, late-stage AD and non-AD neurological disorders and were increased compared to control subjects (Bradley-Whitman et al., 2014), suggesting that DNA oxidation is a general event during AD and likely to present a major threat to neuronal function (Lu et al., 2004).
Base excision repair (BER) is the primary mechanism for repair of small DNA base modifications, e.g. oxidative base lesions and single-strand breaks (Maynard et al., 2009). Mutations in BER genes have been shown to result in higher mutation rates in a variety of organisms, implying that loss of BER can contribute to the development of cancer, neurodegeneration and aging (Sharma and Dianov, 2007; Tell and Demple, 2015; Wilson and Bohr, 2007).
Multiple lines of evidence show that BER plays a role in neurodegeneration. The expression and activity of BER proteins change with aging and AD progression. The expression of uracil DNA glycosylase, 8-oxoguanine DNA glycosylase (OGG1) and DNA Polymerase β (Polβ) are reduced in AD brains (Canugovi et al., 2013). Studies in mice have also provided insight into the role of BER in neurodegeneration and AD. Loss of BER components, including Polβ, apurinic/apyrimidinic endonuclease (APE1) or Ligase III, results in early embryonic or post-natal lethality in mice (Sykora et al., 2013a). In particular, lack of Polβ causes death during embryogenesis and is associated with defects in neuronal development (Sugo et al., 2000). However, the Polβ heterozygotes are viable. Repair of oxidative DNA damage in neurons heavily depends on Polβ (Sykora et al., 2013b; Wei and Englander, 2008). Recently, by generating 3xTg AD mice with a deficiency in Polβ activity, the authors showed that reducing Polβ levels in an AD background was sufficient to accelerate synaptic and cognitive deficits, including neuronal dysfunction and cell death (Sykora et al., 2015).
2.2 DNA strand breaks in AD
In addition to oxidative DNA lesions, ROS also generates DNA strand breaks. Studies have found that there are increased DNA breaks in brains of AD patients (Mullaart et al., 1990) and the damage may precede tangle formation (Su et al., 1997). Also, using sections from hippocampi of AD brains, a high incidence of nuclei with either double-stranded or single-stranded DNA breaks was observed (Adamec et al., 1999). Other studies showed that during physiological brain activity when exploring a novel environment, DNA strand breaks transiently increased in neurons and were rapidly repaired in WT mice, while APP transgenic mice had more severe and prolonged neuronal DNA strand breaks compared to control mice (Suberbielle et al., 2013). The breast and ovarian cancer susceptibility protein 1 (BRCA1) protein plays an important role in DNA double-strand break repair (Zhang and Powell, 2005). In the brains of AD patients, BRCA1 levels are reduced, and likewise DNA repair capacity is reduced. BRCA1 was also found reduced in AD mouse brains and BRCA1 depletion caused learning and memory deficits (Suberbielle et al., 2015). Thus, these results indicate that DNA damage normally occurs during brain activity and that if we can improve double strand break repair capacity in AD brains, aspects of AD may be ameliorated.
Studies showed that 3xTg-AD/Polβ mice exhibit more extensive DSBs, neuronal damage and loss in the hippocampus than 3xTg-AD mice (Sykora et al., 2015), suggesting that Polβ functions in reducing neuronal loss. APE1 protects sensory neurons from cisplatin-induced neurotoxicity, suggesting that APE1 plays a role in improving neuronal function (Kim et al., 2015). Treatment with two inhibitors of critical BER components, the Poly (ADP-Ribose) Polymerase 1 (PARP1) inhibitor ABT-888 and the APE1 inhibitor CRT0044876 in neurons improved synaptic function, indicating that BER functions downstream of DNA oxidation to regulate synaptic transmission (Jagust et al., 2010). Knockdown of BRCA1 in the hippocampus of mice increases neuronal DSBs, alters neuronal structure and function, reduces neuronal size, increases neuronal excitability and impairs LTP, indicating that BRCA1 plays a role in improving synaptic function (Suberbielle et al., 2015). These studies suggest that DNA repair deficiencies can result in neuronal abnormalities, and cognition and memory deficits. Further it underscores the importance that DNA repair makes neuronal cells resistant to stress.
2.3 Mitochondrial DNA damage in AD
Oxidative DNA damage to mitochondrial DNA leads to mutations, deletions, and base substitutions (Mancuso et al., 2007). One AD molecular paradigm is the “amyloid cascade hypothesis”, which is based on studies of rare autosomal dominant variants. Additionally, a mitochondrial cascade hypothesis for AD (Swerdlow et al., 2010; Swerdlow and Khan, 2004) has been proposed and assumes that the inherited, gene-determined make-up of an individual’s electron transport chain determines basal rates of ROS production, which again determines the pace at which acquired mitochondrial DNA, RNA, lipid, and protein damage accumulates. Cells respond to elevated ROS by generating Aβ from APP, which further perturbs mitochondrial function. The accumulation of oxidative stress and formation of Aβ increases mtDNA damage and oxidative stress ultimately leading to neurodegeneration (Swerdlow and Khan, 2004). Researchers have previously implicated mitochondrial dysfunction in AD (Corral-Debrinski et al., 1994; Coskun et al., 2012; Mancuso et al., 2010). Analysis of mtDNA in neurons revealed that Cytochrome c oxidase (COX) deficiency is caused by high levels of mtDNA deletions or damage which accumulate with age (Krishnan et al., 2012). COX deficiency is considered to be a common feature of mitochondrial diseases and is a marker of mtDNA dysfunction. Mitochondrial COX activity is decreased in both brain and platelets of AD patients (Chagnon et al., 1995; Kish et al., 1992; Mutisya et al., 1994; Parker et al., 1990; Parker et al., 1994). Studies showed that mitochondrial DNA deletions cause increased COX deficiency in neurons of patients with sporadic AD compared with age-matched controls (Krishnan et al., 2012). These results suggest that in AD patient brains, COX activity deficiency may be caused by DNA damage (Davis et al., 1997). Another study showed that COX deficiency does not promote the oxidative stress observed in AD but rather is a consequence of the accumulation of Aβ in neurons (Fukui et al., 2007). The studies discussed here indicate that mtDNA deletions are associated with the biochemical deficiency observed in late onset Alzheimer’s disease. However, a causal relationship between mtDNA deletions and AD needs to be researched further.
2.4 Chromosomal deviations in AD
Mutagenic or aberrant DSB repair is a culprit in chromosomal changes (Harvey et al., 1997; Varga and Aplan, 2005). There are some early studies showing cytogenetic and chromosomal aberrations in AD (Buckton et al., 1982; Mark and Brun, 1973; Moorhead and Heyman, 1983; White et al., 1981). Recently, Mosch et al. found that the relative amount of hyperploid neurons was increased in the AD brain (Mosch et al., 2007) Moreover, compared with healthy controls, a significant increase of chromosome 21-specific aneuploidy was detected in the cerebral cortex of postmortem AD (Iourov et al., 2009). Interestingly, X chromosome aneuploidy rates were doubled in neural cells of the hippocampus and prefrontal cortex in AD postmortem brain samples compared to controls (Yurov et al., 2014). Lymphocytes and fibroblasts have been used as surrogate peripheral tissues to investigate damage to the genome in neurodegenerative diseases (Coppede and Migliore, 2009; Migliore et al., 2011). In peripheral blood lymphocytes or skin fibroblasts from AD patients there was an increased frequency of micronuclei and aneuploidy of chromosome 18 and 21 (Geller and Potter, 1999; Migliore et al., 1999; Trippi et al., 2001). Increased trisomy 21 in buccal cells of AD patients has also been reported (Thomas and Fenech, 2008). Thus, many studies report that chromosomal defects are common in AD patients. The main casual explanation for the prevalence of aneuploidy in AD patients is that these patients are vulnerable to neuronal stem cell errors during neurogenesis (Potter, 1991; Taupin, 2011; Yurov et al., 2011; Yurov et al., 2014). If and how these newly generated aneuploid neurons contribute to the pathogenesis of AD still needs additional study.
Chromatin changes occur in response to DNA damage and involves histone modifications, chromatin remodeling, recruiting histone variants and histone chaperones (Avvakumov et al., 2011; Soria et al., 2012). Chromatin modulation through PARylation initiates the DNA damage response and promotes DNA repair. PARylation is mediated by poly-(ADP-ribose) polymerases (PARPs). PARP1 transfers ADP-ribose from NAD+ to protein acceptor sites. Increased PARP1 activity leads to reduced NAD+ levels and cell death. The activation of PARP1 and accumulation of PAR have been demonstrated in post mortem brains of AD patients, particularly in neurons of the frontal and temporal lobes (Love et al., 1999; Strosznajder et al., 2012). Together, these results indicate that chromatin modifications play a major role after DNA damage in AD and suggest that interventions aimed at inhibiting chromatin modification such as PARP1 inhibition, may slow down the progression of AD.
2.5 Epigenetic methylation in AD
Epigenetic mechanisms are involved in learning and memory, behavioral abnormalities and neurodegenerative diseases. Most cases of AD are sporadic AD, which include different susceptible genes/loci and the courses of the disease can be different for each individual (Wang et al., 2016), indicating that epigenetic and environmental factors play an important role in AD etiology. Many studies have shown that methylation plays an essential role in AD occurrence (Zawia et al., 2009). It was found that regions of the human and primate APP promoter displayed tissue and brain region-specific profiles of methylation, which loosely reflects APP expression patterns (Rogaev et al., 1994). An important epigenetic factor affecting AD is folic acid metabolism, also known as one-carbon metabolism, which plays an important role in DNA synthesis, DNA repair and DNA methylation (Coppede, 2010). Interestingly, AD individuals are characterized by having decreased plasma folate values (Clarke et al., 1998; Koseoglu and Karaman, 2007; Malaguarnera et al., 2004; McCaddon et al., 2003; McIlroy et al., 2002; Postiglione et al., 2001; Selley et al., 2002). Animal studies suggest that dietary restriction of folic acid in early life leads to epigenetic modifications increasing PS1 expression, γ-secretase activity, and Aβ levels (Chan and Shea, 2007). Collectively, these data indicate that epigenetic mechanisms play roles in gene expression, DNA repair, and are involved in AD via regulation of APP processing and Aβ production.
3. DNA repair defects in AD
3.1. Aβ and Aβ plaque
According to the amyloid hypothesis (Hardy and Selkoe, 2002), accumulation of Aβ in the brain is a driver of AD pathogenesis. The disease process, including formation of neurofibrillary tangles containing tau protein, is proposed to result from an imbalance between β production and Aβ clearance. Excessive amyloid deposition causes tau hyperphosphorylation and the formation of neurofibrillary tangles which then cause neuronal morphology changes and apoptosis. Amyloid deposition and neuronal apoptosis further induce neuroinflammation, thus creating a vicious cycle.
