Abstract
The diversity and uniqueness of flatworm G protein coupled receptors (GPCRs) provides impetus for identifying ligands useful as tools for studying flatworm biology, or as therapeutics for treating diseases caused by parasitic flatworm infections. To catalyse this discovery process, technologies optimized for mammalian GPCR high throughput screening need be transposed for screening flatworm GPCRs. Here, we demonstrate the utility of a genetically encoded cAMP biosensor for resolving the properties of an abundantly expressed planarian serotonergic GPCR (S7.1R). Application of this methodology resolved the real time kinetics of GPCR modulation by ligands and demonstrated a marked difference in the kinetic action of antagonists at S7.1R. Notably, bromocriptine caused a protracted inhibition of S7.1R activity in vitro and a protracted paralysis of planarian movement, replicating the effect of S7.1R in vivo RNAi. The lengthy inhibition of function caused by bromocriptine at this abundantly expressed GPCR provides a useful tool to ablate serotonergic signaling in vivo, and is a noteworthy feature for exploitation as an anthelmintic vulnerability.
Keywords: 5-HT, Flatworm, cAMP, Ergot alkaloid, Praziquantel
Graphical abstract
Highlights
-
•
Application of a real time cAMP biosensor to study a planarian serotonergic GPCR.
-
•
The biosensor reveals differential kinetics of 5-HT GPCR inhibition by antagonists.
-
•
Bromocriptine causes a persistent signaling inhibition and paralysis of intact worms.
-
•
Bromocriptine action akin to a ‘pharmacological knockout’ of receptor function.
1. Introduction
Parasitic flatworm infections cause a variety of diseases in humans and livestock. Schistosomiasis, a disease which infects over 200 million people worldwide, is the most crippling parasitic worm infection in terms of health and economic impact. Chronic schistosome infections damage internal organs with clinical outcomes spanning gastrointestinal and liver pathologies, genitourinary disease, anemia, undernutrition, retarded pediatric growth and development and a heightened risk for comorbidities (Colley et al., 2014). Cestode infections also present a healthcare concern: a prime example being Taenia solium infections that progress to central nervous system involvement and neurocysticercosis, a leading course of acquired epilepsy in the developing world. Beyond human disease, parasitic flatworm infections of sheep, cattle and fish cause significant agricultural impact. Consequently, it is important that anthelmintic medications continue to be efficacious, and supported by a discovery pipeline harboring novel ligands to anticipate the potential emergence of drug resistance associated with existing treatments.
In this regard, sequencing data has demonstrated the existence of a broad portfolio of G protein coupled receptors in flatworms (∼500 in Schmidtea mediterranea, ∼100 in Schistosoma mansoni, estimates, ∼60 in E. multilocularis (Zamanian et al., 2011, Tsai et al., 2013, Saberi et al., 2016)), the biology and ligand specificities of which are largely unexplored. These GPCRs represent attractive targets for drug design given the precedence for GPCR modulators predominating the human disease pharmacopeia, where a major proportion of marketed drugs are direct ligands, or modulators, of GPCR evoked signals (Roth and Kroeze, 2015). The structural divergence of flatworm GPCR sequences, enhanced by the existence of flatworm-specific clades, highlights the potential for discovering novel GPCR ligands that modulate flatworm biology, and potentially act as novel therapeutics that disrupt parasite GPCR signaling.
To accelerate the discovery of flatworm selective GPCR ligands, it will be necessary to apply high throughput screening (HTS) approaches against flatworm GPCRs. This will require transposition of the same high throughput, scalable reporter technologies that have catalyzed drug development for human GPCRs. Of particular utility are genetically encoded biosensors of second messenger activity, designed to resolve GPCR activity in real time within intact cells. These probes enable resolution of the kinetic modulation of GPCR function over time from a single sample, allowing flexibility in assay design and throughput relative to fixed endpoint methods in broken cell preparations (e.g. radioimmunoassays), and possess sufficient sensitivity to resolve different classes of GPCR ligands. Such genetically-encoded sensors are available for Ca2+ (Kotlikoff, 2007) and cAMP (Fan et al., 2008, Binkowski et al., 2011b), as well as a further toolbox of probes for directly monitoring GPCR function (Clister et al., 2015). However, these approaches have yet to be widely adopted to profile flatworm GPCRs (Chan et al., 2016b).
