Abstract
Praziquantel (PZQ) is effectively the only drug currently available for treatment and control of schistosomiasis, a disease affecting hundreds of millions of people worldwide. Many anthelmintics, likely including PZQ, target ion channels, membrane protein complexes essential for normal functioning of the neuromusculature and other tissues. Despite this fact, only a few classes of parasitic helminth ion channels have been assessed for their pharmacological properties or for their roles in parasite physiology. One such overlooked group of ion channels is the transient receptor potential (TRP) channel superfamily. TRP channels share a common core structure, but are widely diverse in their activation mechanisms and ion selectivity. They are critical to transducing sensory signals, responding to a wide range of external stimuli. They are also involved in other functions, such as regulating intracellular calcium and organellar ion homeostasis and trafficking. Here, we review current literature on parasitic helminth TRP channels, focusing on those in schistosomes. We discuss the likely roles of these channels in sensory and locomotor activity, including the possible significance of a class of TRP channels (TRPV) that is absent in schistosomes. We also focus on evidence indicating that at least one schistosome TRP channel (SmTRPA) has atypical, TRPV1-like pharmacological sensitivities that could potentially be exploited for future therapeutic targeting.
Keywords: Schistosoma, Schistosomiasis, Ion channels, TRP channels, Capsaicin, TRPA1, TRPV1, Vanilloid receptor
Graphical abstract
Highlights
-
•
We provide an overview of transient receptor potential (TRP) channels in schistosomes and other parasitic helminths.
-
•
TRP channels are important for sensory signaling, ion homeostasis, organellar trafficking, and a host of other functions.
-
•
Very little work has been done on TRP channels in parasitic helminths.
-
•
TRPV channels, found throughout the Metazoa, appear not to be present in parasitic platyhelminths.
-
•
TRP channels in schistosomes appear to have atypical pharmacology, perhaps an entrée for therapeutic targeting.
1. Introduction
Trematode flatworms of the genus Schistosoma cause schistosomiasis, a neglected tropical parasitic disease affecting hundreds of millions globally (Colley et al., 2014, King and Dangerfield-Cha, 2008). Pathology in schistosome infections results mainly from immunopathological host responses to parasite egg deposition, with associated morbidity, impaired childhood development and adult productivity, higher susceptibility to other infectious agents such as HIV, and, in an estimated 280,000 people annually, death (Colley et al., 2014, Hotez and Fenwick, 2009, King, 2010, King and Dangerfield-Cha, 2008, Ndeffo Mbah et al., 2013, van der Werf et al., 2003).
In the absence of an effective vaccine, chemotherapeutic intervention remains the main strategy for managing and controlling the spread of schistosomiasis. Praziquantel (PZQ) is the current drug of choice (Danso-Appiah et al., 2013, Kramer et al., 2013), and is in effect the only antischistosomal treatment currently available (Hagan et al., 2004). Reliance on a single drug for a disease of such high prevalence is a dangerous situation, particularly in light of reports of field and experimentally-induced isolates exhibiting PZQ insusceptibility (reviewed by Day and Botros, 2006, Doenhoff and Pica-Mattoccia, 2006, Greenberg, 2013, Wang et al., 2012). Furthermore, immature schistosomes (2–4 weeks post infection) are largely refractory to PZQ, complicating treatment strategies and analysis of efficacy (Aragon et al., 2009, Pica-Mattoccia and Cioli, 2004, Sabah et al., 1986, Xiao et al., 1985). There is clearly an urgent need for new or repurposed therapeutics for schistosomiasis treatment and control.
A large proportion of current anthelmintic drugs, likely including PZQ (Greenberg, 2005), target ion channels of the parasite's neuromuscular system (Greenberg, 2014, Wolstenholme, 2011). However, the functional and pharmacological properties of only a few families of ion channels found in parasitic helminths have been investigated in any detail. One largely unexplored group of ion channels in schistosomes and other parasitic helminths is the transient receptor potential (TRP) channel superfamily. Here, we review the current state of knowledge regarding TRP channels in schistosomes and other platyhelminths, including recent studies that indicate that these channels are important regulators of neuromuscular activity in schistosomes, and also appear to exhibit atypical pharmacology which might be exploitable for therapeutic targeting. We also attempt to articulate some of the many open questions that are available for investigation in this nascent field.
2. TRP channels
TRP channels comprise a large superfamily of (typically non-selective) cation channels that display an extraordinary diversity of functions and activation mechanisms (Nilius and Szallasi, 2014, Venkatachalam and Montell, 2007). Indeed, a single TRP channel can be activated through different, seemingly unrelated, mechanisms. TRP channels were initially discovered and characterized in Drosophila, with later identification of 25–30 mammalian isoforms. Though the full array of physiological functions of these channels is only gradually becoming clear, one unifying theme appears to be their key role in responding to all major classes of external sensory stimuli (eg, light, chemicals, temperature, touch), in large part due to their role in modulating intracellular Ca2+ concentrations (Gees et al., 2010). TRP channels also provide individual cells the capability to sense changes in their environment (eg, alterations in osmolarity), and appear to be involved in the enhanced proliferation, aberrant differentiation, and resistance to apoptosis associated with uncontrolled tumor invasion (Santoni et al., 2011). TRP channels are also under intense investigation for their roles in organellar trafficking and autophagy (Venkatachalam et al., 2015), pain and inflammation (Bautista et al., 2013, Dai, 2015; Julius, 2013), cancer (Gautier et al., 2014, Liberati et al., 2013), metabolic diseases (Zhu et al., 2011), respiratory diseases (Grace et al., 2014), cardiovascular diseases (Yue et al., 2014), and other conditions (Brinkmeier, 2011, Kaneko and Szallasi, 2014, Nilius and Szallasi, 2014). In the free-living model nematode Caenorhabditis elegans, TRP channels mediate a wide range of functions (Venkatachalam et al., 2014, Xiao and Xu, 2011), including, among others, nicotine dependence, lifespan regulation, organelle biogenesis and trafficking, and, as expected, various modalities of sensory transduction.
Based on structural homology, TRP channels fall into 7 (or 8) subfamilies in metazoans (Peng et al., 2015, Venkatachalam and Montell, 2007):
-
A
TRPC (canonical) channels are most similar to Drosophila TRP, the founding member of the TRP superfamily. They are activated by the phospholipase C cascade, among other factors, may sense mechanical stretch, and possibly Ca2+ store depletion.
-
B
TRPV (vanilloid) channels are involved in thermoreception, taste, nociception, and response to inflammatory signaling (Kauer and Gibson, 2009, Vriens et al., 2009). There are several members of the sub-family, including TRPV1, which is found in the vertebrates. TRPV1 is the receptor for capsaicin, an active ingredient in chili peppers, and related compounds (Caterina et al., 1997).
-
C
TRPA (ANKTM) channels contain multiple N-terminal ankyrin domains and are thought to be gated by temperature and noxious mechanical stimuli (Zygmunt and Hogestatt, 2014). They are modulated by many compounds, including pungent electrophilic compounds such as allyl isothiocyanate (AITC; found in mustard oil). TRPA1 is the only mammalian TRPA channel.
Both TRPV1 and TRPA1 are activated by endogenous pro-inflammatory (and other) compounds (Bautista et al., 2013) and are often co-expressed in cells that respond to noxious and pro-inflammatory stimuli (Fernandes et al., 2012). High-resolution structures for both TRPV1 and TRPA have recently been reported (Cao et al., 2013, Liao et al., 2013, Paulsen et al., 2015).
-
D
TRPM (melastatin) channels transduce taste, osmotic swelling, temperature (cold perception), and other sensory stimuli. They respond to many chemical compounds, and there are several subtypes in mammals.
-
E
TRPML (mucolipin) channels are intracellular channels that function in endolysosomal vesicles (Gao et al., 2015, Venkatachalam et al., 2015). They are important for autophagy and nutrient (amino acid) utilization. TRPML channels are permeable to ions such as Fe2+ and Zn2+, and are likely involved in iron flux and organellar ion homeostasis. A loss-of-function mutation in one of the three human subtypes causes endolysosomal storage disease, mucolipidosis type IV, a childhood neurodegenerative disorder.
