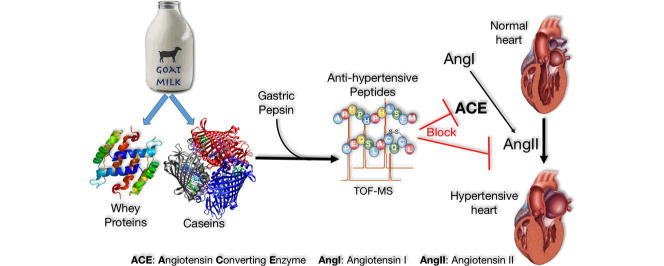
Graphical abstract
Abbreviations: GCP, goat casein proteins; GWP, goat whey proteins; P-GCP, pepsin digested-GCP; P-GWP, pepsin digested-GWP; ACE, angiotensin I-converting enzyme; HHL, hippuryl-histidyl-leucine; HA, hippuric acid; HL, histidyl-leucine; TNBS, 2,4,6-trinitrobenzene sulfonate; TNP-, 2,4,6-trinitrophenyl; MALDI-TOF/MS, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
Keywords: Goat milk, Bioactive peptides, Caseins, Whey, Angiotensin I-converting enzyme (ACE), Anti-hypertension
Abstract
Angiotensin-converting enzyme (ACE) plays a central role in blood pressure regulation by producing the vasoconstrictor angiotensin II. The inhibition of ACE with natural inhibitors, as alternatives to avoid the side effect of synthetic drugs, is a major target in the prevention of hypertension. In this study, we examined the separated caseins and whey proteins of goat milk for the presence of ACE inhibitory peptides. Digestion of isolated whey proteins and caseins of goat milk by gastric pepsin generated soluble hydrolysates exhibiting significant inhibition of ACE compared to weak inhibition by undigested proteins. The hydrolysates were fractionated by size exclusion chromatography, Sephacryl S-100 column, into four fractions (F1–F4). The late-eluting fraction (F4) of either whey or caseins exhibited greater ACE inhibition. Peptides in both F4 fractions, isolated by RP-HPLC, exhibited variable ACE inhibitory activities with the hydrophobic peptide peaks being the most potent ACE inhibitors. MALDI-TOF MS/MS resulted in identification of three potent ACE inhibitory peptides: PEQSLACQCL from β-lactoglobulin (residues 113–122), QSLVYPFTGPI from β-casein (residues 56–66), and ARHPHPHLSFM from κ-casein (residues 96–106). The peptides from whey and caseins exert significant ACE inhibitory activities comparable to that of captopril, an antihypertensive drug, exhibiting IC50 values of 4.45 μM and 4.27 μM, respectively. The results introduce, for the first time, new potent ACE-inhibitory peptides that can be released by gastric pepsin of goat milk whey and caseins and thus may pave the way for their candidacy as anti-hypertensive bioactive peptides and prevention of associated disorders.
Introduction
Milk proteins are the major source of bioactive peptides released upon enzymatic hydrolysis during gastrointestinal transit or food processing. Such peptides are being identified in dairy protein hydrolysates and shown to possess opioid, immune-modulatory, antimicrobial, antithrombotic, growth stimulating or antihypertensive properties [1], [2]. The milk protein-derived bioactive peptides have the potential to be formulated into foods to provide their health promoting effects in human. The major difference between drugs and milk protein-derived bioactive peptides is that synthetic drugs are normally not present within the human body unless they are intentionally administered. On the other hand, bioactive peptides may be present in human as they may arise from digestion of food [2]. These differences may bring additional challenges to the discovery of milk bioactive peptides and to the evaluation of their therapeutic efficacy.
Milk of bovine is the most commonly searched for dairy bioactive peptides. In the past few years, developments in molecular biology, genomics, and proteomics have highlighted the extreme complexity and variability of milk proteins across species [3]. However, in most dairy species, other than bovine, the repertoire of potential milk bioactive proteins or their derived peptides remains to be unraveled. It is one of the greatest challenges facing milk science to provide the basis for health-promoting properties of milk proteins and peptides of dairy species other than bovine. The importance of goat milk is intensifying because cow’s milk is a common cause of food allergy in infants [4], [5]. In addition, goat milk proteins are more digestible and medically is being recommended for newborn when human milk is lacking [6]. In newborns, milk feeding contributes to protect against oxidative stress and the associated diseases such as cardiovascular disorder [7], [8].
Hypertension is recognized as a serious risk factor for cardiovascular disease [9]. Angiotensin I-converting enzyme (ACE) is a key enzyme in regulation of blood pressure through two different reactions in the renin-angiotensin-aldosterone system (RAAS) and the kinin nitric oxide system (KNOS). For this, many synthetic ACE inhibitors, such as captopril, enalapril, fosinopril, lisinopril, and ramipril were identified and used for the treatment of hypertension. However, these synthetic inhibitors have side effects including coughing, taste disturbance and skin rash [10], [11]. Thus, one of the major challenges to today’s world healthcare sectors is to identify ACE inhibitors from natural resources.
