Summary
Sensing the level of oxygen in the external and internal environments is essential for survival. Organisms have evolved multiple mechanisms to sense oxygen. No function in oxygen sensing has been attributed to any mammalian olfactory system. Here, we demonstrate that low environmental oxygen directly activates a subpopulation of sensory neurons in the mouse main olfactory epithelium. These neurons express the soluble guanylate cyclase Gucy1b2 and the cation channel Trpc2. Low oxygen induces calcium influx in these neurons, and Gucy1b2 and Trpc2 are required for these responses. In vivo exposure of a mouse to low environmental oxygen causes Gucy1b2-dependent activation of olfactory bulb neurons in the vicinity of the glomeruli formed by axons of Gucy1b2+ sensory neurons. Low environmental oxygen also induces conditioned place aversion, for which Gucy1b2 and Trpc2 are required. We propose that this chemosensory function enables a mouse to rapidly assess the oxygen level in the external environment.
Highlights
-
•
Sensing the level of oxygen in the external environment is essential for survival
-
•
Low oxygen directly activates sensory neurons in the mouse main olfactory epithelium
-
•
These responses require the guanylate cyclase Gucy1b2 and the cation channel Trpc2
-
•
A mouse could thus rapidly assess the oxygen level in the external environment
Sensing the level of oxygen is essential for survival, but no mammalian olfactory system has been implicated. Bleymehl et al. demonstrate that low oxygen activates mouse sensory neurons that express the soluble guanylate cyclase Gucy1b2 and the cation channel Trpc2.
Introduction
The sense of smell relies predominantly on the repertoire of ∼1,100 odorant receptor genes expressed in canonical olfactory sensory neurons (OSNs) in the main olfactory epithelium (MOE) (Buck and Axel, 1991). The mouse olfactory system comprises a variety of additional subsystems that detect a wide diversity of molecular cues in the external environment (Dey and Stowers, 2016, Munger et al., 2009). We have described a novel subpopulation of sensory neurons in the mouse MOE: type B cells (Omura and Mombaerts, 2014, Omura and Mombaerts, 2015, Saraiva et al., 2015). Type B cells express the transient receptor potential cation channel Trpc2, thought for many years to be expressed exclusively in vomeronasal sensory neurons (VSNs), where it is a critical component of signal transduction (Leypold et al., 2002, Lucas et al., 2003, Stowers et al., 2002). Within the MOE, type B cells are unique in their expression of the soluble guanylate cyclase Gucy1b2, a molecule that is poorly characterized. We refer to Trpc2+ Gucy1b2+ neurons of the MOE as type B cells in order to distinguish them from Trpc2+ Gucy1b2− cells of the MOE (type A cells), some of which express odorant receptor genes (Omura and Mombaerts, 2014).
The functions of type B cells remain enigmatic, and no sensory stimuli have been described. Molecularly, type B cells are fundamentally different from canonical OSNs and VSNs (Omura and Mombaerts, 2014, Omura and Mombaerts, 2015, Saraiva et al., 2015), suggesting that their ligands are also fundamentally different. First, type B cells do not appear to express odorant receptor genes, vomeronasal receptor genes, or trace amine-associated receptor genes. Second, type B cells express the cyclic nucleotide-gated channel subunit Cnga2 (characteristic for OSNs) and Trpc2 (characteristic for VSNs), but not the adenylate cyclase Adcy3 (characteristic for OSNs). Third, the differential, higher expression of 55 genes by type B cells compared to canonical OSNs defines type B cells as a cell type that is molecularly distinct from OSNs.
Here, we report the first sensory stimulus for type B cells: a reduced level of oxygen, hereafter referred to as low environmental oxygen or low oxygen. The behavioral deficits in a novel gene-targeted mouse strain carrying a knockout mutation in the Gucy1b2 locus are consistent with a biological function of type B cells as sensors for low oxygen in the external environment.
Results
Low Oxygen Directly Activates Type B Cells
Type B cells can be visualized in the MOE of mice of the gene-targeted Gucy1b2-IRES-tauGFP strain, in which Gucy1b2 remains intact in its coding region and is coexpressed with a fusion protein of tau and GFP (Omura and Mombaerts, 2015) (Figure 1A). We hereafter abbreviate this mutant allele as Gucy1b2-GFP. To determine the chemical cues that are detected by these neurons, we dissociated the MOE of homozygous (−/−) Gucy1b2-GFP mice. This procedure yielded 0.4%–1.9% GFP-positive cells (GFP+, type B) in the dissociated cell preparations. The remaining cells are GFP negative (GFP−, non-type B). This proportion of GFP+ cells in the dissociated cell preparations is consistent with the sparse distribution of type B cells within the MOE (Omura and Mombaerts, 2014, Omura and Mombaerts, 2015). For comparison, we also analyzed type A cells, which were identified through post hoc immunostaining with Adcy3.
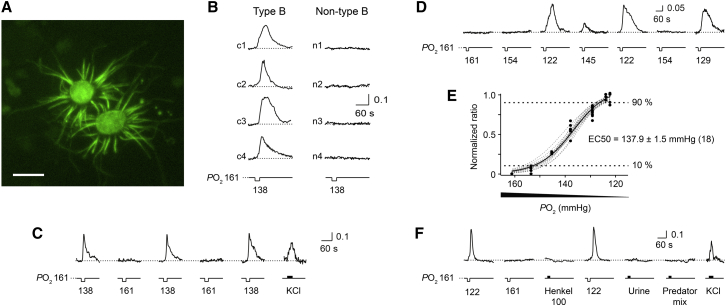
Figure 1.
Type B Cells Respond to Low Oxygen
(A) Confocal image of intrinsic fluorescence of type B cells from a Gucy1b2-GFP −/− mouse (8 weeks) in an ex vivo en face whole-mount preparation. Scale bar, 5 μm.
(B) Decreasing the PO2 from 161 to 138 mmHg in the bath solution evoked a transient rise in intracellular Ca2+ in type B cells within seconds. No responses occurred in non-type B cells. A Ca2+ response is defined as a stimulus-dependent deviation in fluorescence ratio exceeding twice the SD of the mean baseline noise.