DNA damage can directly or indirectly lead to altered Aβ levels and plaque deposition. Oxidative stress and reactive ROS damage also contribute to AD pathophysiology. Previous reports showed that ROS enhances β-secretase activity and exacerbates Aβ aggregation (Guglielmotto et al., 2009; Paola et al., 2000; Tamagno et al., 2002). ROS also influence APP processing, promoting Aβ accumulation through β- and γ-secretase activation (Leuner et al., 2012a; Leuner et al., 2012b; Shen et al., 2008). Plaque formation has been correlated with increased ROS levels, lipid peroxidation, DNA strand breaks, and presence of specific oxidized DNA bases and adducts in AD patient brains (Colurso et al., 2003; Williams et al., 2006). There are antioxidant defense enzymes functioning to protect cells from damage due to ROS. These include the superoxide dismutases (SOD), catalase (CAT), and the peroxidases (GPX1 and GPX4). Moreover, SOD2 knock out AD animal models showed increased amyloid deposition and enhanced Aβ levels (Li et al., 2004). Curiously, it has also been shown that mtDNA damage induced by expressing an inducible mitochondrial-targeted endonuclease (Mito-PstI) in the central nervous system, resulted in less Aβ accumulation and lower plaque burden in adult cortical and hippocampal neurons, suggesting that mtDNA damage is not a primary cause of Aβ production (Pinto et al., 2013). In another study, DNA Polβ deficiency led to exacerbated neurodegeneration and demonstrated that a BER deficiency can reduce extracellular and increase intracellular Aβ deposition (Sykora et al., 2015). DNA repair is important for cellular resistance to endogenous damage generated in AD pathophysiology. Lack of DNA repair may not be the cause of AD pathology, but it contributes to the progression of the disease. In the future, it will be important to clarify how loss of DNA repair and the presence of persistent DNA damage contribute to AD pathology. Table 1 describes some known DNA repair gene interactions in the context of AD.
Table 1.
Correlations between AD phenotypes and DNA repair function.
| AD phenotypes | DNA repair pathway |
DNA repair gene |
DNA repair function | Relation between DNA repair and AD |
Reference |
|---|---|---|---|---|---|
| Amyloid β and plaque | BER | Polβ | 3xTg-AD/Polβ+/− mice increase intracellular Aβ production, but reduce extracellular Aβ production and plaque burden. |
↓DNA repair -↓ ↑Aβ - ↑AD |
Sykora et al.,2015 |
| Mitochondrial DNA damage in a mouse model of Alzheimer’s disease decreases Aβ plaque formation. |
↑DNA damage - ↓Aβ - ↓AD |
Pinto et al., 2013 | |||
| Neuronal loss | BER | Polβ | 3xTg-AD/Polβ+/− mice exhibit more extensive neuronal damage and loss in the hippocampus. |
↓DNA repair - ↑Neuronal loss -↑AD |
Sykora et al., 2015 |
| BER | APE1 | APE1 protects sensory neurons from cisplatin- induced neurotoxicity. |
↑DNA repair - ↓Neurotoxicity - ↓AD |
Kim et al., 2015 | |
| Synaptic dysfunction | BER | PARP1, APE1 |
BER functions downstream of DNA oxidation to regulate synaptic transmission. |
↑DNA repair - ↑Synaptic function - ↓AD |
Yu et al., 2015 |
| HR | BRCA1 | Knocking down neuronal BRCA1 in the dentate gyrus causes increased DNA double-strand breaks, neuronal shrinkage, synaptic plasticity impairments, and learning and memory deficits. |
↓DNA repair - ↓Synaptic function - ↑AD |
Suberbielle et al., 2015 | |
| Abnormal neuroinflammation |
BER | PARP1 | Activation of PARP1 regulates production of inflammatory mediators, including IL-1β, IL-6, TNFα, iNOS and COX-2. |
↓DNA repair - ↑Inflammation -↑AD |
El-Hamoly et al., 2014 |
| Vascular factors | DSBR | HDM2 | Statin, which was used in atherosclerosis to reduce serum cholesterol, promotes DNA repair. |
↑DNA repair - ↓Vascular factors -↓AD |
Mahmoudi et al., 2008 |
| Glutamate signaling | BER | APE1 | Physiological levels of glutamate induced reversible nuclear oxidative DNA damage in cerebral cortical neurons. |
↑DNA repair - ↓Glutamate -↓AD |
Yang et al., 2011 |
| Mitochondrial dysfunction |
BER | PARP1 | DNA repair is important for mitochondrial health as is also many other mitochondrial functions like mitophagy. |
↑DNA repair - ↑Mitochondrial function -↓AD |
Scheibye-Knudsen et al., 2014 |
3.2. Tau and tangles
Tau is a microtubule-associated protein important for assembly and stabilization of microtubules. Tau is abundant in the central nervous system and is mainly an axonal protein. Tau is encoded by MAPT gene. In human brains, there are six isoforms of tau that are generated by alternative splicing of exons 2, 3 and 10. These isoforms differ in containing zero, one, or two amino terminal inserts of 29 amino acids each, and three or four microtubule binding domain repeats (Goedert et al., 1989). Tau is a phosphoprotein and its biological activity is strongly influenced by its degree of phosphorylation (Buee et al., 2000). Neurofibrillary tangles (NFTs) that are intraneuronal aggregates of abnormally phosphorylated, insoluble tau protein, are a pathological hallmark of AD and a group of neurodegenerative diseases collectively designated as tauopathies (Iqbal et al., 2016; Mandelkow and Mandelkow, 2012). Recent reports indicate a critical role for tau in the pathogenesis of AD. AD-related pathological forms of tau were detected in the axon of neurons in the brainstem of very young individuals (Braak and Del Tredici, 2011). Moreover, some evidence directly implicates tau in Aβ toxicity (Ittner et al., 2010).
The presence of tau in the nucleus was reported years ago (Loomis et al., 1990). Tau colocalizes with the nucleolar protein nucleolin and may play a role in nucleolar organization (Sjoberg et al., 2006). Moreover, in mouse brains, one tau isoform was enriched in the nucleus (Liu and Gotz, 2013), suggesting a specific role for tau in murine neuronal genome. This is in line with several reports demonstrating a genome protective function for the tau protein. Tau was shown to protect DNA from oxidative damage probably by binding to the minor groove of DNA (Hua and He, 2003; Wei et al., 2008), and prefibrillar tau oligomerization resulted in the loss of the DNA protective function of tau (Violet et al., 2015). In response to heat shock, tau accumulated in the neuronal nucleus and protected DNA from heat shock induced DNA damage (Sultan et al., 2011; Violet et al., 2014). However, in another study, tau knockout protected neuronal DNA from heat shock induced DNA damage (Miao et al., 2010). The reason for these apparent conflicting observations may be related to the experimental conditions used in these studies and needs further investigation.
Pathological tau has also been shown to affect global chromatin structure (Frost et al., 2014), probably via a mechanism involving lamin dysfunction (Frost et al., 2016). Thus, tau directly via interaction with DNA and indirectly by affecting the structure of cytoskeleton and nucleus plays a key role in genome stability. Taken together, there is strong evidence for a key role of tau in genome protection and DNA metabolism. This suggests that abnormal tau structure and aberrant nuclear function of tau could contribute to neuronal genome instability and the pathology of AD. Tau abnormalities can lead to AD via different mechanisms and given the recent failures of drugs targeting Aβ production, more research is now focusing on tau in AD research.
3.3. Neuroinflammation in AD
Abnormal neuroinflammation, including accumulation of activated microglia and astrocytes, is a pathological characteristic of many neurodegenerative diseases. In the brains of AD patients and in AD transgenic mice, amyloid plaques are surrounded by activated microglia and reactive astrocytes. In response to genetic or environmental challenges, microglia and astrocytes are activated, and this is characterized by morphological changes, altered expression of surface antigens, and production of immune modulators.
PARP1 has a role in the DNA damage response and is important for repair of single- and double-stranded DNA breaks. PARP1 inhibition has been shown to be beneficial in inflammatory diseases, such as diabetes, asthma and atherosclerosis (Ebrahimkhani et al., 2014; Sperling et al., 2013), through a variety of means, including the regulation of transcription factors, cytokines, adhesion factors and inflammatory mediators (Cummings et al., 2014; Jia et al., 2014; Stampfer, 2006). NF-κB, an inflammation mediator, was determined as a target for PARP1 activity (Godyn et al., 2016). Activation of PARP1 regulates production of inflammatory mediators, including IL-1β, IL-6, TNFα, INOS and COX-2 (Rodrigue et al., 2012). Therefore, PARP inhibitors may be able to regulate neuroinflammation, but could also cause increased genome instability; thus, their role in AD needs to be studied further.
3.4 Vascular factors
Besides amyloid β and tau, vascular factors are also commonly considered hallmarks of AD. In recent years, several vascular risk factors have been associated with dementia and AD. Many cardiovascular diseases, such as atrial fibrillation, coronary heart disease and heart failure are common in elderly individuals and predispose individuals to AD. Stroke may also increase the risk of AD (Garcia-Alloza et al., 2011; Leys et al., 2005; Snowdon et al., 1997; Troncoso et al., 2008; Vermeer et al., 2003), as stroke causes loss of neuronal tissue and influences amyloid pathology (Garcia-Alloza et al., 2011; Snowdon et al., 1997). AD is more common amongst patients suffering from cardiovascular diseases. However the association is still controversial since some studies have shown that coronary heart disease is related to AD (Newman et al., 2005; Roberts et al., 2010), while others showed no association (Knopman et al., 2005; Petrovitch et al., 1998).
Increasing evidence suggests that accumulation of DNA damage is a risk factor for cardiovascular diseases such as atherosclerosis (Shah and Mahmoudi, 2015). In vascular disease, there is persistent genomic and mitochondrial DNA damage resulting in inflammation, cell senescence and apoptosis (Uryga et al., 2015). Loss of DNA repair genes in mice can also promote vascular defects. ERCC1 null mice, which are nucleotide excision repair deficient, showed increased vascular cell senescence, accelerated development of vasodilator dysfunction, increased vascular stiffness, and elevated blood pressure at a very young age (Durik et al., 2012). These mice also have dementia (Borgesius et al., 2011). Ku deficient mice, which are compromised for DSB repair, displayed abnormal heart histology and function (Ngo et al., 2015) Since DNA damage can trigger vascular disease, a new prospect for prevention and treatment may be to focus on DNA damage prevention or increasing DNA repair capacity. DNA repair is important in vascular disease (Gray et al., 2015), and augmentation of DNA repair may have therapeutic benefits. For example, statins (HMG-CoA reductase inhibitors) which are used against atherosclerosis to reduce serum cholesterol levels may decrease DNA damage and enhance repair (Mahmoudi et al., 2008). Collectively, these studies suggest that loss of DNA repair can alter vascular functions but also raise the question of whether boosting DNA repair mechanisms can ameliorate vascular disease. Since vascular factors are involved in AD, improvements in DNA repair mechanisms may improve AD phenotypes.
3.5 Glutamate signaling
Progressive derangement of neurotransmission is partially responsible for memory impairment in AD. Glutamate is the major excitatory neurotransmitter in the central nervous system, playing an important role in synaptic plasticity, neurogenesis, neurite outgrowth, neuron survival, learning and memory (Mattson, 2008). Several experimental studies suggest that glutamate signaling may play a considerable role in the pathogenesis of AD (Mattson, 2003). AD pathogenesis disrupts neuronal glutamate homeostasis and increases neuronal sensitivity to glutamate, which can produce chronic toxicity (Yang et al., 2011). The excitotoxic damage from glutamate signaling resulted in high levels of γH2AX (Crowe et al., 2006).
At physiological levels glutamate signaling protects neurons against injury and disease in part through inducing DNA repair capability (Yang et al., 2011; Yang et al., 2010). Studies show that physiological levels of glutamate induced reversible nuclear oxidative DNA damage in cerebral cortical neurons. The DNA damage was prevented by Ca2+/calmodulin-dependent protein kinase (CaMK) and cyclic AMP response element binding protein (CREB) signaling (Yang et al., 2011). This signaling was associated with increased DNA repair capacity, specifically increased APE1 expression. Other studies found that NF-κB was also activated in response to glutamate (Jiang et al., 2003). NF-κB upregulates manganese superoxide dismutase (MnSOD) to reduce DNA damage (Mattson et al., 1997) and enhance DNA repair (Wang et al., 2009), thereby protecting neurons against oxidative stress and excitotoxicity. Thus, glutamate signaling up-regulates BER and protects neurons against neurodegeneration. Future studies on the role of glutamate and DNA repair in AD drug development should be considered.