Milk bioactive peptides constitute alternatives for this, serving directly as ACE inhibitors, or providing a scaffold for the engineering of novel molecules with clinical potential. In earlier work we found that gastric pepsin digestion of goat milk proteins generated various bioactive peptides with potent antioxidant activities [12]. The current study aimed to explore the ACE inhibitory activities of hydrolysates and peptides of separated whey proteins and caseins of goat milk, liberated upon cleavage with gastric pepsin. The structures of goat milk peptides for inhibition of ACE enzyme and the potential of whey proteins, by-products of cheese industry, as a source of new therapeutic peptides against hypertension are discussed.
Material and methods
Materials
Goat milk was obtained from three goats, Egyptian Baladi breed, at the animal station of the South Valley University (Qena, Egypt). Angiotensin I-converting enzyme (ACE) from rabbit lung, its substrate hippuryl-L-histidyl-L-leucine (HHL), and pepsin were purchased from Sigma-Aldrich (Tokyo, Japan). Captopril and 2,4,6-trinitrobenzene sulfonate (TNBS) were products of Nacalai Tesque Inc. Sephacryl S-100 was a product of Amersham-Pharmacia Biotech (Tokyo, Japan). All other reagents were of analytical grade.
Separation of caseins and whey proteins
The raw goat milk was transported from the station and immediately cooled in ice before removing fat. Fat was removed from 250 ml raw milk by centrifugation at 5000 × g for 30 min at 10 °C, and then the skimmed milk was passed through three layers of gauze. Casein was precipitated by adjusting to pH 4.6 with 10% acetic acid and centrifugation at 5000 × g for 10 min. The pellet “caseins” was re-suspended in dH2O. Casein suspension and the supernatant “whey proteins” were dialyzed against dH2O, using 1000 MWCO tubes (Spectra/Por, California, USA) at 4 °C. These fractions were lyophilized and referred to as goat casein proteins (GCP) and goat whey proteins (GWP).
In vitro pepsin digestion
The GCP and GWP were dissolved in milli-Q water and then adjusted to pH 3.0 with HCl. Pepsin in 1 mM HCl was added to the protein solution at enzyme-to-substrate (E/S) ratio of 1:50 (w/w) and incubated for 2 h at 37 °C with mild shaking. Pepsin was inactivated by heating at 85 °C for 5 min and then placed on ice for 5 min. Insoluble solids were removed by centrifugation at 3000 × g for 10 min. The resulting supernatants were adjusted to pH 7.0, to fully inactivate pepsin, and then lyophilized, referred to as pepsin digested-GWP (P-GWP) and pepsin digested-GCP (P-GCP). The GCP and GWP as well as their hydrolysates (P-GCP and P-GWP) were tested for ACE inhibitory activity as described below.
Fractionation of peptides in P-GCP and P-GWP
Pepsin digests P-GWP and P-GCP (12 mg in 2 mL dH2O), were fractionated by size-exclusion chromatography on Sephacryl S-100 column (1.0 × 50 cm), equilibrated and eluted with 12.5 mM pyridine-acetate buffer (pH 5.5), at flow rate of 2 ml/min. Protein elution was monitored at 280 nm. All fractions were lyophilized and tested for ACE inhibitory activity. The peptides in the active fractions (1 mg/mL) of P-GWP and P-GCP were further purified by reversed phase-HPLC, using TSK gel ODS-120T column (7.8 × 300 mm) and a linear gradient was employed using 1–50% acetonitrile over 110 min at flow rate of 0.5 mL/min. Peptide elution was monitored at 215 nm. The purification process by RP-HPLC was repeated to collect enough amounts of each peak. The respective peak from different runs was combined, lyophilized and tested for ACE inhibitory activity.
ACE inhibitory activity
The assay of ACE inhibitory activity is based on specific binding of TNBS to the primary amine of His-Leu dipeptide produced by hydrolytic cleavage from Hip-His-Leu by ACE, forming TNP-His-Leu (TNP-HL) by desulfitation, followed by formation of a yellow complex with sulfite detected at 420 nm [13]. The assay was optimized in 96-well microtiter plate with the capacity to process high number of samples with small volume in short time (Suppl. Fig. 1). The inhibition assay was performed at a final concentration of 16.95 mU/mL ACE and 1.10 mM HHL substrate in the presence of a given concentration of peptides. Briefly, a 5-μL aliquot of ACE solution (200 mU/mL) was added to 31 μL 50 mM sodium borate buffer pH 8.3 containing 0.3 M NaCl (SBBS) in each well of 96-well microplate. A 10-μL aliquot of peptide sample (14–236 μg/mL) or SBBS in control reaction (Ctrl) was added. The reaction was started by the addition of 13 μL substrate HHL solution (5 mM) to the reaction mixture (final volume of 59 μL). Two blanks were prepared: one without ACE and inhibitor peptide (Bi) and another without ACE and HHL (Bs). After incubation for 1 h at 37 °C, 100 μL sodium tetraborate (200 mM), 50 μL sodium sulfite (10 mM) and 50 μL TNBS (3.4 mM) were added to each well. The mixtures were further incubated for 20 min at 37 °C. The absorbance was measured at 420 nm using Ultrospec Biotrak II microplate reader (Amersham-Biosciences) with on-board software and interface packet for Biochrom reader. The assay was performed by using the same samples in triplicate with two wells per sample.