-
F
TRPP (polycystin) channels appear to be mechano- or proton sensors. They are conserved in vertebrates, invertebrates, and yeast. One human subtype is mutated in autosomal dominant polycystic kidney disease (ADPKD), a disorder in which both kidneys show age-dependent massive enlargement.
-
G
TRPN (NompC) channels appear to be mechanosensors and are not found in mammals or schistosomes.
-
H
TRPVL channels have been proposed as a separate TRP channel sub-family found only in cnidarians and polychaete worms (Peng et al., 2015).
For more details on the different classes and the members of those classes, see recent reviews (Nilius and Szallasi, 2014, Peng et al., 2015, Venkatachalam and Montell, 2007).
3. Schistosomes have several TRP channel genes, but none coding for TRPV channels
As noted above, TRP channels have been well characterized in the free-living nematode Caenorhabditis elegans and are implicated in many critical functions in that organism (Venkatachalam et al., 2014, Xiao and Xu, 2011). In contrast, until recently there were virtually no studies on this important group of ion channels in any parasitic helminths, including schistosomes.
TRP channels almost certainly play key roles in a variety of schistosome physiological functions. For example, it is reasonable to predict that TRP channels function in the transduction of the thermal and chemical signals necessary for host-finding in cercariae (McKerrow and Salter, 2002); indeed, another platyhelminth, the free-living planarian Dugesia japonica, appears to use a TRPM channel for thermo-signaling (Inoue et al., 2014). Thermosensory and chemosensory TRP channels may also play critical roles as schistosomes nearly instantaneously adapt to vastly different environmental temperatures and chemical milieus while transitioning from the aquatic environment into the mammalian (and snail) host. Once within the mammalian host, schistosomes also likely require TRP channel sensory and neuromuscular functions to help guide migration of schistosomula and juvenile worms to (and from) the lungs and eventually to their predilection site. In adult worms, TRP channels may be important for maintaining position within the blood vessels, male-female pairing and reproduction, and responses to host factors. TRP channels may also play critical roles in phototropism, host finding, and development in miracidia and snail-stage sporocysts. Normal functioning of the endolysosomal TRPML-like channel is likely to be essential for organellar trafficking, autophagy, nutrient acquisition, and perhaps neuronal development in schistosomes, as it is in other organisms (Gao et al., 2015, Venkatachalam et al., 2015), and may have a role to play in iron transport and utilization in mammalian-stage blood-feeding schistosomes, as in trypanosomes (Taylor et al., 2013).
Analysis of the original Schistosoma mansoni genome predicted representatives of the TRP channel family, with several within the TRPM and TRPC subfamilies, and one each within the TRPA, TRPP, and TRPML subfamilies (Prole and Taylor, 2011, Wolstenholme et al., 2011). We have further queried the revised S. mansoni genome (Protasio et al., 2012) for TRP channel homologs (Fig. 1), and have matched them with their most closely-related human TRP channel subfamily and subtype within that subfamily (eg, TRPC3 vs. TRPC5). Although such subtype comparisons should be treated as a first approximation, as subtype distinctions defined for mammalian ion channels can break down in phyla distant from the mammals (Jeziorski et al., 2000), they may provide some insight into the diversity of TRP channels in schistosomes.
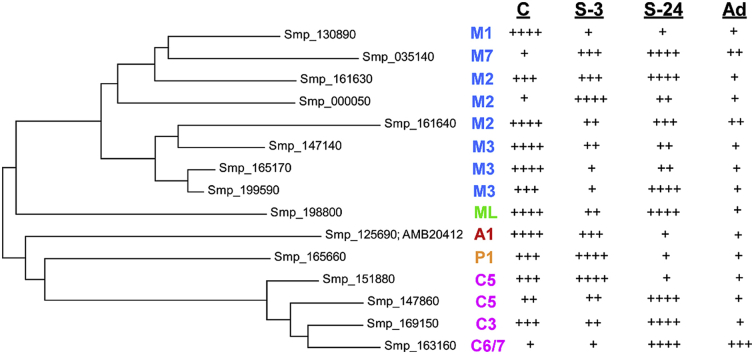
Fig. 1.
Families of TRP channel genes in S. mansoni. Maximum likelihood tree of predicted S. mansoni TRP channel protein sequences, shown with closest human TRP channel subtype (color coded by subfamily). Note the absence of predicted TRPV-like sequences. Relative expression levels (+, ++, +++, ++++) at different parasite stages are derived from transcriptomics data (Protasio et al., 2012) at http://www.genedb.org/Homepage/Smansoni as well as our own analysis using RT-PCR against S. mansoni RNA. C, cercariae; S-3, 3-h schistosomula; S-24, 24-h schistosomula; A, adults. Tree was derived using alignment and tree building software as implemented in MEGA6 (Tamura et al., 2013). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Confirming the published bioinformatics analyses (Prole and Taylor, 2011, Wolstenholme et al., 2011), we find on the order of 15 TRP channel-like genes in S. mansoni distributed amongst the TRPC, TRPM, TRPA, TRPP, and TRPML subfamilies. The most highly represented subfamily is TRPM, with 8 S. mansoni genes, followed by the TRPC subfamily (4 genes). The TRPA, TRPP, and TRPML subfamilies are each represented by a single member in S. mansoni. We find that a similar set of TRP channels also appears in the Schistosoma haematobium and Schistosoma japonicum genomes (not shown). Gene knockout studies of the mammalian orthologues of these schistosome TRP channels produce interesting phenotypes, including sensory and other defects (Wu et al., 2010). Similar phenotypes in schistosomes could have devastating effects on essential sensory signaling and completion of the parasite life cycle. Furthermore, agents that agonize or allosterically potentiate these channels could dysregulate channel activity and also prove highly deleterious to the worms (indeed, most anthelmintics that act on channels activate them inappropriately). Interestingly, there is also precedence for the same TRP channel orthologue from different species exhibiting differing sensitivities to sensory input or pharmacological agents. For example, TRPV3 is activated by heat in mice, but cold in frogs (Saito et al., 2011), and caffeine activates mouse TRPA1, but suppresses human TRPA1 (Nagatomo and Kubo, 2008).
Fig. 1 also depicts relative expression levels of the predicted S. mansoni TRP channels at different stages of the life cycle, based on normalized RNAseq data at the GeneDB website (Protasio et al., 2012). These varying levels of expression suggest that different TRP channels may play distinctive roles at different life cycle stages. Interestingly, the highest relative expression of most schistosome TRP channels occurs in cercariae (C) or schistosomula (S-3, S-24), with reduced expression in adults, perhaps indicating that these larval stages, in which host finding, temperature adaptation, and migration are critical, are particularly dependent on TRP channel function. In addition, using RT-PCR, we find that essentially all of these sequences are also expressed in adult worms. For example, the RNAseq data at GeneDB for SmTRPA (Smp_125690; AMB20412), a TRPA1-like channel from S. mansoni, suggests that it is not expressed in adult worms (Protasio et al., 2012). However, as described (Bais et al., 2015), we can detect SmTRPA RNA expression in adults using qRT-PCR (and can knock it down with siRNA).
