Summary
Acquisition of new ecological opportunities is a major driver of adaptation and species diversification [1, 2, 3, 4]. However, how groups of organisms expand their habitat range is often unclear [3]. We study the Gerromorpha, a monophyletic group of heteropteran insects that occupy a large variety of water surface-associated niches, from small puddles to open oceans [5, 6]. Due to constraints related to fluid dynamics [7, 8, 9] and exposure to predation [5, 10], we hypothesize that selection will favor high speed of locomotion in the Gerromorpha that occupy water-air interface niches relative to the ancestral terrestrial life style. Through biomechanical assays and phylogenetic reconstruction, we show that only species that occupy water surface niches can generate high maximum speeds. Basally branching lineages with ancestral mode of locomotion, consisting of tripod gait, achieved increased speed on the water through increasing midleg length, stroke amplitude, and stroke frequency. Derived lineages evolved rowing as a novel mode of locomotion through simultaneous sculling motion almost exclusively of the midlegs. We demonstrate that this change in locomotory behavior significantly reduced the requirement for high stroke frequency and energy expenditure. Furthermore, we show how the evolution of rowing, by reducing stroke frequency, may have eliminated the constraint on body size, which may explain the evolution of larger Gerromorpha. This correlation between the diversity in locomotion behaviors and niche specialization suggests that changes in morphology and behavior may facilitate the invasion and diversification in novel environments.
Keywords: water surface locomotion, rowing gait, tripod gait, Gerromorpha ecology, evolution
Highlights
-
•
Semi-aquatic bugs are adapted to life on water surface niches worldwide
-
•
Life on the water surface requires high locomotory maximum speed
-
•
Increased speed was achieved through changes in leg length and locomotion behavior
-
•
Derived lineages evolved rowing, an energy-efficient mode of locomotion on water
During evolution, the semi-aquatic bugs colonized a variety of water surface niches from small puddles to open oceans. Crumière et al. show that access to this new habitat is associated with morphological and behavioral changes that determine locomotory speed. Variation in locomotion behavior in distinct lineages is correlated with niche specialization.
Results and Discussion
Phylogeny and Ancestral Habitat Reconstruction
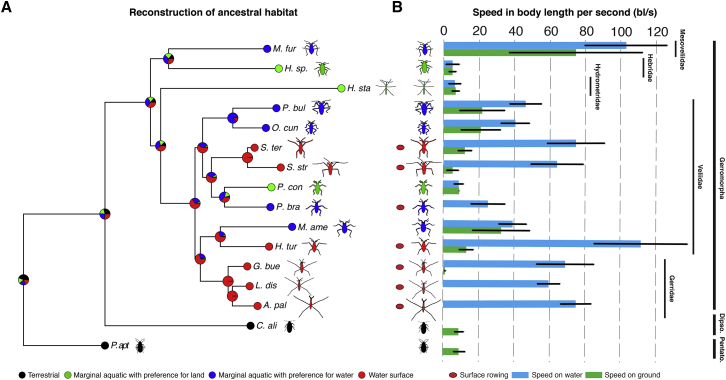
To reconstruct the ancestral habitat, we first built the phylogeny for our sample using 14 molecular markers (Figures 1A and S1A). We then assigned each species to one of four niches based on previous descriptions and our own field observations: terrestrial, marginal aquatic with preference for solid substrates, marginal aquatic with preference for water surface, and open water surface [5, 11, 12, 13, 14]. Our ancestral character state reconstruction suggests a gradual transition from the ancestral terrestrial to the derived open water habitat (Figure 1A), consistent with a previous reconstruction by Andersen [5, 11].
Figure 1.
Ancestral Habitat Reconstruction and the Evolution of Maximum Speed in Relation to Habitat Preference
(A) The pies represent the probability of ancestral habitat.
(B) Speeds, in body lengths per second (bl/s), are given for both water (blue bars) and ground (green bars) locomotion, except for the terrestrial species, which cannot move on water.
Abbreviations are as follows: Pentato, Pentatomomorpha; Dipso, Dipsocoromorpha; P.apt, Pyrrhocoris apterus; C.ali, Cryptostemma alienum; M.fur, Mesovelia furcata; H.sp, Hebrus species; H.sta, Hydrometra stagnorum; S.str, Stridulivelia strigosa; S.ter, Stridulivelia tersa; P. bra, Platyvelia brachialis; O.cun, Oiovelia cunucunumana; P.bul, Paravelia bullialata; P.con, Paravelia conata; H.tur, Husseyella turmalis; M.ame, Microvelia americana; L.dis, Limnoporus dissortis; G.bue, Gerris buenoi; A.pal, Aquarius paludum.