(C) Repeatability of type B cell responses to low oxygen (138 mmHg). KCl, 30 mM.
(D) Examples of Ca2+ responses to various levels of low oxygen (in mmHg) in a type B cell.
(E) Stimulus-response curves obtained from single type B cells (n = 18; dashed gray lines). Each cell was stimulated with 20 s pulses of solution containing distinct oxygen levels (mmHg). Relative changes in peak fluorescence ratio induced by a given stimulus were normalized to the maximum peak fluorescence ratio of a given cell. Curves are fit using the Hill equation combined with an iterative Levenberg-Marquardt nonlinear, least-squares fitting routine, defining EC50, slope (−22.9 ± 3.0), and saturation (1.0 ± 0.05). Solid black curve represents a fit of the mean stimulus-response curve using all measured data points (chi-square 0.201823; p = 0.99). Responses were recorded during a 4 min trial period with a 1 min recovery period between trials (illumination off). Each stimulus was tested one to three times per cell. Numbers are presented as mean ± SD.
(F) Responses of a type B cell to various chemostimuli. Low oxygen (122 mmHg), Henkel 100 (1:100), diluted urine (1:100), predator odor mix (TMT, [2,5-dihydro-2,4,5-trimethylthiazole], PEA [β-phenylethylamine], 2-PT [2-propylthietane], and 2,6-DMP [2,6-dimethylpyrazine], each at 100 μM), and KCl (30 mM).
We loaded the dissociated cells with the Ca2+ indicator fura-2/AM. We performed ratiometric Ca2+ imaging (Pérez-Gómez et al., 2015) in order to visualize neural activity after chemical stimulation. Cells were continuously superfused with oxygenated solution (95% O2, 0% N2, and 5% CO2) yielding an O2 tension (PO2) of 161 mmHg (1 mmHg = 133.3 Pa), which is equivalent to a relative atmospheric O2 level of 21.7% (Figure S1A). Under these conditions, type B cells did not show spontaneous Ca2+ transients but responded to elevated KCl (30 mM) with a Ca2+ rise. Switching for 20 s to a solution with a low oxygen level (60% O2, 35% N2, and 5% CO2; yielding a PO2 of 138 mmHg, equivalent to a relative atmospheric O2 level of 18.6%; Figure S1A) but keeping the CO2 level and pH constant produced a transient rise in intracellular Ca2+ in 69% of type B cells (142/206), whereas 0/110 non-type B cells responded to this stimulus (Figure 1B; Figure S1F). Ca2+ elevations in type B cells to low oxygen levels could be repeated multiple times without obvious desensitization (Figure 1C) and persisted when minutes-long stimuli were applied (Figures S1B and S1C).
Stimulus-response curves derived from 18 single type B cells (Figures 1D and 1E) allowed us to make three inferences about the oxygen-sensing mechanism. First, these curves are nearly identical, suggesting that the cells expressed an oxygen sensor with identical or very similar molecular properties. Second, the stimulus-response curves are extremely steep, with Hill coefficients of 22.95 ± 3.3; hence, their relative dynamic range is narrow, comprising a difference in O2 levels of ∼1.4 ppm (44 μM). Third, the 10%–90% operating range of these cells (153–126 mmHg; EC50 = 137.9 ± 1.5 mmHg) is far above the range of normal (∼100 mmHg) PO2 values in arterial blood or the working range of carotid body chemosensors, the major sensors of blood oxygen in mammals (López-Barneo et al., 2016, Prabhakar, 2016, Semenza, 2011). It is therefore unlikely that type B cells detect the level of oxygen in blood.
Type B cells also generated a Ca2+ rise to stimulation with 3-isobutyl-1-methylxanthine (IBMX, 100 μM), which inhibits endogenous phosphodiesterases, and to 8-bromo-cGMP (8-br-cG, 500 μM), a membrane-permeable cyclic guanosine 3′,5′ monophosphate (cGMP) analog (Figures S1D and S1F). But unlike canonical OSNs, type B cells did not respond to forskolin (50 μM), which activates adenylate cyclase (Figures S1D and S1F). Type A cells did not respond to low oxygen but responded to IBMX with a Ca2+ rise (Figure S1D). Several other chemostimuli failed to produce Ca2+ elevations in type B cells (Figure 1F; Figure S1F), including carbon disulfide (CS2, 13 μM), a volatile semiochemical (Munger et al., 2010); mouse urine (1:100), a rich source of natural pheromones; a mixture containing 100 volatile odorants (Henkel 100, 1:100); and a mixture containing four volatile predator odors (Pérez-Gómez et al., 2015). Moreover, type B cells did not respond to shifting the proton (H+) concentration in the solution to values of pH 6.5 or 7.5 (Figures S1E and S1F). Taken together, low oxygen generates rapid, graded, and seemingly non-adapting responses in type B cells.
Gucy1b2 and Trpc2 Are Required for Type B Cell Responses to Low Oxygen
Next, we investigated the mechanistic basis of low-oxygen-evoked Ca2+ rises in type B cells.
Removal of extracellular Ca2+ abolished the responses in a reversible manner, indicating that responses were triggered primarily by Ca2+ entry into the cells (Figure 2A; Figure S1H). To determine whether Gucy1b2 is essential for the evoked responses in type B cells, we generated a novel mouse strain with a gene-targeted knockout (KO) in the Gucy1b2 gene, Gucy1b2-D-IRES-tauGFP (hereafter abbreviated as Gucy1b2-KO). Because the GFP sequence is spliced out of the mutant transcripts, the cells expressing the mutant allele do not express GFP (Figure S2). Therefore we crossed the Gucy1b2-KO mutation with the gene-targeted Trpc2-IRES-taumCherry mutation (Omura and Mombaerts, 2014), in which Trpc2 is intact in its coding sequence and coexpressed with a fusion of tau and mCherry. Mice of this cross are referred to as Gucy1b2-KO∗, with the asterisk referring to the intrinsic fluorescence of mCherry. Gucy1b2-KO∗ mice can be heterozygous (+/−) or homozygous (−/−) for the Gucy1b2-KO mutation but are always homozygous for Trpc2-IRES-taumCherry and are referred to as, respectively, Gucy1b2-KO∗ +/− or Gucy1b2-KO∗ −/−. In mice of this cross, we performed post hoc immunostaining (Chamero et al., 2011) for Adcy3 to distinguish type B cells (which are Adcy3− and mCherry+) from type A cells (which are Adcy3+ and mCherry+); canonical OSNs are Adcy3+ and mCherry− (Figure S1G). We found that low-oxygen-evoked Ca2+ rises were abolished in type B cells from Gucy1b2-KO∗ −/− mice, whereas responses to 8-bromo-cGMP or KCl remained normal (Figures 2B and 2C).