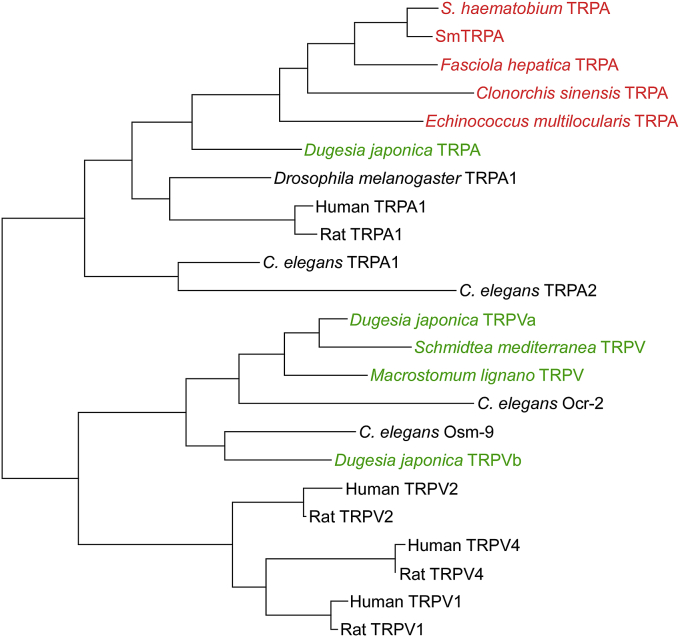
Notably, there appear to be no representatives of the TRPV subfamily in any schistosome genomes, a striking absence given that TRPV channels are found in animals ranging from cnidarians to mammals (Peng et al., 2015). Indeed, as illustrated in Fig. 2, we find no TRPV representatives in the available genomes of any endoparasitic trematodes or cestodes we have examined (at http://parasite.wormbase.org). In contrast, we do find TRPV homologs in the free-living planarians Schmidtea mediterranea,, Dugesia japonica (Inoue et al., 2014), and Macrostomum lignano (Fig. 2), highlighting potentially differential sensory modalities required for a free-living vs. parasitic lifestyle. Thus, parasitic platyhelminths may not require many of the sensory functions associated with TRPV channels, and may have consequently lost all members of the TRPV sub-family as an adaptation to parasitism; those remaining sensory and other functions that would normally be mediated by TRPV channels may be fulfilled instead by TRP channels from other subfamilies. Recent work from our laboratory (Bais et al., 2015) indicates that a TRPA1-like channel in S. mansoni (SmTRPA) may be mediating responses of the parasite to activators and antagonists of mammalian TRPV1 (as well as to TRPA1 modulators).
Fig. 2.
Phylogenetic relationships of TRPV- and TRPA-like sequences from platyhelminths and other organisms. Maximum likelihood analysis on the conserved ion transport domains of a subset of TRPA- and TRPV-like sequences from various organisms, with an emphasis on platyhelminths. Sequences in red are from parasitic platyhelminths; those in green are from free-living platyhelminths. Note the absence of TRPV-like sequences in the parasitic platyhelminths, but several examples in the free-living flatworms. Sequences used (plus accession numbers) are: S. haematobium TRPA (A_03331); SmTRPA (AMB20412); Fasciola hepatica TRPA (wormbase: BN1106_s1338B000219.mRNA-1); Clonorchis sinensis TRPA (GAA49883); Echinococcus multilocularis TRPA (EmuJ_000226200); Dugesia japonica TRPA (BAP91037); Drosophila melanogaster TRPA1 (Q7Z020); Human TRPA1 (O75762); Rat TRPA1 (NP_997491); C. elegans TRPA1 (Q18297); C. elegans TRPA2 (NP_492031); Dugesia japonica TRPVa (BAP40096); Schmidtea mediterranea TRPV (wormbase: mk4.000540.12); Macrostomum lignano TRPV (wormbase: maker-uti_cns_0006599-snap-gene-0.2-mRNA-1); C. elegans Ocr-2 (CCD63561); C. elegans Osm-9 (AAB87064); Dugesia japonica TRPVb (BAP40097); Human TRPV2 (AAH51305); Rat TRPV2 (Q9WUD2); Human TRPV4 (Q9HBA0); Rat TRPV4 (Q9ERZ8); Human TRPV1 (NP_061197); Rat TRPV1 (O35433). Analysis was performed using MEGA6 (Tamura et al., 2013). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Capsaicin and other TRPV1 channel modulators affect schistosome locomotor activity
We have found that modulators of several different TRP channels have effects on locomotor activity in schistosomes. One on which we have focused is capsaicin, an active ingredient in hot peppers, and an activator of TRPV1 (Caterina et al., 1997). TRPV1, also known as the vanilloid receptor (VR), is sensitive to heat (>43 °C), pH, and endogenous and exogenous inflammatory agents, among other factors. Many TRPV1 channels are also activated with high potency by capsaicin and related compounds; indeed, this sensitivity to capsaicin led to the initial discovery of TRPV1 (Caterina et al., 1997). Capsaicin and related compounds show selectivity for TRPV1; other members of the TRPV channel subfamily are not activated by capsaicin (Caterina et al., 1999, Vriens et al., 2009). Furthermore, TRPV1 channels from different species can exhibit large differences in capsaicin sensitivity. Thus, rabbit TRPV1 is ∼100-fold less sensitive to capsaicin than rat or human TRPV1, and avian (chicken) TRPV1 is largely insensitive to capsaicin; these sensitivity differences localize to a few amino acids in the S3 and S4 domains of the TRPV1 sequence (Cao et al., 2013, Chou et al., 2004, Gavva et al., 2004, Jordt and Julius, 2002, Phillips et al., 2004).
Genes for single or multiple TRPV-like channels have been found in many invertebrates, but capsaicin-sensitive TRPV1 channels are found only in the vertebrates (Venkatachalam and Montell, 2007). Nonetheless, a few scattered reports indicate that invertebrates can exhibit sensitivity to capsaicin. These include mollusks and leeches, which show cellular activation and avoidance behaviors in response to relatively high concentrations (>100 μM) of capsaicin (Erdelyi and Such, 1986, Hernadi et al., 1995, Kalil-Gaspar et al., 2007, Summers et al., 2014). Micromolar concentrations of capsaicin and capsaicin-like compounds (N-vanillylnonanamide, N-benzoylmonoethanolamine benzoate) also inhibit substrate attachment of a mollusk (the zebra mussel Dreissena polymorpha) by its byssus, a proteinaceous adhesive apparatus (Angarano et al., 2007). Such byssal attachment of this invasive species is responsible for fouling of aquatic man-made structures. C. elegans does not appear to avoid capsaicin itself, though there is evidence that thermal avoidance behavior may be enhanced by the compound (Wittenburg and Baumeister, 1999), and flies (Drosophila) have been reported to show a positive preference for capsaicin (Al-Anzi et al., 2006).
There are few studies of the effects of capsaicin on schistosomes or other platyhelminths. Capsaicin and the TRPM8 agonist icilin were tested against the free-living planarian Dugesia dorotocephala, and although icilin elicited increased locomotor activity, there was no detectable response to 10 μM capsaicin (Rawls et al., 2007). An earlier study on Dugesia (Baguna et al., 1989) showed that 10−6 M capsaicin stimulated cell division, while 100-fold higher concentrations (10−4 M) appeared to kill the worms. Finally, oil extracts of the leaves and fruit of the Brazilian pepper Capsicum annuum, which likely contain capsaicin, were shown to kill 90–96% of S. mansoni cercariae within 15 min (Frischkorn et al., 1978). However, an oil extract of the marigold pepper Piper marginatum, which is not a chili pepper (genus Capsicum), and presumably does not contain capsaicin (Barceloux, 2009), had a similar effect on cercariae, suggesting that the killing of cercariae by these extracts may be independent of capsaicin.
We have found that capsaicin has powerful effects on schistosome locomotor activity (Bais et al., 2015). Concentrations of capsaicin ≥10 μM, similar to those used for testing on intact mice (Caterina et al., 2000), dramatically stimulate motility in both S. mansoni schistosomula and adults, a surprising finding given the absence of TRPV (and TRPV1) channel genes in this organism. Motility of both male and female adult worms in culture increases 5–10-fold (as measured by changes in pixel gray scale levels in video recordings) in 10 μM capsaicin, and 25–40-fold in 60 μM capsaicin. Resiniferatoxin (RTX), a highly potent plant toxin that activates TRPV1 by binding to the vanilloid binding site (Cao et al., 2013), also increases adult worm motility 5–20-fold at concentrations of 3–10 μM RTX. A selective TRPV1 antagonist (SB 366791) blocks the stimulatory effects of capsaicin on S. mansoni locomotor activity. Thus, in schistosomes, an organism lacking genes for TRPV-like channels, stimulation of motility by TRPV1 activators exhibits TRPV1-like pharmacology.
Another important effect of capsaicin on adult schistosomes is to disrupt worm pairing. In capsaicin, male-female worm pairs separate rapidly, perhaps indicative of a nociceptive response. This finding is significant, because adult schistosomes within the mammalian host reside within the circulatory system in copula, as male-female pairs, and that pairing is essential for normal female development, maturation, and reproduction (LoVerde et al., 2010). Disrupted pairing could therefore have implications for worm survival and maturation, disease transmission, and pathology, since parasite eggs are responsible for the major pathophysiological effects of schistosomiasis.