Samples sizes in terms of number of videos are given in Table S1. Error bars represent standard deviation. See also Figures S1 and S4 and Tables S1 and S2.
Correlation between Locomotion Speed and Niche Preference
To test whether adaptation to locomotion on water has favored high speed in the Gerromorpha, we first measured maximum speed in 16 species with clear differences in niche preference (Figure 1). We used body length per second (bl/s) as a speed unit to account for differences in body size found among species. The terrestrial outgroups and the Gerromorpha that specialize in marginal aquatic niches with preference for solid substrates [5, 11, 12, 14] produce maximum speeds ranging between 6 and 9 bl/s (Figure 1; Table S1). In contrast, Gerromorpha with preference for water surface (marginal aquatic and open water; [5, 11]) produce higher maximum speeds ranging between 27 and 113 bl/s (Figure 1; Table S1). Formal correlation tests, after taking phylogeny into account, revealed a strong correlation between maximum speed and niche preference such that species that occupy solid substrates are slow, whereas those that occupy the water surface are remarkably faster and their maximum speeds were invariably higher on water than on ground (Figures 1, S1B, and S4A; Table S1; Spearman correlation test with phylogenetic independent contrast [PIC] rho = 0.88, adjusted p = 6.5e−5; similar results were obtained with Pearson correlations) [15, 16]. The increase in locomotion speed cannot be explained merely by the plastic nature of animal behavior [2, 17, 18], as lineages with preference for solid substrates were not able to deliver high maximum speeds when tested on water. Altogether, these findings corroborate the hypothesis that water surface lifestyle has selected in favor of increased locomotion speed.
Correlation between Speed, Midleg Length, and Niche Preference
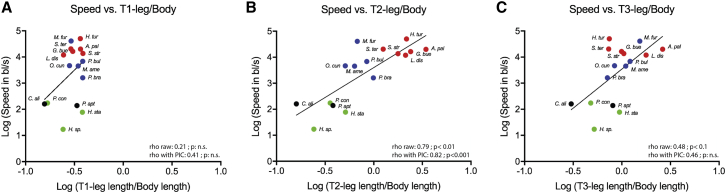
Because speed depends on leg length [19], we tested whether there is a correlation between leg length, speed, and niche preference in the Gerromorpha. The terrestrial outgroups and the species that prefer solid substrates [5, 11, 12, 14] exhibit low values of leg length to body length (Figures 2 and S2; Table S2). Conversely, species that prefer water surface, including the basally branching Mesovelia and the derived Veliidae and Gerridae [5, 11], exhibit higher values of leg length to body length (Figures 2 and S2; Table S2). Pairwise correlation tests detected a strong and significant correlation between the length of the midlegs and both speed and niche preference, but not between the forelegs or hindlegs and these two variables (Figures 2 and S4A; Spearman correlation tests with PIC; midleg and speed: rho = 0.82, adjusted p = 5.3e−4; midleg and habitat: rho = 0.88, adjusted p = 6.5e−5).
Figure 2.
Correlation Tests between Leg Length, Speed, and Habitat Preference
The ratio of leg length by body length is used to take into account the differences in size across species.
(A and C) There is no significant correlation between foreleg length and speed (rho raw: 0.21, adjusted p: 4.9e−1, n.s.; rho PIC: 0.41, adjusted p: 1.8e−1, n.s.) (A) and between hindleg length and speed (rho raw: 0.48, adjusted p: 9.7e−2; rho PIC: 0.46, adjusted p: 1.5e−1, n.s.) (C) using respectively both Spearman correlation test on raw data and with phylogenetic correction.
(B) There is a significant positive correlation between midleg length and speed (rho raw: 0.79, adjusted p: 1.6e−3; rho PIC: 0.82, adjusted p: 5.3e−4) using respectively both Spearman correlation test on raw data and with phylogenetic correlation.
See also Figures S1, S2, and S4 and Tables S1 and S2.
