Abstract
Further increase in energy density of lithium batteries is needed for zero emission vehicles. However, energy density is restricted by unavoidable theoretical limits for positive electrodes used in commercial applications. One possibility towards energy densities exceeding these limits is to utilize anion (oxide ion) redox, instead of classical transition metal redox. Nevertheless, origin of activation of the oxide ion and its stabilization mechanism are not fully understood. Here we demonstrate that the suppression of formation of superoxide-like species on lithium extraction results in reversible redox for oxide ions, which is stabilized by the presence of relatively less covalent character of Mn4+ with oxide ions without the sacrifice of electronic conductivity. On the basis of these findings, we report an electrode material, whose metallic constituents consist only of 3d transition metal elements. The material delivers a reversible capacity of 300 mAh g−1 based on solid-state redox reaction of oxide ions.
Energy storage by metal redox reactions sets strict limits on capacity in metal oxide cathode materials used in lithium-ion batteries. Here authors study stabilization of redox reactions at oxygen sites and demonstrate a cathode with a high reversible capacity enabled by the process.
Rechargeable lithium-ion batteries (LIBs) are widely used in our daily life because LIBs have the highest gravimetric/volumetric energy density among commercial energy storage devices. LIBs are used as a power source for zero emission electric vehicles and are expected to be used for grid energy storage1. LIBs are becoming a key technology enabling a shift from fossil fuel to renewable electric energy, which potentially realizes green and sustainable energy development in the future. Nevertheless, the possibility of further increase in the energy density is severely restricted because of unavoidable theoretical limits for positive electrodes, such as spinel-type oxides and iron phosphates.
In contrast to these materials, which are currently used in the commercial LIB, there is room for further increase in the energy density for layered oxides, such as LiCoO2 (ref. 2), LiNi1/2Mn1/2O2 (ref. 3), Li2MnO3 (ref. 4) and those derivatives5,6,7. Among them, Li2MnO3-based electrode materials have been extensively studied as positive electrode materials in the past decade6,8,9,10,11,12,13,14,15. The reaction mechanism of this material was a controversial subject for a long time. Since the oxidation state of manganese ions is tetravalent, oxidation of manganese ions beyond the tetravalent state is difficult. Instead of manganese ions, negatively charged anions, oxide ions (O2−), donate electrons on charge (electrochemical oxidation). However, oxidation of oxide ions results in partial loss of oxygen as an irreversible process, that is, decomposition reaction8. The loss of oxygen induces the formation of trivalent manganese ions on discharge (electrochemical reduction), leading to the unfavourable phase transition in the layered structure11. Nevertheless, it has been evidenced that reversible solid-state redox for oxide ions is possible for the Li2RuO3-based system, which essentially has the same crystal structure as Li2MnO3, and the contribution of oxide ions has been experimentally evidenced by using an arsenal of characterization techniques14 and theoretical method16. Very recently, the formation of peroxo-like dimers in Li2−xIrO3 has been experimentally visualized by transmission electron microscopy17 whereas recent experimental18 and theoretical19 studies have suggested that an isolated hole is formed on the charge process for lithium-excess electrode materials. Enrichment of lithium ions as the highly ionized cation results in a less covalent character for oxide ions, and thus the oxide ions are more easily oxidized compared with conventional oxides with late transition metals. Therefore, it has been proposed that the holes in the oxygen 2p orbital is effectively stabilized18,19.
The use of anion redox, especially oxide ions, is a crucial strategy to design and develop new electrode materials with high gravimetric/volumetric energy density for LIB. Reversible capacity of electrode materials is potentially further increased by the enrichment of lithium contents with less transition metals in the close-packed structure of oxide ions. Recently, our group has reported that Li3NbO4 (refs 20, 21) and Li4MoO5 (ref. 22), which have higher lithium contents than those of Li2MnO3 and Li2RuO3, are potentially utilized as host structures for a new series of high-capacity electrode materials. Similar concepts are also proposed in the literature23,24. Manganese-substituted Li3NbO4, Li1.3Nb0.3Mn0.4O2 (0.43Li3NbO4–0.57LiMnO2), delivers large reversible capacity (approximately 300 mAh g−1) with reversible solid-state redox reaction of oxide ions20. Similar to pentavalent niobium, a material with pentavalent antimony, Li4FeSbO6, has been recently reported25. Solid-state redox reaction of oxide ions is also activated in Li4FeSbO6, and a reductive coupling mechanism as an irreversible process has been evidenced in this system. As a non-rocksalt system, the use of Co-doped Li2O has been also proposed26. Although many articles now describes the anion redox for battery materials, the border between reversibility and irreversibility for the solid-state redox reaction of oxide ions remains unclear, and it is a critical point to understand the factors affecting reversibility of anion redox.
In this article, we answer these questions through systematic studies on Li3NbO4-LiMeO2 (Me=Fe, Mn and V) binary system. Reversibility of the solid-state redox reaction highly depends on the transition metal elements selected, which correlates with the formation of electrochemically unstable superoxide species because of charge transfer from oxidized oxide ions. Furthermore, on the basis of these findings, we demonstrate a niobium-free high-capacity positive electrode material, which effectively utilizes reversible solid-state redox reaction of oxide ions. These findings can potentially enable high-energy LIBs free of less abundant transition elements, such as cobalt and nickel ions.
Results
Synthesis and characterization of Li3NbO4-LiMeO2 system
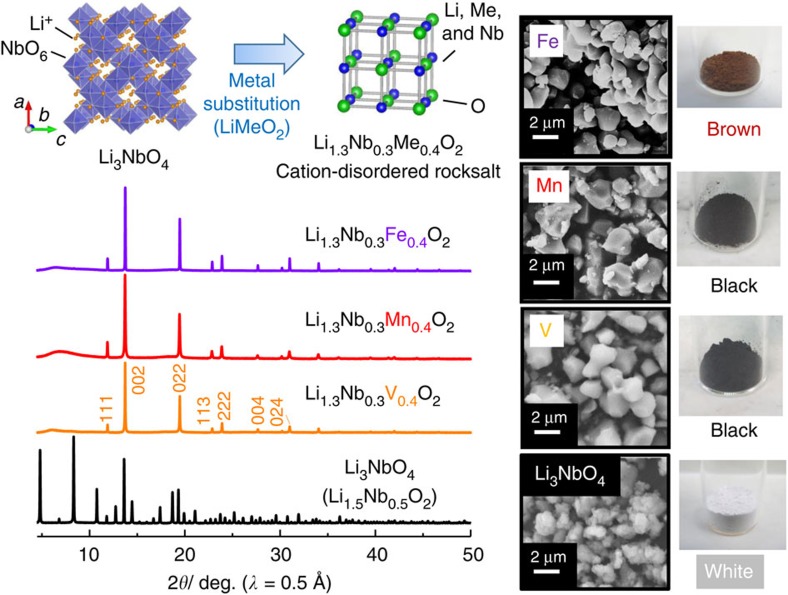
Figure 1 summarizes characterization of Li3NbO4-LiMeO2 (Me=Fe, Mn and V) binary system by synchrotron X-ray diffraction (SXRD) and SEM. Li3NbO4 crystallizes into a cation-ordered rocksalt-type structure while a lack of d electrons in a conduction band results in an insulating character. Substitution of 3d transition metal ions for Nb/Li ions effectively induces conductive electrons, and colour of samples is also changed from white for Li3NbO4 to black for substituted samples with Mn3+ and V3+. Such 3d transition metals can accept electrons from oxide ions. However, a change in the crystal structure is also unavoidable, and formation of a cation-disordered rocksalt-type structure is found. Recently, electrode materials with the cation-disordered rocksalt-type structure, such as Li1.211Mo0.467Cr0.3O2 (ref. 27) and Li1.33V0.67O1.33F0.67 (ref. 28), have been reported as high-capacity electrode materials. Historically, such cation-disordered rocksalt phase had been regarded as electrochemically inactive as electrode materials because of a lack of the Li migration path in a bulk structure. Nevertheless, formation of percolating network for the Li-excess system (Li1+xMe1−xO2) opens the path for Li migration in the cation-disordered rocksalt-type structure27.
