Abstract
There is a substantial need to develop new medicines against parasitic diseases via public-private partnerships. Based on high throughput phenotypic screens of largely protozoal pathogens and bacteria, the Medicines for Malaria Venture (MMV) has recently assembled an open-access ‘Pathogen Box’ containing 400 well-curated chemical compounds. In the present study, we tested these compounds for activity against parasitic stages of the nematode Haemonchus contortus (barber's pole worm). In an optimised, whole-organism screening assay, using exsheathed third-stage (xL3) and fourth-stage (L4) larvae, we measured the inhibition of larval motility, growth and development of H. contortus. We also studied the effect of the ‘hit’ compound on mitochondrial function by measuring oxygen consumption. Among the 400 Pathogen Box compounds, we identified one chemical, called tolfenpyrad (compound identification code: MMV688934) that reproducibly inhibits xL3 motility as well as L4 motility, growth and development, with IC50 values ranging between 0.02 and 3 μM. An assessment of mitochondrial function showed that xL3s treated with tolfenpyrad consumed significantly less oxygen than untreated xL3s, which was consistent with specific inhibition of complex I of the respiratory electron transport chain in arthropods. Given that tolfenpyrad was developed as a pesticide and has already been tested for absorption, distribution, excretion, biotransformation, toxicity and metabolism, it shows considerable promise for hit-to-lead optimisation and/or repurposing for use against H. contortus and other parasitic nematodes. Future work should assess its activity against hookworms and other pathogens that cause neglected tropical diseases.
Keywords: Haemonchus contortus, Anthelmintic screening, Pathogen Box, Tolfenpyrad, Mitochondrial respiratory chain
Graphical abstract
Highlights
-
•
We screened compounds in the ‘Pathogen Box’ for activity against the parasitic nematode Haemonchus contortus.
-
•
Tolfenpyrad, a pyrazole-5-carboxamide-based insecticide, has significant anthelmintic activity.
-
•
Tolfenpyrad inhibits oxygen consumption in parasitic larvae of H. contortus.
1. Introduction
Compounded by massive global food and water shortages and climate change, parasitic illnesses, including neglected tropical diseases (NTDs; WHO, 2015), have a devastating, long-term impact on human and animal health and welfare worldwide, and thus represent a major global challenge. Together, NTDs infect more than one billion people worldwide, resulting in an estimated loss of 26 million disability-adjusted life years (Hotez et al., 2014).
Despite their adverse socioeconomic impact, there are major limitations in the diagnosis, treatment and control of NTDs. Currently, there are no commercial vaccines available against most of these diseases (Pedrique et al., 2013, Hotez et al., 2016), diagnostic methods frequently suffer from insufficient specificity and sensitivity (Utzinger et al., 2012, Assefa et al., 2014), and treatments are often not highly effective and/or are toxic (Castro et al., 2006, Witschel et al., 2012, Molina et al., 2015). In addition, often the small numbers of drugs (or drug classes) frequently used, limited use of combination drug therapies and the implementation of mass drug administration programs bear the risk of drug resistance emerging in some groups of target pathogens (Humphries et al., 2012, Witschel et al., 2012, Webster et al., 2014). Therefore, the development of new drugs is crucial to ensure effective and sustained treatment and control into the future.
In spite of some success through the discovery of, for example, monepantel (Kaminsky et al., 2008, Prichard and Geary, 2008) and derquantel (Little et al., 2011), progress in discovering new drugs against parasitic worms of animal health importance has been relatively poor. Likely reasons for limited success beyond the lack of resources include an over-confidence in the validation of molecular targets (enzymes and receptors) and in studying an inappropriate developmental stage of a pathogen. However, key gaps include a lack of readily available curated sets of compounds for targeted screening and subsequent evaluation, limited cooperation among different areas (including parasitology, drug discovery, medicinal chemistry and safety evaluation) which are essential to find starting points for drug discovery, and to bring them to tangible and translational outcomes and outputs.
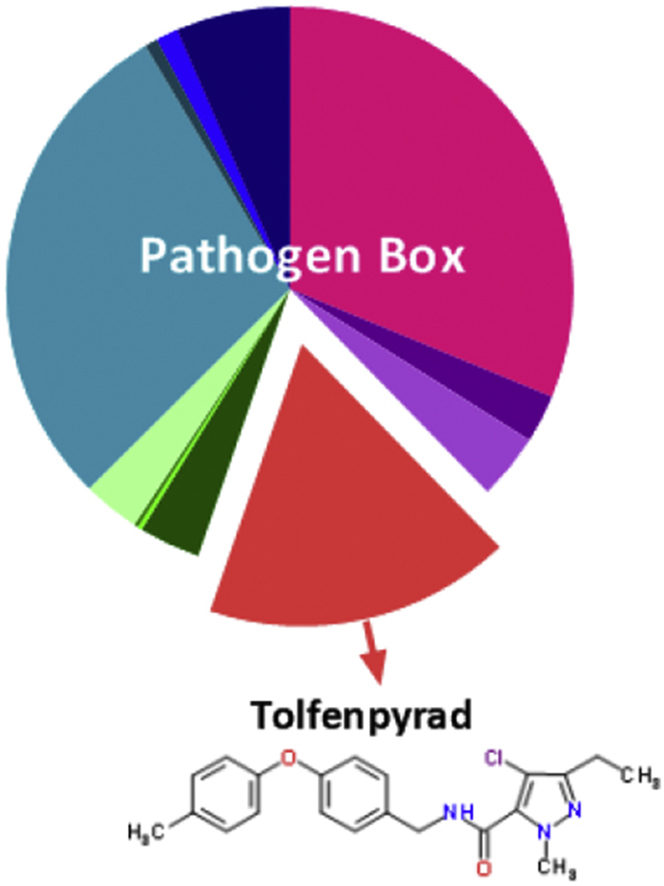
In the late 1990s, an innovative collaboration model for research and development for neglected diseases emerged in the form of public-private partnerships (PPPs) that came to be known as product development partnerships (PDPs). A key example is the Medicines for Malaria Venture (MMV), created from a desire to catalyse the discovery development and delivery of new medicines against malaria. Over the last decade, almost seven million compounds have been tested in phenotypic assays against malaria, and this has resulted in a solid pipeline of new preclinical and clinical candidates. In addition, an open science initiative has made many of these structures available, and a collection of 400 key malaria phenotypic ‘hits’, called the ‘Malaria Box’, was launched in 2013. Building on this model, in December 2015, MMV took this a stage further, with an initiative to stimulate the discovery of drugs for neglected parasitic diseases. The ‘Pathogen Box’ (www.pathogenbox.org), contains 400 diverse drug-like molecules, and is provided at no cost to research groups.