The percentage of ACE inhibitory activity was calculated according to the following equation:
where C, S, Bi and Bs represent the absorbance of control (100% activity), sample (inhibitor peptide), blank inhibitor (HHL alone) and blank sample (peptide alone). The blank sample (Bs) is included to distinguish the value of the free amino groups of the inhibitor peptide from that of substrate (HHL) released upon cleavage of hippuric acid by the ACE. For the inhibitory activity of captopril (final concentration of 2–10 μg/mL), blank sample (Bs) was not included because it does not contain free amino groups or produce yellow color with TNBS. The IC50 value (the concentration of inhibitor resulting in a 50% reduction of ACE activity) was calculated by regression analysis from ACE inhibition curve obtained with increasing amounts of inhibitor.
MALDI-TOF MS/MS analysis
The peptide peak of RP-HPLC (1 μL) was directly spotted, in triplicates, onto a steel MALDI target plate and allowed to air-dry at room temperature. Then 2 μL of MALDI matrix (10 mg/mL of α-cyano-4-hydroxy-cinnamic acid [α-HCCA] in 50% acetonitrile-2.5% trifluoroacetic acid; Bruker Daltonics) was added to the dried peptide spots. After drying, MALDI-TOF MS/MS analyses were performed with Autoflex Speed TOF/TOF (Bruker Daltonics) in positive reflector mode, with an accelerating voltage of 20,000 V, in the mass range of 1000–3200 Da. Between 100 and 200 shots/spot were acquired with 1 kHz repetition rate using SmartBeam laser of the original instrument configuration. For the MS/MS mode, argon gas was used as collision gas at a pressure of 2 × 10−6 mbar. The spectral analysis was carried out with FlexAnalysis 3.3 and ProteinScape software (Bruker Daltonics). Calibration was done by using peptides calibration standard that covers the mass range 1000–4000 Da (Bruker Daltonics). The analysis was performed in two independent experiments with triplicate spots per sample.
Results
ACE inhibitory activity proteins and digests
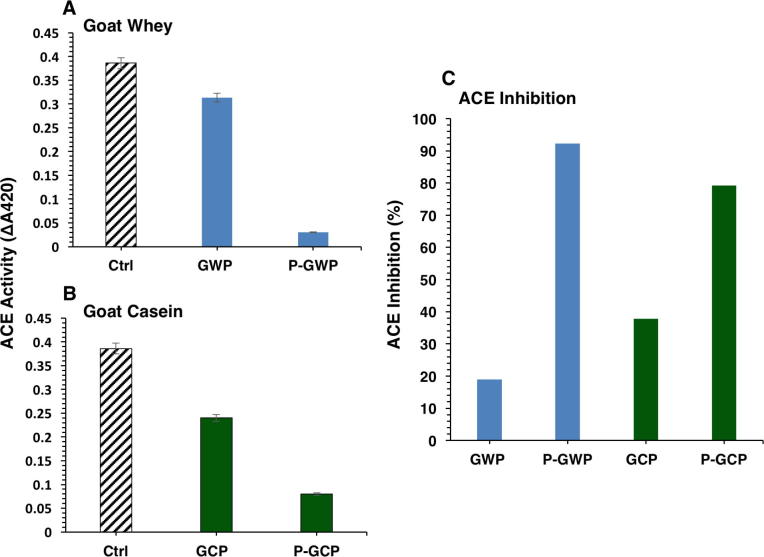
Proteins of goat milk were fractionated into caseins (GCP) and acid whey proteins (GWP), and then digested with pepsin to generate pepsin hydrolysates of goat milk caseins (P-GCP) and whey proteins (P-GWP). Fig. 1 shows the effect of GWP (A) and GCP (B) and their pepsin hydrolysates (P-GWP and P-GCP) at concentration of 10 μg/mL on ACE activity. Although undigested proteins (GWP and GCP) exhibited ACE inhibitory activity, their pepsin hydrolysates (P-GWP and P-GCP) exhibited significantly higher ACE inhibitory activities (Fig. 1C). Both hydrolysates (P-GWP and P-GCP) showed dose-dependent inhibition of ACE activity up to a peptides concentration of 40 μg/mL (Fig. 2). Although at 40 μg/mL both P-GWP (Fig. 2A) and P-GCP (Fig. 2B) produced over 95% ACE inhibition, P-GWP showed more pronounced dose-dependency and potency of ACE inhibition (Fig. 2A).
Fig. 1.
The effect of goat milk whey proteins (A) and caseins (B) as well as their pepsin hydrolysates on ACE activity. (C) The ACE inhibitory activity of GWP, GCP, and their hydrolysates (P-GWP and P-GCP) at final concentration of 10 μg/mL. ACE activity is presented as change in absorbance (A420) of TNP-HL. ACE inhibitory activity presented as percentage calculated as described in Materials and Methods. The data are representative of three experiments with two wells per sample.
Fig. 2.