Figure 1. Crystal structures and particle morphology of Li1.3Nb0.3Me0.4O2.
Synchrotron X-ray diffraction (SXRD) patterns of as-prepared 0.43 Li3NbO4–0.57 LiMeO2 (Me=Fe3+, Mn3+, and V3+) samples, which are reformulated as Li1.3Nb0.3Me0.4O2 based on the formulation of α-NaFeO2-type layered structure, are shown. SXRD patterns of the samples, Me=Fe3+ and Mn3+, were also shown in our previous work20. The samples crystallize into the cation disordered rocksalt structure with lattice parameters of 4.19 Å for Fe3+, 4.21 Å for Mn3+ and 4.17 Å for V3+. Structural analysis was carried out using RIETAN-FP (ref. 41). Substitution of Fe3+, Mn3+ and V3+ for Nb5+/Li+ results in the donation of d electrons in a conduction band, and thus colour of the samples changes from white for Li3NbO4. Data of Li3NbO4 (Li1.5Nb0.5O2) are also shown for comparison. Schematic illustrations of the crystal structures were drawn using the program VESTA (ref. 42).
Electrochemistry of Li3NbO4-LiMeO2 system in half-cells
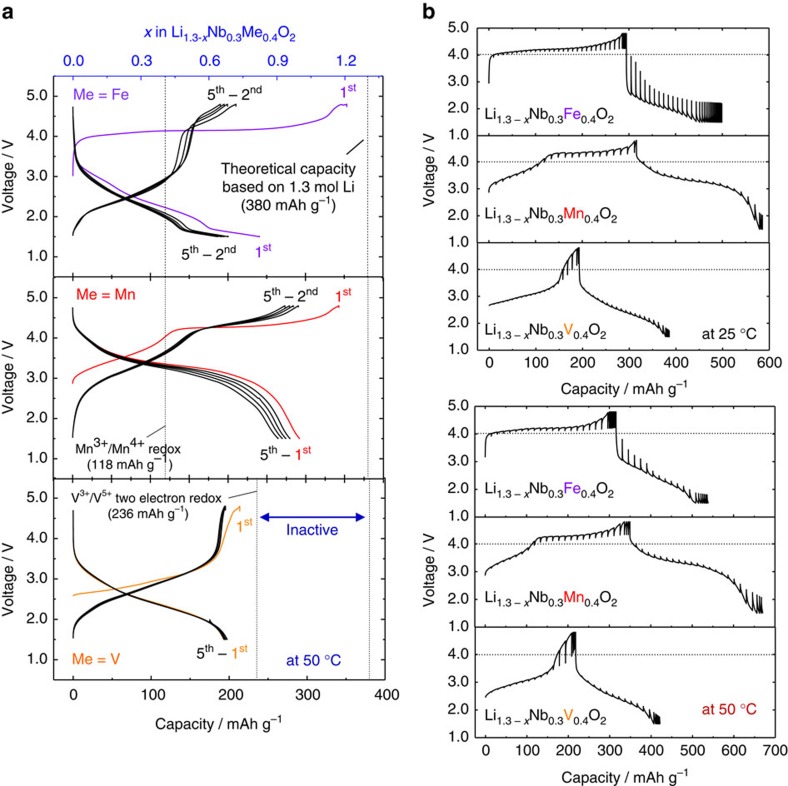
Although as-prepared samples with primary particle size of 2–3 μm show insufficient electrode performance20, mechanical ball milling with carbon (reduction of particle size with uniform mixing with carbon is achieved in Supplementary Fig. 1) effectively improves the electrode performance of the samples in Li cells. Electrode performance is further improved at elevated temperature (50 °C), and three cation-disordered rocksalt samples, Li1.3Nb0.3Me0.4O2 (Me=Fe3+, Mn3+ and V3+), deliver large reversible capacities in Li cells, as shown in Fig. 2. It is noted that three Li3NbO4-based samples with different 3d transition metals show quite different electrochemical behaviour. The Fe system shows a large initial charge (oxidation) capacity of 350 mAh g−1 that is quite close to that of the theoretical capacity (383 mAh g−1) as defined by the extraction of all Li+ ions (1.3 moles) from the crystal lattice. However, a clear change in a voltage profile is found on the second charge process, which is totally different from the first charge process. A well-defined voltage plateau is observed on the initial charge, but an S-shaped profile is noted after first discharge process. Polarization is small for the S-shaped profile centred at 2.5 V, which will be discussed in the later section. The voltage plateau is not observed for the V system, and the sample shows S-shaped profile from the initial charge process. The observed reversible capacity is comparable to that of a theoretical capacity based on V3+/V5+ two-electron redox (236 mAh g−1) and is much smaller than that expected from lithium contents in the structure. The Mn system delivers a large discharge capacity of 300 mAh g−1 with the appearance of voltage plateau as reported in our literature20. Capacity retention of the samples is compared in Supplementary Fig. 2 at room temperature. Although the Mn system shows large reversible capacity, the V and Fe systems show better capacity retention in Li cells. Quasi-open-circuit voltage (QOCV) of the samples at 25 and 50 °C are also compared in Fig. 2b. The Fe system shows quite small polarization with flat QOCV at 4 V on charge whereas huge polarization (> 1.5 V) is observed on discharge at 25 °C. The polarization is significantly reduced at 50 °C. The V system shows the smallest polarization on charge/discharge with an S-shaped voltage profile as expected from galvanostatic curves (Fig. 2a). Two different regions are observed for the Mn system on charge; relatively small polarization for the sloping region from 3 to 4 V and slightly large polarization for the plateau region at 4.2 V. On the discharge process, two regions are not distinguished and a continuous S-shaped profile on QOCV is observed. Such behaviour originates from the hysteresis of oxidation/reduction reaction for solid-state redox of oxide ions.
Figure 2. Electrochemical properties of Li1.3Nb0.3Me0.4O2 in Li cells.
(a) Galvanostatic oxidation/reduction curves of Li1.3Nb0.3Me0.4O2 (Me=Fe3+, Mn3+ and V3+) samples in Li cells at 10 mA g−1 at 50 °C. Quasi open circuit voltage (QOCV) of the samples measured by galvanostatic intermittent titration technique (GITT) is also plotted in (b); charge for 1 h at 10 mA g−1 at room temperature and 50 °C and rest for 5 h.