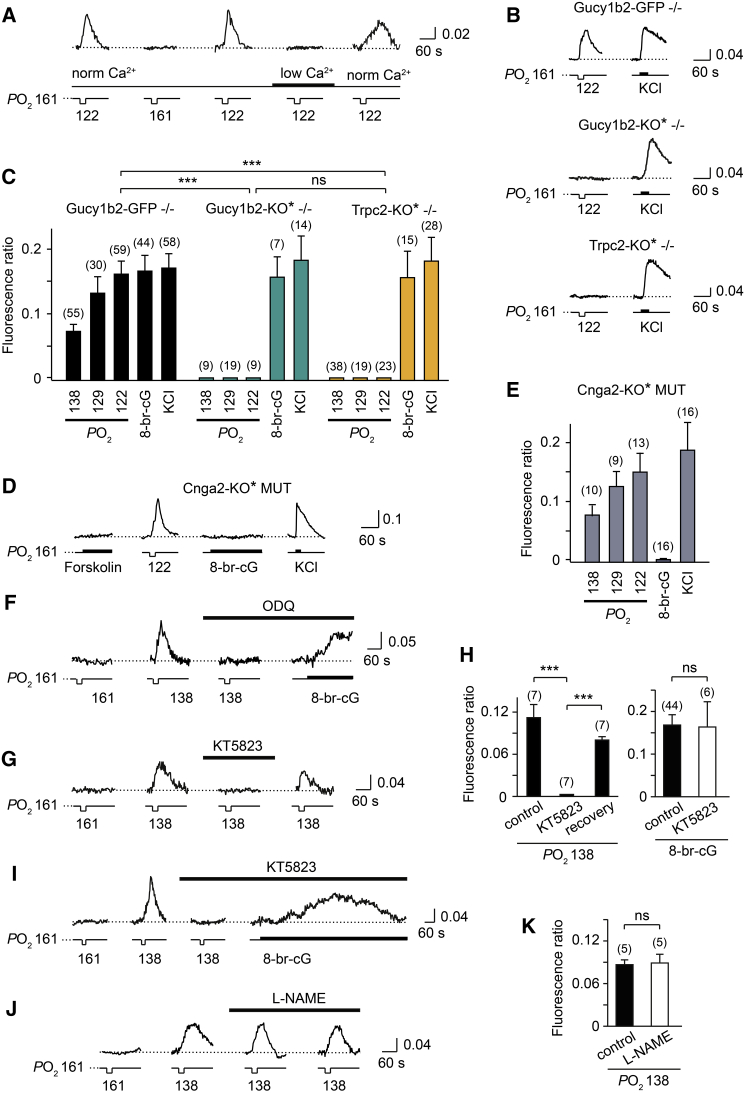
Figure 2.
Low-Oxygen-Evoked Ca2+ Responses of Type B Cells Require Ca2+ Entry, Gucy1b2, and Trpc2
(A) Recording example indicating that Ca2+ responses to low oxygen (122 mmHg) depend on Ca2+ entry into type B cells. Removal of external Ca2+ eliminated the response in a reversible manner.
(B) Recording examples of Ca2+ responses in type B cells from Gucy1b2-GFP −/− mice, Gucy1b2-KO∗ −/− mice, and Trpc2-KO∗ −/− mice. Cells were stimulated with low oxygen (122 mmHg) or KCl (30 mM).
(C) Group data indicating that the Ca2+ responses to low oxygen require Gucy1b2 (Gucy1b2-KO∗ −/−, n = 4 mice) and Trpc2 (Trpc2-KO∗ −/−, n = 5 mice) (PO2: 138, 129, or 122 mmHg) (ANOVA: F(14,398) = 12.791, p < 0.0001; LSD: ∗p < 0.05, ∗∗p < 0.001, ∗∗∗p < 0.0001). Responses to 8-bromo-cGMP (8-br-cG; 500 μM) or KCl (30 mM) do not differ between the three genotypes (LSD: p = 0.66–0.71). Maximum fluorescence ratios are plotted; number of cells for each genotype and stimulus condition is indicated above each bar. No significant difference was observed between Gucy1b2-GFP −/− mice and Gucy1b2-KO∗ +/− mice in the responsiveness of their type B cells (n = 6; LSD: p = 0.55).
(D and E) Recording example (D) and group data (E) (n = 3 mice) indicating that type B cells in Cnga2-KO∗ MUT mice have normal Ca2+ responses to low oxygen but lack responsiveness to 8-br-cGMP (500 μM). Number of cells is indicated above each bar.
(F) Recording example indicating that Ca2+ responses to low oxygen in a type B cell (Gucy1b2-GFP −/− mouse) are eliminated after a 10 min treatment with ODQ (10 μM) (paired t test: t = 3.835, ∗p < 0.05), whereas the response to 8-br-cGMP (500 μM) is not.
(G–I) Recording examples (G and I) and group data (H) showing the effects of the PKG inhibitor KT5823 (10 μM) on type B cell Ca2+ responses to low oxygen (138 mmHg) or 8-br-cGMP (50 μM). Responses to low oxygen are blocked by KT5823 in a reversible manner (ANOVA: F(2,20) = 26.856, p < 0.0001; LSD: ∗∗∗p < 0.0001), whereas responses to 8-br-cGMP remain unaffected (p = 0.17). Number of independent recordings is indicated above each bar.
(J and K) Lack of effect of the NOS inhibitor L-NAME (100 μM) on type B cell Ca2+ responses to low oxygen (138 mmHg) (paired t test: t = 2.359, p = 0.078).