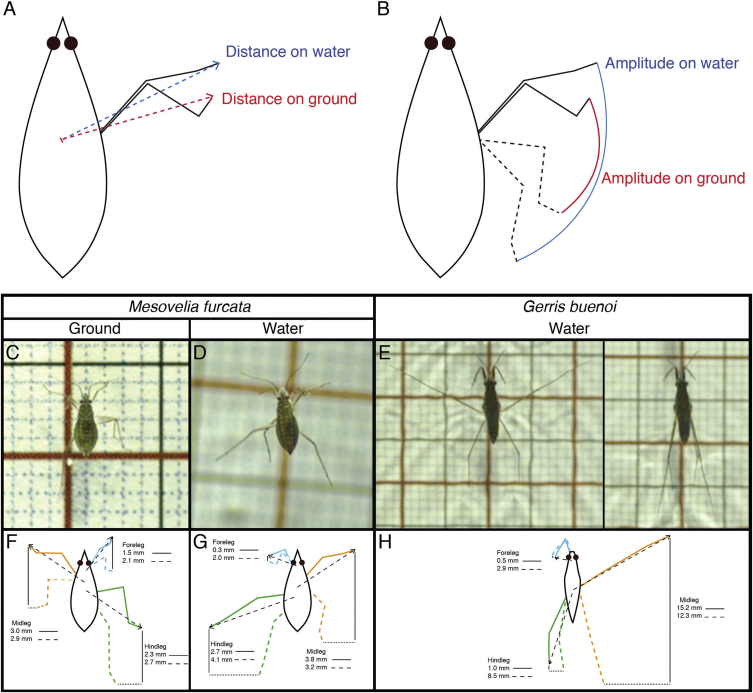
Increased leg length is known to contribute to generating faster movement through increasing the amplitude of strokes [20, 21]. When we analyzed how the legs act during locomotion on fluid compared to solid substrates, we failed to detect any difference in the amplitude of leg strokes between water and ground locomotion in species with short midlegs, preference for solid substrates, and that use the tripod gait (Hebrus) (Figures 3A and 3B; Table S3). However, in species with elongated midlegs, with preference for water surface, and that also use the tripod gait (Microvelia and Mesovelia; Movie S1), we detected a significant increase in the amplitude of midleg strokes when these species moved on water compared to when they moved on solid substrates (Figures 3C, 3D, 3F, and 3G; Table S3). Finally we tested Gerris, a species that employs rowing (Movie S1) and that generates movement through distortion of the water surface and generation and shedding of vortices [8, 22]. In this species, we found that the midlegs stretched in a straight shape and executed considerably large amplitudes of stroke, whereas the amplitudes of the forelegs and hindlegs were minimal if any (Figures 3E–3H; Table S3). Altogether, these results suggest that the preference for various water surface niches have favored increased speed through increasing the length and the amplitude of stroke of the midlegs.
Figure 3.
Analysis of Leg Movement Parameters between Ground and Water Surface Locomotion
(A and B) Representation of the measurements of leg deployment (A) and the amplitude of leg movements (B) during locomotion on ground and on water.
(C–H) Analysis of locomotion for Mesovelia furcata on ground (C and F) and on water (D and G) and for Gerris buenoi on water (E and H) with leg deployment (dashed line) and amplitude of movement (solid line).
Results are showed in Table S3. See also Figures S3 and S4, Table S3, and Movie S1.
The Tripod Gait Employs High Frequency of Leg Strokes on Water
Stroke frequency is another key biomechanical parameter that determines speed [21]. During locomotion, leg motion pattern includes two timescales representing a stance phase (leg pushing against substrate) and a swing phase (leg loses contact with substrate; [23, 24]). We therefore compared stroke frequency (number of steps per second) and pattern of leg motion (stance and swing phases separately) between 16 extant species (Figures 4A, 4B, and S3). Exclusively terrestrial species in addition to all Gerromorpha that occupy solid substrates move with a highly similar stroke frequency (ranging between 14 and 24 strokes per second [st/s]), and their pattern of motion is characterized by a longer stance phase (Figures 4A, 4B, and S3). Some of these species (Hebrus and Hydrometra) employed the same pattern of leg motion on water as the pattern they used on ground (Figures 4A, 4B, and S3).