Capsaicin also affects motility of S. mansoni cercariae, but in a manner seemingly different than in adults and schistosomula. Cercariae exposed to capsaicin initially, but briefly, appear to become hyperactive, then subsequently enter a phase in which they remain in a single spot, continuing to move, but more in the pulsatile fashion of schistosomula than in the free-swimming manner of cercariae (see S2, S3 videos in Bais et al., 2015). The cercariae do not lose their tails, as they do in PZQ (Andrews, 1978), and seem to retain the capacity to swim about, but appear to exhibit “uncertainty” about where to go, almost as if they are receiving contradictory sensory signals (though clearly that interpretation will need to be tested). This response, which appears to differ from the sustained hyperactivity seen with adults and schistosomula, may reflect either the presence of a different repertoire of channels and receptors expressed by cercariae, or the triggering by capsaicin of different downstream pathways that produce distinct outputs at the different stages. Alternatively, both types of responses may in fact simply reflect different manifestations of either a high level of sensory “confusion” elicited by inappropriate and excessive activation of sensory inputs by capsaicin, or a nociceptive response.
5. The effects of capsaicin on worm motility are dependent on expression of SmTRPA, a S. mansoni TRPA1-like channel
As discussed, the S. mansoni genome predicts no TRPV homologs, which raises the question of how the effects of capsaicin and other selective TRPV1 modulators are being transduced in these parasites. The answer could have important implications for understanding schistosome physiology and possible development of novel therapeutics. There are at least three possibilities: 1) The effects of capsaicin on worm motility could represent a nonspecific response to the compound, though that would not explain the TRPV1-like pharmacological sensitivities we observe; 2) Schistosomes may express a novel capsaicin receptor that is unrelated to TRP channels (eg, a G protein-coupled receptor). Such a novel receptor would be an exciting finding in itself and could possibly lead to compounds that selectively target this parasite receptor. It also does not exclude the participation of a TRP channel acting as a receptor-operated channel; 3) Capsaicin and other TRPV1 modulators could be interacting directly with a non-TRPV-like TRP channel. This third alternative could, like the second mechanism, have implications for selective drug targeting, and might also provide new insights into TRP channel structure-function relationships and evolutionary links.
In order to test this third possibility, we initiated RNAi knockdown experiments to determine if a schistosome TRP channel might be required for the worm's response to capsaicin. We focused on SmTRPA, the only TRPA1-like gene found in the S. mansoni genome (though we tested other schistosome TRP channels as well). We postulated that SmTRPA would be an appropriate candidate, even though mammalian TRPA1 channels are not activated by capsaicin (Shintaku et al., 2012, Story et al., 2003). TRPA and TRPV channels are frequently found co-expressed, often have overlapping functions, can functionally and physically interact with one another, and are thought to be closely related phylogenetically (reviewed by Fernandes et al., 2012, Nilius et al., 2012, Peng et al., 2015, Venkatachalam et al., 2014).
Our knockdown experiments showed that suppressing SmTRPA expression almost entirely eliminated the effects of capsaicin on locomotor activity in both male and female adult worms (Bais et al., 2015). This result indicates that schistosome sensitivity to TRPV1 ligands is SmTRPA-dependent. Whether the role of SmTRPA in capsaicin sensitivity is as an ionotropic channel, or as another part of a pathway remains to be determined. However, knockdown of SmTRPA does not significantly reduce the well-characterized (Hillman and Senft, 1973, Mellin et al., 1983, Ribeiro and Geary, 2010) serotonin-induced stimulation of locomotor activity (Bais et al., 2015), indicating that knockdown does not generally compromise the parasite neuromuscular system or its ability to respond to stimulation and that the role played by SmTRPA in capsaicin sensitivity is relatively specific.
In addition to affecting worm responses to capsaicin, suppression of SmTRPA expression also attenuates stimulation of motor activity by the TRPA1 activator AITC (Bais et al., 2015). The most parsimonious explanation of these results is that SmTRPA exhibits mixed TRPV1/TRPA1-like pharmacology. On the other hand, our data are also consistent with a model in which SmTRPA, though required for the action of both TRPV1 and TRPA1 modulators on worm activity, is not the primary receptor, but is instead one essential component of a pathway linked to worm myoactivity, perhaps as a receptor-operated channel. If so, that leaves open the question of the identity of the schistosome receptor(s) responding to these compounds, as well as the individual constituents of that pathway.
6. Conclusions and future questions
Considering that TRP channels, because of their diverse and critical functions, are the subject of intense scrutiny in other organisms, and that parasite ion channels are validated therapeutic targets, the paucity of research on these channels in parasitic helminths is somewhat surprising. On the other hand, the current situation leaves the door wide open for unanticipated and potentially significant new findings. Indeed, our evidence suggesting novel pharmacology of the S. mansoni TRPA1 channel was unexpected, but, if confirmed by heterologous expression and functional studies, could have important implications for development of new antischistosomal therapeutics, and for illuminating phylogenetic relationships of TRP channels. If SmTRPA is indeed found to be responding directly to TRPV1 ligands, determining the structural correlates of that activity could provide new clues about how a TRPA1 channel with mixed TRPA1/TRPV1 pharmacology functions, and how it might be selectively targeted. As pointed out (Bais et al., 2015), residues thought to be critical for constituting the vanilloid receptor site of TRPV1 are not well conserved in SmTRPA.
There are several compounds that have been shown to act on TRP channels that also have deleterious effects on schistosomes or other platyhelminths (killing, paralysis, hyperactivity), or that appear to be upregulated by the host in response to schistosome infection (Table 1). Some of these agents are already approved for clinical use and could potentially be repurposed for treatment of parasite infections or used as starting scaffolds for new antischistosomals that selectively target schistosome TRP channels. Interestingly, most of the compounds listed in Table 1 have other, better characterized targets, but have more recently been shown also to modulate TRP channels, some with very high affinity. For example, clotrimazole is a widely-used antifungal drug that inhibits cytochrome P-450 enzymes. It has recently been shown to have relatively potent anti-schistosomal activity (Ziniel et al., 2015). However, clotrimazole also acts on a variety of mammalian TRP channels, and is in fact one of the most potent inhibitors of human TRPM8, a cold and menthol receptor, with a reported IC50 of 200 nM (Meseguer et al., 2008).
Table 1.
Selected TRP channel modulators with schistosome (or other platyhelminth)-related effects.
| Compound | Vertebrate TRP channel activity | Schistosome-related effects | References |
|---|---|---|---|
| Capsaicin | ↑ TRPV1 |
S. mansoni: Adults and schistosomula: increased locomotor activity in vitro Cercariae: inhibition of swimming |
Bais et al. (2015) |
| Resiniferatoxin (RTX) | ↑ TRPV1 | Increased locomotor activity in S. mansoni adults | Bais et al. (2015) |
| SB 366791 | ↓ TRPV1 | Blocks capsaicin effects on locomotor activity in S. mansoni adults | Bais et al. (2015) |
| Anandamide | ↑ TRPV1 | Increased host expression in S. japonicum-infected mice | Liu et al. (2009) |
| Linalool | ↑ TRPV3 | Cercaricidal (S. japonicum) | Yang et al. (2014) |
| Arachidonic acid (indirect, via metabolites) | ↑ TRPV4 | Lethal to all intramammalian S. mansoni, worm stages, S. haematobium adults | El Ridi et al. (2010) |
| Camphor | ↑ TRPV1 ↑ TRPV3 |
Possibly cercaricidal (S. mansoni) | Nevine et al. (2016) |
| Ruthenium red | ↓ TRPVs | Inhibits S. mansoni egg hatching | Katsumata et al. (1988) |
| Allicin | ↑ TRPV1 ↑ TRPA1 |
Motility changes, tegumental damage in S. mansoni adults | Lima et al. (2011) |
| Bradykinin | ↑ TRPV1 ↑ TRPA1 (indirect, via GPCR) |
Potent attractant for S. mansoni skin-stage schistosomula; substrate for degradation by S. mansoni tegumental peptidase |
Grabe and Haas (2004) Fajtova et al. (2015) |
| Clotrimazole | ↑ TRPV1 ↑ TRPA1 ↓ TRPM2 ↓ TRPM8 |
Killing of S. mansoni schistosomula and adults in vitro | Ziniel et al. (2015) |
| AITC | ↑ TRPA1 | Increased locomotor activity in S. mansoni adults | Bais et al. (2015) |
| Auronofin | ↑ TRPA1 | Lethal to schistosomes in vitro, reduces worm, egg burden in vivo; lethal to cestodes in vitro |
Kuntz et al. (2007) Song et al. (2012) Bonilla et al. (2008) Martinez-Gonzalez et al. (2010) |
| Apomorphine | ↑ or ↓ TRPA1 (concentration-dependent) | Lengthening of S. mansoni adults and impaired contractility in schistosomula Disrupted regenerative polarity in free-living D. japonica |
Mellin et al. (1983) Chan et al. (2014) |
| Chlorpromazine | ↑ or ↓ TRPA1 (V-dependent) ↓ TRPC5 |
S. mansoni: Sporocysts: increased motility Schistosomula: overactive Adults: transiently overactive; altered carbohydrate metabolism |
Boyle et al. (2000) Harder et al. (1987) Abdulla et al. (2009) |
| Nifedipine | ↑ TRPM3 |
S. mansoni: Schistosomula: reduced viability Adults: impaired motility, tegumental lesions, intense contractility |
Silva-Moraes et al. (2013) |
| Genistein | ↑ TRPC5 | Impairment of miracidia-to-sporocyst transition, S. mansoni | Walker and Rollinson (2008) |
Note that most of these compounds act on non TRP channel targets as well. ↑, activation or sensitization; ↓, inhibition. List of selected TRP channel modulators and targets adapted from Fernandez-Carvajal et al., 2015, Schaefer, 2014, Alexander et al., 2015.