Charge compensation mechanisms in Li3NbO4-LiMeO2 system
Three different samples show quite different electrochemical behaviour in Li cells, as shown in Fig. 2. Such behaviour is expected to originate from differences in charge compensation mechanisms depending on the 3d transition metals (Fe, Mn and V) in the Li3NbO4 framework. Charge compensation mechanisms in Li cells were, therefore, examined using a combination of different characterization methods: synchrotron SXRD, hard/soft X-ray absorption spectroscopy (XAS) and X-ray photoelectron spectroscopy (XPS) with the assist of density functional theory (DFT) calculations. Results are described in detail in Supplementary Figs 3–10, and DFT calculations in Fig. 3, Supplementary Figs 11–15 and Supplementary Table 1.
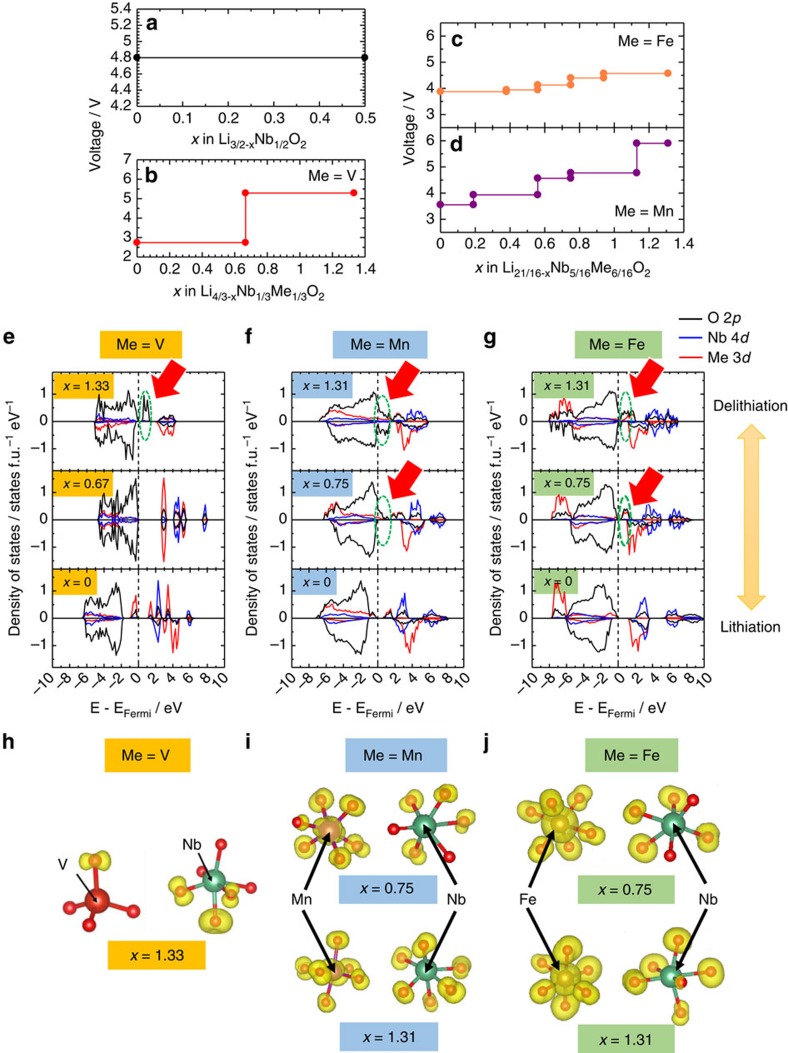
Figure 3. Summary of DFT calculations for Li-Nb-Me-O system.
Calculated voltage profiles for (a) Li3NbO4 (Li1.5NbO2), (b) Li4/3V1/3Nb1/3O2, (c) Li21/16−xFe6/16Nb5/16O2 and (d) Li21/16−xMn6/16Nb5/16O2, and partial density of states (PDOS) are also shown in e–g. PDOS of Li3−xNbO4 is shown in Supplementary Fig. 15. Transition metal ions are mainly oxidized at an early stage of Li removal, whereas oxide ions are responsible for charge compensation at a late stage. The highest voltage is seen for M=Fe, followed in order by Mn and V, at an early stage of Li removal. Fe 3d orbital strongly hybridized with O 2p orbital at x=0.75 while weak hybridization is observed for the Mn system. V 3d orbital is localized at above O 2p level. These are supported by the PDOS diagrams and calculated partial charge density of electron at the hole state near to Fermi level, as shown in h–j).
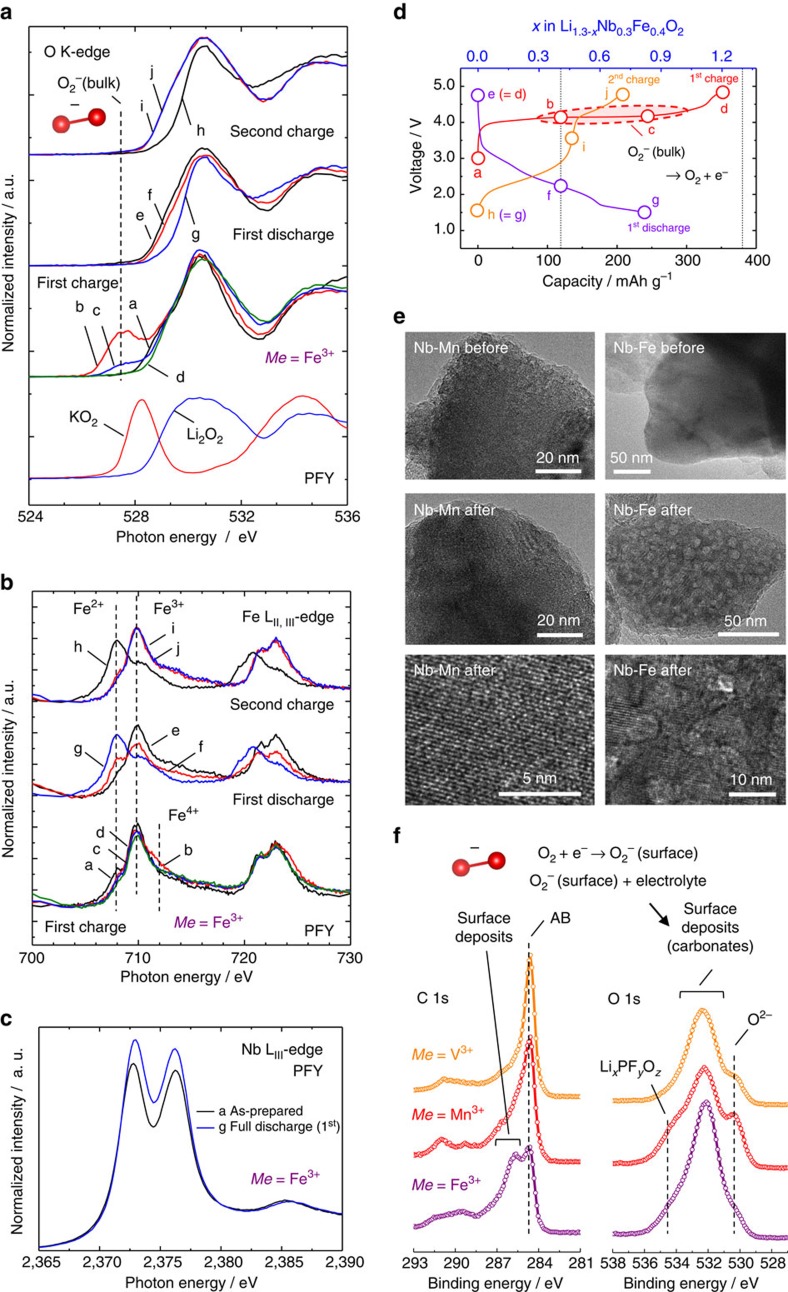
Important findings are summarized as follows: (1) In the Mn system, reversible oxidation of oxide ions, coupled with Mn3+/Mn4+ redox, is realized as reported in our previous work20. (2) In the V system, V3+/V5+ two-electron redox is active, but the oxidation of oxide ions is not evidenced. (3) In the Fe system, the formation of superoxide (O2–) (ref. 29) is observed from the measurement of O K-edge XAS, which is further supported by the DFT study with COOP analysis (Supplementary Fig. 14). Since the most significant changes on charge/discharge curves were observed for the Fe system, changes in soft XAS spectra were examined on the initial cycle and second charge at 50 °C (Fig. 4). Formation of superoxide is further pronounced on charge at 50 °C, and superoxide is stabilized in the bulk of particle rather than the surface (Supplementary Fig. 9). However, superoxide species are electrochemically oxidized and decomposed by further charge. The superoxide species disappears as seen in the O K-edge spectra after charge to 4.8 V. This process inevitably results in the oxygen loss and structural reconstruction process (the latter is also supported by transmission electron microscopy as the formation of nanosize grains, as shown in Fig. 4e). Origin of the formation of superoxide is discussed in the later section. The oxygen loss results in the reduction of Fe3+ to Fe2+ and accumulation of surface deposits on discharge. The voltage plateau is significantly shortened on the second charge, and the S-shaped voltage profile centred at 2.5 V is observed with relatively small polarization. This reaction mainly originates from Fe2+/Fe3+ redox, as shown in Fig. 4b, and the formation of superoxide is not found in the second charge. In the conventional Li-excess system, Li1+xNiyCozMn(1–x–y–z)O2, a clear voltage plateau is observed at 4.6 V at the initial charge process, but not for the second charge. An S-shaped voltage profile without the plateau region is observed from the second charge with activated Mn3+/Mn4+ redox8,9,11,12. The reversible contribution of oxide ions for charge compensation is less pronounced in these systems. Oxygen loss on charge is further supported by XPS (Fig. 4f and Supplementary Fig. 8). Oxygen molecules released in the cells are electrochemically reduced on discharge, leading to the formation of superoxide (on the surface of electrode), which further reacts with electrolyte11,30,31. This process is clearly evidenced, especially for the Fe system, as the accumulation of surface deposits on AB and active materials (Fig. 4f and Supplementary Fig. 8).