3.6 Mitochondrial dysfunction
Mitochondrial dysfunction occurs early in AD and can cause AD pathology. Mitochondrial dysfunction alters energy metabolism, free radical generation, signaling pathways, cellular calcium homeostasis, reactive oxygen species production and has been implicated in the pathogenesis of AD (Coskun et al., 2012; Davis et al., 1997; Devi et al., 2006; Floyd and Hensley, 2002; Hirai et al., 2001; Leon et al., 2016; Sheehan et al., 1997; Yan and Stern, 2005; Yao et al., 2011; Yao et al., 2009). Both nuclear and mitochondrial DNA damage may lead to mitochondrial dysfunction because nuclear DNA encodes the majority of proteins expressed in mitochondria. In addition, some studies show that Aβ progressively accumulates in the brain mitochondria of AD patients and in transgenic mouse models of AD, exerting deleterious effects on mitochondrial function, including energy failure, altered mitochondrial properties, and elevation of ROS production (Caspersen et al., 2005; Du et al., 2008; Hansson Petersen et al., 2008; Yao et al., 2009). However, the underlying mechanisms of how Aβ impairs mitochondrial function, which might include DNA replication, repair and transcription, are still unclear.
It is generally thought that improving mitochondrial function may ameliorate some AD pathology. Studies show that blockade of Aβ Peptide-Binding Alcohol Dehydrogenase (ABAD)-Aβ interactions improves mitochondrial function, attenuates mitochondrial ROS production, and improves spatial learning and memory in transgenic mAPP mice (Yao et al., 2011). Some antioxidants and mitochondrial targeted compounds also benefit AD, such as methylene blue, piracetam, ginkgo, curcumin and omega-3 polyunsaturated fatty acids (Eckert et al., 2012; Gardner and Boles, 2011; Hroudova et al., 2014). Those antioxidants might improve mitochondrial function by enhancing mitochondrial membrane potential and ATP production as well as by reducing sensitivity to apoptosis in cells and animal models of AD (Leuner et al., 2010). Taken together, improving mitochondrial dysfunction is a new direction of drug development for AD.
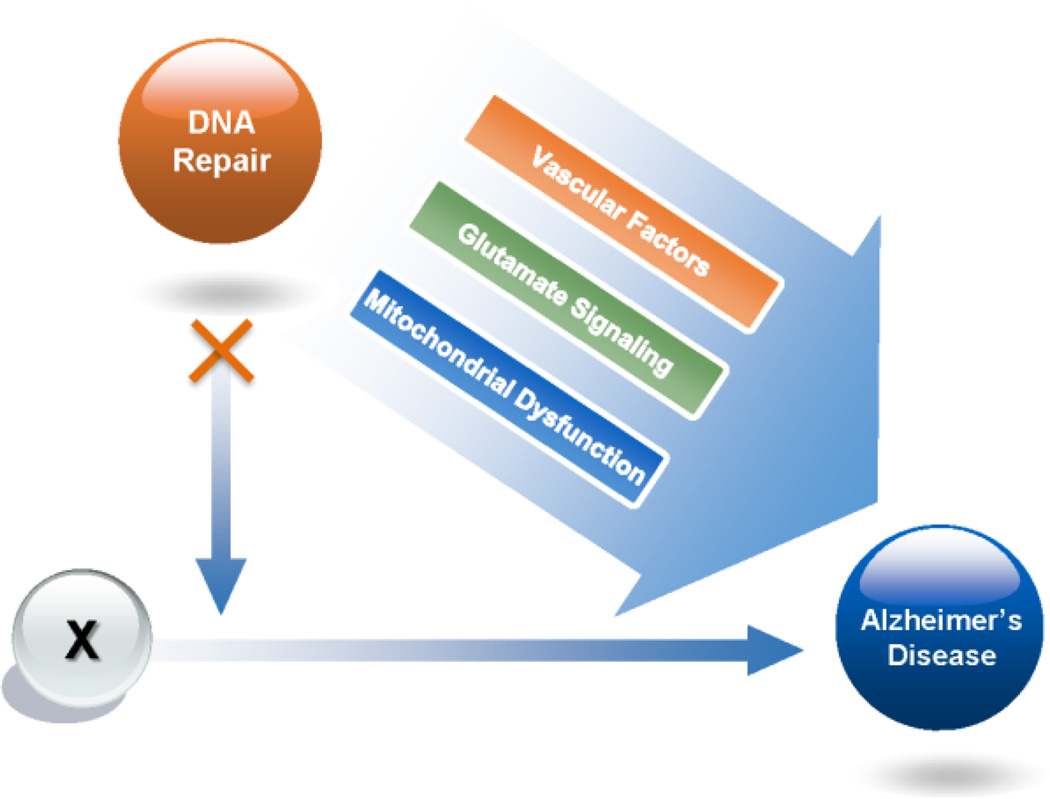
Evidence is emerging that persistent DNA damage alters cellular metabolism and mitochondria homoeostasis, each of which may contribute to the progression of AD. DNA repair fixes damage and improves mitochondrial function (Mandavilli et al., 2002). Cell lines vary in their efficiency in repairing oxidative mtDNA damage. For example, some cell lines such as vascular endothelial and muscle cells are more efficient at repair than neuronal and glial cells. (Hollensworth et al., 2000; Ledoux et al., 1998). DNA repair in mitochondria relies on BER enzymes such as Polβ, APE1, Ligase III etc. and is important for mitochondrial health. Using in vitro and in vivo studies, defective mitophagy was found in Xeroderma pigmentosum group A, a nucleotide excision DNA repair disorder with severe neurodegeneration. Mitophagy, the selective clearance of dysfunctional mitochondria, functions in promoting a healthy pool of mitochondria and in preventing neurodegeneration and premature aging. (Fang et al., 2014; Scheibye-Knudsen et al., 2014). Thus, efficient DNA repair plays an essential role in maintaining mitochondrial function thereby becoming a potential line of treatment for AD (Figure 2).
Figure 2. How DNA repair plays a role in sporadic Alzheimer’s disease.
Since there are several different hypotheses about the origins of AD, we have used an “X” to represent the initiation of AD. Loss of DNA repair may exacerbate Alzheimer’s disease through multiple pathways including changes in vascular factors, glutamate signaling, nuclear and mitochondria DNA damage and repair pathways.
4. Polymorphisms of DNA repair genes in Alzheimer’s disease
One important approach to understanding the impact of DNA repair on AD pathogenesis is the analysis of single nucleotide polymorphisms (SNPs) in DNA repair genes in AD patients. Thus far, no GWAS studies have found an association between DNA repair factors and AD, and therefore it is still unclear whether loss of DNA repair is a risk factor for AD onset. However, it is our proposal that the rate of AD progression maybe more directly impacted by loss of DNA repair capacity than AD initiation.
As discussed earlier, many studies have found that DNA damage is significantly increased in postmortem brains from AD patients (Lovell et al., 1999; Lovell and Markesbery, 2007a; Weissman et al., 2009; Weissman et al., 2007; Yang et al., 2008). Additionally, previous clinical studies have shown that BER deficiencies are associated with the pathogenesis of AD (Lovell et al., 2000). Several studies have analyzed the impact of BER gene polymorphisms on the risk of developing late-onset AD (Table 2).
Table 2.
Polymorphisms of DNA repair genes in AD.
| Gene | Role in DNA repair |
Number of cases/controls |
Studied Polymorphisms |
Major findings | Reference |
|---|---|---|---|---|---|
| OGG1 | BER | 178 / 146 | Ser326Cys | No significant association | Coppede et al., 2007 |
| BER | 91 / 93 | Ser326Cys | No significant association | Parildar-Karpuzoglu et al., 2008 | |
| BER | 41 / 51 | Ser326Cys and Arg46Gln |
The CG Ser326Cys genotype seems to have a tendency to be associated with AD. The Arg46Gln polymorphism is not associated with the pathogenesis of AD. |
Dorszewska et al., 2009 | |
| MUTYH | BER | 120 / 110 | rs3219489 (Gln324His) |
No significant association | Kwiatkowski et al., 2015 |
| APE1 | BER | 91 / 93 | Asp148Glu | No significant association | Parildar-Karpuzoglu et al., 2008 |
| XRCC1 | BER | 98 / 101 | Arg194Trp | The variants seemed to be at two fold risk of AD, although no significant association. |
Dogru-Abbasoglu et al., 2007 |
| BER | 212 / 203 | Arg194Trp | No significant association | Qian et al., 2010 | |
| BER | 91 / 93 | Arg280His, Arg399Gln |
No significant association | Parildar-Karpuzoglu et al., 2008 | |
| BER | 120 / 110 | rs1799782 (Arg194Trp), rs25487 (Arg399Gln) |
Positive association between AD risk and the presence of G/A genotype variant of the Arg399Gln polymorphism. The presence of the A/A genotype of this polymorphism reduced the risk of AD. |
Kwiatkowski et al., 2015 | |
| XPD (ERCC2) |
NER | 97 / 101 | Arg156Arg, Lys751Gln |
No significant association | Dogru-Abbasoglu et al., 2007 |
| XPF (ERCC4) |
NER | 97 / 101 | Ser824Ser | No significant association | Dogru-Abbasoglu et al., 2007 |
| PARP1 | Various | 120 / 110 | rs1136410 (Val762Ala) |
T/C variant increases while the T/T variant reduces risk of AD. |
Kwiatkowski et al., 2015 |
| Various | 120 / 111 | Asp81Asp, Val762Ala |
No significant association in single polymorphisms, but two halotypes were significantly associated with AD. |
Liu et al., 2010 |
Abbreviations: OGG1: 8-oxoguanine DNA Glycosylase; MUTYH: mutY DNA glycosylase; APE1: Apurinic/apyrimidinic endonuclease 1; XRCC1: X-Ray Repair Cross-Complementing Group 1; XPD: Xeroderma pigmentosum group D; XPF: Xeroderma pigmentosum group F; PARP1: Poly (ADP-Ribose) Polymerase 1.
OGG1 is a key enzyme of the BER pathway, which removes 8-OHdG (Nakabeppu, 2001; Nishioka et al., 1999). Studies found no significant associations between the Ser326Cys polymorphism in OGG1 and AD (Coppede et al., 2007; Dorszewska et al., 2009; Parildar-Karpuzoglu et al., 2008), although there are some reports on OGG1 mutations (A53T and A288V) in AD patients (Jacob et al., 2013; Mao et al., 2007). MUTYH, an adenine DNA glycosylase that removes adenine opposite 8-oxodG base pairs (Oka and Nakabeppu, 2011), has also been evaluated and no significant associations between MUTYH and late-onset AD risk identified (Kwiatkowski et al., 2015). APE1 is an endonuclease involved in removal of abasic site in DNA during BER. However, there were no significant associations between the Asp148Glu polymorphisms in APE1 and late-onset AD risk (Parildar-Karpuzoglu et al., 2008). The following three types of polymorphisms in X-ray repair cross-complementing group 1 (XRCC1) have also been investigated: Arg194Trp, Arg280His and Arg399Gln. Studies showed no significant association between these polymorphisms and late-onset AD risk (Dogru-Abbasoglu et al., 2007; Ginsberg et al., 2011; Parildar-Karpuzoglu et al., 2008; Qian et al., 2010); however, there was a positive association between AD risk and the G/A genotype variant of the rs25487 (Arg399Gln) polymorphism while the A/A genotype of Arg399Gln reduced the risk of AD (Kwiatkowski et al., 2015).