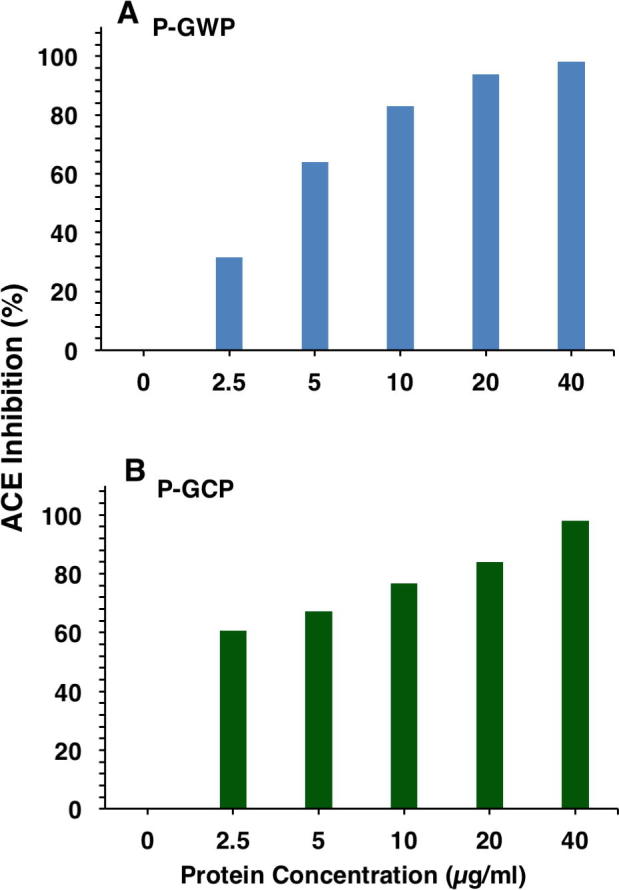
Dose-dependent ACE inhibitory activities of goat milk P-GWP (A) and P-GCP (B) hydrolysates. ACE inhibitory activity is presented as percentage. The data are representative of three experiments with two wells per sample.
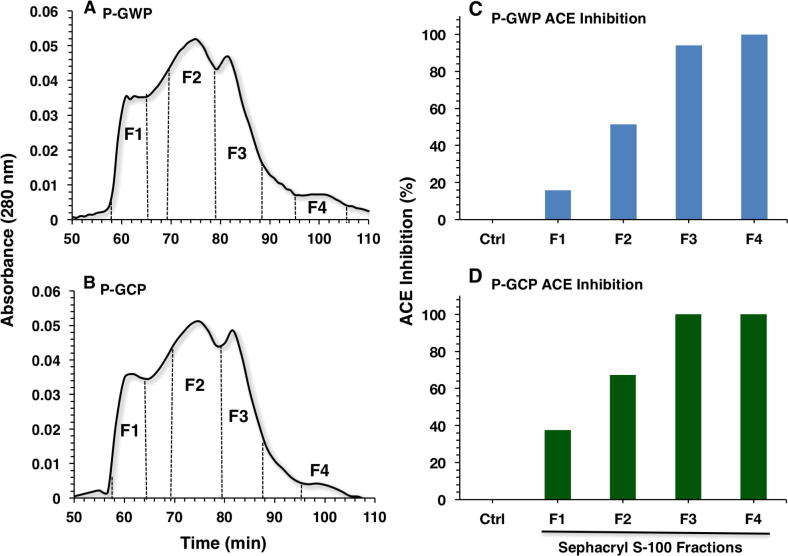
The hydrolysates were fractionated using size-exclusion column (Sephacryl S-100). Both P-GWP (Fig. 3A) and P-GCP (Fig. 3B) hydrolysates were pooled into four fractions (F1–F4). Fig. 3C and D shows the ACE inhibitory activity of the fractions of P-GWP (Fig. 3C) and P-GCP (Fig. 3D). The early-eluting fraction (F1) of P-GCP exhibited higher ACE inhibition than that of P-GWP, whereas the degree of ACE inhibition increased linearly as the elution time slow (Fig. 3C). Fractions of P-GCP exhibited similar trend of ACE inhibition whereas the late-eluting fractions (F3 and F4) exhibited equally the highest ACE inhibitory activity (Fig. 3D). At concentration of 10 μg/mL, the late-eluting fraction (F4) of either whey (Fig. 3C) or caseins (Fig. 3D) showed 100% ACE inhibition.
Fig. 3.
ACE inhibitory activity of peptide fractions of goat milk P-GWP (A) and P-GCP (B) hydrolysates separated by size exclusion chromatography using Sephacryl S-100 column. ACE activity of each fraction (F1–F4) was at final concentration of 10 μg/mL. ACE inhibitory activity of P-GWP (C) and P-GCP (D) fractions is presented as percentage. The data are representative of three experiments done in duplicates.