Obviously, several questions remain regarding the properties and biological roles of TRP channels in schistosomes and other parasitic platyhelminths. For example, although we have some idea of the expression patterns of the different S. mansoni TRP channel RNAs at different stages of the life cycle, we do not know the specific cells and tissues in which these channels are preferentially expressed. Nor do we know the repertoire of TRP channels expressed in individual cells and tissues, how they might interact, nor what signaling and gene expression pathways are impacted by their activation. We do not know if any other schistosome TRP channels also display atypical pharmacological sensitivities, though we have some suggestive preliminary evidence of unusual responses of worms to TRP channel ligands. We do not know which sensory signals are transduced by different TRP channels or whether or how TRP channel diversity increases due to expression of splice variants that may serve distinct functions. Assays of motility are useful, but do not have the resolution required to properly dissect TRP channel sensory functions such as chemotaxis and thermotaxis; adaptation of published assays and development of new ones will be required to better establish the roles of these channels in sensory function. Furthermore, why do schistosomes and other parasitic platyhelminths lack TRPV channels, and do other schistosome TRP channels such as SmTRPA serve some of the functions (as well as exhibiting the pharmacology) normally associated with TRPV channels?
In addition to exogenous TRP modulators, there are several endogenous agents that modulate mammalian TRP channels (Palazzo et al., 2013). Many of these compounds target TRPV1 and TRPA1, activating them either directly or through second messenger pathways. Can these endogenous TRP channel activators influence schistosome migratory, evasive, and other behaviors through the parasite's TRP channels? For example, anandamide, a host endocannabinoid TRPV1 activator, is upregulated in response to schistosome infection and associated with schistosome-induced liver fibrosis (Table 1; Liu et al., 2009); it will be interesting to determine whether this compound has effects on schistosomes that are mediated by SmTRPA (or another schistosome TRP channel). Several pro-inflammatory compounds also act on mammalian TRP channels (Julius, 2013, Palazzo et al., 2013, Radresa et al., 2013), both directly (eg, 4-hydroxynoneal on TRPA1, leukotriene B4 on TRPV1) and indirectly (eg, bradykinin for both TRPA1 and TRPV1). Host inflammation and its regulation are important components of an environment that is permissive for schistosome development and maturation (Riner et al., 2013). Do schistosomes respond to these compounds through their TRP channels, and does interference with such signaling have consequences for the parasite?
In addition to the TRP channels associated with sensory signaling, the (presumably) intracellular TRPML-like channel may also be important for schistosome survival and development. In other organisms, TRPML channels are critical for endolysosomal trafficking, as well as autophagy, nutrient acquisition, and iron utilization (Gao et al., 2015, Venkatachalam et al., 2015). Are similar functions associated with the S. mansoni TRPML channel? Can this channel be selectively targeted to disrupt some of these essential functions?
TRP channels have already been validated in other organisms as critical components of several physiological functions. Further studies on this key ion channel family in parasitic helminths are likely to provide further surprises, and may lead to novel therapeutics.
Acknowledgments
Much of the work summarized in this review was supported by National Institutes of Health grant R21 AI100505 to RMG.
References
- Abdulla M.-H., Ruelas D.S., Wolff B., Snedecor J., Lim K.-C., Xu F., Renslo A.R., Williams J., McKerrow J.H., Caffrey C.R. Drug discovery for schistosomiasis: hit and lead compounds identified in a library of known drugs by medium-throughput phenotypic screening. PLoS Negl. Trop. Dis. 2009;3:e478. doi: 10.1371/journal.pntd.0000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Anzi B., Tracey W.D., Benzer S. Response of Drosophila to wasabi is mediated by painless, the fly homolog of mammalian TRPA1/ANKTM1. Curr. Biol. 2006;16:1034–1040. doi: 10.1016/j.cub.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Alexander S.P., Catterall W.A., Kelly E., Marrion N., Peters J.A., Benson H.E., Faccenda E., Pawson A.J., Sharman J.L., Southan C., Davies J.A., CGTP Collaborators The concise guide to PHARMACOLOGY 2015/16: voltage-gated ion channels. Br. J. Pharmacol. 2015;172:5904–5941. doi: 10.1111/bph.13349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. Effect of praziquantel on the free living stages of Schistosoma mansoni. Z. Parasitenkd. 1978;56:99–106. doi: 10.1007/BF00925943. [DOI] [PubMed] [Google Scholar]
- Angarano M.B., McMahon R.F., Hawkins D.L., Schetz J.A. Exploration of structure-antifouling relationships of capsaicin-like compounds that inhibit zebra mussel (Dreissena polymorpha) macrofouling. Biofouling. 2007;23:295–305. doi: 10.1080/08927010701371439. [DOI] [PubMed] [Google Scholar]
- Aragon A.D., Imani R.A., Blackburn V.R., Cupit P.M., Melman S.D., Goronga T., Webb T., Loker E.S., Cunningham C. Towards an understanding of the mechanism of action of praziquantel. Mol. Biochem. Parasitol. 2009;164:57–65. doi: 10.1016/j.molbiopara.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguna J., Salo E., Romero R. Effects of activators and antagonists of the neuropeptides substance P and substance K on cell proliferation in planarians. Int. J. Dev. Biol. 1989;33:261–266. [PubMed] [Google Scholar]
- Bais S., Churgin M.A., Fang-Yen C., Greenberg R.M. Evidence for novel pharmacological sensitivities of transient receptor potential (TRP) channels in Schistosoma mansoni. PLoS Negl. Trop. Dis. 2015;9:e0004295. doi: 10.1371/journal.pntd.0004295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barceloux D.G. Pepper and capsaicin (Capsicum and Piper species) Dis. Mon. 2009;55:380–390. doi: 10.1016/j.disamonth.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Bautista D.M., Pellegrino M., Tsunozaki M. TRPA1: a gatekeeper for inflammation. Annu. Rev. Physiol. 2013;75:181–200. doi: 10.1146/annurev-physiol-030212-183811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla M., Denicola A., Novoselov S.V., Turanov A.A., Protasio A., Izmendi D., Gladyshev V.N., Salinas G. Platyhelminth mitochondrial and cytosolic redox homeostasis is controlled by a single thioredoxin glutathione reductase and dependent on selenium and glutathione. J. Biol. Chem. 2008;283:17898–17907. doi: 10.1074/jbc.M710609200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J.P., Zaide J.V., Yoshino T.P. Schistosoma mansoni: effects of serotonin and serotonin receptor antagonists on motility and length of primary sporocysts in vitro. Exp. Parasitol. 2000;94:217–226. doi: 10.1006/expr.2000.4500. [DOI] [PubMed] [Google Scholar]
- Brinkmeier H. TRP channels in skeletal muscle: gene expression, function and implications for disease. Adv. Exp. Med. Biol. 2011;704:749–758. doi: 10.1007/978-94-007-0265-3_39. [DOI] [PubMed] [Google Scholar]