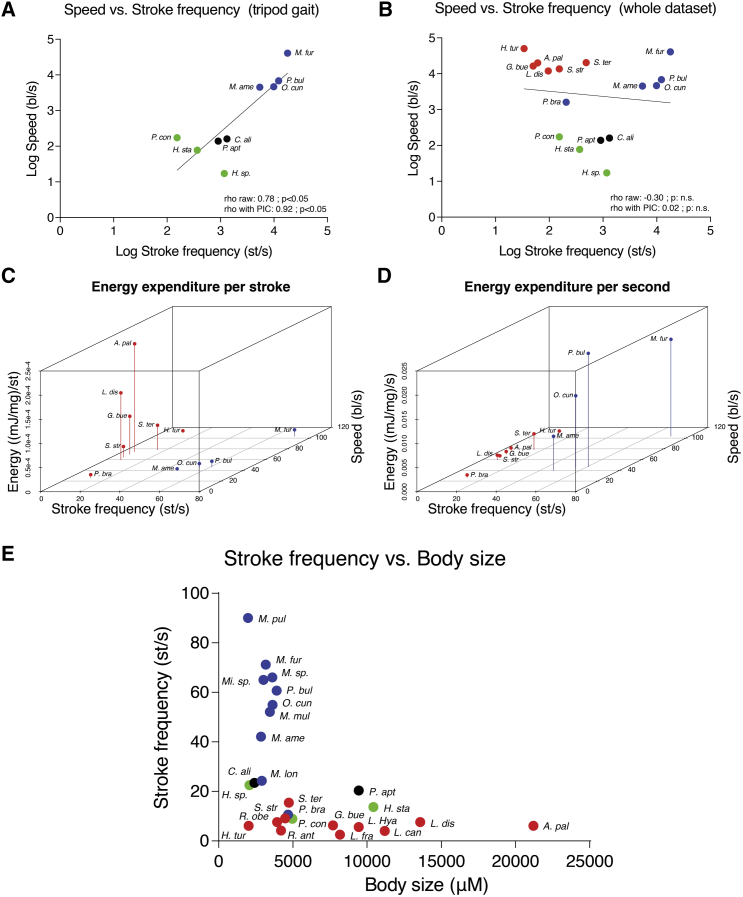
Figure 4.
Correlation Tests between Stroke Frequency, Speed, and Habitat Preference with Associated Energy Expenditure and Relation with Body Size
(A) A significant positive correlation is observed between stroke frequency and speed in species employing the tripod gait using both Spearman correlation test on raw data (rho raw: 0.78, adjusted p: 3.8e−2) and with phylogenetic correlation (rho PIC: 0.92, adjusted p: 3.6e−3).
(B) There is no correlation between stroke frequency and speed when we add species employing the rowing gait to the dataset using both Spearman correlation test on raw data (rho raw: −0.30, adjusted p: 3.3e−1, n.s.) and with phylogenetic correlation (rho PIC: 0.02, adjusted p: 0.97, n.s.). Sample sizes in term of number of videos are given in Table S1.
(C) Species using tripod gait (blue dots) spend less energy per stroke (p: 0.08, n.s.; Student’s t test).
(D) However, when normalized to the number of stroke employed per second, species using the rowing gait spend less energy (Student’s t test; p: 4.5e−5). Sample sizes and energy expenditure are given in the Supplemental Experimental Procedures and Table S1, respectively.
(E) High stroke frequency can be observed only in animals with small body size.
See also Figures 3, S3, and S4 and Tables S1 and S2.
The Gerromorpha that have preference for water surface habitats and that also use the tripod gait move both on water and on ground with a much higher stroke frequency (varying between 42 and 71 st/s; Figures 4A, 4B, and S3A). Their motion pattern on ground resembled that of species that prefer solid substrates, except for Mesovelia furcata (Figure S3B). Interestingly, when they moved on water, these species were able to change their motion pattern such that the stance and the swing phases were executed with the same duration (Figure S3C). In addition, we detected a strong correlation between stroke frequency, speed, and preference for water surface in the Gerromorpha that retain the ancestral tripod gait (Figures 4A and S4B; Spearman correlation tests with PIC; stroke frequency and speed: rho = 0.92, adjusted p = 3.6e−3; stroke frequency and habitat: rho = 0.83, adjusted p = 0.013). These findings are surprising because there is a trade-off between leg length and stroke frequency such that stroke frequency decreases with increasing leg length [25]. The Gerromorpha that move on water using the ancestral tripod gait show a significant increase in both leg length and stroke frequency, thus indicating that this trade-off was overcome during the transition to water surface-dwelling lifestyle.
In species that employ rowing as a mode of water surface locomotion (three Gerridae and three Veliidae), stroke frequency was dramatically low (varying between 6 and 16 st/s) (Figures 4B and S3A; Table S1) despite the high speed that they were able to generate (varying between 27 and 113 bl/s; Figures 1B and 4B; Table S1). In addition, the pattern of leg motion was reversed in most rowing species compared to species that specialize in solid substrates, such that the stance phase is now shorter than the swing phase (Figure S3C). Therefore, niche specialization across the Gerromorpha was accompanied by changes in the mode of locomotion, leg length, patterns of leg motion, and stroke frequency.