Purification of ACE inhibitory peptides
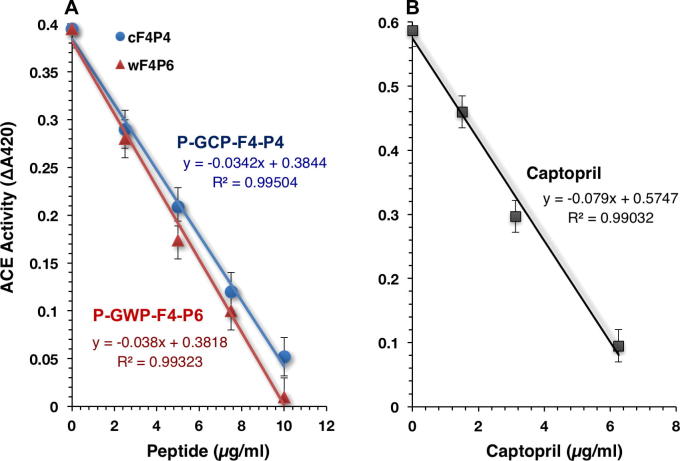
The two most potent late-eluting fractions (F4) of both P-GWP and P-GCP were subjected to preparative reversed phase-HPLC using TSK-Gel ODS-120T column. Six peptide peaks (P1–P6) were collected from F4 of P-GWP (Fig. 4A) and six peptide peaks (P1–P6) from F4 of P-GCP (Fig. 4B). The isolated peptides have molecular masses ranging from 1660 to 871 Da, in agreement with earlier study [12]. At a concentration as low as 10 μg/mL, all P-GWP-F4-derived peptide peaks showed ACE inhibition ranging from 40% to 100% (Fig. 4C). Among the P-GWP-F4-derived peaks, P6 (Fig. 4C) exhibited the most potent ACE inhibition (100%). The six P-GCP-F4-derived peptide peaks showed ACE inhibitory activities ranging from 20% to 100%, whereas P4 was the most potent ACE inhibitory peptide fraction (Fig. 4D), at concentration as low as 10 μg/mL. As shown in Fig. 5A, the whey-derived peak 6, P-GWP-F4-P6 (wF4P6), displayed strong ACE inhibitory activity in a dose-dependent manner, having half maximal inhibitory concentration (IC50) values of 4.85 μg/mL (4.45 μM/L). The casein-derived peptide peak, P-GCP-F4-P4 (cF4P4), contained two peptides and exhibited high ACE inhibitory activity with IC50 values of 5.46 μg/mL corresponding to mixed-type 4.27 μM/L. Captopril was used as the positive control (Fig. 5B). The IC50 of captopril was 3.56 μg/mL (16.38 μM/L), under the assay condition employed in this study. The linear dose-response relationship indicates the profound ACE inhibitory activity of these peptide peaks, which is largely comparable to the inhibitory effect of captopril on weight-basis.
Fig. 4.
Purification of peptides using RP-HPLC from the active fractions (F4) of pepsin hydrolysates P-GWP (A) and P-GCP (B). ACE inhibitory activity of the purified peptides of P-GWP (C) and P-GCP (D) was tested at final concentration of 10 μg/mL. The data are representative of 3 replicates.
Fig. 5.
Dose-dependent inhibition of ACE activity by RP-HPLC-derived peptide peaks from whey (P-GWP-F4-P6) and caseins (P-GCP-F4-P4) hydrolysates of goat milk. The inhibition assay was performed as a function of concentration of peptides (A) and captopril (B). ACE activity is presented as change in absorbance (A420) of TNP-HL. Values are representative of three experiments.
Identification of the ACE inhibitory peptides
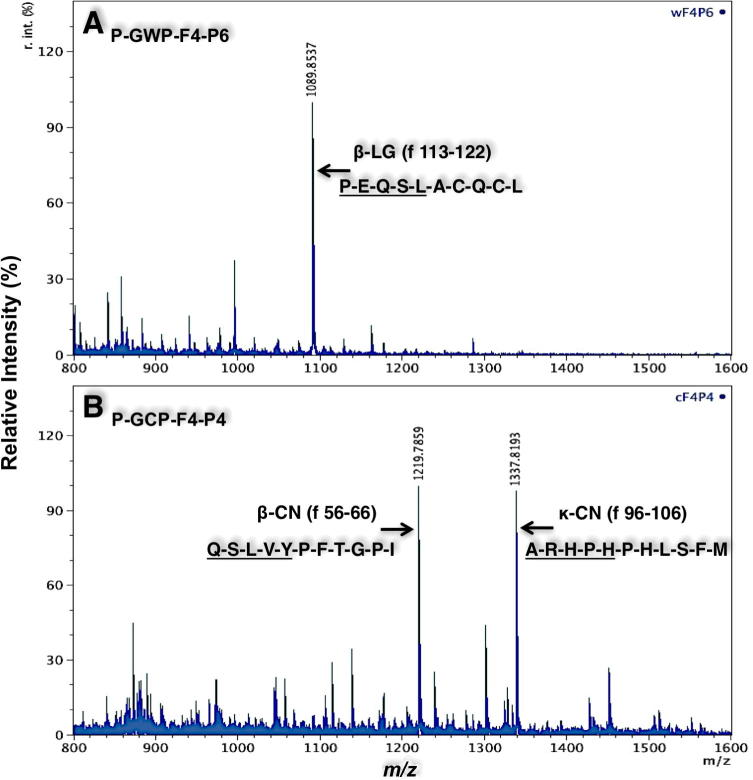
The most potent ACE inhibitory peptides in whey (wF4P6), and in casein (cF4P4) were subjected to MALDI-TOF MS/MS analysis to identify their sizes and amino acid sequences (Fig. 6). Only one major peptide in the RP-HPLC derived P6 of whey was identified as PEQSLACQCL originated from β-lactoglobulin, residues 113–122 (Fig. 6A). As shown in Fig. 6B, two major peptides were identified in the RP-HPLC derived peak 4 of caseins, ARHPHPHLSFM originated from κ-casein (residues 96–106) and QSLVYPFTGPI originated from β-casein (residues 56–66). These peptides possess high contents of hydrophobic amino acid and also relative abundance of Pro residues within their sequences. These characteristics have previously been highlighted to result in potent ACE inhibitory peptides [14]. Although an ACE-inhibitory peptide corresponding to β-casein fragment 58–65 (LVYPFPGP) was reported in goat sodium caseinate hydrolysates [15], to the best of authors’ knowledge, none of these peptides have previously been reported with such potent ACE inhibitory activity, which may provide potential therapeutic candidates for treatment of hypertension.