- Cao E., Liao M., Cheng Y., Julius D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature. 2013;504:113–118. doi: 10.1038/nature12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina M.J., Leffler A., Malmberg A.B., Martin W.J., Trafton J., Petersen-Zeitz K.R., Koltzenburg M., Basbaum A.I., Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Caterina M.J., Rosen T.A., Tominaga M., Brake A.J., Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chan J.D., Agbedanu P.N., Zamamian M., Gruba S.M., Haynes C.L., Day T.A., Marchant J.S. “Death and axes”: unexpected Ca2+ entry phenologs predict new anti-schistosomal agents. PLoS Pathog. 2014;10:e1003942. doi: 10.1371/journal.ppat.1003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou M.Z., Mtui T., Gao Y.D., Kohler M., Middleton R.E. Resiniferatoxin binds to the capsaicin receptor (TRPV1) near the extracellular side of the S4 transmembrane domain. Biochemistry. 2004;43:2501–2511. doi: 10.1021/bi035981h. [DOI] [PubMed] [Google Scholar]
- Colley D.G., Bustinduy A.L., Secor W.E., King C.H. Human schistosomiasis. Lancet. 2014;383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y. TRPs and pain. Semin. Immunopathol. 2015;38:277–291. doi: 10.1007/s00281-015-0526-0. [DOI] [PubMed] [Google Scholar]
- Danso-Appiah A., Olliaro P.L., Donegan S., Sinclair D., Utzinger J. Drugs for treating Schistosoma mansoni infection. Cochrane Database Syst. Rev. 2013;2013:CD000528. doi: 10.1002/14651858.CD000528.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day T.A., Botros S. Drug resistance in schistosomes. In: Maule A., Marks N.J., editors. Parasitic Flatworms: Molecular Biology, Biochemistry, Immunology and Physiology. CAB International; Oxfordshire, UK: 2006. pp. 256–268. [Google Scholar]
- Doenhoff M.J., Pica-Mattoccia L. Praziquantel for the treatment of schistosomiasis: its use for control in areas with endemic disease and prospects for drug resistance. Expert Rev. Anti infect. Ther. 2006;4:199–210. doi: 10.1586/14787210.4.2.199. [DOI] [PubMed] [Google Scholar]
- El Ridi R., Aboueldahab M., Tallima H., Salah M., Mahana N., Fawzi S., Mohamed S.H., Fahmy O.M. In vitro and in vivo activities of arachidonic acid against Schistosoma mansoni and Schistosoma haematobium. Antimicrob. Agents Chemother. 2010;54:3383–3389. doi: 10.1128/AAC.00173-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdelyi L., Such G. Effects of capsaicin on molluscan neurons: a voltage clamp study. Comp. Biochem. Physiol. C. 1986;85:319–327. doi: 10.1016/0742-8413(86)90201-x. [DOI] [PubMed] [Google Scholar]
- Fajtova P., Stefanic S., Hradilek M., Dvorak J., Vondrasek J., Jikova A., Ulrychova L., McKerrow J.H., Caffrey C.R., Mares M., Horn M. Prolyl oligopeptidase from the blood fluke Schistosoma mansoni: from functional analysis to anti-schistosomal inhibitors. PLoS Negl. Trop. Dis. 2015;9:e0003827. doi: 10.1371/journal.pntd.0003827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes E.S., Fernandes M.A., Keeble J.E. The functions of TRPA1 and TRPV1: moving away from sensory nerves. Br. J. Pharmacol. 2012;166:510–521. doi: 10.1111/j.1476-5381.2012.01851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Carvajal A., Fernandez-Ballester G., Gonzalez-Muniz R., Ferrer-Montiel A. Pharmacology of TRP channels. In: Madrid R., Bacigalupo J., editors. TRP Channels in Sensory Transduction. Springer; Switzerland: 2015. pp. 41–71. [Google Scholar]
- Frischkorn C.G., Frischkorn H.E., Carrazonni E. Cercaricidal activity of some essential oils of plants from Brazil. Die Naturwiss. 1978;65:480–483. doi: 10.1007/BF00702834. [DOI] [PubMed] [Google Scholar]
- Gao Q., Zhang X., Xu H. TRPML channels. In: Zheng J., Trudeau M.C., editors. Handbook of Ion Channels. CRC Press; Boca Raton FL: 2015. pp. 453–462. [Google Scholar]
- Gautier M., Dhennin-Duthille I., Ay A.S., Rybarczyk P., Korichneva I., Quadid-Ahidouch H. New insights into pharmacological tools to TR(i)P cancer up. Br. J. Pharmacol. 2014;171:2582–2592. doi: 10.1111/bph.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavva N.R., Klionsky L., Qu Y., Shi L., Tamir R., Edenson S., Zhang T.J., Viswanadhan V.N., Toth A., Pearce L.V., Vanderah T.W., Porreca F., Blumberg P.M., Lile J., Sun Y., Wild K., Louis J.C., Treanor J.J. Molecular determinants of vanilloid sensitivity in TRPV1. J. Biol. Chem. 2004;279:20283–20295. doi: 10.1074/jbc.M312577200. [DOI] [PubMed] [Google Scholar]
- Gees M., Colsoul B., Nilius B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb. Perspect. Biol. 2010;2:a003962. doi: 10.1101/cshperspect.a003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabe K., Haas W. Navigation within host tissues: Schistosoma mansoni and Trichobilharzia ocellata schistosomula respond to chemical gradients. Int. J. Parasitol. 2004;34:927–934. doi: 10.1016/j.ijpara.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Grace M.S., Baxter M., Dubuis E., Birrell M.A., Belvisi M.G. Transient receptor potential (TRP) channels in the airway: role in airway disease. Br. J. Pharmacol. 2014;171:2593–2607. doi: 10.1111/bph.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg R.M. Are Ca2+ channels targets of praziquantel action? Int. J. Parasitol. 2005;35:1–9. doi: 10.1016/j.ijpara.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Greenberg R.M. New approaches for understanding mechanisms of drug resistance in schistosomes. Parasitology. 2013;140:1534–1546. doi: 10.1017/S0031182013000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg R.M. Ion channels and drug transporters as targets for anthelmintics. Curr. Clin. Microbiol. Rep. 2014;1:51–60. doi: 10.1007/s40588-014-0007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan P., Appleton C.C., Coles G.C., Kusel J.R., Tchuem-Tchuente L.A. Schistosomiasis control: keep taking the tablets. Trends Parasitol. 2004;20:92–97. doi: 10.1016/j.pt.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Harder A., Andrews P., Thomas H. Chlorpromazine, other amphiphilic cationic drugs and praziquantel: effects on carbohydrate metabolism of Schistosoma mansoni. Parasitol. Res. 1987;73:245–249. doi: 10.1007/BF00578512. [DOI] [PubMed] [Google Scholar]
- Hernadi L., Erdelyi L., Parducz A., Szabadi H., Such G., Jansco G. In vitro capsaicin-induced cytological changes and alteration in calcium distribution in giant serotonergic neurons of the snail Helix pomatia: a light- and electron-microscopic study. Cell Tissue Res. 1995;282:445–453. doi: 10.1007/BF00318876. [DOI] [PubMed] [Google Scholar]
- Hillman G.R., Senft A.W. Schistosome motility measurements: response to drugs. J. Pharmacol. Exp. Ther. 1973;185:177–184. [PubMed] [Google Scholar]
- Hotez P.J., Fenwick A. Schistosomiasis in Africa: an emerging tragedy in our new global health decade. PLoS Negl. Trop. Dis. 2009;3:e485. doi: 10.1371/journal.pntd.0000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T., Yamashita T., Agata K. Thermosensory signaling by TRPM is processed by brain serotonergic neurons to produce planarian thermotaxis. J. Neurosci. 2014;34:15701–15714. doi: 10.1523/JNEUROSCI.5379-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeziorski M.C., Greenberg R.M., Anderson P.A. The molecular biology of invertebrate voltage-gated Ca2+ channels. J. Exp. Biol. 2000;203:841–856. doi: 10.1242/jeb.203.5.841. [DOI] [PubMed] [Google Scholar]