Results in histograms are presented as mean ± SEM.
Next, we performed similar experiments in mice that are homozygous for Gucy1b2-GFP and homozygous for a knockout mutation in the Trpc2 gene (Trpc2-KO) (Omura and Mombaerts, 2014). These doubly homozygous mice are referred to as Trpc2-KO∗ −/− mice, with the asterisk referring to the intrinsic fluorescence of GFP. Here, too, we found that low-oxygen-evoked Ca2+ rises were abolished in type B cells from Trpc2-KO∗ −/− mice, whereas responses to 8-bromo-cGMP or KCl remained normal (Figures 2B and 2C). These results identify both Gucy1b2 and Trpc2 as essential molecular components of the signal transduction machinery that underlies the sensing of low oxygen by type B cells.
To determine whether Cnga2 is required for low-oxygen-evoked Ca2+ elevations in type B cells, we performed experiments in mice that are homozygous for Trpc2-IRES-taumCherry and hemizygous (males) or homozygous (females) for a knockout mutation in the Cnga2 gene, which is located on the X chromosome (Zheng et al., 2000). Mice of this cross are referred to as Cnga2-KO∗ MUT, with the asterisk referring to the intrinsic fluorescence of mCherry and MUT referring collectively to mice that are hemizygous or homozygous for the Cnga2 knockout mutation. Low-oxygen-evoked Ca2+ rises and responses to KCl remained normal in type B cells of Cnga2-KO∗ MUT mice, whereas responses to 8-bromo-cGMP were absent (Figures 2D and 2E). Thus, Cnga2 is not required for the sensing of low oxygen by type B cells but is required for producing a Ca2+ elevation to 8-bromo-cGMP application. Therefore, Trpc2, but not Cnga2, is the critical ion channel for the generation of low-oxygen-evoked Ca2+ responses in type B cells.
Atypical soluble guanylate cyclases of invertebrates contain heme-nitric oxide and oxygen-binding domains that can form a stable complex with oxygen (Derbyshire and Marletta, 2012, Gray et al., 2004, Morton, 2004). We applied ODQ (10 μM), a chemical that targets such domains and inhibits soluble guanylate cyclases by oxidation of the ferrous iron in the heme cofactor (Derbyshire and Marletta, 2012). We observed that low-oxygen-evoked responses of type B cells were eliminated following ODQ treatment (Figure 2F; Figure S1J). Thus, heme-nitric oxide and oxygen-binding domains are required for the sensing of low oxygen by type B cells, consistent with the requirement for Gucy1b2.
Rat Gucy1b2, which exhibits a high amino acid sequence identity to mouse Gucy1b2, can be enzymatically active and produce cGMP in the absence of another subunit (Koglin et al., 2001). One potential route of how cGMP formation can evoke neuronal excitation is through phosphorylation via a cGMP-activated protein kinase (PKG). Low-oxygen-evoked responses of type B cells were eliminated in a reversible manner following treatment with the PKG inhibitor KT5823 (10 μM) (Figures 2G and 2H). This result indicates that a PKG is required for the sensing of low oxygen in type B cells and also argues that a rise in intracellular cGMP concentration underlies this response. Interestingly, the Ca2+ elevation to 8-bromo-cGMP remained normal after KT5823 treatment (Figures 2I and 2H), consistent with a model in which Trpc2 and Cnga2 are part of two independent signaling pathways in type B cells (see Discussion).
We assessed a role of endogenously produced nitric oxide (NO) in the response to low oxygen by inhibiting NO synthases (NOS) with the cell-permeable NOS inhibitor L-NAME (100 μM). This treatment had no effect on low-oxygen-evoked responses in type B cells (Figures 2J and 2K). Additionally, single-cell RNA sequencing (RNA-seq) analyses of type B cells revealed that these neurons do not express Nos1, Nos2, and Nos3 (Saraiva et al., 2015). Together, these results rule out an involvement of endogenous NO produced through NOS activity in the sensing of low oxygen by type B cells.
Low Oxygen Activates Olfactory Bulb Neurons in the Vicinity of Type B Cell Glomeruli in Freely Breathing Mice
Based on the results of the single-cell experiments, we surmised that type B cells serve as sensitive detectors of reduced oxygen levels in the ambient air—environmental hypoxia. The axons of type B cells terminate in approximately three glomeruli on the posteroventral surface of the main olfactory bulb near the necklace glomeruli (Omura and Mombaerts, 2014, Omura and Mombaerts, 2015), where they synapse onto olfactory bulb neurons. Type B cell glomeruli can be visualized in mice that are heterozygous or homozygous for the Gucy1b2-GFP mutation (Omura and Mombaerts, 2015). We assessed activation of postsynaptic olfactory bulb neurons in the vicinity of type B cell glomeruli following exposure of mice to low environmental oxygen by using immunohistochemistry for the immediate early gene product c-Fos (Pérez-Gómez et al., 2015) (Figure 3). Singly housed mice of three genotypes (Gucy1b2-GFP −/− mice used as reference mice, Gucy1b2-KO∗ +/− mice, and Gucy1b2-KO∗ −/− mice) were habituated to 20% O2 in a testing arena for 30 min each day for 3 consecutive days. On day 4, mice were exposed to 20% O2 for 30 min and subsequently to either 16% or 20% O2 for an additional 10 min. Gucy1b2-GFP −/− mice responded to low oxygen exposure with a robust increase in the number of c-Fos immunoreactive cells that reside in the vicinity of type B cell glomeruli (Figures 3A, 3B, and 3G). Similar results were obtained in Gucy1b2-KO∗ +/− mice (Figures 3C, 3D, and 3G), but this response was abolished in Gucy1b2-KO∗ −/− mice (Figures 3E–3G).