Rowing Gait Is Significantly More Efficient on the Water Surface Than Tripod Gait
Species that use the derived rowing gait show a substantially lower stroke frequency when compared to those using the ancestral tripod gait. Therefore, we tested whether the evolution of rowing could be associated with increased efficiency during water surface locomotion. First, we inferred (see Supplemental Experimental Procedures) the amount of energy spent in the two veliids Stridulivelia strigosa and Paravelia bullialata, which have a similar body mass (Table S1), generate comparable speeds, but differ in their mode of locomotion (Figure 1). Stridulivelia strigosa stroked 9 times to generate a speed of 64 body lengths (28 cm) in 1 s, whereas Paravelia bullialata stroked 61 times to generate a speed of 47 body lengths (18 cm) in 1 s (Figures 1B and 4D). We calculated that Stridulivelia strigosa spent 2.21e−5 mJ/mg/st (millijoule per milligram per stroke), and Paravelia bullialata spent 1.17e−5 mJ/mg/st (Figure 4C; Table S1). Therefore, by taking into account the number of strokes per unit of time, Stridulivelia strigosa (rowing) spent 2.9e−4 mJ/mg/s (millijoule per milligram per second), and Paravelia bullialata (tripod) spent 2.35e−2 mJ/mg/s, which is over 80-fold higher in the latter (Figure 4D; Table S1). When we extended this analysis to the entire sample of the Gerromorpha that transited to the derived water surface habitat, we found that the rowing species consistently spent less energy to generate movement than species that employ the ancestral tripod gait (Figure 4D; Table S1; Student’s t test; p = 4.6e−4). Species using the ancestral tripod gait on water spend much of the time on aquatic plants and would only execute bursts of fast movement when crossing free water patches, presumably, to minimize the risk of capture by bottom-striking predators such as fish [5, 10]. Therefore, the maintenance of the tripod gait may have been advantageous, despite the high-energy demand, as it allows these animals to be versatile. Rowing species, however, spend much more time on the open water where they forage, mate, and interact with predators [5, 10]. This lifestyle may have increased demands on frequent, fast, and energy-efficient locomotion. These results indicate that the derived mode of water surface locomotion through rowing gait, characteristic of derived species that specialize in open water, is more energy efficient when compared to the ancestral mode using the tripod gait.
Reduced Stroke Frequency Is Associated with Increased Body Size in Derived Gerromorpha
Another important trade-off in walking systems, including arthropods, exists between body size and stroke frequency, such that stroke frequency decreases with increasing body size [21, 26]. Because the evolution of rowing in derived lineages is associated with a dramatic decrease in stroke frequency, we hypothesized that this decrease may have removed the constraint on body size [27]. To test this hypothesis, we plotted body size by stroke frequency in a total of 25 species that exhibit large variation in these two traits (Figure 4E). We found that species with small body size (<5,000 μm) may exhibit either low or high stroke frequency (Figure 4E). Conversely, all species with larger body size (>5,000 μm) showed a significantly low stroke frequency (Figure 4E). In our dataset, we could not find any species with both high stroke frequency and large body size (Figure 4E). Litterbugs (Dipsocoromorpha; Figure 1A; [28]) are characteristically small [29], and similarly the ancestral state of body size in the Gerromorpha has been undoubtedly small to very small [5]. It is possible that because of their small body size, the ancestors of the Gerromorpha were free from the constraint imposed on larger bugs, such that stroke frequency increased and facilitated the transition to water surface life. Subsequently, the evolution of rowing combined with the significant increase in the length of the driving midlegs [10, 30, 31, 32, 33], and most likely changes in the associated innervation and musculature [22, 34, 35], may have contributed to reducing stroke frequency while maintaining high speeds. These structural and behavioral changes may have contributed to the evolution of larger bodies including the largest semi-aquatic bug known, the Gerrid Gigantometra gigas, whose body length is over 3 cm and leg span over 25 cm [36].