Each of the 400 compounds in the ‘Pathogen Box’ has confirmed activity against one or more key pathogens that cause some of the most socioeconomically important diseases worldwide, including tuberculosis, malaria, sleeping sickness, leishmaniasis, schistosomiasis, hookworm disease, toxoplasmosis and cryptosporidiosis. In addition, all compounds have been tested for cytotoxicity, with compounds included in the library being at least 5-fold more selective for the pathogen than its mammalian host. The complete set of compounds is dispatched to laboratories around the world to boost drug discovery efforts. This initiative provided us with a unique opportunity to assess these curated compounds for nematocidal activity in a recently developed whole-organism screening assay (Preston et al., 2015, Preston et al., 2016). Our aim was to rapidly screen all 400 compounds against parasitic stages of the barber's pole worm, Haemonchus contortus, and to identify hit compounds and characterise/assess them for further evaluation as nematocidal candidates. This worm was used because it is one of the best-studied members of a large order (Strongylida) of socioeconomically important nematodes of animals, including humans, because there is extensive information available on its biology and molecular biology, and because its genome and developmental transcriptome have been characterised in detail (Gasser and von Samson-Himmelstjerna, 2016), providing a foundation for drug discovery efforts.
2. Materials and methods
2.1. Procurement of H. contortus
The Haecon-5 strain of Haemonchus contortus, which is partially resistant to benzimidazoles (Dr Jody Zawadzki, personal communication), was maintained in experimental sheep as described previously (Schwarz et al., 2013, Preston et al., 2015) and in accordance with the institutional animal ethics guidelines (permit no. 1413429; The University of Melbourne, Australia). L3s were produced from H. contortus eggs by incubating faeces from infected sheep at 27 °C for 1 week (Preston et al., 2015), sieved through nylon mesh (pore size: 20 μm) to remove debris or dead larvae and then stored at 10 °C for a maximum of 3 months. For screening and basal oxygen consumption measurements (see following sub-sections), L3s were exsheathed and sterilised in 0.15% v/v sodium hypochlorite (NaClO) at 37 °C for 20 min (Preston et al., 2015). Thereafter, xL3s were washed five times in sterile physiological saline by centrifugation at 600 g (5 min) at 22–24 °C. Then, xL3s were immediately suspended in Luria Bertani medium [LB: 10 g of tryptone (cat no. LP0042; Oxoid, England), 5 g of yeast extract (cat no. LP0042; Oxoid) and 5 g of NaCl (cat. no. K43208004210; Merck, Denmark)] in 1 l of reverse-osmosis deionised water). LB was autoclaved and supplemented with 100 IU/ml of penicillin, 100 μg/ml of streptomycin and 2.5 μg/ml of amphotericin (Fungizone, antibiotic – antimycotic; cat. no. 15240-062; Gibco, USA); this supplemented LB was designated LB*. Fourth-stage larvae (L4s) were produced from xL3s in vitro for 7 days at 38 °C and 10% CO2, as described by Preston et al., 2015, Preston et al., 2016.