Through its elimination of bulky DNA damage, nucleotide excision repair (NER) serves as an important DNA repair pathway. Studies show no significant associations between polymorphisms in Xeroderma pigmentosum group D or group F and late-onset AD risk (Dogru-Abbasoglu et al., 2006).
PARP1 is involved in the regulation of numerous cellular processes, including multiple DNA repair pathways (Burkle, 2006; Gibson and Kraus, 2012). Previous studies examined two silent polymorphisms, c.414C>T and c.2456T>C, in the PARP1 gene of 120 AD patients and 111 control subjects. No association was found between PARP1 single polymorphisms and late-onset AD risk. Interestingly, they found two haplotypes, 414T-2456T and 414C-2456C, were significantly overrepresented in the AD patients whereas the haplotype 414T-2456C showed a protective effect (Liu et al., 2010). Another study showed that in the PARP1 rs1136410 polymorphism, the T/C variant increases AD risk (OR = 4.159, 95% CI: 1.978–8.745) while the T/T variant reduces risk (OR = 0.240, 95% CI: 0.114–0.556) of AD (Kwiatkowski et al., 2015).
Polymorphic variants in DNA repair genes have not generally been evaluated for their association with AD risk, except the few noted above. However, we believe DNA repair alters a cell’s resiliency to stress. Thus, risk analysis may not be the most appropriate analysis to find an associated between DNA repair and AD. Instead, we would argue that the rate of disease progression relative to DNA repair gene polymorphisms is what needs to be measured. If an individual lacking BER capacity is subjected to unremitting neuroinflammation, does a lack of BER alter the progression or speed of neurodegeneration, Aβ deposition, or tau pathophysiology? This type of rate of disease progression versus DNA repair genotyping would provide useful insight.
5. DNA repair in neurological disease
Maintaining genomic stability in the nervous system is important because mutations in DNA repair factors cause many congenital diseases of the nervous system. Mutations in NER components results in syndromes such as Xeroderma Pigmentosum (XP), Cockayne Syndrome (CS), and Trichothiodystrophy (TTD), all of which have severe neurological features. About a quarter of XP patients show neurological abnormalities including microcephaly, mental retardation, and deafness (Mimaki et al., 1986). CS patients exhibit developmental failure, premature aging, and progressive neurodegeneration. Most TTD patients show neurological abnormalities. Mutations in the ATM gene, which cause Ataxia Telangiectasia and are involved in double strand break repair also cause neurological problems (Shiloh and Ziv, 2013). While AT is a multisystem disease, its hallmark features are neurological including movement and coordination defects, cerebellar atrophy, lack of natural eye movements and slurred speech (Biton et al., 2008). DNA repair of single strand breaks and blocked DNA ends is also essential to the nervous system. Discovery of two diseases called ataxia with occulomotor apraxia-1 (AOA1) and spinocerebellar ataxia with axonal neuropathy (SCAN1) accentuate this point. The presentation of these diseases is limited to the nervous system, and the findings show that, single strand break repair is extremely important to preserve neurological health. It is our belief that DNA repair functions are essential to the nervous system and modulation of DNA repair could potentially benefit AD patients.
6. Summary and future prospects
In summary, several types of DNA damage are found in AD patients and AD animal models, and these include oxidation DNA damage, DNA strand breaks, mitochondrial DNA damage and others. This suggests that the DNA damage response in AD may be deficient, and further that DNA damage may be exacerbating AD progression. Neuronal vulnerability to a particular type of DNA damage, along with a subsequent decrease in repair of that damage, may cause these adducts to accumulate with age and promote brain aging and neurodegeneration. Oxidative DNA damage and DNA strand breaks may be causative in AD or may be subsequent to other events, but is likely an important factor to consider. While identification of causal factors for AD is clearly important, it is also important task is to identify genes and environmental triggers that contribute to the rate of AD progression. AD is a multi-factorial disease with millions of people afflicted so identification of genes, like DNA repair genes, that may modify the rate of disease progression needs to be carried out. Further, this raises the question of whether stimulation of DNA repair may increase the resiliency of AD neurons and forestall elements of AD neurodegeneration.
Highlights.
DNA damage and defects in DNA repair processing may exacerbate AD progression.
DNA repair likely plays a role in ameliorating AD pathogenesis directly and indirectly.
Polymorphic variants of DNA repair genes may be associated with AD risk.
Neuronal vulnerability to a particular type of DNA damage, along with a subsequent decrease in repair, may cause these adducts to accumulate with age and promote brain aging and neurodegeneration.
Acknowledgments
This work was supported entirely by Intramural Research Program of National Institute on Aging, National Institutes of Health. We thank Qiping Lu and Stephanie Cordonnier for critically reading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamec E, Vonsattel JP, Nixon RA. DNA strand breaks in Alzheimer’s disease. Brain research. 1999;849:67–77. doi: 10.1016/s0006-8993(99)02004-1. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s A. 2015 Alzheimer’s disease facts and figures. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Avvakumov N, Nourani A, Cote J. Histone chaperones: modulators of chromatin marks. Molecular cell. 2011;41:502–514. doi: 10.1016/j.molcel.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Biton S, Barzilai A, Shiloh Y. The neurological phenotype of ataxia-telangiectasia: solving a persistent puzzle. DNA repair. 2008;7:1028–1038. doi: 10.1016/j.dnarep.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Borgesius NZ, de Waard MC, van der Pluijm I, Omrani A, Zondag GC, van der Horst GT, Melton DW, Hoeijmakers JH, Jaarsma D, Elgersma Y. Accelerated age-related cognitive decline and neurodegeneration, caused by deficient DNA repair. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:12543–12553. doi: 10.1523/JNEUROSCI.1589-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta neuropathologica. 2011;121:171–181. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- Bradley-Whitman MA, Timmons MD, Beckett TL, Murphy MP, Lynn BC, Lovell MA. Nucleic acid oxidation: an early feature of Alzheimer’s disease. Journal of neurochemistry. 2014;128:294–304. doi: 10.1111/jnc.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholtz N, Demuth I. DNA-repair in mild cognitive impairment and Alzheimer’s disease. DNA repair. 2013;12:811–816. doi: 10.1016/j.dnarep.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Buckton KE, Whalley LJ, Lee M, Christie JE. Chromosome aneuploidy in Alzheimer’s disease. Experimental brain research Suppl. 1982;5:58–63. doi: 10.1007/978-3-642-68507-1_9. [DOI] [PubMed] [Google Scholar]
- Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain research. Brain research reviews. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- Burkle A. DNA repair and PARP in aging. Free radical research. 2006;40:1295–1302. doi: 10.1080/10715760600915288. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid beta-peptide. Trends in molecular medicine. 2001;7:548–554. doi: 10.1016/s1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- Caldecott KW. Single-strand break repair and genetic disease. Nature reviews. Genetics. 2008;9:619–631. doi: 10.1038/nrg2380. [DOI] [PubMed] [Google Scholar]
- Canugovi C, Misiak M, Ferrarelli LK, Croteau DL, Bohr VA. The role of DNA repair in brain related disease pathology. DNA repair. 2013;12:578–587. doi: 10.1016/j.dnarep.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canugovi C, Shamanna RA, Croteau DL, Bohr VA. Base excision DNA repair levels in mitochondrial lysates of Alzheimer’s disease. Neurobiology of aging. 2014;35:1293–1300. doi: 10.1016/j.neurobiolaging.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, McKhann G, Yan SD. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:2040–2041. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- Chagnon P, Betard C, Robitaille Y, Cholette A, Gauvreau D. Distribution of brain cytochrome oxidase activity in various neurodegenerative diseases. Neuroreport. 1995;6:711–715. doi: 10.1097/00001756-199503270-00002. [DOI] [PubMed] [Google Scholar]
- Chan A, Shea TB. Folate deprivation increases presenilin expression, gamma-secretase activity, and Abeta levels in murine brain: potentiation by ApoE deficiency and alleviation by dietary S-adenosyl methionine. Journal of neurochemistry. 2007;102:753–760. doi: 10.1111/j.1471-4159.2007.04589.x. [DOI] [PubMed] [Google Scholar]
- Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Archives of neurology. 1998;55:1449–1455. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- Colurso GJ, Nilson JE, Vervoort LG. Quantitative assessment of DNA fragmentation and beta-amyloid deposition in insular cortex and midfrontal gyrus from patients with Alzheimer’s disease. Life sciences. 2003;73:1795–1803. doi: 10.1016/s0024-3205(03)00512-5. [DOI] [PubMed] [Google Scholar]
- Coppede F. One-carbon metabolism and Alzheimer’s disease: focus on epigenetics. Current genomics. 2010;11:246–260. doi: 10.2174/138920210791233090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppede F, Mancuso M, Lo Gerfo A, Manca ML, Petrozzi L, Migliore L, Siciliano G, Murri L. A Ser326Cys polymorphism in the DNA repair gene hOGG1 is not associated with sporadic Alzheimer’s disease. Neuroscience letters. 2007;414:282–285. doi: 10.1016/j.neulet.2006.12.035. [DOI] [PubMed] [Google Scholar]
- Coppede F, Migliore L. DNA damage and repair in Alzheimer’s disease. Current Alzheimer research. 2009;6:36–47. doi: 10.2174/156720509787313970. [DOI] [PubMed] [Google Scholar]
- Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, McKee AC, Beal MF, Graham BH, Wallace DC. Marked changes in mitochondrial DNA deletion levels in Alzheimer brains. Genomics. 1994;23:471–476. doi: 10.1006/geno.1994.1525. [DOI] [PubMed] [Google Scholar]
- Coskun P, Wyrembak J, Schriner SE, Chen HW, Marciniack C, Laferla F, Wallace DC. A mitochondrial etiology of Alzheimer and Parkinson disease. Biochimica et biophysica acta. 2012;1820:553–564. doi: 10.1016/j.bbagen.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe SL, Movsesyan VA, Jorgensen TJ, Kondratyev A. Rapid phosphorylation of histone H2A.X following ionotropic glutamate receptor activation. The European journal of neuroscience. 2006;23:2351–2361. doi: 10.1111/j.1460-9568.2006.04768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL, Morstorf T, Zhong K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimer’s research & therapy. 2014;6:37. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RE, Miller S, Herrnstadt C, Ghosh SS, Fahy E, Shinobu LA, Galasko D, Thal LJ, Beal MF, Howell N, Parker WD., Jr Mutations in mitochondrial cytochrome c oxidase genes segregate with late-onset Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4526–4531. doi: 10.1073/pnas.94.9.4526. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogru-Abbasoglu S, Aykac-Toker G, Hanagasi HA, Gurvit H, Emre M, Uysal M. The Arg194Trp polymorphism in DNA repair gene XRCC1 and the risk for sporadic late-onset Alzheimer’s disease. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2007;28:31–34. doi: 10.1007/s10072-007-0744-x. [DOI] [PubMed] [Google Scholar]