Conclusions
Understanding how the evolution of behavior and morphology can be associated with niche expansion and species diversification is a major challenge in evolutionary biology [1, 3, 17, 18]. We have shown that lineages that remained in the ancestral habitat, composed of solid substrates, produce low maximum speeds and that lineages that specialize in open water surface niches generate significantly higher maximum speeds. This increase in locomotion speed is associated with the evolution of increased midleg length, changes in leg motion patterns, and increased frequency of leg strokes. The subsequent evolution of rowing, as a novel mode of locomotion on the water, removed the requirement for high stroke frequency, reduced energy expenditure, and enabled derived lineages to specialize in open water zones. Finally, we have shown how the evolution of rowing, by removing the requirement for high stroke frequency, may have led to the evolution of larger Gerromorpha. By uncovering the link between the ecology of the semi-aquatic bugs and the variety of phenotypes they exhibit, this work draws a comprehensive picture of how a combination of behavioral and structural changes can impact the evolutionary trajectory of groups of animals and can be associated with niche expansion and lineage diversification.
Experimental Procedures
Insect Sampling and Culture
Extant specimens were collected during fieldwork in the locations indicated in Table S2. All species were kept in water tanks at 25°C, 50% humidity, and 14 hr of daylight and fed on live crickets.
Phylogenetic Reconstruction
Phylogeny reconstruction was conducted using 14 molecular markers with the software Geneious version 7.1.9 using plugins MrBayes version 3.2.6 [37] (one million generations; 25% burnin) and PhyML version 3.0 [38], using GTR model with 100 bootstraps. More details can be found in the Supplemental Experimental Procedures.
Habitat Classification
To enable reconstruction, we consolidated Andersen’s habitat classes [5, 11] into the following four: terrestrial, marginal aquatic with preference for solid substrates, marginal aquatic with preference for water surface, and open water surface. Each species was assigned to one of these four classes based on previous descriptions [5, 11, 12, 13, 14] and on the environment where we caught them.
Video Acquisition, Quantification of Speed, and Stroke Frequency
A set of adult individuals for each species were filmed at 2,000 frames per second, both on water surface and on a solid substrate with a grid paper in the background as a calibration scale. To calculate speed, a mean value was extracted from a defined interval plateau phase from velocity curve along each video. This interval represents the maximum speed during the run of the individual. Stroke frequency was determined as the number of steps performed by the individuals during a given locomotion duration and converted into number of strokes per second. Details of sample sizes and calculations can be found in the Supplemental Experimental Procedures.
Reconstruction of Ancestral Character State
Reconstruction of ancestral character states was performed in Rstudio version 0.99.486 using a maximum likelihood method adapted to discrete characters (ace, package ape; [16]) and represented using phytools [39]. Details about methods of reconstruction and scripts used can be found in the Supplemental Experimental Procedures.
Inference of Energy Consumption
We inferred the amount of energy spent per stroke based on the procedure from [40]. Kinetic energy (Ek in joules) used during a stroke is determined using the following expression: Ek = 0.5mv2, where m is the mass of the insect in grams, and v is the velocity generated during one stroke in meters per second. The analyses were performed using speed data extracted from the high-speed movies. Calculations and sample sizes are detailed in the Supplemental Experimental Procedures.
Statistical Analyses
Details and R script used for statistical analyses are provided in the Supplemental Experimental Procedures.
Author Contributions
Conceptualization, A.J.J.C. and A.K.; Methodology, A.J.J.C. and A.K.; Investigation, A.J.J.C.; Formal Analysis, A.J.J.C., M.E.S., and M.S.; Resources, A.J.J.C., M.E.S., D.A., F.F.F.M., and A.K.; Data Curation, D.A. and M.E.S.; Writing – Original Draft, A.J.J.C. and A.K.; Writing – Review & Editing, A.J.J.C., M.E.S., M.S., D.A., F.F.F.M., and A.K.; Visualization, A.J.J.C. and A.K.; Supervision, A.K.; Funding Acquisition, A.K.
Acknowledgments
We thank W. Salzburger, E. Abouheif, F. Bonneton, S. Viala, A. Decaras, and W. Toubiana for comments and discussion; H. Labrique and J.C. Streito for help with the Dipsocoromorpha; and M. Burrows for advice on inferring energy expenditure. This work was funded by ERC-CoG #616346, ATIP-Avenir, and CNPq-PVE #400751/2014-3 to A.K. and a postdoctoral fellowship by the Swiss National Science Foundation to M.E.S. Specimens from Brazil were collected under SISBIO permit #43105-1.
Published: December 8, 2016
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, four figures, three tables, and one movie and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2016.09.061.
Accession Numbers
Accession numbers for the sequences and alignment of concatenated sequences reported in this paper are available in Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.134c4).
Supplemental Information
Mesovelia furcata using the tripod gait (top) and Gerris buenoi using the rowing gait (bottom) during water surface locomotion. Videos were taken on a grid for size reference.