2.2. Screening of compounds
The Pathogen Box contains 400 compounds representing compounds that are active against one or more of 12 distinct pathogens (http://www.pathogenbox.org/about-pathogen-box/supporting-information). Individual compounds had only been tested to confirm activity against the pathogen for which the compounds were first reported to be active, and have not been tested against the other pathogens represented in the Pathogen Box. All compounds have been tested for cytotoxicity; typically, they are five-fold less potent against a human fibroblast cell line (MRC-5) than the pathogen (cf. Table 1); toxicity values are within levels considered acceptable for an initial drug discovery programme (www.pathogenbox.org/about-pathogen-box/supporting-information). Each of the 400 compounds was prepared as described previously (Preston et al., 2015) and screened (in triplicate) at a concentration of 20 μM on xL3s of H. contortus in 96-well microculture plates using two reference-control compounds, moxidectin and monepantel (Preston et al., 2015, Preston et al., 2016). In brief, compounds were dissolved to a stock concentration of 10 mM in dimethyl sulfoxide (DMSO, Ajax Finechem, Australia). Compounds were individually diluted to the final concentration of 20 μM using LB*, and dispensed (in triplicate) into wells of the microculture plates using a multichannel pipette. In addition, the negative-controls (LB* and LB* + 0.5% DMSO; six wells each), and positive-controls (final concentration of 20 μM of monepantel [Zolvix, Novartis Animal Health, Switzerland] and 20 μM of moxidectin [cydectin, Virbac, France]) were dispensed in triplicate wells. Then, xL3s (∼300/well) were dispensed into wells of the plate using an automated multichannel pipette (Viaflo Assist/II, Integra Biosciences, Switzerland). Following an incubation for 72 h at 38 °C and 10% CO2, a video recording (5 s) was taken of each well of the 96-well microculture plate (containing xL3s) using a grey-scale camera (Rolera bolt, Q imaging Scientific Coms, Canada), and a motorised X-Y axis stage (BioPoint 2, Ludl Electronics Products, USA). Individual videos were processed to calculate a motility index (MI) using an algorithm described previously (Preston et al., 2015, Preston et al., 2016). MIs were normalised to the positive- and negative-controls (to remove plate-to-plate variation) using the program Prism (v.6 GraphPad Software, USA). A compound was recorded as having activity if it reduced xL3 motility by ≥ 70% after 72 h of incubation.
Table 1.
In vitro activity of tolfenpyrad on the motility of exsheathed third-stage (xL3) and fourth-stage (L4) larvae of Haemonchus contortus (after 24 h, 48 h and 72 h of exposure) and on the development of the L4 stage (after 7 days of exposure), in comparison with values for monepantel and moxidectin, as well as cytotoxicity data for tolfenpyrad.
| Bioassay | Time | Half maximum inhibitory concentration (IC50; μM) |
||
|---|---|---|---|---|
| Tolfenpyrad | Monepantel | Moxidectin | ||
| xL3 motility | 24 h | 2.3 | 52 | 2.5 |
| 48 h | 3.0 | 6 | 2.5 | |
| 72 h | 3.0 | 0.4 | 2.3 | |
| L4 development | 7 days | 0.06 | 0.4 | 12.3 |
| L4 motility | 24 h | 0.13 | 4.3 | 2.2 |
| 48 h | 0.06 | 2.2 | 0.6 | |
| 72 h | 0.02 | 3 | 0.005 | |
| Cytotoxicity on human fibroblast (MRC-5) cellsa | 56 | na | na | |
Data from the Laboratory of Microbiology, Parasitology and Hygiene (LMPH), University of Antwerp, provided to Medicines for Malaria Venture (MMV) to accompany the Pathogen Box. na = not applicable.
2.3. Dose-response assessments of active compounds on xL3 and L4 motility, and L4 growth and development
Anti-xL3 activity of any ‘hit’ compound was confirmed, and half maximum inhibitory concentration (IC50) values estimated from dose-response curves (24 h, 48 h and 72 h). Compounds that reduced the motility of xL3s were also tested for their ability to inhibit the development of xL3s to L4s, the motility of L4s and/or their ability to retard L4 growth, as described previously (Preston et al., 2015, Preston et al., 2016). In brief, growth retardation and morphological alterations in L4s exposed for 48 h to LB* containing either 1% DMSO (negative-control), 100 μM of each tolfenpyrad (test compound), moxidectin (positive-control) or monepantel (positive-control) were assessed microscopically (20–100× magnification). For each treatment, the mean width of 30 L4s ± the standard error of the mean (SEM) was calculated, and a non-parametric (Kruskal-Wallis) one-way ANOVA and Dunn's multiple comparison test was used to calculate statistical difference between treatments. All assays (xL3 motility, and L4 development, growth and motility) were performed in triplicate, between 3 and 5 times on different days. To determine IC50 values, the data from each assay (xL3 motility, L4 motility and development) were converted to a percentage with reference to the negative-control (LB* + 0.5% DMSO), and IC50 values determined using a variable slope four-parameter equation, constraining the top value to 100% and using a least squares (ordinary) fit model (v.6 GraphPad Software). Selectivity indices (SIs) were calculated using a recognised formula (SI = human fibroblast (MRC-5) cells IC50/H. contortus IC50; Fisher et al., 2014) employing cytotoxicity data linked to the Pathogen Box compounds.
2.4. Measurement of basal oxygen consumption
Following standardization using an established protocol, the basal oxygen consumption (‘respiratory’) rate in xL3s was measured using the Seahorse XF24 flux analyser (Seahorse Biosciences, USA) (McGee et al., 2011). In brief, 450 μl of LB* + 1% DMSO containing 100 μM of tolfenpyrad (‘hit’ compound) or 100 μM of moxidectin (control; not known to inhibit respiration), or LB* + 1% DMSO alone (untreated control) were transferred in quadruplicate to the wells of a 24-well plate (Seahorse XF24). Then, 1000 xL3s in 50 μl LB* were added to the wells, and the plates pre-incubated for 2 h, equilibrated for 30 min and oxygen consumption measured using the flux analyser. Seven measurements were taken over a 1 h-period (protocol: 2 min-mix, 2 min-pause and 4 min-measure; McGee et al., 2011). Experiments were repeated twice. A two-way repeated measures ANOVA with a Dunnett's multiple comparison test (v.6 GraphPad Prism) was used to assess the statistical difference in oxygen consumption between treated and untreated xL3s.