Figure 4. Changes in electronic structures for Li1.3−xNb0.3Fe0.4O2−δ on initial charge/discharge and second charge.
Changes in the O K-edge (a) Fe LII, III-edge XAS spectra (b), Nb LIII-edge XAS spectra (c), and the points where XAS spectra have been collected in (d). XAS spectra of KO2 (superoxide) and Li2O2 (peroxide) are also shown in (a) for comparison. Niobium is not responsible for charge compensation process (other data sets of Nb are shown in Supplementary Fig. 10). (e) TEM images of Li1.3Nb0.3Fe0.4O2 and Li1.3Nb0.3Mn0.4O2 particles before and after the electrochemical cycle at 50 °C. Oxygen loss for the Fe system results in the formation of nanosized grains in the single particle, and not for the Mn system. Lattice fringes are clearly observed after electrochemical cycle for the Mn system. (f) Surface structures observed by X-ray photoelectron (XPS) spectroscopy after discharge to 1.5 V. Oxygen molecules released on charge result in the electrochemical reduction on discharge, leading to the formation of surface deposits because of chemical reaction between active superoxide with electrolyte, as shown in our previous literature11.
In contrast, for Li1.3Nb0.3Mn0.4O2, reversible changes in O K-edge XAS spectra are observed (Supplementary Fig. 6). Moreover, a clear voltage plateau is observed even in the ‘second' charge (Fig. 2), indicating that solid-state redox reaction is a reversible process. Note that DFT calculations also support these findings. Formation energy of the charged Mn system is energetically stable, but segregation (decomposition) to LiFeO2 and LiNbO3 accompanying O2 gas evolution is energetically preferable for the Fe system (see equations (s3) and (s4) in Supplementary Methods). Nevertheless, the plateau gradually becomes shorter in the continuous cycles probably because of the increase in polarization. Cyclability as the electrode material is expected to be further improved through the optimization of battery components, for example, electrolyte, binder, surface coating of particle and so on, and controlling charge conditions, as shown in our previous work20.
A question remains in the V system. Why are not oxide ions experimentally oxidized in this system? Theoretical prediction in Fig. 3 indicates that (1) two-electron redox of V3+/V5+ occurs coupled with vanadium migration to tetrahedral sites, which is consistent with experimental finding (see the Supplementary Methods for theoretical and experimental evidences) and (2) oxidation of oxide ions is also possible in the V system, which was not experimentally observed on charge to 4.8 V, as shown in Fig. 2. This inconsistency for the oxide ion redox simply originates from the difficultly of the electron transfer from oxide ions to V5+ for the fully charged state, namely kinetic limitation. DFT calculation clearly supports this fact. Oxide ions are theoretically oxidized, but the hole induced in oxygen 2p orbital is isolated in the structure, as shown in Fig. 3. Vanadium ions in Li0.5Nb5+0.3V5+0.4O2 (fully charged state), therefore, cannot transfer electrons from oxide ions and oxidation of oxide ions in the V system is kinetically restricted. Similar situation is observed for Li3−xNbO4 as a model material. Calculated voltage by DFT study is estimated to be 4.8 V for Li3NbO4 (Li3/2Nb1/2O2, Fig. 3a). Corresponding partial density of states diagram and partial electron density are also shown in Supplementary Fig. 15. Oxidation of O2− ions is indicated during delithiation by DFT study since creation of O 2p hole level is clearly visible just above Fermi level at x=0.5 in Li3/2−xNb1/2O2. However, holes are completely isolated in the structure, and thus electron transfer between O 2p and Nb5+ is kinetically limited as observed in the experimental study20.