- Dogru-Abbasoglu S, Inceoglu M, Parildar-Karpuzoglu H, Hanagasi HA, Karadag B, Gurvit H, Emre M, Aykac-Toker G, Uysal M. Polymorphisms in the DNA repair genes XPD (ERCC2) and XPF (ERCC4) are not associated with sporadic late-onset Alzheimer’s disease. Neuroscience letters. 2006;404:258–261. doi: 10.1016/j.neulet.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Dorszewska J, Kempisty B, Jaroszewska-Kolecka J, Rozycka A, Florczak J, Lianeri M, Jagodzinski PP, Kozubski W. Expression and polymorphisms of gene 8-oxoguanine glycosylase 1 and the level of oxidative DNA damage in peripheral blood lymphocytes of patients with Alzheimer’s disease. DNA and cell biology. 2009;28:579–588. doi: 10.1089/dna.2009.0926. [DOI] [PubMed] [Google Scholar]
- Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nature medicine. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durik M, Kavousi M, van der Pluijm I, Isaacs A, Cheng C, Verdonk K, Loot AE, Oeseburg H, Bhaggoe UM, Leijten F, van Veghel R, de Vries R, Rudez G, Brandt R, Ridwan YR, van Deel ED, de Boer M, Tempel D, Fleming I, Mitchell GF, Verwoert GC, Tarasov KV, Uitterlinden AG, Hofman A, Duckers HJ, van Duijn CM, Oostra BA, Witteman JC, Duncker DJ, Danser AH, Hoeijmakers JH, Roks AJ. Nucleotide excision DNA repair is associated with age-related vascular dysfunction. Circulation. 2012;126:468–478. doi: 10.1161/CIRCULATIONAHA.112.104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimkhani MR, Daneshmand A, Mazumder A, Allocca M, Calvo JA, Abolhassani N, Jhun I, Muthupalani S, Ayata C, Samson LD. Aag-initiated base excision repair promotes ischemia reperfusion injury in liver, brain, and kidney. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E4878–E4886. doi: 10.1073/pnas.1413582111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert GP, Renner K, Eckert SH, Eckmann J, Hagl S, Abdel-Kader RM, Kurz C, Leuner K, Muller WE. Mitochondrial dysfunction--a pharmacological target in Alzheimer’s disease. Molecular neurobiology. 2012;46:136–150. doi: 10.1007/s12035-012-8271-z. [DOI] [PubMed] [Google Scholar]
- Fang EF, Scheibye-Knudsen M, Brace LE, Kassahun H, SenGupta T, Nilsen H, Mitchell JR, Croteau DL, Bohr VA. Defective mitophagy in XPA via PARP-1 hyperactivation and NAD(+)/SIRT1 reduction. Cell. 2014;157:882–896. doi: 10.1016/j.cell.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Floyd RA, Hensley K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiology of aging. 2002;23:795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Forestier A, Douki T, Sauvaigo S, De Rosa V, Demeilliers C, Rachidi W. Alzheimer’s disease-associated neurotoxic peptide amyloid-beta impairs base excision repair in human neuroblastoma cells. International journal of molecular sciences. 2012;13:14766–14787. doi: 10.3390/ijms131114766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B, Bardai FH, Feany MB. Lamin Dysfunction Mediates Neurodegeneration in Tauopathies. Current biology : CB. 2016;26:129–136. doi: 10.1016/j.cub.2015.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B, Hemberg M, Lewis J, Feany MB. Tau promotes neurodegeneration through global chromatin relaxation. Nature neuroscience. 2014;17:357–366. doi: 10.1038/nn.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui H, Diaz F, Garcia S, Moraes CT. Cytochrome c oxidase deficiency in neurons decreases both oxidative stress and amyloid formation in a mouse model of Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14163–14168. doi: 10.1073/pnas.0705738104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbita SP, Lovell MA, Markesbery WR. Increased nuclear DNA oxidation in the brain in Alzheimer’s disease. Journal of neurochemistry. 1998;71:2034–2040. doi: 10.1046/j.1471-4159.1998.71052034.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Gregory J, Kuchibhotla KV, Fine S, Wei Y, Ayata C, Frosch MP, Greenberg SM, Bacskai BJ. Cerebrovascular lesions induce transient beta-amyloid deposition. Brain : a journal of neurology. 2011;134:3697–3707. doi: 10.1093/brain/awr300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner A, Boles RG. Beyond the serotonin hypothesis: mitochondria, inflammation and neurodegeneration in major depression and affective spectrum disorders. Progress in neuro-psychopharmacology & biological psychiatry. 2011;35:730–743. doi: 10.1016/j.pnpbp.2010.07.030. [DOI] [PubMed] [Google Scholar]
- Geller LN, Potter H. Chromosome missegregation and trisomy 21 mosaicism in Alzheimer’s disease. Neurobiology of disease. 1999;6:167–179. doi: 10.1006/nbdi.1999.0236. [DOI] [PubMed] [Google Scholar]
- Gibson BA, Kraus WL. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nature reviews. Molecular cell biology. 2012;13:411–424. doi: 10.1038/nrm3376. [DOI] [PubMed] [Google Scholar]
- Ginsberg G, Angle K, Guyton K, Sonawane B. Polymorphism in the DNA repair enzyme XRCC1: utility of current database and implications for human health risk assessment. Mutation research. 2011;727:1–15. doi: 10.1016/j.mrrev.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Godyn J, Jonczyk J, Panek D, Malawska B. Therapeutic strategies for Alzheimer’s disease in clinical trials. Pharmacological reports : PR. 2016;68:127–138. doi: 10.1016/j.pharep.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Potier MC, Ulrich J, Crowther RA. Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. The EMBO journal. 1989;8:393–399. doi: 10.1002/j.1460-2075.1989.tb03390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz J, Ittner A, Ittner LM. Tau-targeted treatment strategies in Alzheimer’s disease. British journal of pharmacology. 2012;165:1246–1259. doi: 10.1111/j.1476-5381.2011.01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray K, Kumar S, Figg N, Harrison J, Baker L, Mercer J, Littlewood T, Bennett M. Effects of DNA damage in smooth muscle cells in atherosclerosis. Circulation research. 2015;116:816–826. doi: 10.1161/CIRCRESAHA.116.304921. [DOI] [PubMed] [Google Scholar]
- Guglielmotto M, Tamagno E, Danni O. Oxidative stress and hypoxia contribute to Alzheimer’s disease pathogenesis: two sides of the same coin. TheScientificWorldJournal. 2009;9:781–791. doi: 10.1100/tsw.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Steiner H. Alzheimer disease gamma-secretase: a complex story of GxGD-type presenilin proteases. Trends in cell biology. 2002;12:556–562. doi: 10.1016/s0962-8924(02)02394-2. [DOI] [PubMed] [Google Scholar]
- Hansson Petersen CA, Alikhani N, Behbahani H, Wiehager B, Pavlov PF, Alafuzoff I, Leinonen V, Ito A, Winblad B, Glaser E, Ankarcrona M. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:13145–13150. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Harvey AN, Costa ND, Savage JR, Thacker J. Chromosomal aberrations induced by defined DNA double-strand breaks: the origin of achromatic lesions. Somatic cell and molecular genetics. 1997;23:211–219. doi: 10.1007/BF02721372. [DOI] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer’s disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollensworth SB, Shen C, Sim JE, Spitz DR, Wilson GL, LeDoux SP. Glial cell type-specific responses to menadione-induced oxidative stress. Free radical biology & medicine. 2000;28:1161–1174. doi: 10.1016/s0891-5849(00)00214-8. [DOI] [PubMed] [Google Scholar]
- Hroudova J, Singh N, Fisar Z. Mitochondrial dysfunctions in neurodegenerative diseases: relevance to Alzheimer’s disease. BioMed research international. 2014:175062. doi: 10.1155/2014/175062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Q, He RQ. Tau could protect DNA double helix structure. Biochimica et biophysica acta. 2003;1645:205–211. doi: 10.1016/s1570-9639(02)00538-1. [DOI] [PubMed] [Google Scholar]
- Iourov IY, Vorsanova SG, Liehr T, Yurov YB. Aneuploidy in the normal, Alzheimer’s disease and ataxia-telangiectasia brain: differential expression and pathological meaning. Neurobiology of disease. 2009;34:212–220. doi: 10.1016/j.nbd.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Liu F, Gong CX. Tau and neurodegenerative disease: the story so far. Nature reviews. Neurology. 2016;12:15–27. doi: 10.1038/nrneurol.2015.225. [DOI] [PubMed] [Google Scholar]
- Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, Wolfing H, Chieng BC, Christie MJ, Napier IA, Eckert A, Staufenbiel M, Hardeman E, Gotz J. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- Jacob KD, Noren Hooten N, Tadokoro T, Lohani A, Barnes J, Evans MK. Alzheimer’s disease-associated polymorphisms in human OGG1 alter catalytic activity and sensitize cells to DNA damage. Free radical biology & medicine. 2013;63:115–125. doi: 10.1016/j.freeradbiomed.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust WJ, Bandy D, Chen K, Foster NL, Landau SM, Mathis CA, Price JC, Reiman EM, Skovronsky D, Koeppe RA Alzheimer’s Disease Neuroimaging, I. The Alzheimer’s Disease Neuroimaging Initiative positron emission tomography core. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2010;6:221–229. doi: 10.1016/j.jalz.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen DK, Bohr VA, Stevnsner T. DNA repair deficiency in neurodegeneration. Prog Neurobiol. 2011;94:166–200. doi: 10.1016/j.pneurobio.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Q, Deng Y, Qing H. Potential therapeutic strategies for Alzheimer’s disease targeting or beyond beta-amyloid: insights from clinical trials. BioMed research international. 2014:837157. doi: 10.1155/2014/837157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Zhu D, Okagaki P, Lipsky R, Wu X, Banaudha K, Mearow K, Strauss KI, Marini AM. N-methyl-D-aspartate and TrkB receptor activation in cerebellar granule cells: an in vitro model of preconditioning to stimulate intrinsic survival pathways in neurons. Annals of the New York Academy of Sciences. 2003;993:134–145. doi: 10.1111/j.1749-6632.2003.tb07522.x. discussion 159–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Muller-Hill B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kim HS, Guo C, Thompson EL, Jiang Y, Kelley MR, Vasko MR, Lee SH. APE1, the DNA base excision repair protein, regulates the removal of platinum adducts in sensory neuronal cultures by NER. Mutation research. 2015;779:96–104. doi: 10.1016/j.mrfmmm.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Bergeron C, Rajput A, Dozic S, Mastrogiacomo F, Chang LJ, Wilson JM, DiStefano LM, Nobrega JN. Brain cytochrome oxidase in Alzheimer’s disease. Journal of neurochemistry. 1992;59:776–779. doi: 10.1111/j.1471-4159.1992.tb09439.x. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Annals of neurology. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Petersen RC, Cha RH, Edland SD, Rocca WA. Coronary artery bypass grafting is not a risk factor for dementia or Alzheimer disease. Neurology. 2005;65:986–990. doi: 10.1212/01.wnl.0000171954.92119.c7. [DOI] [PubMed] [Google Scholar]