3. Results
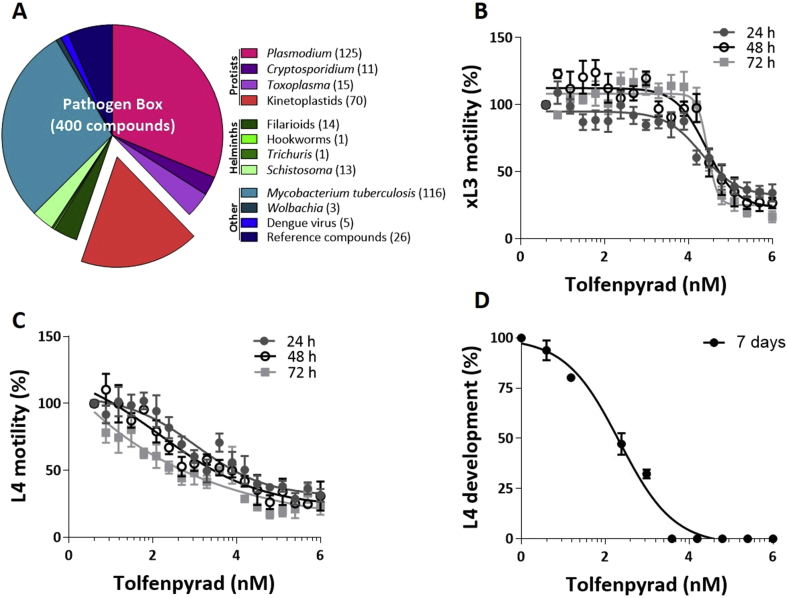
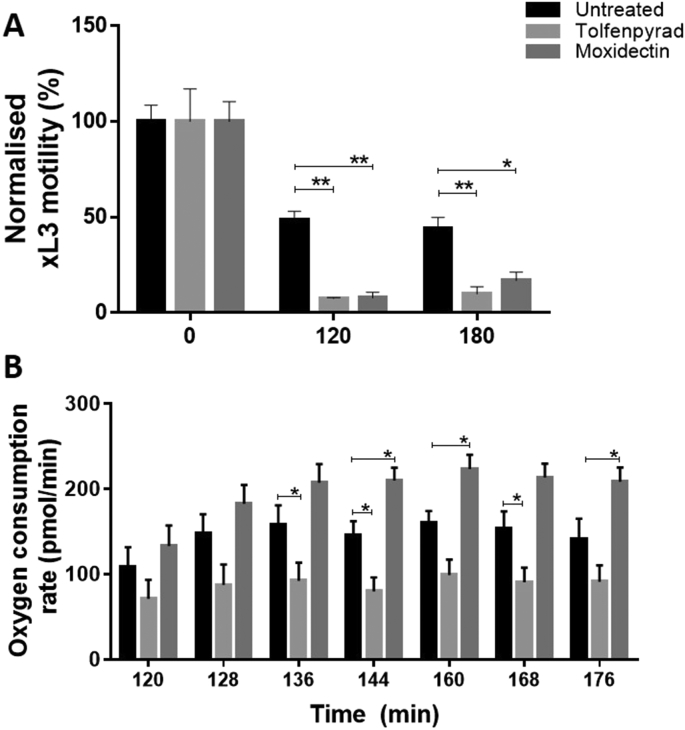
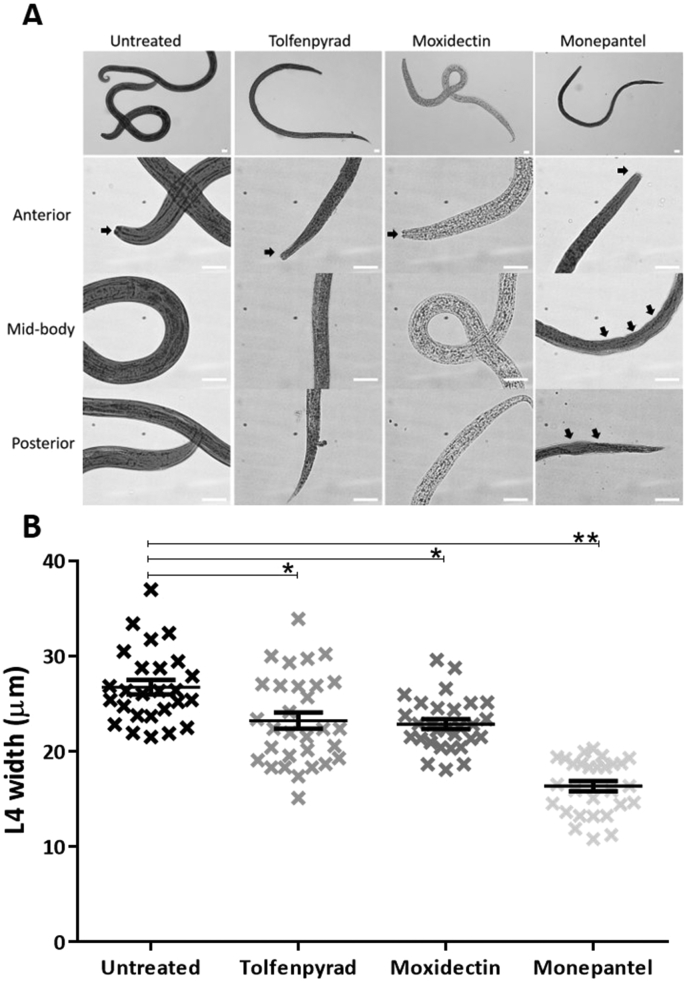
In the primary screen of the 400 compounds (Fig. 1; Supplementary file 1), one compound (tolfenpyrad; Compound ID: MMV688934; batch: MMV688934-01; pubchem.ncbi.nlm.nih.gov/compound/10110536) was recorded to inhibit xL3 motility by ≥ 70%. Although there are benzimidazole-based compounds in the Pathogen Box, nematocidal activity was not detected using this ≥70% threshold, because the Haecon-5 strain of H. contortus is partially resistant to this class of chemicals. Subsequent assays using xL3s and L4s of H. contortus showed that the potency of tolfenpyrad, measured as IC50 values, ranged from 0.02 to 3 μM (Fig. 1; Table 1). In comparison to moxidectin and monepantel, tolfenpyrad was able to reduce motility earlier than monepantel, with inhibition occurring after 2 h of exposure and an IC50 value of 2.3 μM at 24 h compared with 52.1 μM for monepantel (Fig. 3). Tolfenpyrad and moxidectin were found to have a similar inhibitory effect on xL3 motility at the time points tested (Table 1). Furthermore, when examining the inhibitory activity of the compounds on L4 motility, tolfenpyrad and moxidectin had lower IC50 values at 24 h, 48 h and 72 h compared with that of monepantel (Table 1). In the L4 development assay, tolfenpyrad had a greater inhibitory effect on the development of xL3 to L4 (IC50 of 0.06 μM) than did moxidectin and monepantel (IC50 of 12.3 μM and 0.4 μM, respectively). Light microscopic examination of parasitic larvae revealed morphological damage (shrivelled and granulated appearance) in L4s following exposure in vitro (48 h) to tolfenpyrad and monepantel (Fig. 2), but not in xL3s (data not shown). L4s exposed to tolfenpyrad were significantly thinner than ‘untreated’ controls, and had a similar width to moxidectin-exposed, but not as pronounced as monepantel-exposed larvae (Fig. 2). Finally, it was assessed whether tolfenpyrad would inhibit respiration in H. contortus xL3s, as it does in arthropods by targeting complex I of the respiratory electron transport chain (Song et al., 2013), by measuring oxygen consumption over time (Fig. 3). The results showed that tolfenpyrad-treated xL3s consumed substantially (P < 0.05) less oxygen than both moxidectin-treated and untreated xL3s (Fig. 3) (Table 1).
Fig. 1.