- Jordt S.E., Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- Julius D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- Kalil-Gaspar P., Marcuzzo S., Ribon P., Molina C.G., Achaval M. Capsaicin-induced avoidance behavior in the terrestrial Gastropoda Megalobulimus abbreviatus: evidence for TRPV-1 signaling and opioid modulation in response to chemical noxious stimuli. Comp. Biochem. Physiol. A. 2007;148:286–291. doi: 10.1016/j.cbpa.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Kaneko Y., Szallasi A. Transient receptor potential (TRP) channels: a clinical perspective. Br. J. Pharmacol. 2014;171:2474–2507. doi: 10.1111/bph.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumata T., Shimada M., Sato K., Aoki Y. Possible involvement of calcium ions in the hatching of Schistosoma mansoni eggs in water. J. Parasitol. 1988;74:1040–1041. [PubMed] [Google Scholar]
- Kauer J.A., Gibson H.E. Hot flash: TRPV channels in the brain. Trends Neurosci. 2009;32:215–224. doi: 10.1016/j.tins.2008.12.006. [DOI] [PubMed] [Google Scholar]
- King C.H. Parasites and poverty: the case of schistosomiasis. Acta Trop. 2010;113:95–104. doi: 10.1016/j.actatropica.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C.H., Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic Illn. 2008;4:65–79. doi: 10.1177/1742395307084407. [DOI] [PubMed] [Google Scholar]
- Kramer C.V., Zhang F., Sinclair D., Olliaro P.L. Drugs for treating urinary schistosomiasis. Cochrane Db Syst. Rev. 2013;2013:CD000053. doi: 10.1002/14651858.CD000053.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz A.N., Davioud-Charvet E., Sayed A.A., Califf L.L., Dessolin J., Arner E.S., Williams D.L. Thioredoxin glutathione reductase from Schistosoma mansoni: an essential parasite enzyme and a key drug target. PLoS Med. 2007;4:e206. doi: 10.1371/journal.pmed.0040206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M., Cao E., Julius D., Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504:107–112. doi: 10.1038/nature12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati S., Morelli M.B., Nabissi M., Santoni M., Santoni G. Oncogenic and anti-oncogenic effects of transient receptor potential channels. Curr. Top. Med. Chem. 2013;13:344–366. doi: 10.2174/1568026611313030011. [DOI] [PubMed] [Google Scholar]
- Lima C.M., Freitas F.I., Morais L.C., Cavalcanti M.G., Silva L.S., Padilha R.J., Barbosa C.G., Santos F.A., Alves L.C., Diniz Mde F. Ultrastructural study on the morphological changes to male worms of Schistosoma mansoni after in vitro exposure to allicin. Rev. Soc. Bras. Med. Trop. 2011;44:327–330. doi: 10.1590/s0037-86822011005000023. [DOI] [PubMed] [Google Scholar]
- Liu H., Gao X., Duan R., Yang Q., Zhang Y., Cheng Y., Guo Y., Tang W. Endocannabinoids anandamide and its cannabinoid receptors in liver fibrosis after murine schistosomiasis. J. Huazhong Univ. Sci. Technol. Med. Sci. 2009;29:182–186. doi: 10.1007/s11596-009-0209-y. [DOI] [PubMed] [Google Scholar]
- LoVerde P.T., Andrade L.F., Oliveira G. Signal transduction regulates schistosome reproductive biology. Curr. Opin. Microbiol. 2010;12:422–428. doi: 10.1016/j.mib.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Gonzalez J.J., Guevara-Flores A., Alvarez G., Rendon-Gomez J.L., Del Arenal I.P. In vitro killing action of auranofin on Taenia crassiceps metacestode (cysticerci) and inactivation of thioredoxin-glutathione reductase (TGR) Parasitol. Res. 2010;107:227–231. doi: 10.1007/s00436-010-1867-1. [DOI] [PubMed] [Google Scholar]
- McKerrow J.H., Salter J. Invasion of skin by Schistosoma cercariae. Trends Parasitol. 2002;18:193–195. doi: 10.1016/s1471-4922(02)02309-7. [DOI] [PubMed] [Google Scholar]
- Mellin T.N., Busch R.D., Wang C.C., Kath G. Neuropharmacology of the parasitic trematode, Schistosoma mansoni. Am. J. Trop. Med. Hyg. 1983;32:83–93. doi: 10.4269/ajtmh.1983.32.83. [DOI] [PubMed] [Google Scholar]
- Meseguer V., Karashima Y., Talavera K., D'Hoedt D., Donovan-Rodriguez T., Viana F., Nilius B., Voets T. Transient receptor potential channels in sensory neurons are targets of the antimycotic agent clotrimazole. J. Neurosci. 2008;28:576–586. doi: 10.1523/JNEUROSCI.4772-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatomo K., Kubo Y. Caffeine activates mouse TRPA1 channels but suppresses human TRPA1 channels. Proc. Natl. Acad. Sci. 2008;105:17373–17378. doi: 10.1073/pnas.0809769105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndeffo Mbah M.L., Poolman E.M., Atkins K.E., Orenstein E.W., Meyers L.A., Townsend J.P., Galvani A.P. Potential cost-effectiveness of schistosomiasis treatment for reducing HIV transmission in Africa – the case of Zimbabwean women. PLoS Negl. Trop. Dis. 2013;7:e2346. doi: 10.1371/journal.pntd.0002346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevine N.G., Mahmoud S., Metwally M., Sabri H., Zalat R. Potential effect of camphor oil as a local cercaricidal agent. Glob. Adv. Res. J. Educ. Res. Rev. 2016;5:32–37. [Google Scholar]
- Nilius B., Appendino G., Owsianik G. The transient receptor potential channel TRPA1: from gene to pathophysiology. Pflug. Arch. 2012;464:425–458. doi: 10.1007/s00424-012-1158-z. [DOI] [PubMed] [Google Scholar]
- Nilius B., Szallasi A. Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine. Pharmacol. Rev. 2014;66:676–814. doi: 10.1124/pr.113.008268. [DOI] [PubMed] [Google Scholar]
- Palazzo E., Rossi F., de Novellis V., Maione S. Endogenous modulators of TRP channels. Curr. Top. Med. Chem. 2013;13:398–407. doi: 10.2174/1568026611313030014. [DOI] [PubMed] [Google Scholar]
- Paulsen C.E., Armache J.P., Gao Y., Cheng Y., Julius D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature. 2015;520:511–517. doi: 10.1038/nature14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng G., Shi X., Kadowaki T. Evolution of TRP channels inferred by their classification in diverse animal species. Mol. Phylogenet. Evol. 2015;84:145–157. doi: 10.1016/j.ympev.2014.06.016. [DOI] [PubMed] [Google Scholar]
- Phillips E., Reeve A., Bevan S., McIntyre P. Identification of species-specific determinants of the action of the antagonist capsazepine and the agonist PPAHV on TRPV1. J. Biol. Chem. 2004;279:17165–17172. doi: 10.1074/jbc.M313328200. [DOI] [PubMed] [Google Scholar]
- Pica-Mattoccia L., Cioli D. Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int. J. Parasitol. 2004;34:527–533. doi: 10.1016/j.ijpara.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Prole D.L., Taylor C.W. Identification of intracellular and plasma membrane calcium channel homologues in pathogenic parasites. PLoS One. 2011;6:e26218. doi: 10.1371/journal.pone.0026218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protasio A.V., Tsai I.J., Babbage A., Nichol S., Hunt M., Aslett M.A., De Silva N., Velarde G.S., Anderson T.J., Clark R.C., Davidson C., Dillon G.P., Holroyd N.E., LoVerde P.T., Lloyd C., McQuillan J., Oliveira G., Otto T.D., Parker-Manuel S.J., Quail M.A., Wilson R.A., Zerlotini A., Dunne D.W., Berriman M. A systematically improved high quality genome and transcriptome of the human blood fluke Schistosoma mansoni. PLoS Negl. Trop. Dis. 2012;6:e1455. doi: 10.1371/journal.pntd.0001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radresa O., Pare M., Albert J.S. Multiple roles of transient receptor potential (TRP) channels in inflammatory conditions and current status of drug development. Curr. Top. Med. Chem. 2013;13:367–385. doi: 10.2174/1568026611313030012. [DOI] [PubMed] [Google Scholar]