- Koseoglu E, Karaman Y. Relations between homocysteine, folate and vitamin B12 in vascular dementia and in Alzheimer disease. Clinical biochemistry. 2007;40:859–863. doi: 10.1016/j.clinbiochem.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Krishnan KJ, Ratnaike TE, De Gruyter HL, Jaros E, Turnbull DM. Mitochondrial DNA deletions cause the biochemical defect observed in Alzheimer’s disease. Neurobiology of aging. 2012;33:2210–2214. doi: 10.1016/j.neurobiolaging.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D, Czarny P, Galecki P, Bachurska A, Talarowska M, Orzechowska A, Bobinska K, Bielecka-Kowalska A, Pietras T, Szemraj J, Maes M, Sliwinski T. Variants of Base Excision Repair Genes MUTYH , PARP1 and XRCC1 in Alzheimer’s Disease Risk. Neuropsychobiology. 2015;71:176–186. doi: 10.1159/000381985. [DOI] [PubMed] [Google Scholar]
- Leandro GS, Lobo RR, Oliveira DV, Moriguti JC, Sakamoto-Hojo ET. Lymphocytes of patients with Alzheimer’s disease display different DNA damage repair kinetics and expression profiles of DNA repair and stress response genes. International journal of molecular sciences. 2013;14:12380–12400. doi: 10.3390/ijms140612380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux SP, Shen CC, Grishko VI, Fields PA, Gard AL, Wilson GL. Glial cell-specific differences in response to alkylation damage. Glia. 1998;24:304–312. [PubMed] [Google Scholar]
- Leon J, Sakumi K, Castillo E, Sheng Z, Oka S, Nakabeppu Y. 8-Oxoguanine accumulation in mitochondrial DNA causes mitochondrial dysfunction and impairs neuritogenesis in cultured adult mouse cortical neurons under oxidative conditions. Scientific reports. 2016;6:22086. doi: 10.1038/srep22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner K, Kurz C, Guidetti G, Orgogozo JM, Muller WE. Improved mitochondrial function in brain aging and Alzheimer disease - the new mechanism of action of the old metabolic enhancer piracetam. Frontiers in neuroscience. 2010:4. doi: 10.3389/fnins.2010.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner K, Muller WE, Reichert AS. From mitochondrial dysfunction to amyloid beta formation: novel insights into the pathogenesis of Alzheimer’s disease. Molecular neurobiology. 2012a;46:186–193. doi: 10.1007/s12035-012-8307-4. [DOI] [PubMed] [Google Scholar]
- Leuner K, Schutt T, Kurz C, Eckert SH, Schiller C, Occhipinti A, Mai S, Jendrach M, Eckert GP, Kruse SE, Palmiter RD, Brandt U, Drose S, Wittig I, Willem M, Haass C, Reichert AS, Muller WE. Mitochondrion-derived reactive oxygen species lead to enhanced amyloid beta formation. Antioxidants & redox signaling. 2012b;16:1421–1433. doi: 10.1089/ars.2011.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys D, Henon H, Mackowiak-Cordoliani MA, Pasquier F. Poststroke dementia. The Lancet. Neurology. 2005;4:752–759. doi: 10.1016/S1474-4422(05)70221-0. [DOI] [PubMed] [Google Scholar]
- Li F, Calingasan NY, Yu F, Mauck WM, Toidze M, Almeida CG, Takahashi RH, Carlson GA, Flint Beal M, Lin MT, Gouras GK. Increased plaque burden in brains of APP mutant MnSOD heterozygous knockout mice. Journal of neurochemistry. 2004;89:1308–1312. doi: 10.1111/j.1471-4159.2004.02455.x. [DOI] [PubMed] [Google Scholar]
- Liu C, Gotz J. Profiling murine tau with 0N, 1N and 2N isoform-specific antibodies in brain and peripheral organs reveals distinct subcellular localization, with the 1N isoform being enriched in the nucleus. PloS one. 2013;8:e84849. doi: 10.1371/journal.pone.0084849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HP, Lin WY, Wu BT, Liu SH, Wang WF, Tsai CH, Lee CC, Tsai FJ. Evaluation of the poly(ADP-ribose) polymerase-1 gene variants in Alzheimer’s disease. Journal of clinical laboratory analysis. 2010;24:182–186. doi: 10.1002/jcla.20379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis PA, Howard TH, Castleberry RP, Binder LI. Identification of nuclear tau isoforms in human neuroblastoma cells. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:8422–8426. doi: 10.1073/pnas.87.21.8422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love S, Barber R, Wilcock GK. Increased poly(ADP-ribosyl)ation of nuclear proteins in Alzheimer’s disease. Brain : a journal of neurology. 1999;122(Pt 2):247–253. doi: 10.1093/brain/122.2.247. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Gabbita SP, Markesbery WR. Increased DNA oxidation and decreased levels of repair products in Alzheimer’s disease ventricular CSF. Journal of neurochemistry. 1999;72:771–776. doi: 10.1046/j.1471-4159.1999.0720771.x. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Markesbery WR. Oxidative damage in mild cognitive impairment and early Alzheimer’s disease. Journal of neuroscience research. 2007a;85:3036–3040. doi: 10.1002/jnr.21346. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Markesbery WR. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer’s disease. Nucleic acids research. 2007b;35:7497–7504. doi: 10.1093/nar/gkm821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell MA, Soman S, Bradley MA. Oxidatively modified nucleic acids in preclinical Alzheimer’s disease (PCAD) brain. Mechanisms of ageing and development. 2011;132:443–448. doi: 10.1016/j.mad.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell MA, Xie C, Markesbery WR. Decreased base excision repair and increased helicase activity in Alzheimer’s disease brain. Brain research. 2000;855:116–123. doi: 10.1016/s0006-8993(99)02335-5. [DOI] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Madabhushi R, Pan L, Tsai LH. DNA damage and its links to neurodegeneration. Neuron. 2014;83:266–282. doi: 10.1016/j.neuron.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi M, Gorenne I, Mercer J, Figg N, Littlewood T, Bennett M. Statins use a novel Nijmegen breakage syndrome-1-dependent pathway to accelerate DNA repair in vascular smooth muscle cells. Circulation research. 2008;103:717–725. doi: 10.1161/CIRCRESAHA.108.182899. [DOI] [PubMed] [Google Scholar]
- Malaguarnera M, Ferri R, Bella R, Alagona G, Carnemolla A, Pennisi G. Homocysteine, vitamin B12 and folate in vascular dementia and in Alzheimer disease. Clinical chemistry and laboratory medicine. 2004;42:1032–1035. doi: 10.1515/CCLM.2004.208. [DOI] [PubMed] [Google Scholar]
- Mancuso M, Coppede F, Murri L, Siciliano G. Mitochondrial cascade hypothesis of Alzheimer’s disease: myth or reality? Antioxidants & redox signaling. 2007;9:1631–1646. doi: 10.1089/ars.2007.1761. [DOI] [PubMed] [Google Scholar]
- Mancuso M, Orsucci D, LoGerfo A, Calsolaro V, Siciliano G. Clinical features and pathogenesis of Alzheimer’s disease: involvement of mitochondria and mitochondrial DNA. Advances in experimental medicine and biology. 2010;685:34–44. doi: 10.1007/978-1-4419-6448-9_4. [DOI] [PubMed] [Google Scholar]
- Mandavilli BS, Santos JH, Van Houten B. Mitochondrial DNA repair and aging. Mutation research. 2002;509:127–151. doi: 10.1016/s0027-5107(02)00220-8. [DOI] [PubMed] [Google Scholar]
- Mandelkow EM, Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harbor perspectives in medicine. 2012;2:a006247. doi: 10.1101/cshperspect.a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao G, Pan X, Zhu BB, Zhang Y, Yuan F, Huang J, Lovell MA, Lee MP, Markesbery WR, Li GM, Gu L. Identification and characterization of OGG1 mutations in patients with Alzheimer’s disease. Nucleic acids research. 2007;35:2759–2766. doi: 10.1093/nar/gkm189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark J, Brun A. Chromosomal deviations in Alzheimer’s disease compared to those in senescence and senile dementia. Gerontologia clinica. 1973;15:253–258. doi: 10.1159/000245464. [DOI] [PubMed] [Google Scholar]
- Markesbery WR, Lovell MA. DNA oxidation in Alzheimer’s disease. Antioxidants & redox signaling. 2006;8:2039–2045. doi: 10.1089/ars.2006.8.2039. [DOI] [PubMed] [Google Scholar]
- Martin LJ. DNA damage and repair: relevance to mechanisms of neurodegeneration. Journal of neuropathology and experimental neurology. 2008;67:377–387. doi: 10.1097/NEN.0b013e31816ff780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin Prince AW, Guerchet Maëlenn, Ali Gemma-Claire, Wu Yu-Tzu, Prina Matthew. World Alzheimer Report 2015. The global impact of dementia. An analysis of prevalence, incidence, cost and trends. London, UK: Alzheimer’s Disease International; 2015. [Google Scholar]
- Mattson MP. Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolecular medicine. 2003;3:65–94. doi: 10.1385/NMM:3:2:65. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Glutamate and neurotrophic factors in neuronal plasticity and disease. Annals of the New York Academy of Sciences. 2008;1144:97–112. doi: 10.1196/annals.1418.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Goodman Y, Luo H, Fu W, Furukawa K. Activation of NF-kappaB protects hippocampal neurons against oxidative stress-induced apoptosis: evidence for induction of manganese superoxide dismutase and suppression of peroxynitrite production and protein tyrosine nitration. Journal of neuroscience research. 1997;49:681–697. doi: 10.1002/(SICI)1097-4547(19970915)49:6<681::AID-JNR3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Maynard S, Schurman SH, Harboe C, de Souza-Pinto NC, Bohr VA. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis. 2009;30:2–10. doi: 10.1093/carcin/bgn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaddon A, Hudson P, Hill D, Barber J, Lloyd A, Davies G, Regland B. Alzheimer’s disease and total plasma aminothiols. Biological psychiatry. 2003;53:254–260. doi: 10.1016/s0006-3223(02)01451-8. [DOI] [PubMed] [Google Scholar]
- McIlroy SP, Dynan KB, Lawson JT, Patterson CC, Passmore AP. Moderately elevated plasma homocysteine, methylenetetrahydrofolate reductase genotype, and risk for stroke, vascular dementia, and Alzheimer disease in Northern Ireland. Stroke; a journal of cerebral circulation. 2002;33:2351–2356. doi: 10.1161/01.str.0000032550.90046.38. [DOI] [PubMed] [Google Scholar]
- McKinnon PJ. DNA repair deficiency and neurological disease. Nature reviews. Neuroscience. 2009;10:100–112. doi: 10.1038/nrn2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecocci P, MacGarvey U, Beal MF. Oxidative damage to mitochondrial DNA is increased in Alzheimer’s disease. Annals of neurology. 1994;36:747–751. doi: 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- Miao Y, Chen J, Zhang Q, Sun A. Deletion of tau attenuates heat shock-induced injury in cultured cortical neurons. Journal of neuroscience research. 2010;88:102–110. doi: 10.1002/jnr.22188. [DOI] [PubMed] [Google Scholar]
- Migliore L, Botto N, Scarpato R, Petrozzi L, Cipriani G, Bonuccelli U. Preferential occurrence of chromosome 21 malsegregation in peripheral blood lymphocytes of Alzheimer disease patients. Cytogenetics and cell genetics. 1999;87:41–46. doi: 10.1159/000015389. [DOI] [PubMed] [Google Scholar]
- Migliore L, Coppede F, Fenech M, Thomas P. Association of micronucleus frequency with neurodegenerative diseases. Mutagenesis. 2011;26:85–92. doi: 10.1093/mutage/geq067. [DOI] [PubMed] [Google Scholar]
- Mimaki T, Itoh N, Abe J, Tagawa T, Sato K, Yabuuchi H, Takebe H. Neurological manifestations in xeroderma pigmentosum. Annals of neurology. 1986;20:70–75. doi: 10.1002/ana.410200112. [DOI] [PubMed] [Google Scholar]
- Moorhead PS, Heyman A. Chromosome studies of patients with Alzheimer disease. American journal of medical genetics. 1983;14:545–556. doi: 10.1002/ajmg.1320140319. [DOI] [PubMed] [Google Scholar]