Fig. 6.
MALDI-TOF mass spectra of the RP-HPLC-derived peptide peak 6 from whey, P-GWP-F4-P6 (A) and of peptide peak 4 from casein, P-GCP-F4-P4 (B). The MS-MS sequences of the peptides are shown depicting the origin of the fragment originating from β-lactoglobulin, β-LG (A) and fragments within the source proteins, β-casein, β-CN, and κ-casein, κ-CN (B). Underline indicates sequence obtained by de novo sequencing of the fragments and the rest of peptide sequence was deduced from peptide molecular mass and assignment to protein database.
Discussion
Bioactive peptides or hydrolysates of milk proteins are being considered as possible approach for use in nutraceuticals and pharmaceuticals for prevention and treatment of hypertension [1], [16], [17]. In the present work, we have shown that ACE inhibitory peptides can be released from goat milk caseins and whey proteins after gastric pepsin digestion. The inhibitory activity was greatly higher in the small peptides-containing fractions of size exclusion chromatography (Fig. 3) and increased as the peptides were further separated based on hydrophobicity, using RP-HPLC, whereas the hydrophobic peptides showed the strongest ACE inhibition (Fig. 4).
The most active peptides in goat milk are one peptide from whey β-lactoglobulin, PEQSLACQCL fragment 113–122 and two peptides from caseins, ARHPHPHLSFM (fragment 96–106 κ-casein), and QSLVYPFTGPI (fragment 56–66 β-casein). These peptides displayed high ACE inhibitory activity which compares favorably with the activity of captopril, an ACE inhibitor, on weight basis. Previously reported MKP peptide from bovine αs2-casein showed IC50 values of 0.12 μg/mL, 0.3 μM [18] and IVY peptide from wheat germ exhibited IC50 values of 0.48 μM [19]. However, most reported food-derived peptides exhibited IC50 values ranging from 32.9 to 128 μM [14], [20], [21], which are much higher than the values of goat peptides found in this study.
ACE-inhibitory activity of peptides seems to rely on a balance between their amino acid sequences and further breaks down into inactive peptides by gastrointestinal enzymes. Many of the known bioactive peptides have been produced in vitro using gastrointestinal enzymes, usually pepsin and trypsin or α-chymotrypsin. While trypsin preferentially cleaves at the carboxyl side of lysine and arginine, α-chymotrypsin preferentially cleaves peptide amide bonds at the carboxyl side of tyrosine, tryptophan, and phenylalanine. The two peptides, PEQSLACQCL and QSLVYPFTGPI of goat β-lactoglobulin and β-casein found in this study, are not expected to be cleaved by either trypsin or α-chymotrypsin, as the sequences could be protected from proteolysis because of its high hydrophobicity and the presence of proline residues [22]. For the peptide ARHPHPHLSFM, cleavage at arginine (by trypsin) and phenylalanine (by α-chymotrypsin) will produce a more hydrophobic peptide (HPHPHLSF) rich in proline and histidine, which are well known to contribute to ACE inhibitory action [23]. It is worth noting that the ACE inhibitory peptides from goat milk found in this study possess C-terminal hydrophobic amino acid residues. Studies have indicated that binding of inhibitory peptides to ACE is strongly influenced by the C-terminal sequence [24], [25]. Hydrophobic amino acid residues with aromatic or branched side chains or proline residues at one or more positions in the C-terminal region are common features among potent peptide inhibitors [23]. Peptides containing proline and hydroxy proline residues have also been found to be resistant to hydrolysis [26]. Residues such as tyrosine, phenylalanine and tryptophan are also present at the C-terminal of many potent ACE inhibitors [27]. The three goat milk ACE inhibitory peptides found in this study possess many hydrophobic residues and rich in proline and histidine, characteristics which are known to contribute to ACE inhibitory action [2]. It has been reported that many of hydrophobic antioxidant peptides also present antihypertensive activity through inhibition of ACE, suggesting the existence of multifunctional peptides [28], [29].
Conclusions
We identified three new peptides from goat milk exhibiting potent inhibition of ACE, which herald a fascinating opportunity as nutraceutical or therapeutic application. The results also suggest a beneficial impact of whey proteins, the by-product of goat cheese industry, as source of natural peptides with potential antihypertensive effect. Further studies verifying the transport mechanism and the ability of these peptides in reducing the hypertension in vivo would provide better insight into their potential in the management of hypertension. For application as nutraceuticals or in therapy, carriers (e.g., emulsions, liposomes, nanoemulsions, and nanoparticles) used in the pharmaceutical sector for protection and delivery of peptides as well as encapsulation strategies may help to enhance bioavailability in human of bioactive peptides [30]. The excellent results of this study shed insights, for the first time, into the potential of new multifunctional bioactive peptides as therapeutic alternatives in the treatment of hypertension as they additionally exert antioxidant activities [12]. Further, the amino acid sequence of the three ACE inhibitory peptides found in goat milk may also form the basis for the design of analogues with therapeutic potential.