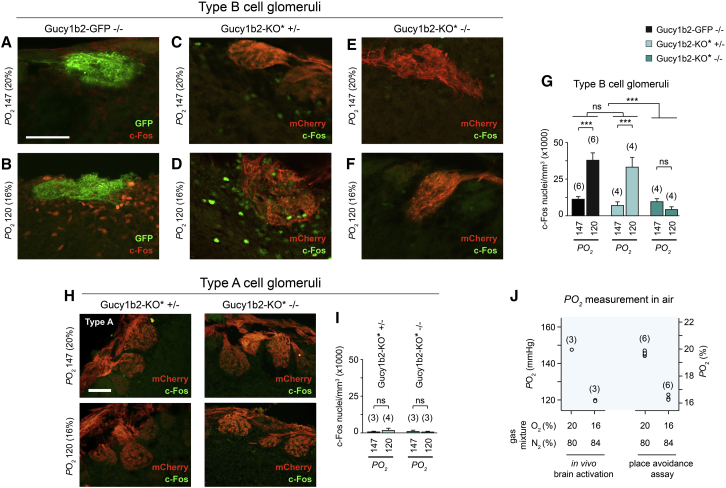
Figure 3.
Low Environmental Oxygen Causes Gucy1b2-Dependent Activation of Olfactory Bulb Neurons in the Vicinity of Type B Cell Glomeruli in Freely Breathing Mice
(A–F) c-Fos immunoreactivity in the olfactory bulbs of Gucy1b2-GFP −/− mice (A and B), of Gucy1b2-KO∗ +/− mice (C and D), and of Gucy1b2-KO∗ −/− mice (E and F). Mice were exposed to normal (147 mmHg, 20%) (A, C, and E) or low (120 mmHg, 16%) (B, D, and F) oxygen. Sagittal sections were derived from olfactory bulbs. The intrinsic fluorescence of GFP or the immunoreactivity for mCherry identifies presynaptic axons and outlines the dense glomerular neuropil. The c-Fos immunoreactivity denotes the activity of postsynaptic olfactory bulb neurons. Scale bar, 50 μm.
(G) Quantification of c-Fos+ nuclei reveals that exposure to low environmental oxygen caused a significant increase in neuronal activity in the vicinity of type B cell glomeruli of Gucy1b2-GFP −/− mice (used as reference mice) and Gucy1b2-KO∗ +/− mice. Responses in these two genotypes were indistinguishable from each other (LSD: p = 0.95). By contrast, this response was absent in Gucy1b2-KO∗ −/− mice (LSD: p = 0.14). ANOVA genotype: F(2,229) = 28.767, p < 0.0001; ANOVA treatment: F(1,229) = 105.600, p < 0.0001; LSD: ∗∗∗p < 0.0001. The number of sections analyzed ranged from 23 to 57 per genotype and treatment. Number of mice is indicated above each bar.
(H) c-Fos immunoreactivity in the vicinity of type A cell glomeruli in the olfactory bulbs of Gucy1b2-KO∗ +/− mice (left panels) and Gucy1b2-KO∗ −/− mice (right panels). Mice were exposed to normal (147 mmHg, 20%) or low (120 mmHg, 16%) oxygen. Scale bar, 50 μm. No c-Fos immunoreactivity was detetectable under these conditions.
(I) Quantification of c-Fos+ nuclei reveals that exposure to low oxygen caused no significant increase in neuronal activity in the vicinity of type A cell glomeruli of Gucy1b2-KO∗ +/− or Gucy1b2-KO∗ −/− mice (ANOVA: F(1,251) = 0.134, p = 0.72). Basal c-Fos immunoreactivity was very low, and the two genotypes were indistinguishable from each other (ANOVA: F(1,251) = 2.712, p = 0.10). The number of sections analyzed ranged from 18 to 124 per genotype and treatment. Number of mice is indicated above each bar.
(J) Plot of the oxygen levels measured in the ambient air of the behavioral chambers as used during in vivo experiments of activation of olfactory bulb neurons and conditioned place aversion. Concentrations were measured in percentage and converted into PO2 (mmHg). Number of independent measurements is indicated. PCO2 was <0.1% (0.76 mmHg).
Results in histograms are presented as mean ± SEM.
We also assessed the specificity of type B cell glomeruli versus type A cell glomeruli in their responsivity to low oxygen exposure. Type A cell glomeruli reside at rostral positions on the ventral surface of the main olfactory bulb and can be visualized in heterozygous or homozygous Trp2-IRES-taumCherry mice (Omura and Mombaerts, 2014, Omura and Mombaerts, 2015). In contrast to type B cell glomeruli, we found no evidence that postsynaptic olfactory bulb neurons in the vicinity of type A cell glomeruli were activated following in vivo exposure to low oxygen either in Gucy1b2-KO∗ +/− or in Gucy1b2-KO∗ −/− mice (Figures 3H and 3I). These in vivo results are consistent with our Ca2+ imaging experiments on single type A cells (Figure S1D) and confirm and extend our observations that low-oxygen-evoked responses occur in type B cells, but not in type A cells.
These results demonstrate the physiological relevance of low oxygen detection by type B cells in awake, behaving mice and link the sensing of low environmental oxygen to type B cells in a biologically relevant context.
Gucy1b2 and Trpc2 Are Required for Conditioned Place Aversion of a Low-Oxygen Environment
Given the importance of the olfactory system in mediating a variety of aversive behaviors (Li and Liberles, 2015, Pérez-Gómez et al., 2015), we reasoned that exposure to low environmental oxygen may evoke an aversive response. We developed a conditioned place aversion assay in order to determine whether low oxygen has negative (aversive) motivational effects. A conditioned place aversion assay is a Pavlovian conditioning task established to capture the negative reinforcement that is associated with a given stimulus or environmental cue (Cunningham et al., 2006). We determined that this test can reveal an aversive quality of low (16%) versus normal (20%) environmental oxygen levels as follows. Mice were tested in a two-chamber apparatus (Figures 4A and 4B) in which they were trained daily for 3 days to associate 16% O2 with one chamber. On the testing day, their preference of the 16%-paired chamber (but now at 20% O2) was quantified. Gucy1b2-GFP −/− mice (used as reference mice) and Gucy1b2-KO +/− mice spent significantly more time in the 20%-paired chamber than in the 16%-paired chamber; this difference in dwell time yields a negative preference index, which represents an aversion of the low-oxygen environment (Figures 4B–4D). By contrast, Gucy1b2-KO −/− mice failed to show a preference between the two chambers, indicating that Gucy1b2 is required for conditioned place aversion of low oxygen (Figures 4B–4D). Trpc2-KO∗ −/− mice also failed to display a preference for the 16%- versus the 20% O2-paired chamber in the assay, whereas Trpc2-KO∗ +/− mice showed a significant preference for the 20% O2-paired chamber (Figures 4E and 4F). The requirement for Gucy1b2 and Trpc2 in this conditioned place aversion is consistent with a biological role of type B cells in sensing a low oxygen level in the ambient air.