- Mosch B, Morawski M, Mittag A, Lenz D, Tarnok A, Arendt T. Aneuploidy and DNA replication in the normal human brain and Alzheimer’s disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:6859–6867. doi: 10.1523/JNEUROSCI.0379-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullaart E, Boerrigter ME, Ravid R, Swaab DF, Vijg J. Increased levels of DNA breaks in cerebral cortex of Alzheimer’s disease patients. Neurobiology of aging. 1990;11:169–173. doi: 10.1016/0197-4580(90)90542-8. [DOI] [PubMed] [Google Scholar]
- Mutisya EM, Bowling AC, Beal MF. Cortical cytochrome oxidase activity is reduced in Alzheimer’s disease. Journal of neurochemistry. 1994;63:2179–2184. doi: 10.1046/j.1471-4159.1994.63062179.x. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y. Regulation of intracellular localization of human MTH1, OGG1, and MYH proteins for repair of oxidative DNA damage. Progress in nucleic acid research and molecular biology. 2001;68:75–94. doi: 10.1016/s0079-6603(01)68091-7. [DOI] [PubMed] [Google Scholar]
- Newman AB, Fitzpatrick AL, Lopez O, Jackson S, Lyketsos C, Jagust W, Ives D, Dekosky ST, Kuller LH. Dementia and Alzheimer’s disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. Journal of the American Geriatrics Society. 2005;53:1101–1107. doi: 10.1111/j.1532-5415.2005.53360.x. [DOI] [PubMed] [Google Scholar]
- Ngo J, Matsuyama M, Kim C, Poventud-Fuentes I, Bates A, Siedlak SL, Lee HG, Doughman YQ, Watanabe M, Liner A, Hoit B, Voelkel N, Gerson S, Hasty P, Matsuyama S. Cell death & disease. 2015. Bax deficiency extends the survival of Ku70 knockout mice that develop lung and heart diseases;6:e1706. doi: 10.1038/cddis.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka K, Ohtsubo T, Oda H, Fujiwara T, Kang D, Sugimachi K, Nakabeppu Y. Expression and differential intracellular localization of two major forms of human 8-oxoguanine DNA glycosylase encoded by alternatively spliced OGG1 mRNAs. Molecular biology of the cell. 1999;10:1637–1652. doi: 10.1091/mbc.10.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obulesu M, Rao DM. DNA damage and impairment of DNA repair in Alzheimer’s disease. Int J Neurosci. 2010;120:397–403. doi: 10.3109/00207450903411133. [DOI] [PubMed] [Google Scholar]
- Oka S, Nakabeppu Y. DNA glycosylase encoded by MUTYH functions as a molecular switch for programmed cell death under oxidative stress to suppress tumorigenesis. Cancer science. 2011;102:677–682. doi: 10.1111/j.1349-7006.2011.01869.x. [DOI] [PubMed] [Google Scholar]
- Oka S, Ohno M, Tsuchimoto D, Sakumi K, Furuichi M, Nakabeppu Y. Two distinct pathways of cell death triggered by oxidative damage to nuclear and mitochondrial DNAs. The EMBO journal. 2008;27:421–432. doi: 10.1038/sj.emboj.7601975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paola D, Domenicotti C, Nitti M, Vitali A, Borghi R, Cottalasso D, Zaccheo D, Odetti P, Strocchi P, Marinari UM, Tabaton M, Pronzato MA. Oxidative stress induces increase in intracellular amyloid beta-protein production and selective activation of betaI and betaII PKCs in NT2 cells. Biochemical and biophysical research communications. 2000;268:642–646. doi: 10.1006/bbrc.2000.2164. [DOI] [PubMed] [Google Scholar]
- Parildar-Karpuzoglu H, Dogru-Abbasoglu S, Hanagasi HA, Karadag B, Gurvit H, Emre M, Uysal M. Single nucleotide polymorphisms in base-excision repair genes hOGG1, APE1 and XRCC1 do not alter risk of Alzheimer’s disease. Neuroscience letters. 2008;442:287–291. doi: 10.1016/j.neulet.2008.07.047. [DOI] [PubMed] [Google Scholar]
- Parker WD, Jr, Filley CM, Parks JK. Cytochrome oxidase deficiency in Alzheimer’s disease. Neurology. 1990;40:1302–1303. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- Parker WD, Jr, Mahr NJ, Filley CM, Parks JK, Hughes D, Young DA, Cullum CM. Reduced platelet cytochrome c oxidase activity in Alzheimer’s disease. Neurology. 1994;44:1086–1090. doi: 10.1212/wnl.44.6.1086. [DOI] [PubMed] [Google Scholar]
- Petrovitch H, White L, Masaki KH, Ross GW, Abbott RD, Rodriguez BL, Lu G, Burchfiel CM, Blanchette PL, Curb JD. Influence of myocardial infarction, coronary artery bypass surgery, and stroke on cognitive impairment in late life. The American journal of cardiology. 1998;81:1017–1021. doi: 10.1016/s0002-9149(98)00082-4. [DOI] [PubMed] [Google Scholar]
- Pinto M, Pickrell AM, Fukui H, Moraes CT. Mitochondrial DNA damage in a mouse model of Alzheimer’s disease decreases amyloid beta plaque formation. Neurobiology of aging. 2013;34:2399–2407. doi: 10.1016/j.neurobiolaging.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postiglione A, Milan G, Ruocco A, Gallotta G, Guiotto G, Di Minno G. Plasma folate, vitamin B(12), and total homocysteine and homozygosity for the C677T mutation of the 5,10-methylene tetrahydrofolate reductase gene in patients with Alzheimer’s dementia. A case-control study. Gerontology. 2001;47:324–329. doi: 10.1159/000052822. [DOI] [PubMed] [Google Scholar]
- Potter H. Review and hypothesis: Alzheimer disease and Down syndrome--chromosome 21 nondisjunction may underlie both disorders. American journal of human genetics. 1991;48:1192–1200. [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Chen W, Wu J, Tao T, Bi L, Xu W, Qi H, Wang Y, Guo L. Association of polymorphism of DNA repair gene XRCC1 with sporadic late-onset Alzheimer’s disease and age of onset in elderly Han Chinese. Journal of the neurological sciences. 2010;295:62–65. doi: 10.1016/j.jns.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Roberts RO, Knopman DS, Geda YE, Cha RH, Roger VL, Petersen RC. Coronary heart disease is associated with non-amnestic mild cognitive impairment. Neurobiology of aging. 2010;31:1894–1902. doi: 10.1016/j.neurobiolaging.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue KM, Kennedy KM, Devous MD, Sr Rieck JR, Hebrank AC, Diaz-Arrastia R, Mathews D, Park DC. beta-Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology. 2012;78:387–395. doi: 10.1212/WNL.0b013e318245d295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogaev EI, Lukiw WJ, Lavrushina O, Rogaeva EA, St George-Hyslop PH. The upstream promoter of the beta-amyloid precursor protein gene (APP) shows differential patterns of methylation in human brain. Genomics. 1994;22:340–347. doi: 10.1006/geno.1994.1393. [DOI] [PubMed] [Google Scholar]
- Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, Fripp J, Tochon-Danguy H, Morandeau L, O’Keefe G, Price R, Raniga P, Robins P, Acosta O, Lenzo N, Szoeke C, Salvado O, Head R, Martins R, Masters CL, Ames D, Villemagne VL. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiology of aging. 2010;31:1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Ryan SM, Nolan YM. Neuroinflammation negatively affects adult hippocampal neurogenesis and cognition: can exercise compensate? Neuroscience and biobehavioral reviews. 2015;61:121–131. doi: 10.1016/j.neubiorev.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Scheibye-Knudsen M, Fang EF, Croteau DL, Bohr VA. Contribution of defective mitophagy to the neurodegeneration in DNA repair-deficient disorders. Autophagy. 2014;10:1468–1469. doi: 10.4161/auto.29321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. The molecular pathology of Alzheimer’s disease. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: genotypes, phenotypes, and treatments. Science. 1997;275:630–631. doi: 10.1126/science.275.5300.630. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Deciphering the genesis and fate of amyloid beta-protein yields novel therapies for Alzheimer disease. The Journal of clinical investigation. 2002;110:1375–1381. doi: 10.1172/JCI16783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. SnapShot: pathobiology of Alzheimer’s disease. Cell. 2013;154:468–468. e461. doi: 10.1016/j.cell.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Selley ML, Close DR, Stern SE. The effect of increased concentrations of homocysteine on the concentration of (E)-4-hydroxy-2-nonenal in the plasma and cerebrospinal fluid of patients with Alzheimer’s disease. Neurobiology of aging. 2002;23:383–388. doi: 10.1016/s0197-4580(01)00327-x. [DOI] [PubMed] [Google Scholar]
- Shah NR, Mahmoudi M. The role of DNA damage and repair in atherosclerosis: A review. Journal of molecular and cellular cardiology. 2015;86:147–157. doi: 10.1016/j.yjmcc.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Sharma RA, Dianov GL. Targeting base excision repair to improve cancer therapies. Molecular aspects of medicine. 2007;28:345–374. doi: 10.1016/j.mam.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Sheehan JP, Swerdlow RH, Miller SW, Davis RE, Parks JK, Parker WD, Tuttle JB. Calcium homeostasis and reactive oxygen species production in cells transformed by mitochondria from individuals with sporadic Alzheimer’s disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:4612–4622. doi: 10.1523/JNEUROSCI.17-12-04612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Chen Y, Liu H, Zhang K, Zhang T, Lin A, Jing N. Hydrogen peroxide promotes Abeta production through JNK-dependent activation of gamma-secretase. The Journal of biological chemistry. 2008;283:17721–17730. doi: 10.1074/jbc.M800013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiloh Y, Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nature reviews. Molecular cell biology. 2013;14:197–210. [PubMed] [Google Scholar]
- Sinha RN. Make dementia a public health priority in India. Indian journal of public health. 2011;55:67–69. doi: 10.4103/0019-557X.85234. [DOI] [PubMed] [Google Scholar]
- Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Davis D, Doan M, Dovey HF, Frigon N, Hong J, Jacobson-Croak K, Jewett N, Keim P, Knops J, Lieberburg I, Power M, Tan H, Tatsuno G, Tung J, Schenk D, Seubert P, Suomensaari SM, Wang S, Walker D, Zhao J, McConlogue L, John V. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- Sjoberg MK, Shestakova E, Mansuroglu Z, Maccioni RB, Bonnefoy E. Tau protein binds to pericentromeric DNA: a putative role for nuclear tau in nucleolar organization. Journal of cell science. 2006;119:2025–2034. doi: 10.1242/jcs.02907. [DOI] [PubMed] [Google Scholar]
- Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. Jama. 1997;277:813–817. [PubMed] [Google Scholar]
- Soria G, Polo SE, Almouzni G. Prime, repair, restore: the active role of chromatin in the DNA damage response. Molecular cell. 2012;46:722–734. doi: 10.1016/j.molcel.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Johnson KA, Doraiswamy PM, Reiman EM, Fleisher AS, Sabbagh MN, Sadowsky CH, Carpenter A, Davis MD, Lu M, Flitter M, Joshi AD, Clark CM, Grundman M, Mintun MA, Skovronsky DM, Pontecorvo MJ, Group AAS. Amyloid deposition detected with florbetapir F 18 ((18)F-AV-45) is related to lower episodic memory performance in clinically normal older individuals. Neurobiology of aging. 2013;34:822–831. doi: 10.1016/j.neurobiolaging.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer MJ. Cardiovascular disease and Alzheimer’s disease: common links. Journal of internal medicine. 2006;260:211–223. doi: 10.1111/j.1365-2796.2006.01687.x. [DOI] [PubMed] [Google Scholar]
- Strosznajder JB, Czapski GA, Adamczyk A, Strosznajder RP. Poly(ADP-ribose) polymerase-1 in amyloid beta toxicity and Alzheimer’s disease. Molecular neurobiology. 2012;46:78–84. doi: 10.1007/s12035-012-8258-9. [DOI] [PubMed] [Google Scholar]
- Su JH, Deng G, Cotman CW. Neuronal DNA damage precedes tangle formation and is associated with up-regulation of nitrotyrosine in Alzheimer’s disease brain. Brain research. 1997;774:193–199. doi: 10.1016/s0006-8993(97)81703-9. [DOI] [PubMed] [Google Scholar]