Conflict of Interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jare.2016.12.002.
Appendix A. Supplementary material
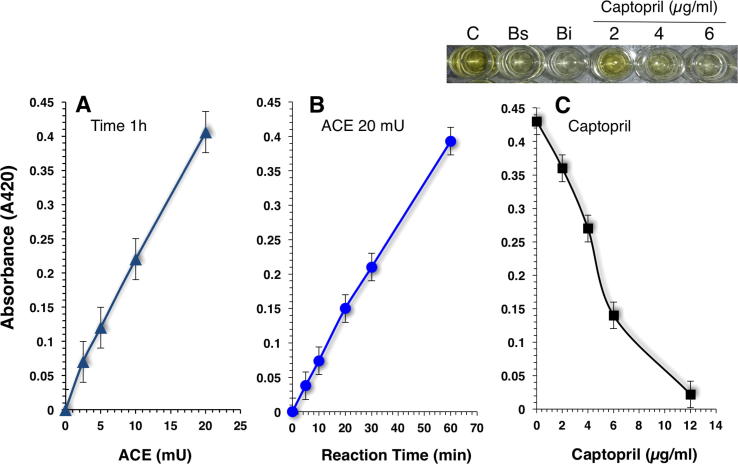
Supplementary Fig. 1.
Linearity of the reaction as a function of ACE concentrations (A) and reaction time (B). The color development of TNP-HL was monitored for 60 min under ACE concentrations ranging from 2.5 to 20 mU/mL (A) or with 20 mU ACE/mL for various lengths of time (B). ACE inhibitory activity of captopril obtained with ACE concentration of 20 mU/mL and an incubation time of 60 min (C). Upper panel shows the yellow color development of control (C), Blanks (Bs and Bi) and three dilutions of captopril (2, 4 and 6 μg/mL).
References
- 1.Brandelli A., Daroit D.J., Corrêa A.P.F. Whey as a source of peptides with remarkable biological activities. Food Res Int. 2015;73:149–161. [Google Scholar]
- 2.Nongonierma A.B., FitzGerald R.J. Strategies for the discovery, identification and validation of milk protein-derived bioactive peptides. Trends Food Sci Technol. 2016;50:26–43. [Google Scholar]
- 3.Martin P., Cebo C., Miranda G. Milk proteins|inter-species comparison of milk proteins: quantitative variability and molecular diversity A2 - fuquay. In: John W., editor. Encyclopedia of dairy sciences. 2nd ed. Academic Press; San Diego: 2011. pp. 821–842. [Google Scholar]
- 4.Park Y.W. Bioactive components in milk and dairy products. Wiley-Blackwell; 2009. Bioactive components in goat milk; pp. 43–81. [Google Scholar]
- 5.Simos Y., Metsios A., Verginadis I., D’Alessandro A.G., Loiudice P., Jirillo E. Antioxidant and anti-platelet properties of milk from goat, donkey and cow: an in vitro, ex vivo and in vivo study. Int Dairy J. 2011;21:901–906. [Google Scholar]
- 6.Carver J.D. Advances in nutritional modifications of infant formulas. Am J Clin Nutr. 2003;77:1550S–1554S. doi: 10.1093/ajcn/77.6.1550S. [DOI] [PubMed] [Google Scholar]
- 7.Chessex P., Watson C., Kaczala G.W., Rouleau T., Lavoie M.E., Friel J. Determinants of oxidant stress in extremely low birth weight premature infants. Free Radic Biol Med. 2010;49:1380–1386. doi: 10.1016/j.freeradbiomed.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Correa A.P.F., Daroit D.J., Coelho J., Meira S.M.M., Lopes F.C., Segalin J. Antioxidant, antihypertensive and antimicrobial properties of ovine milk caseinate hydrolyzed with a microbial protease. J Sci Food Agric. 2011;91:2247–2254. doi: 10.1002/jsfa.4446. [DOI] [PubMed] [Google Scholar]
- 9.Collins R., MacMahon S. Blood pressure, antihypertensive drug treatment and the risks of stroke and of coronary heart disease. Brit Med Bull. 1994;50:272–298. doi: 10.1093/oxfordjournals.bmb.a072892. [DOI] [PubMed] [Google Scholar]
- 10.Angelo A., Marco C. Drug-induced angioedema without urticaria. Drug Safety. 2001;24:599–606. doi: 10.2165/00002018-200124080-00004. [DOI] [PubMed] [Google Scholar]
- 11.Chen J., Wang Y., Ye R., Wu Y., Xia W. Comparison of analytical methods to assay inhibitors of angiotensin I-converting enzyme. Food Chem. 2013;141:3329–3334. doi: 10.1016/j.foodchem.2013.06.048. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed S.A., El-Bassiony T., Elmalt L.M., Ibrahim H.R. Identification of potent antioxidant bioactive peptides from goat milk proteins. Food Res Int. 2015;74:80–88. doi: 10.1016/j.foodres.2015.04.032. [DOI] [PubMed] [Google Scholar]
- 13.Matsui T., Matsufuji H., Osajima Y. Colorimetric measurement of angiotensin I-converting enzyme inhibitory activity with trinitrobenzene sulfonate. Biosci Biotechnol Biochem. 1992;56:517–518. doi: 10.1271/bbb.56.517. [DOI] [PubMed] [Google Scholar]
- 14.Norris R., Poyarkov A., O’Keeffe M.B., FitzGerald R.J. Characterisation of the hydrolytic specificity of Aspergillus niger derived prolyl endoproteinase on bovine beta-casein and determination of ACE inhibitory activity. Food Chem. 2014;156:29–36. doi: 10.1016/j.foodchem.2014.01.056. [DOI] [PubMed] [Google Scholar]