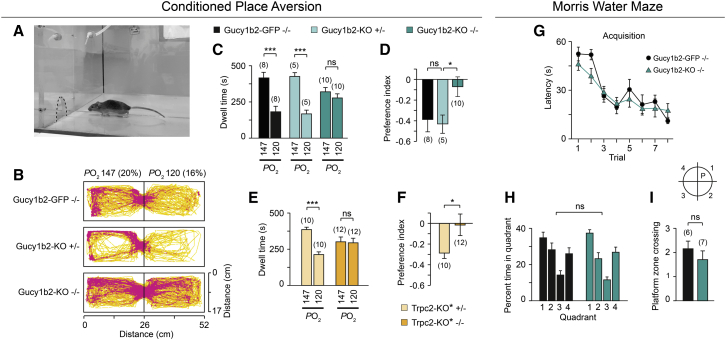
Figure 4.
Gucy1b2 and Trpc2 Are Required for Conditioned Place Aversion of a Low-Oxygen Environment
(A) Behavioral arena. The opening between the two chambers is indicated with a stipple line. Experiments were performed under infrared illumination.
(B) Heatmap graphs obtained from trajectory plots of all tested mice in the conditioned place aversion assay demonstrating their preference of the 20%-paired chamber versus the 16%-paired chamber.
(C and D) Average dwell times and preference index of Gucy1b2-GFP −/− mice (used as reference mice), Gucy1b2-KO +/− mice and Gucy1b2-KO −/− mice for the 20%- versus 16%-paired chamber. ANOVA: F(5,22) = 3.728, p < 0.042; LSD: ∗∗p < 0.001; n.s., p = 0.79 (C); ANOVA: F(5,45) = 10.256, p < 0,002; LSD: ∗∗p < 0.001; n.s., p = 0.28) (D).
(E and F) Average dwell times and preference index of Trpc2-KO∗ +/− mice and Trpc2-KO∗ −/− mice for the 20%- versus the 16%-paired chamber. ANOVA: F(3,40) = 6.7629, p < 0.001; LSD: ∗∗∗p < 0.001; n.s., p = 0.81 (E); paired t test: t(20) = 2.2353, ∗p < 0.05 (F). The number of mice is indicated at each bar.
(G) Plot of latency-to-find platform versus trial number in a Morris water maze assay for Gucy1b2-GFP −/− mice (n = 6) and Gucy1b2-KO −/− mice (n = 7). ANOVA genotype: F(1,103) = 1.22, p = 0.27.
(H and I) Analysis of reference memory in a Morris water maze assay using single probe trials in the absence of the platform (P) for Gucy1b2-GFP −/− mice (n = 6) and Gucy1b2-KO −/− mice (n = 7). Percentage of time spent in each quadrant (H; ANOVA genotype: F(1,51) = 0.043, p = 0.84) and number of platform zone crossings in quadrant 1 (I; t test: t(11) = 0.364, p = 0.72) were analyzed.
Results are presented as mean ± SEM.
To exclude that this behavioral phenotype may be caused by learning and memory deficits, we performed a Morris water maze assay (Vorhees and Williams, 2006) using Gucy1b2-GFP −/− mice and Gucy1b2-KO −/− mice. We analyzed the acquisition performance measured as latency to reach the platform (Figure 4G). We observed no significant difference in the performance between the two genotypes in this test. We also assessed the reference memory using single probe trials in the absence of the platform. We analyzed the time spent in each quadrant (Figure 4H) and the number of times crossing the area where the platform used to be located (Figure 4I). Again, there was no significant difference between the two genotypes. Therefore, Gucy1b2-KO −/− mice perform normally in a place learning assay, form and keep spatial memories, and exhibit proper spatial navigation.
If Gucy1b2 expression were to also occur in neurons of the central nervous system, it may influence the interpretation of the behavioral experiments with the Gucy1b2-KO −/− mice. We analyzed selected brain regions for intrinsic GFP fluorescence using coronal sections from Gucy1b2-GFP −/− mice. This anatomical analysis included the piriform cortex, subdivisons of the amygdala, various hypothalamic nuclei, the hippocampus, the bed nucleus of the stria terminalis, the periaqueductal gray, and the nucleus accumbens (Figure S3). The absence of GFP expression in these brain regions strengthens the functional link between the activation of type B cells by low environmental oxygen and conditioned place aversion.
Last, we performed whole-body plethysmography (Macías et al., 2014) to analyze a variety of standard respiratory parameters using unrestrained mice of three genotypes: Gucy1b2-KO −/− mice, Trpc2-KO −/− mice, and wild-type 129S6/SvEvTac mice used as reference mice (Figure S4). We found that Gucy1b2 and Trpc2 are not required for the hyperventilation response to low environmental oxygen (10% O2), indicating that Gucy1b2 and Trpc2 are not required for carotid body responses.
Discussion
Here, we have revealed a novel and unexpected role of the mouse olfactory system in sensing a low oxygen level in the ambient air. We have identified a neuronal subpopulation of the MOE with previously unknown function, type B cells, as sensors of low oxygen in the external environment. The physiological responses of these cells rely on signal transduction mechanisms that require Gucy1b2 and Trpc2 and that differ fundamentally from all previously identified oxygen sensors in mammals, including the glomus cells of the carotid body. Similar to what is the case for some invertebrate soluble guanylate cyclases, a heme domain of Gucy1b2 may bind molecular oxygen, but heterologous expression or functional gene transfer, combined with mutagenesis, will be required to test this hypothesis.