- Suberbielle E, Djukic B, Evans M, Kim DH, Taneja P, Wang X, Finucane M, Knox J, Ho K, Devidze N, Masliah E, Mucke L. DNA repair factor BRCA1 depletion occurs in Alzheimer brains and impairs cognitive function in mice. Nature communications. 2015;6:8897. doi: 10.1038/ncomms9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suberbielle E, Sanchez PE, Kravitz AV, Wang X, Ho K, Eilertson K, Devidze N, Kreitzer AC, Mucke L. Physiologic brain activity causes DNA double-strand breaks in neurons, with exacerbation by amyloid-beta. Nature neuroscience. 2013;16:613–621. doi: 10.1038/nn.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugo N, Aratani Y, Nagashima Y, Kubota Y, Koyama H. Neonatal lethality with abnormal neurogenesis in mice deficient in DNA polymerase beta. The EMBO journal. 2000;19:1397–1404. doi: 10.1093/emboj/19.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan A, Nesslany F, Violet M, Begard S, Loyens A, Talahari S, Mansuroglu Z, Marzin D, Sergeant N, Humez S, Colin M, Bonnefoy E, Buee L, Galas MC. Nuclear tau, a key player in neuronal DNA protection. The Journal of biological chemistry. 2011;286:4566–4575. doi: 10.1074/jbc.M110.199976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, Burns JM, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis. Journal of Alzheimer’s disease : JAD. 2010;2(20 Suppl):S265–S279. doi: 10.3233/JAD-2010-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, Khan SM. A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Medical hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- Sykora P, Misiak M, Wang Y, Ghosh S, Leandro GS, Liu D, Tian J, Baptiste BA, Cong WN, Brenerman BM, Fang E, Becker KG, Hamilton RJ, Chigurupati S, Zhang Y, Egan JM, Croteau DL, Wilson DM, 3rd, Mattson MP, Bohr VA. DNA polymerase beta deficiency leads to neurodegeneration and exacerbates Alzheimer disease phenotypes. Nucleic acids research. 2015;43:943–959. doi: 10.1093/nar/gku1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykora P, Wilson DM, 3rd, Bohr VA. Base excision repair in the mammalian brain: implication for age related neurodegeneration. Mechanisms of ageing and development. 2013a;134:440–448. doi: 10.1016/j.mad.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykora P, Yang JL, Ferrarelli LK, Tian J, Tadokoro T, Kulkarni A, Weissman L, Keijzers G, Wilson DM, 3rd, Mattson MP, Bohr VA. Modulation of DNA base excision repair during neuronal differentiation. Neurobiology of aging. 2013b;34:1717–1727. doi: 10.1016/j.neurobiolaging.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T. The role of presenilin cofactors in the gamma-secretase complex. Nature. 2003;422:438–441. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Bardini P, Obbili A, Vitali A, Borghi R, Zaccheo D, Pronzato MA, Danni O, Smith MA, Perry G, Tabaton M. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiology of disease. 2002;10:279–288. doi: 10.1006/nbdi.2002.0515. [DOI] [PubMed] [Google Scholar]
- Taupin P. Neurogenesis, NSCs, pathogenesis and therapies for Alzheimer’s disease. Frontiers in bioscience. 2011;3:178–190. doi: 10.2741/s143. [DOI] [PubMed] [Google Scholar]
- Tell G, Demple B. Base excision DNA repair and cancer. Oncotarget. 2015;6:584–585. doi: 10.18632/oncotarget.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanan R, Oikawa S, Hiraku Y, Ohnishi S, Ma N, Pinlaor S, Yongvanit P, Kawanishi S, Murata M. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. International journal of molecular sciences. 2015;16:193–217. doi: 10.3390/ijms16010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Fenech M. Chromosome 17 and 21 aneuploidy in buccal cells is increased with ageing and in Alzheimer’s disease. Mutagenesis. 2008;23:57–65. doi: 10.1093/mutage/gem044. [DOI] [PubMed] [Google Scholar]
- Trippi F, Botto N, Scarpato R, Petrozzi L, Bonuccelli U, Latorraca S, Sorbi S, Migliore L. Spontaneous and induced chromosome damage in somatic cells of sporadic and familial Alzheimer’s disease patients. Mutagenesis. 2001;16:323–327. doi: 10.1093/mutage/16.4.323. [DOI] [PubMed] [Google Scholar]
- Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O’Brien RJ. Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Annals of neurology. 2008;64:168–176. doi: 10.1002/ana.21413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uryga A, Gray K, Bennett M. DNA Damage and Repair in Vascular Disease. Annual review of physiology. 2015 doi: 10.1146/annurev-physiol-021115-105127. [DOI] [PubMed] [Google Scholar]
- Varga T, Aplan PD. Chromosomal aberrations induced by double strand DNA breaks. DNA repair. 2005;4:1038–1046. doi: 10.1016/j.dnarep.2005.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. The New England journal of medicine. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- Violet M, Chauderlier A, Delattre L, Tardivel M, Chouala MS, Sultan A, Marciniak E, Humez S, Binder L, Kayed R, Lefebvre B, Bonnefoy E, Buee L, Galas MC. Prefibrillar Tau oligomers alter the nucleic acid protective function of Tau in hippocampal neurons in vivo. Neurobiology of disease. 2015;82:540–551. doi: 10.1016/j.nbd.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Violet M, Delattre L, Tardivel M, Sultan A, Chauderlier A, Caillierez R, Talahari S, Nesslany F, Lefebvre B, Bonnefoy E, Buee L, Galas MC. A major role for Tau in neuronal DNA and RNA protection in vivo under physiological and hyperthermic conditions. Frontiers in cellular neuroscience. 2014;8:84. doi: 10.3389/fncel.2014.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HZ, Bi R, Hu QX, Xiang Q, Zhang C, Zhang DF, Zhang W, Ma X, Guo W, Deng W, Zhao L, Ni P, Li M, Fang Y, Li T, Yao YG. Validating GWAS-Identified Risk Loci for Alzheimer’s Disease in Han Chinese Populations. Molecular neurobiology. 2016;53:379–390. doi: 10.1007/s12035-014-9015-z. [DOI] [PubMed] [Google Scholar]
- Wang J, Jacob NK, Ladner KJ, Beg A, Perko JD, Tanner SM, Liyanarachchi S, Fishel R, Guttridge DC. RelA/p65 functions to maintain cellular senescence by regulating genomic stability and DNA repair. EMBO reports. 2009;10:1272–1278. doi: 10.1038/embor.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in mild cognitive impairment. Journal of neurochemistry. 2006;96:825–832. doi: 10.1111/j.1471-4159.2005.03615.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Xiong S, Xie C, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer’s disease. Journal of neurochemistry. 2005;93:953–962. doi: 10.1111/j.1471-4159.2005.03053.x. [DOI] [PubMed] [Google Scholar]
- Wei W, Englander EW. DNA polymerase beta-catalyzed-PCNA independent long patch base excision repair synthesis: a mechanism for repair of oxidatively damaged DNA ends in post-mitotic brain. Journal of neurochemistry. 2008;107:734–744. doi: 10.1111/j.1471-4159.2008.05644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Qu MH, Wang XS, Chen L, Wang DL, Liu Y, Hua Q, He RQ. Binding to the minor groove of the double-strand, tau protein prevents DNA from damage by peroxidation. PloS one. 2008;3:e2600. doi: 10.1371/journal.pone.0002600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman L, de Souza-Pinto NC, Mattson MP, Bohr VA. DNA base excision repair activities in mouse models of Alzheimer’s disease. Neurobiology of aging. 2009;30:2080–2081. doi: 10.1016/j.neurobiolaging.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman L, Jo DG, Sorensen MM, de Souza-Pinto NC, Markesbery WR, Mattson MP, Bohr VA. Defective DNA base excision repair in brain from individuals with Alzheimer’s disease and amnestic mild cognitive impairment. Nucleic acids research. 2007;35:5545–5555. doi: 10.1093/nar/gkm605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BJ, Crandall C, Goudsmit J, Morrow CH, Alling DW, Gajdusek DC, Tijio JH. Cytogenetic studies of familial and sporadic Alzheimer disease. American journal of medical genetics. 1981;10:77–89. doi: 10.1002/ajmg.1320100110. [DOI] [PubMed] [Google Scholar]
- Williams TI, Lynn BC, Markesbery WR, Lovell MA. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer’s disease. Neurobiology of aging. 2006;27:1094–1099. doi: 10.1016/j.neurobiolaging.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Wilson DM, 3rd, Bohr VA. The mechanics of base excision repair, and its relationship to aging and disease. DNA repair. 2007;6:544–559. doi: 10.1016/j.dnarep.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Winslow BT, Onysko MK, Stob CM, Hazlewood KA. Treatment of Alzheimer disease. American family physician. 2011;83:1403–1412. [PubMed] [Google Scholar]
- Wu MF, Yin JH, Hwang CS, Tang CM, Yang DI. NAD attenuates oxidative DNA damages induced by amyloid beta-peptide in primary rat cortical neurons. Free radical research. 2014;48:794–805. doi: 10.3109/10715762.2014.907889. [DOI] [PubMed] [Google Scholar]
- Yan SD, Stern DM. Mitochondrial dysfunction and Alzheimer’s disease: role of amyloid-beta peptide alcohol dehydrogenase (ABAD) International journal of experimental pathology. 2005;86:161–171. doi: 10.1111/j.0959-9673.2005.00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Sykora P, Wilson DM, 3rd, Mattson MP, Bohr VA. The excitatory neurotransmitter glutamate stimulates DNA repair to increase neuronal resiliency. Mechanisms of ageing and development. 2011;132:405–411. doi: 10.1016/j.mad.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Tadokoro T, Keijzers G, Mattson MP, Bohr VA. Neurons efficiently repair glutamate-induced oxidative DNA damage by a process involving CREB-mediated up-regulation of apurinic endonuclease 1. The Journal of biological chemistry. 2010;285:28191–28199. doi: 10.1074/jbc.M109.082883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Weissman L, Bohr VA, Mattson MP. Mitochondrial DNA damage and repair in neurodegenerative disorders. DNA repair. 2008;7:1110–1120. doi: 10.1016/j.dnarep.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Du H, Yan S, Fang F, Wang C, Lue LF, Guo L, Chen D, Stern DM, Gunn Moore FJ, Xi Chen J, Arancio O, Yan SS. Inhibition of amyloid-beta (Abeta) peptide-binding alcohol dehydrogenase-Abeta interaction reduces Abeta accumulation and improves mitochondrial function in a mouse model of Alzheimer’s disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:2313–2320. doi: 10.1523/JNEUROSCI.4717-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurov YB, Vorsanova SG, Iourov IY. The DNA replication stress hypothesis of Alzheimer’s disease. TheScientificWorldJournal. 2011;11:2602–2612. doi: 10.1100/2011/625690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurov YB, Vorsanova SG, Liehr T, Kolotii AD, Iourov IY. X chromosome aneuploidy in the Alzheimer’s disease brain. Molecular cytogenetics. 2014;7:20. doi: 10.1186/1755-8166-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawia NH, Lahiri DK, Cardozo-Pelaez F. Epigenetics, oxidative stress, and Alzheimer disease. Free radical biology & medicine. 2009;46:1241–1249. doi: 10.1016/j.freeradbiomed.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Powell SN. The role of the BRCA1 tumor suppressor in DNA double-strand break repair. Molecular cancer research : MCR. 2005;3:531–539. doi: 10.1158/1541-7786.MCR-05-0192. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Wu HH, Wang Y, Gu GJ, Zhang W, Xia R. Neural stem cell transplantation decreases neuroinflammation in a transgenic mouse model of Alzheimer’s disease. Journal of neurochemistry. 2015 doi: 10.1111/jnc.13413. [DOI] [PubMed] [Google Scholar]