- 15.Minervini F., Algaron F., Rizzello C.G., Fox P.F., Monnet V., Gobbetti M. Angiotensin I-converting-enzyme-inhibitory and antibacterial peptides from Lactobacillus helveticus PR4 proteinase-hydrolyzed caseins of milk from six species. Appl Environ Microbiol. 2003;69:5297–5305. doi: 10.1128/AEM.69.9.5297-5305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groziak S.M., Miller G.D. Natural bioactive substances in milk and colostrum: effects on the arterial blood pressure system. Brit J Nutr. 2000;84:119–125. doi: 10.1017/s0007114500002348. [DOI] [PubMed] [Google Scholar]
- 17.Asoodeh A., Memarpoor Y., Mina C., Jamshid K. Purification and characterisation of angiotensin I converting enzyme inhibitory peptides from lysozyme hydrolysates. Food Chem. 2012;131:291–295. [Google Scholar]
- 18.Yamada A., Sakurai T., Ochi A., Matsuyama E., Yamauchi K., Abe F. Novel angiotensin I-converting enzyme inhibitory peptide derived from bovine casein. Food Chem. 2013;141:3781–3789. doi: 10.1016/j.foodchem.2013.06.089. [DOI] [PubMed] [Google Scholar]
- 19.Matsui T., Li C.H., Osajima Y. Preparation and characterization of novel bioactive peptides responsible for angiotensin I-converting enzyme inhibition from wheat germ. J Pept Sci. 1999;5:289–297. doi: 10.1002/(SICI)1099-1387(199907)5:7<289::AID-PSC196>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Himaya S.W.A., Ngo D.H., Ryu B., Kim S.K. An active peptide purified from gastrointestinal enzyme hydrolysate of Pacific cod skin gelatin attenuates angiotensin-1 converting enzyme (ACE) activity and cellular oxidative stress. Food Chem. 2012;132:1872–1882. [Google Scholar]
- 21.Ko S.C., Kang N., Kim E.A., Kang M.C., Lee S.H., Kang S.M. A novel angiotensin I-converting enzyme (ACE) inhibitory peptide from a marine Chlorella ellipsoidea and its antihypertensive effect in spontaneously hypertensive rats. Process Biochem. 2012;47:2005–2011. [Google Scholar]
- 22.Meisel H. Multifunctional peptides encrypted in milk proteins. BioFactors. 2004;21:55–61. doi: 10.1002/biof.552210111. [DOI] [PubMed] [Google Scholar]
- 23.Cheung H.S., Wang F.L., Ondetti M.A., Sabo E.F., Cushma D.W. Binding of peptide substrate and inhibitors of angiotensin-converting enzyme. J Biol Chem. 1980;255:401–407. [PubMed] [Google Scholar]
- 24.Castellano P., Aristoy M.C., Sentandreu M.A., Vignolo G., Toldra F. Peptides with angiotensin I converting enzyme (ACE) inhibitory activity generated from porcine skeletal muscle proteins by the action of meat-borne Lactobacillus. J Proteom. 2013;89:183–190. doi: 10.1016/j.jprot.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 25.Fujita H., Yokoyama K., Yoshikawa M. Classification and antihypertensive activity of angiotensin I-converting enzyme inhibitory peptides derived from food proteins. J Food Sci. 2000;65:564–569. [Google Scholar]
- 26.Tavares T., del Mar Contreras M., Amorim M., Pintado M., Recio I., Malcata F.X. Novel whey-derived peptides with inhibitory effect against angiotensin-converting enzyme: in vitro effect and stability to gastrointestinal enzymes. Peptides. 2011;32:1013–1019. doi: 10.1016/j.peptides.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Meisel H., Walsh D.J., Murray B.A. ACE inhibitory peptides. In: Mine Y., Shahidi F., editors. Nutraceutical proteins and peptides in health and disease. CRC Press, Taylor and Francis Group; New York (New York): 2006. pp. 269–315. [Google Scholar]
- 28.Aredes Fernández P.A., Stivala M.G., Rodríguez Vaquero M.J. Increase in antioxidant and antihypertensive activity by Oenococcus oeni in a yeast autolysis wine model. Biotechnol Lett. 2011;33:359–364. doi: 10.1007/s10529-010-0446-y. [DOI] [PubMed] [Google Scholar]
- 29.Hernández-Ledesma B., Miralles B., Amigo L., Ramos M., Recio I. Identification of antioxidant and ACE-inhibitory peptides in fermented milk. J Sci Food Agric. 2005;85:1041–1048. [Google Scholar]
- 30.Moutinho C.G., Matos C.M., Teixeira J.A., Balcao V.M. Nanocarrier possibilities for functional targeting of bioactive peptides and proteins: state-of-the-art. J Drug Target. 2012;20:114–141. doi: 10.3109/1061186X.2011.628397. [DOI] [PubMed] [Google Scholar]