Design of high-capacity electrode materials with anion redox
As shown in this article, oxidation of oxide ions is not difficult, and only electron transfer from oxide ions to 3d transition metals is a necessary condition. However, the stabilization of oxidation reaction of oxide ions is not easy. In many materials, oxidation of oxide ions is possible, but this process induces oxygen loss, as in the cases of Li2MnO3 (ref. 4), Li4FeSbO6 (ref. 32), Li4NiMoO6 (ref. 22) and so on8,24. The reversible contribution of oxide ions for charge compensation has been reported only in limited electrode materials, for example, Li2Ru1−yMeyO3 (Me=Ru, Sn and Ti) (refs 14, 33), and the Nb-Mn system shown in this study. In general, peroxide/superoxide species are stabilized for sp elements without valence electrons (K+, Ca2+ and so on ) and d10 closed-shell (Zn2+, Cd2+ and so on). In contrast, transition metal oxides often decompose these species. One typical example is a disproportionation reaction of H2O2 catalysed by MnO2. This reaction is triggered by electron transfer between peroxide ions and surface manganese ions. Nb5+ also has no valence electron, and therefore a similar role is anticipated with the sp elements. Among 3d transition metal elements, Ti4+ has a similar electronic configuration with Nb5+. These ions most probably screen off electrons of unstable ‘oxidized' oxide ions and thus suppresses charge transfer. In addition, Nb5+ and Ti4+ are highly ionized ions compared with late transition metal ions with oxide ions, and thus the mixing between metal d orbital and oxygen 2p orbital is less pronounced. Therefore, similar to the lithium enrichment18,19, a character of oxide ions becomes more ionic (approaches two minus as a net charge) because of electron donation from Nb and Ti, and this fact would be beneficial to stabilize the oxidation of oxide ions.
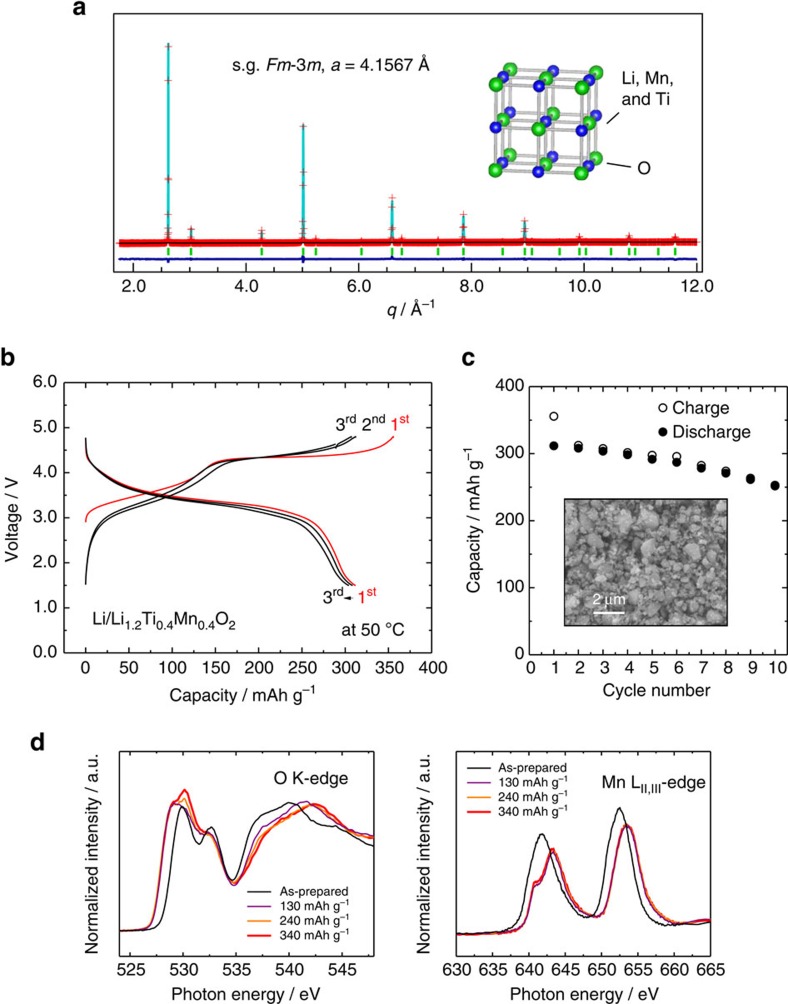
To test this hypothesis, a binary system of Li2TiO3−LiMnO2 has been examined. One to one composition between Li2TiO3 and LiMnO2 has been synthesized, which is reformulated as Li1.2Ti0.4Mn0.4O2. A mole fraction of Mn was adjusted to be the same with Li1.3Nb0.3Mn0.4O2. Similar to Li3NbO4, the cation-disordered rocksalt phase is obtained for Li1.2Ti0.4Mn0.4O2 as a single phase, and uniform size (2–3 μm) of primary particles is found by SEM (Supplementary Fig. 16). A result of structural analysis by neutron scattering is also provided in Fig. 5a and Supplementary Table 2. A mole fraction of Li in the cation-disordered rocksalt phase is inevitably lowered by the use of Ti4+. This fact results in the increase in penalty for percolating Li migration in the structure27,34. However, percolation probability of electron migration through the bonding between oxide ions and manganese ions (40% in the structure) is comparable with that of Li1.3Nb0.3Mn0.4O2. Li1.2Ti0.4Mn0.4O2 was mixed with 10 wt% acetylene black (HS-100, Denka Co. Ltd) and ball-milled to enhance the electrode performance. Thus prepared sample shows a large reversible capacity, as shown in Fig. 5b, and the Nb-free sample delivers more than 300 mAh g−1 at 50 °C. A voltage profile of Li1.2−xTi0.4Mn0.4O2 quite resembles that of Li1.3−xNb0.3Mn0.4O2. Available energy density of Li1.2−xTi0.4Mn0.4O2 exceeds 1,000 mWh g−1 as a positive electrode material. Capacity retention is much better than that of pure Li2MnO3 (ref. 35) and slightly improved in comparison to Li1.3−xNb0.3Mn0.4O2, as shown in Fig. 5c. To examine whether oxide ions are oxidized and stabilized in Li1.2−xTi0.4Mn0.4O2, reaction mechanisms were examined by soft XAS with fluorescence yield in the synchrotron facility36. On the initial charge, Mn3+ is oxidized to Mn4+ for the slope region to 4 V as evidenced from Mn L-edge XAS spectra, and this fact also influences the profile of O K-edge XAS spectra (Fig. 5d). No change in Mn L-edge XAS spectra is observed for the plateau region at 4.2 V. A change in electronic structures is also not evidenced for Ti L-edge XAS spectra (Supplementary Fig. 17). Similar to Li1.3−xNb0.3Mn0.4O2, a new peak appears at ca. 530 eV, (see Supplementary Fig. 6 for more details), which is further intensified as increase in the charge capacity for the plateau region, and the formation of superoxide is not evidenced. Similar observation is also noted for the recent study for the conventional Li-excess system18, but the change is more clearly pronounced in the Ti-Mn system. Although the energy of the new peak is consistent with that of Li2O2, further study is needed to understand the factor affecting the profile of XAS spectra. Nevertheless, the possibility of the formation of superoxide species can be excluded. Such change potentially originates from two possibilities: the formation of isolated holes18,19 and/or σ-hybridization as theoretically proposed in the Ru-Sn system after delithiation19. An almost identical profile with the pristine sample is observed after discharge to 1.5 V, suggesting the reversible process. Very recently, electrode performance and changes in O K-edge XAS spectra on charge have been reported for Li1.19Ti0.38Fe0.57O2 (ref. 37) and Li1.42Mo0.29Fe0.29O2 (ref. 38). Similar to Li1.3−xNb0.3Fe0.4O2 clear evidence for the formation of superoxide and large polarization on charge/discharge have been reported for both samples.