References
- 1.Losos J.B. University of California Press; 2009. Lizards in an Evolutionary Tree: Ecology and Adaptive Radiation of Anoles. [Google Scholar]
- 2.Mayr E. Belknap Press of Harvard University Press; 1963. Animal Species and Evolution. [Google Scholar]
- 3.Schluter D. Oxford University Press; 2000. The Ecology of Adaptive Radiation. [Google Scholar]
- 4.Yoder J.B., Clancey E., Des Roches S., Eastman J.M., Gentry L., Godsoe W., Hagey T.J., Jochimsen D., Oswald B.P., Robertson J. Ecological opportunity and the origin of adaptive radiations. J. Evol. Biol. 2010;23:1581–1596. doi: 10.1111/j.1420-9101.2010.02029.x. [DOI] [PubMed] [Google Scholar]
- 5.Andersen N.M. Volume 3. Scandinavian Science Press LTD; 1982. (The Semiaquatic Bugs (Hemiptera: Gerromorpha). Entomonograph). [Google Scholar]
- 6.Ikawa T., Okabe H., Cheng L.N. Skaters of the seas - comparative ecology of nearshore and pelagic Halobates species (Hemiptera: Gerridae), with special reference to Japanese species. Mar. Biol. Res. 2012;8:915–936. [Google Scholar]
- 7.Denny M.W. Princeton University Press; 1993. Air and Water: The Biology and Physics of Life’s Media. [Google Scholar]
- 8.Hu D.L., Bush J.W.M. The hydrodynamics of water-walking arthropods. J. Fluid Mech. 2010;644:5–33. [Google Scholar]
- 9.Suter R., Rosenberg O., Loeb S., Long H. Locomotion on the water surface: propulsive mechanisms of the fisher spider. J. Exp. Biol. 1997;200:2523–2538. doi: 10.1242/jeb.200.19.2523. [DOI] [PubMed] [Google Scholar]
- 10.Armisén D., Refki P.N., Crumière A.J., Viala S., Toubiana W., Khila A. Predator strike shapes antipredator phenotype through new genetic interactions in water striders. Nat. Commun. 2015;6:8153. doi: 10.1038/ncomms9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen N.M. Phylogenetic inference as applied to the study of evolutionary diversification of semiaquatic bugs (Hemiptera, Gerromorpha) Syst. Zool. 1979;28:554–578. [Google Scholar]
- 12.Dias-Silva K., Moreira F.F.F., Giehl N.F.D., Nóbrega C.C., Cabette H.S.R. Gerromorpha (Hemiptera: Heteroptera) of eastern Mato Grosso State, Brazil: checklist, new records, and species distribution modeling. Zootaxa. 2013;3736:201–235. doi: 10.11646/zootaxa.3736.3.1. [DOI] [PubMed] [Google Scholar]
- 13.Heiss E., Pericart J. Fédération Française des Sociétés de Sciences naturelles; 2007. Faune n° 91 – Hémiptères Aradidae, Piesmatidae et Dipsocoromorphes. [Google Scholar]
- 14.Schuh R.T., Slater J.A. Comstock Publishing Associates; 1995. True Bugs of the World (Hemiptera: Heteroptera): Classification and Natural History. [Google Scholar]
- 15.Kembel S.W., Cowan P.D., Helmus M.R., Cornwell W.K., Morlon H., Ackerly D.D., Blomberg S.P., Webb C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics. 2010;26:1463–1464. doi: 10.1093/bioinformatics/btq166. [DOI] [PubMed] [Google Scholar]
- 16.Paradis E., Claude J., Strimmer K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- 17.Duckworth R.A. The role of behavior in evolution: a search for mechanism. Evol. Ecol. 2009;23:513–531. [Google Scholar]
- 18.West-Eberhard M.J. Oxford University Press; 2003. Developmental Plasticity and Evolution. [Google Scholar]
- 19.Zaaf A., Van Damme R., Herrel A., Aerts P. Spatio-temporal gait characteristics of level and vertical locomotion in a ground-dwelling and a climbing gecko. J. Exp. Biol. 2001;204:1233–1246. doi: 10.1242/jeb.204.7.1233. [DOI] [PubMed] [Google Scholar]
- 20.Baudouin A., Hawkins D. A biomechanical review of factors affecting rowing performance. Br. J. Sports Med. 2002;36:396–402. doi: 10.1136/bjsm.36.6.396. discussion 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu G.C., Wright J.C., Whitaker D.L., Ahn A.N. Kinematic evidence for superfast locomotory muscle in two species of teneriffiid mites. J. Exp. Biol. 2010;213:2551–2556. doi: 10.1242/jeb.024463. [DOI] [PubMed] [Google Scholar]