- Rawls S.M., Gomez T., Ding Z., Raffa R.B. Differential behavioral effect of the TRPM8/TRPA1 channel agonist icilin (AG-3-5) Eur. J. Pharmacol. 2007;575:103–104. doi: 10.1016/j.ejphar.2007.07.060. [DOI] [PubMed] [Google Scholar]
- Ribeiro P., Geary T.G. Neuronal signaling in schistosomes: current status and prospects for postgenomics. Can. J. Zool. 2010;88:1–22. [Google Scholar]
- Riner D.K., Ferragine C.E., Maynard S.K., Davies S.J. Regulation of innate responses during pre-patent schistosome infection provides an immune environment permissive for parasite development. PLoS Pathog. 2013;9:e1003708. doi: 10.1371/journal.ppat.1003708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabah A.A., Fletcher C., Webbe G., Doenhoff M.J. Schistosoma mansoni: chemotherapy of infections of different ages. Exp. Parasitol. 1986;61:294–303. doi: 10.1016/0014-4894(86)90184-0. [DOI] [PubMed] [Google Scholar]
- Saito S., Fukuta N., Shingai R., Tominaga M. Evolution of vertebrate transient receptor potential vanilloid 3 channels: opposite temperature sensitivity between mammals and western clawed frogs. PLoS Genet. 2011;7:e1002041. doi: 10.1371/journal.pgen.1002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoni G., Farfariello V., Amantini C. TRPV channels in tumor growth and progression. Adv. Exp. Med. Biol. 2011;704:947–967. doi: 10.1007/978-94-007-0265-3_49. [DOI] [PubMed] [Google Scholar]
- Schaefer M. TRPs: modulation by drug-like compounds. Handb. Exp. Pharmacol. 2014;223:1077–1106. doi: 10.1007/978-3-319-05161-1_15. [DOI] [PubMed] [Google Scholar]
- Shintaku K., Uchida K., Suzuki Y., Zhou Y., Fushiki T., Wantanabe T., Yazawa S., Tominaga M. Activation of transient receptor potential A1 by a non-pungent capsaicin-like compound, capsiate. Br. J. Pharmacol. 2012;165:1476–1486. doi: 10.1111/j.1476-5381.2011.01634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Moraes V., Couto F.F., Vasconcelos M.M., Araujo N., Coelho P.M., Katz N., Grenfell R.F. Antischistosomal activity of a calcium channel antagonist on schistosomula and adult Schistosoma mansoni worms. Mem. Inst. Oswaldo Cruz. 2013;108:600–604. doi: 10.1590/0074-0276108052013011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L., Li J., Xie S., Qian C., Wang J., Zhang W., Yin X., Hua Z., Yu C. Thioredoxin glutathione reductase as a novel drug target: evidence from Schistosoma japonicum. PLoS One. 2012;7:e31456. doi: 10.1371/journal.pone.0031456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story G.M., Peier A.M., Reeve A.J., Eid S.R., Mosbacher J., Hricik T.R., Earley T.J., Hergarden A.C., Andersson D.A., Hwang S.W., McIntyre P., Jegla T., Bevan S., Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Summers T., Holec S., Burrell B.D. Physiological and behavioral evidence of a capsaicin-sensitive TRPV-like channel in the medicinal leech. J. Exp. Biol. 2014;217:4167–4173. doi: 10.1242/jeb.110049. [DOI] [PubMed] [Google Scholar]
- Taylor M.C., McLatchie A.P., Kelly J.M. Evidence that transport of iron from the lysosome to the cytosol in African trypanosomes is mediated by a mucolipin orthologue. Mol. Microbiol. 2013;89:420–432. doi: 10.1111/mmi.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werf M.J., de Vlas S.J., Brooker S., Looman C.W., Nagelkerke N.J., Habbema J.D., Engels D. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86:125–139. doi: 10.1016/s0001-706x(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K., Luo J., Montell C. Evolutionarily conserved, multitasking TRP channels: lessons from worms and flies. Handb. Exp. Pharmacol. 2014;223:937–962. doi: 10.1007/978-3-319-05161-1_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K., Montell M. TRP channels. Annu. Rev. Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K., Wong C.O., Zhu M.X. The role of TRPMLs in endolysosomal trafficking and function. Cell Calcium. 2015;58:48–56. doi: 10.1016/j.ceca.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriens J., Appendino G., Nilius B. Pharmacology of vanilloid transient receptor potential cation channels. Mol. Phamacol. 2009;75:1262–1279. doi: 10.1124/mol.109.055624. [DOI] [PubMed] [Google Scholar]
- Wang W., Wang L., Liang Y.S. Susceptibility or resistance of praziquantel in human schistosomiasis: a review. Parasitol. Res. 2012;111:1871–1877. doi: 10.1007/s00436-012-3151-z. [DOI] [PubMed] [Google Scholar]
- Walker A.J., Rollinson D. Specific tyrosine phosphorylation induced in Schistosoma mansoni miracidia by haemolymph from schistosome susceptible, but not resistant, Biomphalaria glabrata. Parasitology. 2008;135:337–345. doi: 10.1017/S0031182007003964. [DOI] [PubMed] [Google Scholar]
- Wittenburg N., Baumeister R. Thermal avoidance in Caenorhabditis elegans: an approach to the study of nociception. Proc. Natl. Acad. Sci. 1999;96:10477–10482. doi: 10.1073/pnas.96.18.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme A. Ion channels and receptor as targets for the control of parasitic nematodes. Int. J. Parasitol. Drugs Drug Resist. 2011;1:2–13. doi: 10.1016/j.ijpddr.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme A.J., Williamson S.M., Reaves B.J. TRP channels in parasites. Adv. Exp. Med. Biol. 2011;704:358–371. doi: 10.1007/978-94-007-0265-3_20. [DOI] [PubMed] [Google Scholar]
- Wu L.J., Sweet T.B., Clapham D.E. International Union of Basic and Clinical Pharmacology LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol. Rev. 2010;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R., Xu X.Z. C. elegans TRP channels. Adv. Exp. Med. Biol. 2011;704:323–339. doi: 10.1007/978-94-007-0265-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S.H., Catto B.A., Webster L.T., Jr. Effects of praziquantel on different developmental stages of Schistosoma mansoni in vitro and in vivo. J. Infect. Dis. 1985;151:1130–1137. doi: 10.1093/infdis/151.6.1130. [DOI] [PubMed] [Google Scholar]
- Yang F., Long E., Wen J., Cao L., Zhu C., Hu H., Ruan Y., Okanurak K., Hu H., Wei X., Yang X., Zhang L., Wang X., Ji P., Zheng H., Wu Z., Lv Z. Linalool, derived from Cinnamomum camphora (L.) Presl leaf extracts, possesses molluscicidal activity against Oncomelania hupensis and inhibits infection of Schistosoma japonicum. Parasites Vectors. 2014;7:407. doi: 10.1186/1756-3305-7-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z., Xie J., Yu A.S., Stock J., Du J., Yue L. Role of TRP channels in the cardiovascular system. Am. J. Physiol. Heart Circ. Physiol. 2014;308:H157–H182. doi: 10.1152/ajpheart.00457.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Luo Z., Ma S., Liu D. TRP channels and their implications in metabolic diseases. Pflug. Arch. 2011;46:211–223. doi: 10.1007/s00424-010-0902-5. [DOI] [PubMed] [Google Scholar]
- Ziniel P.D., Karumundi B., Barnard A.H., Fisher E.M., Thatcher G.R., Podust L.M., Williams D.L. The Schistosoma mansoni cytochrome P450 (CYP3050A1) is essential for worm survival and egg development. PLoS Negl. Trop. Dis. 2015;29:e0004279. doi: 10.1371/journal.pntd.0004279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt P.M., Hogestatt E.D. TRPA1. Handb. Exp. Pharmacol. 2014;222:583–630. doi: 10.1007/978-3-642-54215-2_23. [DOI] [PubMed] [Google Scholar]