Figure 5. Li1.2Ti0.4Mn0.4O2 with a cation disordered rocksalt structure.
(a) Neutron diffraction pattern of the as-prepared sample; (b) charge/discharge curves in a Li cell at a rate of 5 mA g−1 at 50 °C; (c) discharge capacity retention and an SEM image of the ball-milled sample and (d) changes in O K-edge and Mn LII, III-edge XAS spectra on charge in Li cells. The samples used in (d) were prepared at 50 °C in Li cells.
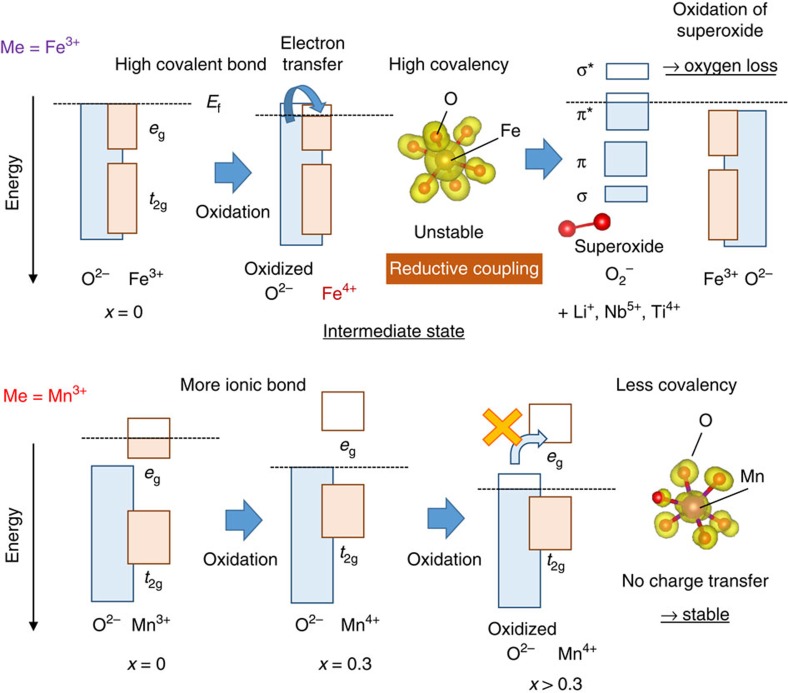
The selection of 3d transition metals, which accept electrons from oxide ions on charge, is an essential key to determine whether to stabilize oxidation of oxide ions (the formation and stabilization of isolated holes as proposed in the literature18,19) or to form unstable superoxide-like species. Stabilization/destabilization mechanisms for Mn/Fe are proposed in Fig. 6. For the case of Nb(Ti)-Fe, the DFT data in Fig. 3 suggest that both oxide and iron ions are simultaneously oxidized on charge (namely oxidation of high covalent Fe-O bond). However, only trace amount of Fe4+ is experimentally found in the Fe L-edge spectra. This fact suggests that electron transfer occurs from ‘oxidized' oxide ions (or a hole induced in O 2p) to Fe4+ because energy levels of both ions are similar for each other, namely highly covalent. Electrons are donated from oxidized oxide ions to neighbouring Fe4+ ions, leading to the formation of superoxide and Fe3+. This process is also called as the reductive coupling14,16. The formation of superoxide is further supported by the DFT study with COOP analysis (Supplementary Fig. 14). Such superoxide species would be stabilized by coupling with Li+ and Nb5+ (Ti4+) ions. Similar results are expected for Ni3+/4+ and Co3+/4+ with heavily hybridized characters for oxide ions near the Fermi level and indeed oxygen loss is experimentally observed for Li-excess electrode materials with these elements. In contrast, oxide ions do not donate electrons to less covalent Mn4+ with the d3 configuration (as the high-spin configuration) associated with energy gap between filled t2g and empty eg bands, as shown in Figs 3 and 6. Ru5+ also have a similar electronic configuration. Since the energy level of t2g orbital for Mn4+ is high enough than that of Fermi level, oxide ions are solely oxidized on further oxidation. The presence of electrons in t2g orbital is also essential without the sacrifice of electronic conductivity in bulk. Moreover, this reaction is further stabilized by the presence of Nb5+ and Ti4+, which donates electrons to oxide ions because of high ionic characters as cations and completely suppresses charge transfer from oxidized oxide ions.
Figure 6. Proposed stabilization/destabilization mechanisms for solid-state redox reaction of oxide ions.
Charge compensation mechanisms on charge in Li cells are compared for Li1.3−xNb0.3Me0.4O2 (Me=Fe3+ and Mn3+). A similar stabilization mechanism is anticipated for Li1.2−xTi0.4Mn0.4O2.
In conclusion, the use of solid-state redox reaction of oxide ions is an effective strategy to further increase energy density of LIBs. We have demonstrated that the stabilization of redox reaction for oxide ions is possible using a combination of only 3d transition metals as the metallic constituents. This serves as a significant proof-of-concept towards practical applications. We expect that by relaxing the constraints posed on materials design by the conventional concept of transition metal redox, many new positive electrode materials with high capacity will appear, similar to Li1.2Ti0.4Mn0.4O2.
Methods
Synthesis of materials
Li3NbO4 was prepared by solid-state reaction from stoichiometric amounts of Li2CO3 (> 98.5%; Kanto Kagaku) and Nb2O5 (99.9%; Wako Pure Chemical Industries) at 950 °C for 24 h in air. Li1.3Nb0.3Me0.4O2 (Me=Fe3+, Mn3+ and V3+) samples were prepared from Li2CO3, Nb2O5 and precursors containing each transition metal: Mn2O3, Fe2O3 (99.9%; Wako Pure Chemical Industries), V2O3 (98%; Sigma-Aldrich Japan). Mn2O3 was obtained by heating of MnCO3 (Kishida Chemical) at 700 °C for 12 h. The precursors were thoroughly mixed by wet mechanical ball milling and then dried in air. Thus obtained mixtures of the samples were pressed into pellets. The pellets were heated at 900 °C for 12 h in air (Fe3+) or inert atmosphere (Mn3+ and V3+). The samples were stored in an Ar-filled glove box until use.
Li1.2Ti0.4Mn0.4O2 was prepared from Li2CO3, TiO2 (Anatase, 98.5%; Wako Pure Chemical Industries), and Mn2O3. The precursors were thoroughly mixed by wet mechanical ball milling and the mixture was heated at 900 °C for 12 h in inert atmosphere. Particle morphology of the samples was observed using a scanning electron microscope (JCM-6000, JEOL) with acceleration voltage of 15 keV.