- 22.Andersen N.M. A comparative study of locomotion on the water surface in semiaquatic bugs (Insecta, Hemiptera, Gerromorpha) Vidensk. Meddr dansk naturh. Foren. 1976:337–396. [Google Scholar]
- 23.Alexander R.M. Princeton University Press; 2003. Principles of Animal Locomotion. [Google Scholar]
- 24.Mendes C.S., Bartos I., Akay T., Márka S., Mann R.S. Quantification of gait parameters in freely walking wild type and sensory deprived Drosophila melanogaster. eLife. 2013;2:e00231. doi: 10.7554/eLife.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanhooydonck B., Van Damme R., Aerts P. Speed and stamina trade-off in lacertid lizards. Evolution. 2001;55:1040–1048. doi: 10.1554/0014-3820(2001)055[1040:sastoi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 26.Heglund N.C., Taylor C.R., McMahon T.A. Scaling stride frequency and gait to animal size: mice to horses. Science. 1974;186:1112–1113. doi: 10.1126/science.186.4169.1112. [DOI] [PubMed] [Google Scholar]
- 27.Arnold S.J. Constraints on phenotypic evolution. Am. Nat. 1992;140(Suppl 1):S85–S107. doi: 10.1086/285398. [DOI] [PubMed] [Google Scholar]
- 28.Li M., Tian Y., Zhao Y., Bu W. Higher level phylogeny and the first divergence time estimation of Heteroptera (Insecta: Hemiptera) based on multiple genes. PLoS ONE. 2012;7:e32152. doi: 10.1371/journal.pone.0032152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weirauch C., Schuh R.T. Systematics and evolution of Heteroptera: 25 years of progress. Annu. Rev. Entomol. 2011;56:487–510. doi: 10.1146/annurev-ento-120709-144833. [DOI] [PubMed] [Google Scholar]
- 30.Khila A., Abouheif E., Rowe L. Evolution of a novel appendage ground plan in water striders is driven by changes in the Hox gene Ultrabithorax. PLoS Genet. 2009;5:e1000583. doi: 10.1371/journal.pgen.1000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khila A., Abouheif E., Rowe L. Comparative functional analyses of ultrabithorax reveal multiple steps and paths to diversification of legs in the adaptive radiation of semi-aquatic insects. Evolution. 2014;68:2159–2170. doi: 10.1111/evo.12444. [DOI] [PubMed] [Google Scholar]
- 32.Refki P.N., Armisén D., Crumière A.J.J., Viala S., Khila A. Emergence of tissue sensitivity to Hox protein levels underlies the evolution of an adaptive morphological trait. Dev. Biol. 2014;392:441–453. doi: 10.1016/j.ydbio.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Refki P.N., Khila A. Key patterning genes contribute to leg elongation in water striders. Evodevo. 2015;6:14. doi: 10.1186/s13227-015-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burrows M. Local circuits for the control of leg movements in an insect. Trends Neurosci. 1992;15:226–232. doi: 10.1016/0166-2236(92)90040-f. [DOI] [PubMed] [Google Scholar]
- 35.Gleeson T.T., Harrison J.M. Muscle composition and its relation to sprint running in the lizard Dipsosaurus dorsalis. Am. J. Physiol. 1988;255:R470–R477. doi: 10.1152/ajpregu.1988.255.3.R470. [DOI] [PubMed] [Google Scholar]
- 36.Tseng M., Rowe L. Sexual dimorphism and allometry in the giant water strider Gigantometra gigas. Can. J. Zool. 1999;77:923–929. [Google Scholar]
- 37.Huelsenbeck J.P., Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 38.Guindon S., Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 39.Revell L.J. phytools: an R package for phylogenetic comparative biology (and other things) Methods Ecol. Evol. 2012;3:217–223. [Google Scholar]
- 40.Burrows M., Dorosenko M. Jumping mechanisms in lacewings (Neuroptera, Chrysopidae and Hemerobiidae) J. Exp. Biol. 2014;217:4252–4261. doi: 10.1242/jeb.110841. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mesovelia furcata using the tripod gait (top) and Gerris buenoi using the rowing gait (bottom) during water surface locomotion. Videos were taken on a grid for size reference.