Our results suggest a model in which Trpc2 and Cnga2 are part of two independent signaling pathways. One pathway is activated by low oxygen and requires Gucy1b2, PKG, and Trpc2, and another pathway requires Cnga2, with unknown upstream signaling elements and stimulus detection properties. As both pathways probably depend on cGMP signaling, they may operate in spatially segregated compartments of type B cells. The finding that 8-bromo-cGMP application did not mimic activation of the Gucy1b2-dependent pathway may be explained by the fact that not all PKG isozymes are activated effectively by 8-bromo-cGMP (Francis et al., 2010). An obvious advantage of such a parallel signaling arrangement is that it would enable a multitasking capacity of the molecular networks formed by Gucy1b2, Trpc2, and Cnga2. We speculate that other chemical cues may be sensed by these neurons as well.
In freely breathing, awake, behaving mice, we find that a low oxygen level in the ambient air mediates an aversive behavioral response—conditioned place aversion—for which Gucy1b2 and Trpc2 are required. Conditional knockouts of Gucy1b2 or Trpc2 exclusively in type B cells will be needed to demonstrate that this behavior is mediated exclusively by these cells. Such experiments await the identification of a type B cell-specific promoter or locus to generate a Cre driver mouse strain with the appropriate specificity. For now, our interpretation of the lack of discrimination between 16% and 20% in Gucy1b2 knockout mice and in Trpc2 knockout mice is that these behavioral deficits are fully consistent with the biological role of type B cells that we propose in detecting a reduced oxygen level in the ambient air. This aversive behavior would enable a mouse to assess external environments with differing oxygen levels and to stay within a preferred environment. As virtually all rodents, including mice, display burrowing behavior and use burrows as a defense against predators and as a safe place to rear young (Deacon, 2006), peripheral sensing of low oxygen by type B cells may contribute to identifying and remembering appropriate locations for burrowing and nest building.
In invertebrates, changes in O2 levels in the external environment are detected by atypical soluble guanylate cyclases (Scott, 2011). In the nematode Caenorhabditis elegans, distinct guanylate cyclase genes (gcy-35/36 and gcy-31/33) are expressed in two pairs of sensory neurons that function in opposing directions to select an environment with a preferred O2 level; the cGMP-generating enzymes are activated by O2 increases or decreases, respectively (Cheung et al., 2005, Gray et al., 2004, Zimmer et al., 2009). In Drosophila melanogaster, O2-sensitive guanylate cyclaces mediate feeding and escape behaviors to hypoxic or hyperoxic environments (Vermehren-Schmaedick et al., 2010). Thus, nematodes and fruitflies sense O2 levels below and above a particular setpoint or range and have developed sensors for hypoxia and hyperoxia. In terrestrial mammals, such as mice, the preferred O2 level is presumably the atmospheric level of 21%, so it makes evolutionarily sense to develop sensors only for hypoxic conditions. We note that GUCY1B2 and TRPC2 are pseudogenes in human.
Conclusion
Type B cells may enable a mouse to rapidly assess the oxygen level in the external environment well before its arterial blood has become hypoxic and its survival is compromised. This peripheral oxygen-sensing system may influence certain social behaviors, such as nest building and other parenting behaviors, because newborns are particularly liable to suffer from hypoxia. Our study also establishes the first physiological and biological function for Trpc2 expression outside VSNs.
Experimental Procedures
All mouse experiments and animal care were performed in accordance with institutional and national guidelines and regulations.
Author Contributions
M.O., T.L.-Z., F.Z., and P.M. conceived the project; K.B. performed calcium imaging and post hoc immunostaining; A.P.-G. and A.M.-P. carried out c-Fos analysis; A.P.-G. performed behavioral analysis; A.P.-G. and T.L.-Z. carried out analysis of brain sections; M.O. and Z.B. carried out the generation of Gucy1b2 knockout mice and anatomical analysis; M.O. performed en face imaging; D.M. performed whole-body plethysmography; R.S.J., T.L.-Z., F.Z., and P.M. provided supervision, expertise, and data analysis. F.Z. and P.M. wrote the manuscript.
Acknowledgments
We thank Pablo Chamero for assistance during an early phase of this project and Eugenia Eckstein and Andreas Schmid for help with confocal imaging. This work was supported by Deutsche Forschungsgemeinschaft Grants SFB 894 (to T.L.-Z. and F.Z.), SFB-Transregio 152 (to T.L.-Z. and F.Z.), International Graduate School GK1326 (to F.Z.), and Schwerpunktprogramm 1392 (to T.L.-Z., F.Z., and P.M.); the Volkswagen Foundation (to T.L.-Z.; VolkswagenStiftung I/82781); the Wellcome Trust (to R.S.J.; WT092738MA); and the European Research Council Advanced Grant ORGENECHOICE (to P.M.) and the Max Planck Society (to P.M.). T.L.-Z. is a Lichtenberg Professor of the Volkswagen Foundation.
Published: December 1, 2016
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and four figures and can be found with this article online at http://dx.doi.org/10.1016/j.neuron.2016.11.001.
Contributor Information
Frank Zufall, Email: frank.zufall@uks.eu.
Peter Mombaerts, Email: peter.mombaerts@gen.mpg.de.