The ‘Pathogen Box’ from the Medicines for Malaria Venture (MMV) (Panel A) contains 400 diverse drug-like molecules with confirmed activity against one or more key pathogens that cause some of the most socioeconomically important diseases worldwide, including malaria, toxoplasmosis, cryptosporidiosis, trypanosomiasis, leishmaniasis, hookworm disease, trichuriasis, schistosomiasis; numbers of chemicals active against different pathogen/pathogen groups are indicated in parentheses. The graphs (Panels B to D) show the activity of tolfenpyrad on the motility of exsheathed third-stage (xL3) and fourth-stage (L4) larvae of Haemonchus contortus (after 24 h, 48 h and 72 h of exposure) and on the development of the L4 stage (after 7 days of exposure), respectively.
Fig. 3.
Panel A. Motility of exsheathed third-stage larvae (xL3s) of Haemonchus contortus incubated in culture medium (LB* + 1% DMSO) (untreated), and in the presence of 100 μM of either tolfenpyrad (‘hit’ compound) or moxidectin (control) at three distinct time points; at each data point, motility was normalised to time (0 min). Panel B. Based on results in panel A, oxygen consumption was measured seven times (8 min each) over a period of 1 h in untreated xL3s, and xL3s treated with the same concentration (100 μM) of tolfenpyrad (‘hit’ compound) or moxidectin (control) using a flux analyser. The mean values from 10 replicates (motility) and 8 replicates (oxygen consumption) from two separate experiments are shown, with variation between measurements displayed as a standard error of the mean (SEM). */** indicate values (mean ± SEM) which are significantly different from one another (P < 0.05 and P < 0.01, respectively), determined using a two-way ANOVA and Dunnett's multiple comparison test.
Fig. 2.
Panel A. Images of fourth-stage larvae (L4) exposed for 48 h to LB* containing either 1% dimethyl sulfoxide (untreated, negative control), 100 μM of each tolfenpyrad, moxidectin (positive-control) or monepantel (positive-control). Displayed are whole worm (L4) or anterior, mid-body and posterior regions (20–100× magnification; white scale bar: 20 μm). Solid, black arrows show structural damage to the cuticle and mouth of L4s. Panel B. Graph showing the mean widths of L4s under the same experimental conditions. The data points represent the mean width of 30 individual L4s ± the standard error of the mean (SEM). A representative graph of two separate experiments is shown. Significance between values (mean ± SEM) was determined using a non-parametric (Kruskal-Wallis) one-way ANOVA and Dunn's multiple comparison test. */** indicate which values are significantly different from one another (P < 0.05 and P < 0.01, respectively).