Electrochemical characterization
Electrode performance of the samples was examined for the carbon composite samples prepared by ball-milling. As-prepared Li1.3Nb0.3Me0.4O2 (Me=Fe3+, Mn3+ and V3+) were mixed with acetylene black by using a planetary ball mill (PULVERISETTE 7; FRITSCH) at 300 r.p.m with a zirconia container and balls. The samples used in Fig. 2, Supplementary Figs 2 and 8 were mixed with 10 wt% carbon. Composite electrodes consisted of 76.5 wt% active materials, 13.5 wt% acetylene black and 10 wt% poly(vinylidene fluoride), pasted on aluminium foil as a current collector. Rest of the data were collected using the samples with 20 wt% carbon. Composite electrodes consisted of 72 wt% active materials, 18 wt% acetylene black and 10 wt% poly(vinylidene fluoride). Metallic lithium (Honjo Metal) was used as a negative electrode. The electrolyte solution used was 1.0 mol dm−3 LiPF6 dissolved in ethylene carbonate:dimethyl carbonate (1:1 by volume) (Kishida Chemical). A polyolefin microporous membrane was used as a separator. R2032-type coin cells (Hosen Corp.) or TJ-AC (Tomcell Japan) were assembled in the Ar-filled glove box. The cells were cycled at a rate of 10 mA g−1 at room temperature or 50 °C.
Materials characterization
Soft X-ray absorption (XAS) spectra were collected at BL-11 (O K-edge and Me LII, III-edges) in the synchrotron facility of Ritsumeikan University (Synchrotron Radiation Center)36. The absorption spectra were collected with fluorescence yield and electron yield modes. Similar to the measurements for hard XAS, the samples were prepared in the Ar-filled glove box, and thus prepared samples were set on the spectrometer using a laboratory-made transfer vessel without air exposure. Normalization of the XAS spectra was carried out using the program code IFEFFIT (ref. 39). The postedge background was determined using a cubic spline procedure.
XPS measurements were carried out with VG ESCALAB 250 spectrometer (Thermo Fisher Scientific K.K.) using monochromatized Al Kα X-ray radiation (1486.6 eV). The system was operated at 15 kV and 200 W. The base pressure of the analysis chamber was less than 10−8 Pa. These characterizations were carried our using a laboratory-made transfer vessel to avoid the sample exposure to moisture/air.
transmission electron microscopy observation was conducted by using JEM-ARM200F (JEOL) operated at 200 keV. The samples were dispersed in dimethyl carbonate and then supported on a copper grid.
A neutron diffraction pattern was collected at BL09 (SPICA) in the Material and Life science Facility (MLF) of the Japan Proton Accelerator Research Complex (J-PARC) (ref. 40).
Data availability
The data supporting the main findings of this study are available from the corresponding authors on request.
Additional information
How to cite this article: Yabuuchi N, et al. Origin of stabilization and destabilization in solid-state redox reaction of oxide ions for lithium-ion batteries. Nat. Commun. 7, 13814 doi: 10.1038/ncomms13814 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary Figures, Supplementary Tables, Supplementary Methods and Supplementary References
Acknowledgments
This research has been partly supported by Advanced Low Carbon Technology Research and Development Program of the Japan Science and Technology Agency (JST) Special Priority Research Area ‘Next-Generation Rechargeable Battery'. The synchrotron X-ray diffraction experiments were made possible through the support of the Japanese Ministry of Education, Science, Sports and Culture, Nanotechnology Support Project (Proposals 2013B1752) with the approval of the Japan Synchrotron Radiation (SR) Research Institute. The hard X-ray absorption work was done under the approval of the Photon Factory Program Advisory Committee (Proposals 2014G055) and the soft X-ray absorption work was done at the SR Center of Ritsumeikan University under the ‘Project for Creation of Research Platforms and Sharing of Advanced Research Infrastructure' (Proposal R1506 and R1549). M.N. is grateful for financial support from the JST Precursory Research for Embryonic Science and Technology program. We also thank the Information Technology Center of Nagoya University for providing computing resources (CX400). The neutron scattering experiment was approved by the Neutron Scattering Program Advisory Committee of IMSS, KEK (Proposal No. 2014S10). TEM observation was supported by Dr Keisuke Shinoda, NIMS, Battery Research Platform of the ALCA program. N.Y. thanks Dr Kimihiko Ito, NIMS, for the courtesy and permission to reproduce the data of O K-edge XAS spectra for KO2.
Footnotes
Author contributions N.Y. and M.N. conceived and directed the project. M.T. and K.S. synthesized Li3NbO4-based samples. Y.K. synthesized Li1.2Ti0.4Mn0.4O2. M.T., K.S. and Y.K. measured electrochemical properties of samples. Y.H., T.M. and H.S. conducted DFT calculations. A.N. carried out XPS measurement. M.Y. measured and analysed neutron diffraction data. K.Y, K.M. and T.O. collected and analysed soft XAS spectra. N.Y., M.N., M.Y., S.K. and T.O. wrote the manuscript.
References
- Dunn B., Kamath H. & Tarascon J.-M. electrical energy storage for the grid: a battery of choices. Science 334, 928–935 (2011). [DOI] [PubMed] [Google Scholar]
- Mizushima K., Jones P. C., Wiseman P. J. & Goodenough J. B. LIXCOO2 (0<x≤1)—a new cathode material for batteries of high-energy density. Mater. Res. Bull. 15, 783–789 (1980). [Google Scholar]
- Ohzuku T. & Makimura Y. Layered lithium insertion material of LiNi1/2Mn1/2O2: a possible alternative to LiCoO2 for advanced lithium-ion batteries. Chem. Lett. 30, 744–745 (2001). [Google Scholar]
- Robertson A. D. & Bruce P. G. Mechanism of electrochemical activity in Li2MnO3. Chem. Mater. 15, 1984–1992 (2003). [Google Scholar]
- Lu Z. H., MacNeil D. D. & Dahn J. R. Layered cathode materials Li[NixLi(1/3−2x/3)Mn(2/3−x/3)]O2 for lithium-ion batteries. Electrochem. Solid State Lett. 4, A191–A194 (2001). [Google Scholar]
- Lu Z. H. & Dahn J. R. Understanding the anomalous capacity of Li/Li[NixLi(1/3−2x/3)Mn(2/3−x/3]O2 cells using in situ X-ray diffraction and electrochemical studies. J. Electrochem. Soc. 149, A815–A822 (2002). [Google Scholar]
- Johnson C. S. et al. The significance of the Li2MnO3 component in ‘composite' xLi2MnO3 center dot (1-x)LiMn0.5Ni0.5O2 electrodes. Electrochem. Commun. 6, 1085–1091 (2004). [Google Scholar]
- Armstrong A. R. et al. Demonstrating oxygen loss and associated structural reorganization in the lithium battery cathode Li[Ni0.2Li0.2Mn0.6]O2. J. Am. Chem. Soc. 128, 8694–8698 (2006). [DOI] [PubMed] [Google Scholar]
- Jiang M., Key B., Meng Y. S. & Grey C. P. Electrochemical and structural study of the layered, ‘Li-excess' lithium-ion battery electrode material Li[Li1/9Ni1/3Mn5/9]O2. Chem. Mater. 21, 2733–2745 (2009). [Google Scholar]