Supplemental Information
References
- Buck L., Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Chamero P., Katsoulidou V., Hendrix P., Bufe B., Roberts R., Matsunami H., Abramowitz J., Birnbaumer L., Zufall F., Leinders-Zufall T. G protein G(α)o is essential for vomeronasal function and aggressive behavior in mice. Proc. Natl. Acad. Sci. USA. 2011;108:12898–12903. doi: 10.1073/pnas.1107770108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung B.H., Cohen M., Rogers C., Albayram O., de Bono M. Experience-dependent modulation of C. elegans behavior by ambient oxygen. Curr. Biol. 2005;15:905–917. doi: 10.1016/j.cub.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Cunningham C.L., Gremel C.M., Groblewski P.A. Drug-induced conditioned place preference and aversion in mice. Nat. Protoc. 2006;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Deacon R.M. Burrowing in rodents: a sensitive method for detecting behavioral dysfunction. Nat. Protoc. 2006;1:118–121. doi: 10.1038/nprot.2006.19. [DOI] [PubMed] [Google Scholar]
- Derbyshire E.R., Marletta M.A. Structure and regulation of soluble guanylate cyclase. Annu. Rev. Biochem. 2012;81:533–559. doi: 10.1146/annurev-biochem-050410-100030. [DOI] [PubMed] [Google Scholar]
- Dey S., Stowers L. Think you know how smell works? Sniff again. Cell. 2016;165:1566–1567. doi: 10.1016/j.cell.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Francis S.H., Busch J.L., Corbin J.D., Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 2010;62:525–563. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J.M., Karow D.S., Lu H., Chang A.J., Chang J.S., Ellis R.E., Marletta M.A., Bargmann C.I. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430:317–322. doi: 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- Koglin M., Vehse K., Budaeus L., Scholz H., Behrends S. Nitric oxide activates the β 2 subunit of soluble guanylyl cyclase in the absence of a second subunit. J. Biol. Chem. 2001;276:30737–30743. doi: 10.1074/jbc.M102549200. [DOI] [PubMed] [Google Scholar]
- Leypold B.G., Yu C.R., Leinders-Zufall T., Kim M.M., Zufall F., Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc. Natl. Acad. Sci. USA. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Liberles S.D. Aversion and attraction through olfaction. Curr. Biol. 2015;25:R120–R129. doi: 10.1016/j.cub.2014.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Barneo J., González-Rodríguez P., Gao L., Fernández-Agüera M.C., Pardal R., Ortega-Sáenz P. Oxygen sensing by the carotid body: mechanisms and role in adaptation to hypoxia. Am. J. Physiol. Cell Physiol. 2016;310:C629–C642. doi: 10.1152/ajpcell.00265.2015. [DOI] [PubMed] [Google Scholar]
- Lucas P., Ukhanov K., Leinders-Zufall T., Zufall F. A diacylglycerol-gated cation channel in vomeronasal neuron dendrites is impaired in TRPC2 mutant mice: mechanism of pheromone transduction. Neuron. 2003;40:551–561. doi: 10.1016/s0896-6273(03)00675-5. [DOI] [PubMed] [Google Scholar]
- Macías D., Fernández-Agüera M.C., Bonilla-Henao V., López-Barneo J. Deletion of the von Hippel-Lindau gene causes sympathoadrenal cell death and impairs chemoreceptor-mediated adaptation to hypoxia. EMBO Mol. Med. 2014;6:1577–1592. doi: 10.15252/emmm.201404153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton D.B. Atypical soluble guanylyl cyclases in Drosophila can function as molecular oxygen sensors. J. Biol. Chem. 2004;279:50651–50653. doi: 10.1074/jbc.C400461200. [DOI] [PubMed] [Google Scholar]
- Munger S.D., Leinders-Zufall T., Zufall F. Subsystem organization of the mammalian sense of smell. Annu. Rev. Physiol. 2009;71:115–140. doi: 10.1146/annurev.physiol.70.113006.100608. [DOI] [PubMed] [Google Scholar]
- Munger S.D., Leinders-Zufall T., McDougall L.M., Cockerham R.E., Schmid A., Wandernoth P., Wennemuth G., Biel M., Zufall F., Kelliher K.R. An olfactory subsystem that detects carbon disulfide and mediates food-related social learning. Curr. Biol. 2010;20:1438–1444. doi: 10.1016/j.cub.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura M., Mombaerts P. Trpc2-expressing sensory neurons in the main olfactory epithelium of the mouse. Cell Rep. 2014;8:583–595. doi: 10.1016/j.celrep.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Omura M., Mombaerts P. Trpc2-expressing sensory neurons in the mouse main olfactory epithelium of type B express the soluble guanylate cyclase Gucy1b2. Mol. Cell. Neurosci. 2015;65:114–124. doi: 10.1016/j.mcn.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Gómez A., Bleymehl K., Stein B., Pyrski M., Birnbaumer L., Munger S.D., Leinders-Zufall T., Zufall F., Chamero P. Innate predator odor aversion driven by parallel olfactory subsystems that converge in the ventromedial hypothalamus. Curr. Biol. 2015;25:1340–1346. doi: 10.1016/j.cub.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar N.R. O2 and CO2 detection by the carotid and aortic bodies. In: Zufall F., Munger S.D., editors. Chemosensory Transduction: The Detection of Odors, Tastes, and Other Chemostimuli. Academic Press; 2016. pp. 321–338. [Google Scholar]
- Saraiva L.R., Ibarra-Soria X., Khan M., Omura M., Scialdone A., Mombaerts P., Marioni J.C., Logan D.W. Hierarchical deconstruction of mouse olfactory sensory neurons: from whole mucosa to single-cell RNA-seq. Sci. Rep. 2015;5:18178. doi: 10.1038/srep18178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K. Out of thin air: sensory detection of oxygen and carbon dioxide. Neuron. 2011;69:194–202. doi: 10.1016/j.neuron.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G.L. Oxygen sensing, homeostasis, and disease. N. Engl. J. Med. 2011;365:537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- Stowers L., Holy T.E., Meister M., Dulac C., Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Vermehren-Schmaedick A., Ainsley J.A., Johnson W.A., Davies S.A., Morton D.B. Behavioral responses to hypoxia in Drosophila larvae are mediated by atypical soluble guanylyl cyclases. Genetics. 2010;186:183–196. doi: 10.1534/genetics.110.118166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees C.V., Williams M.T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C., Feinstein P., Bozza T., Rodriguez I., Mombaerts P. Peripheral olfactory projections are differentially affected in mice deficient in a cyclic nucleotide-gated channel subunit. Neuron. 2000;26:81–91. doi: 10.1016/s0896-6273(00)81140-x. [DOI] [PubMed] [Google Scholar]
- Zimmer M., Gray J.M., Pokala N., Chang A.J., Karow D.S., Marletta M.A., Hudson M.L., Morton D.B., Chronis N., Bargmann C.I. Neurons detect increases and decreases in oxygen levels using distinct guanylate cyclases. Neuron. 2009;61:865–879. doi: 10.1016/j.neuron.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.