4. Discussion
The screening of the Pathogen Box compounds identified one chemical, tolfenpyrad, with major activity against parasitic larval stages (xL3 and L4) of H. contortus in vitro; IC50 values were comparable with those of two commercially available anthelmintics, monepantel and moxidectin. Tolfenpyrad, a pyrazole-5-carboxamide insecticide, is the International Organization for Standardization – approved name for 4-chloro-3-ethyl-1-methyl-N-[4-(p-tolyloxy)benzyl]pyrazole-5-carboxamide (International Union of Pure and Applied Chemistry, IUPAC), which has the Chemical Abstracts Service number 129558-76-5. Tolfenpyrad was included in the MMV ‘Pathogen Box’ based on results from a compound screen of agro-chemicals, which showed that tolfenpyrad has potent and selective in vitro activity against Trypanosoma spp. (Witschel et al., 2012). As a pesticide, tolfenpyrad has relatively broad activity against egg, larval, nymphal and adult stages of various arthropods (including Hemiptera, Coleoptera, Diptera, Lepidoptera, Thysanoptera and Acarina), and has been applied to various infested crops (Song et al., 2012, Yamaguchi et al., 2012). This chemical was developed in Japan and was first approved in 2002; it has been registered for commercial use in several countries other than Japan, including the Dominican Republic, Thailand, the United Arab Emirates, Indonesia and the USA (Yamaguchi et al., 2012).
In the present study, tolfenpyrad was shown to be more potent against L4s than xL3s based on IC50 values (motility and development), with substantial cuticular damage to the L4 but not to the xL3 stage. Compared with untreated-control L4s, the width of tolfenpyrad-treated L4s were significantly reduced as were moxidectin- and monepantel-treated control L4s, indicating that treatment suppresses parasite growth and development. It is unclear whether the difference in potency of tolfenpyrad between the two larval stages tested is due to variation in drug uptake, as at the L4 stage has a functioning pharynx (Sommerville, 1966), or the mode of action. The mode of action of this chemical (as a contact insecticide and fungicide) is likely linked to the specific inhibition of complex I of the respiratory electron transport chain in mitochondria (Lummen, 1998, Song et al., 2013), such that it is effective against various pests that are resistant to insecticides, including organophosphates and carbamates, which have modes of action that are entirely distinct from tolfenpyrad.
To explore whether tolfenpyrad might act as an inhibitor of the electron transport chain in H. contortus, oxygen consumption was measured in xL3s in the presence or absence of tolfenpyrad following a pre-exposure for 2 h to the compound. This approach is routinely used to evaluate mitochondrial function in Caenorhabditis elegans and mammalian tissue by detecting oxygen consumption in real-time using oxygen-sensitive probes (McGee et al., 2011, Andreux et al., 2014). In the present study, we elected to pre-treat xL3s of H. contortus for 2 h prior to the initial measurement of oxygen consumption, as both tolfenpyrad and moxidectin significantly inhibit motility following acute exposure. The results revealed a significant decrease in oxygen consumption in xL3s exposed to tolfenpyrad compared with untreated controls. Similarly, oxygen consumption was lower in tolfenpyrad-treated than moxidectin-treated xL3s, which was also associated with reduced motility following the exposure for 2 h. This discrepancy in oxygen consumption between these treatments indicates that tolfenpyrad is also most likely acting as a complex I mitochondrial electron transport inhibitor in H. contortus.
Although mainly used against agricultural pests, tolfenpyrad had not been assessed previously for use against endoparasites, such as parasitic worms, of animals. A report was prepared by the Joint FAO/WHO Meeting on Pesticide Residues at the request of the Codex Committee on Pesticide Residues in 2013 (FAO, 2013). This report extensively reviewed and appraised many aspects of tolfenpyrad, including: (i) acceptable daily intake; (ii) absorption, distribution, excretion and biotransformation; (iii) toxicity studies (acute; short and long-term; carcinogenicity; genotoxicity; reproductive, developmental and neural toxicity), and (iv) studies of metabolites. The main conclusions from this report were: (i) the acceptable daily intake of tolfenpyrad is 0–0.006 mg/kg in mammals; (ii) following oral administration, tolfenpyrad is rapidly absorbed, widely distributed and metabolized by the liver, with 88–93% being excreted in the faeces, and (iii) tolfenpyrad was not found to be carcinogenic, genotoxic or neurotoxic, but was found to have some reproductive and developmental toxicity. Although some intoxications (associated with excessive exposure/ingestion) have been recorded in humans (e.g., Yamaguchi et al., 2012, Hikiji et al., 2013), well-controlled risk assessment studies in monogastric mammals (mouse, rat, rabbit and dog) have shown that the no-observed-adverse-effect levels (NOAELs) of tolfenpyrad are between 1 and 1.5 mg/kg body weight per day, with lowest-observed-adverse-effect levels (LOAEL) of 5–21 mg/kg body weight per day (FAO, 2013). Therefore, based on the evidence presented in this report and associated literature (see FAO, 2013) as well as the high selectivity of tolfenpyrad for H. contortus (see Table 1), we propose to test tolfenpyrad in vivo in sheep against H. contortus and/or related parasitic nematodes at dosages between 1 and 5 mg/kg body weight. We also propose to assess its activity in vitro against the hookworms Ancylostoma ceylanicum and Necator americanus of humans and other NTD pathogens (London Declaration, 2012). Nonetheless, it will be important to also extend medicinal chemistry work to establish whether a safer and more effective derivative of this chemical might be synthesized. Given that tolfenpyrad was developed as a pesticide and has already been tested for absorption, distribution, excretion, biotransformation, toxicity and metabolism, there is considerable promise for the repurposing of this chemical for use against H. contortus and other parasitic nematodes. Further work should now focus on assessing the activity of tolfenpyrad against hookworms and other worms that cause NTDs.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The present study was funded by the National Health and Medical Research Council of Australia (NHMRC), the Australian Research Council (ARC) and the Wellcome Trust (RBG), and supported by a Victoria Life Sciences Computation Initiative, Australia (VLSCI; grant no. VR0007) on its Peak Computing Facility at The University of Melbourne, Australia, an initiative of the Victorian Government, Australia. Animal ethics approval (AEC no. 0707258) was granted by The University of Melbourne. We thank our colleagues at MMV, especially Angelique Doy, for their support.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ijpddr.2016.07.004.