- Ohzuku T., Nagayama M., Tsuji K. & Ariyoshi K. High-capacity lithium insertion materials of lithium nickel manganese oxides for advanced lithium-ion batteries: toward rechargeable capacity more than 300 mA h g−1. J. Mater. Chem. 21, 10179–10188 (2011). [Google Scholar]
- Yabuuchi N., Yoshii K., Myung S.-T., Nakai I. & Komaba S. Detailed studies of a high-capacity electrode material for rechargeable batteries, Li2MnO3−LiCo1/3Ni1/3Mn1/3O2. J. Am. Chem. Soc. 133, 4404–4419 (2011). [DOI] [PubMed] [Google Scholar]
- Koga H. et al. Li1.20Mn0.54Co0.13Ni0.13O2 with different particle sizes as attractive positive electrode materials for lithium-ion batteries: insights into their structure. J. Phys. Chem. B 116, 13497–13506 (2012). [Google Scholar]
- Oishi M. et al. Charge compensation mechanisms in Li1.16Ni0.15Co0.19Mn0.50O2 positive electrode material for Li-ion batteries analyzed by a combination of hard and soft X-ray absorption near edge structure. J. Power Sources 222, 45–51 (2013). [Google Scholar]
- Sathiya M. et al. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat. Mater. 12, 827–835 (2013). [DOI] [PubMed] [Google Scholar]
- Oishi M. et al. Direct observation of reversible charge compensation by oxygen ion in Li-rich manganese layered oxide positive electrode material, Li1.16Ni0.15Co0.19Mn0.50O2. J. Power Sources 276, 89–94 (2015). [Google Scholar]
- Saubanere M., McCalla E., Tarascon J. M. & Doublet M. L. The intriguing question of anionic redox in high-energy density cathodes for Li-ion batteries. Energy Environ. Sci. 9, 984–991 (2016). [Google Scholar]
- McCalla E. et al. Visualization of O-O peroxo-like dimers in high-capacity layered oxides for Li-ion batteries. Science 350, 1516–1521 (2015). [DOI] [PubMed] [Google Scholar]
- Luo K. et al. Charge-compensation in 3d-transition-metal-oxide intercalation cathodes through the generation of localized electron holes on oxygen. Nat. Chem. 8, 684–691 (2016). [DOI] [PubMed] [Google Scholar]
- Seo D.-H. et al. The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials. Nat. Chem. 8, 692–697 (2016). [DOI] [PubMed] [Google Scholar]
- Yabuuchi N. et al. High-capacity electrode materials for rechargeable lithium batteries: Li3NbO4-based system with cation-disordered rocksalt structure. Proc. Natl Acad. Sci. USA 112, 7650–7655 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuuchi N. et al. Synthesis and electrochemical properties of Li1.3Nb0.3V0.4O2 as a positive electrode material for rechargeable lithium batteries. Chem. Commun. 52, 2051–2054 (2016). [DOI] [PubMed] [Google Scholar]
- Yabuuchi N., Tahara Y., Komaba S., Kitada S. & Kajiya Y. Synthesis and electrochemical properties of Li4MoO5-NiO binary system as positive electrode materials for rechargeable lithium batteries. Chem. Mater. 28, 416–419 (2016). [Google Scholar]
- Wang R. et al. A disordered rock-salt Li-excess cathode material with high capacity and substantial oxygen redox activity: Li1.25Nb0.25Mn0.5O2. Electrochem. Commun. 60, 70–73 (2015). [Google Scholar]
- Lee J. et al. A new class of high capacity cation-disordered oxides for rechargeable lithium batteries: Li-Ni-Ti-Mo oxides. Energy Environ. Sci. 8, 3255–3265 (2015). [Google Scholar]
- McCalla E. et al. Novel complex stacking of fully-ordered transition metal layers in Li4FeSbO6 materials. Chem. Mater. 27, 1699–1708 (2015). [Google Scholar]
- Okuoka S. et al. A new sealed lithium-peroxide battery with a Co-doped Li2O cathode in a superconcentrated lithium bis(fluorosulfonyl)amide electrolyte. Sci. Rep. 4, 5684 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. et al. Unlocking the potential of cation-disordered oxides for rechargeable lithium batteries. Science 343, 519–522 (2014). [DOI] [PubMed] [Google Scholar]
- Chen R. et al. Li+ intercalation in isostructural Li2VO3 and Li2VO2F with O2− and mixed O2−/F− anions. PCCP 17, 17288–17295 (2015). [DOI] [PubMed] [Google Scholar]
- Ruckman M. W. et al. Interpreting the near edges of O2 and O2− in alkali-metal superoxides. Phys. Rev. Lett. 67, 2533–2536 (1991). [DOI] [PubMed] [Google Scholar]
- Hong J. et al. Critical role of oxygen evolved from layered Li-excess metal oxides in lithium rechargeable batteries. Chem. Mater. 24, 2692–2697 (2012). [Google Scholar]
- Yabuuchi N., Kubota K., Aoki Y. & Komaba S. Understanding particle-size-dependent electrochemical properties of Li2MnO3-based positive electrode materials for rechargeable lithium batteries. J. Phys. Chem. C 120, 875–885 (2016). [Google Scholar]
- McCalla E. et al. Understanding the roles of anionic redox and oxygen release during electrochemical cycling of Lithium-rich layered Li4FeSbO6. J. Am. Chem. Soc. 137, 4804–4814 (2015). [DOI] [PubMed] [Google Scholar]
- Sathiya M. et al. Origin of voltage decay in high-capacity layered oxide electrodes. Nat. Mater. 14, 230–238 (2015). [DOI] [PubMed] [Google Scholar]
- Urban A., Lee J. & Ceder G. The configurational space of rocksalt-type oxides for high-capacity lithium battery electrodes. Adv. Energy Mater. 4, 1400478 (2014). [Google Scholar]
- Yu D. Y. W., Yanagida K., Kato Y. & Nakamura H. Electrochemical activities in Li2MnO3. J. Electrochem. Soc. 156, A417–A424 (2009). [Google Scholar]
- Ishii H., Nakanishi K., Watanabe I., Ohta T. & Kojima K. Improvement of ultra soft X-ray absorption spectroscopy and photoelectron spectroscopy beamline for studies on related materials and cathodes of lithium ion batteries. e-J. Surf. Sci. Nanotechnol. 9, 416–421 (2011). [Google Scholar]
- Glazier S. L., Li J., Zhou J., Bond T. & Dahn J. R. Characterization of disordered Li(1+x)Ti2xFe(1–3x)O2 as positive electrode materials in Li-Ion batteries using percolation theory. Chem. Mater. 27, 7751–7756 (2015). [Google Scholar]
- Matsuhara T. et al. Synthesis and electrode performance of Li4MoO5-LiFeO2 binary system as positive electrode materials for rechargeable lithium batteries. Electrochemistry 84, 797–801 (2016). [Google Scholar]
- Newville M. IFEFFIT: interactive XAFS analysis and FEFF fitting. Journal of Synchrotron Radiat. 8, 322–324 (2001). [DOI] [PubMed] [Google Scholar]
- Yonemura M. et al. Development of SPICA, new dedicated neutron powder diffractometer for battery studies. J. Phys.: Conf. Ser. 502, 012053 (2014). [Google Scholar]
- Izumi F. & Momma K. Three-dimensional visualization in powder diffraction. Solid State Phenom. 130, 15–20 (2007). [Google Scholar]
- Momma K. & Izumi F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures, Supplementary Tables, Supplementary Methods and Supplementary References
Data Availability Statement
The data supporting the main findings of this study are available from the corresponding authors on request.