Contributor Information
Sarah Preston, Email: sarah.preston@unimelb.edu.au.
Yaqing Jiao, Email: yaqingj@student.unimelb.edu.au.
Abdul Jabbar, Email: jabbara@unimelb.edu.au.
Sean L. McGee, Email: sean.mcgee@deakin.edu.au.
Benoît Laleu, Email: laleub@mmv.org.
Paul Willis, Email: willisp@mmv.org.
Timothy N.C. Wells, Email: wellst@mmv.org.
Robin B. Gasser, Email: robinbg@unimelb.edu.au.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Andreux P.A., Mouchiroud L., Wang X., Jovaisaite V., Mottis A., Bichet S., Moullan N., Houtkooper R.H., Auwerx J. A method to identify and validate mitochondrial modulators using mammalian cells and the worm C. elegans. Sci. Rep. 2014;4:5285. doi: 10.1038/srep05285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assefa L.M., Crellen T., Kepha S., Kihara J.H., Njenga S.M., Pullan R.L., Brooker S.J. Diagnostic accuracy and cost-effectiveness of alternative methods for detection of soil-transmitted helminths in a post-treatment setting in Western Kenya. PLoS. Negl. Trop. Dis. 2014;8:e2843. doi: 10.1371/journal.pntd.0002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro J.A., de Mecca M.M., Bartel L.C. Toxic side effects of drugs used to treat Chagas' disease (American trypanosomiasis) Hum. Exp. Toxicol. 2006;25:471–479. doi: 10.1191/0960327106het653oa. [DOI] [PubMed] [Google Scholar]
- FAO . 2013. 2013. Pesticide Residues in Food 2013. Report from the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues.http://www.fao.org/publications/card/en/c/299ca869-ae51-5093-8407-9cb30782b9f5 Geneva, Switzerland (17 to 26 September 2013. Accessed 19 03 2016. [Google Scholar]
- Fisher G.M., Tanpure A.D., Andrews K.T., Poulsen S.A. Synthesis and evaluation of antimalarial properties of novel 4-aminoquinoline hybrid compounds. Chem. Biol. Drug Des. 2014;84:462–472. doi: 10.1111/cbdd.12335. [DOI] [PubMed] [Google Scholar]
- Gasser R.B., von Samson-Himmelstjerna G. Haemonchus contortus and Haemonchosis-Past, Present and Future Trends. Adv. Parasitol. 2016;93 [Google Scholar]
- Hikiji W., Yamaguchi K., Saka K., Hayashida M., Ohno Y., Fukunaga T. Acute fatal poisoning with tolfenpyrad. J. Forensic Leg. Med. 2013;8:962–964. doi: 10.1016/j.jflm.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Hotez P.J., Bottazzi M.E., Strych U. New vaccines for the world's poorest people. Annu. Rev. Med. 2016;67:405–417. doi: 10.1146/annurev-med-051214-024241. [DOI] [PubMed] [Google Scholar]
- Hotez P.J., Alvarado M., Basáñez M.G., Bolliger I., Bourne R., Boussinesq M., Brooker S.J., Brown A.S., Buckle G., Budke C.M., Carabin H., Coffeng L.E., Fèvre E.M., Fürst T., Halasa Y.A., Jasrasaria R., Johns N.E., Keiser J., King C.H., Lozano R., Murdoch M.E., O'Hanlon S., Pion S.D.S., Pullan R.L., Ramaiah K.D., Roberts T., Shepard D.S., Smith J.L., Stolk W.A., Undurraga E.A., Utzinger J., Wang M., Murray C.J.L., Naghavi M. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl. Trop. Dis. 2014;8:e2865. doi: 10.1371/journal.pntd.0002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries D., Nguyen S., Boakye D., Wilson M., Cappello M. The promise and pitfalls of mass drug administration to control intestinal helminth infections. Curr. Opin. Infect. Dis. 2012;25:584–589. doi: 10.1097/QCO.0b013e328357e4cf. [DOI] [PubMed] [Google Scholar]
- Kaminsky R., Ducray P., Jung M., Clover R., Rufener L., Bouvier J., Weber S.S., Wenger A., Wieland-Berghausen S., Goebel T., Gauvry N., Pautrat F., Skripsky T., Froelich O., Komoin-Oka C., Westlund B., Sluder A., Maser P. A new class of anthelmintics effective against drug-resistant nematodes. Nature. 2008;452:176–180. doi: 10.1038/nature06722. [DOI] [PubMed] [Google Scholar]
- Little P.R., Hodge A., Maeder S.J., Wirtherle N.C., Nicholas D.R., Cox G.G., Conder G.A. Efficacy of a combined oral formulation of derquantel–abamectin against the adult and larval stages of nematodes in sheep, including anthelmintic-resistant strains. Vet. Parasitol. 2011;181:180–193. doi: 10.1016/j.vetpar.2011.05.008. [DOI] [PubMed] [Google Scholar]
- London Declaration . 2012. Uniting to Combat Neglected Tropical Diseases.http://unitingtocombatntds.org/resource/london-declaration Accessed 27 06 2016. [Google Scholar]
- Lummen P. Complex I inhibitors as insecticides and acaricides. Biochem. Biophys. Acta. 1998;1364:287–296. doi: 10.1016/s0005-2728(98)00034-6. [DOI] [PubMed] [Google Scholar]
- McGee S.L., Sadli N., Morrison S., Swinton C., Suphioglu C. DHA protects against zinc mediated alterations in neuronal cellular bioenergetics. Cell. Physiol. Biochem. 2011;28:157–162. doi: 10.1159/000331724. [DOI] [PubMed] [Google Scholar]
- Molina I., Salvador F., Sanchez-Montalva A., Trevino B., Serre N., Sao Aviles A., Almirante B. Toxic profile of benznidazole in patients with chronic Chagas disease: risk factors and comparison of the product from two different manufacturers. Antimicrob. Agents Chemother. 2015;59:6125–6131. doi: 10.1128/AAC.04660-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrique B., Strub-Wourgaft N., Some C., Olliaro P., Trouiller P., Ford N., Pecoul B., Bradol J.H. The drug and vaccine landscape for neglected diseases (2000-11): a systematic assessment. Lancet Glob. Health. 2013;1:e371–e379. doi: 10.1016/S2214-109X(13)70078-0. [DOI] [PubMed] [Google Scholar]
- Preston S., Jabbar A., Nowell C., Joachim A., Ruttkowski B., Baell J., Cardno T., Korhonen P.K., Piedrafita D., Ansell B.R., Jex A.R., Hofmann A., Gasser R.B. Low cost whole-organism screening of compounds for anthelmintic activity. Int. J. Parasitol. 2015;45:333–343. doi: 10.1016/j.ijpara.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Preston S., Jabbar A., Nowell C., Joachim A., Ruttkowski B., Cardno T., Hofmann A., Gasser R.B. Practical and low cost whole-organism motility assay: a step-by-step protocol. Mol. Cell. Probes. 2016;30:13–17. doi: 10.1016/j.mcp.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Prichard R.K., Geary T.G. Drug discovery: fresh hope to can the worms. Nature. 2008;452:157–158. doi: 10.1038/452157a. [DOI] [PubMed] [Google Scholar]
- Schwarz E.M., Korhonen P.K., Campbell B.E., Young N.D., Jex A.R., Jabbar A., Hall R.S., Mondal A., Howe A.C., Pell J., Hofmann A., Boag P.R., Zhu X.Q., Gregory T.R., Loukas A., Williams B.A., Antoshechkin I., Brown C.T., Sternberg P.W., Gasser R.B. The genome and developmental transcriptome of the strongylid nematode Haemonchus contortus. Genome Biol. 2013;14:R89. doi: 10.1186/gb-2013-14-8-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerville R.I. The development of Haemonchus contortus to the fourth stage in vitro. J. Parasitol. 1966;52:127–136. [PubMed] [Google Scholar]
- Song H., Liu Y., Xiong L., Li Y., Yang N., Wang Q. Design, synthesis, and insecticidal activity of novel pyrazole derivatives containing alpha-hydroxymethyl-N-benzyl carboxamide, alpha-chloromethyl-N-benzyl carboxamide, and 4,5-dihydrooxazole moieties. J. Agric. Food Chem. 2012;60:1470–1479. doi: 10.1021/jf204778v. [DOI] [PubMed] [Google Scholar]
- Song H., Liu Y., Xiong L., Li Y., Yang N., Wang Q. Design, synthesis, and insecticidal evaluation of new pyrazole derivatives containing imine, oxime ether, oxime ester, and dihydroisoxazoline groups based on the inhibitor binding pocket of respiratory complex I. J. Agric. Food Chem. 2013;61:8730–8736. doi: 10.1021/jf402719z. [DOI] [PubMed] [Google Scholar]
- Utzinger J., Becker S.L., Knopp S., Blum J., Neumayr A.L., Keiser J., Hatz C.F. Neglected tropical diseases: diagnosis, clinical management, treatment and control. Swiss Med. Wkly. 2012;142 doi: 10.4414/smw.2012.13727. w.13727. [DOI] [PubMed] [Google Scholar]
- Webster J.P., Molyneux D.H., Hotez P.J., Fenwick A. The contribution of mass drug administration to global health: past, present and future. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2014;369 doi: 10.1098/rstb.2013.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . - Investing to Overcome the Global Impact of Neglected Tropical Diseases. 2015. Third report on neglected tropical diseases (reference: WHO/HTM/NTD/2015.1http://www.who.int/neglected_diseases/9789241564861/en/ Geneva. ISBN: 9789241564861. Accessed 19 03 2016. [Google Scholar]
- Witschel M., Rottmann M., Kaiser M., Brun R. Agrochemicals against malaria, sleeping sickness, leishmaniasis and Chagas disease. PLoS Negl. Trop. Dis. 2012;6:e1805. doi: 10.1371/journal.pntd.0001805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Hikiji W., Takino M., Saka K., Hayashida M., Fukunaga T. Analysis of tolfenpyrad and its metabolites in plasma in a tolfenpyrad poisoning case. J. Anal. Toxicol. 2012;36:529–537. doi: 10.1093/jat/bks060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.