Here we demonstrate the use of a genetically encoded cAMP biosensor to resolve the properties and ligand binding specificity of different flatworm GPCRs. First, we exploit the real time kinetic resolution of this technology to demonstrate an unusually protracted inhibition of signaling at an abundant planarian serotonergic GPCR elicited by the ergot alkaloid bromocriptine. This behavior likely contributes to the protracted paralysis of intact planarian worms exposed to bromocriptine, and represents an intriguing and exploitable aspect of receptor phenomenology for anthelmintic drug design. Second, in the companion paper (Chan et al., 2016a), we demonstrate the utility of this technology for characterizing the interaction of a group of structurally related aporphine ligands with a schistosome serotonergic GPCR (Sm.5HTRL). Collectively, both studies evidence the capacity to characterize flatworm GPCR properties with a reporter technology compatible with HTS campaigns.
2. Materials and methods
2.1. Chemicals
Drugs for GPCR assays and planarian mobility experiments were obtained from Sigma Aldrich: bromocriptine (B2134), cyproheptadine (C3280000), serotonin (H9523), praziquantel (P4668), mianserin (M2525) and 3-Isobutyl-1-methylxanthine (IBMX, I5879).
2.2. Cell culture and cAMP assays
Low passage (∼5–25) HEK293 cells (ATCC CRL-1573.3) were cultured in growth medium (DMEM, 10% heat inactivated fetal bovine serum, penicillin (100 units/ml), streptomycin (100 μg/ml), and L-glutamine (290 μg/ml)). For GPCR functional assays, adherent HEK293 cells cultured in growth medium without penicillin and streptomycin were transiently transfected (Lipofectamine 2000, Thermo Fischer) at 80% confluence approximately 16 h after seeding in T-25 cell-culture flasks. Transfections consisted of a human codon optimized S7.1R construct (Dugesia japonica S7.1R, Dj-S7.1R, GenBank accession number AB004540.1) subcloned into the pCS2(−) mammalian expression vector and the pGloSensor™-22F cAMP construct (Promega, Cat. #E2301) delivered at a 1:1 ratio. One day after transfection, cells were trypsinized and plated in 96 well, solid white plates (Corning, cat # 3917) in DMEM supplemented with 1% heat inactivated dialyzed FBS (Gibco). The following day, media was replaced with 100 μL/well of assay buffer (HBSS supplemented with 0.1% BSA, 20 mM HEPES (pH 7.4) and GloSensor™ reagent). Plates were equilibrated at room temperature for two hours prior to luminescence assays. Luminescence detection was measured on a GloMax®-Multi Detection System plate reader (Promega). All assays were performed in the presence of IBMX (200 μM). Ligands were added at a concentration of 20x per well (i.e. 5 μL drug stock to 100 μL media/well). Assays for long lasting effects of antagonists on S7.1R were performed on cellular suspensions. Transfections were performed as described above, scaled to 80% confluent T-75 cell culture flasks. The following day, cells were trypsinized, centrifuged (300 RCF/5 min) and resuspended in 10 mL DMEM supplemented with 1% heat inactivated dialyzed FBS. Following treatment with test compounds (1hr), cells were centrifuged (300 RCF/5 min) and the media was exchanged for DMEM. Re-suspension and centrifugation were repeated washing a total of three times. After the last wash, cells were resuspended in the assay buffer described above and gently rotated to prevent settling over the course of the equilibration period. Cells were then plated in 96 well, solid white plates (100μL/well) in order to assay 5-HT responsiveness.
2.3. Planarian mobility assays
A clonal line of Dugesia japonica (GI strain) was used for these experiments. Planarian husbandry was performed as described previously (Chan and Marchant, 2011) with worms maintained at room temperature and fed weekly. For mobility experiments, worms were transferred to a glass watchglass (50 mm diameter, Fisher Scientific) centered over a LED backlit light (Edmund Optics, #83-873). Behavior was recorded using an immobilized digital video camera (Canon VIXIA HF R400). For ease of evaluation, experiments are presented as minimal intensity projections to provide a qualitative visual readout of worm motion over the timecourse of the entire experiment. Between recordings, worms were returned to petri dishes. For experiments examining the onset of paralysis, filming started immediately after exposure of worms to drug-containing solution in the glass watchglass. Movement videos were processed using custom written algorithms in Ctrax to track the motility of individual worms (Branson et al., 2009). Motion was scored by quantifying total distance travelled (mm) over the fixed recording interval and averaged for the 10 worms in each assay. Errors in tracking were corrected using the Fix Errors MATLAB Toolbox and descriptive statistics were computed using scripts in the Behavioral Microarray MATLAB Toolbox and custom written algorithms in MATLAB.
2.4. Planarian RNAi
Knockdown of Dugesia japonica S7.1R was performed by a feeding protocol described previously (Chan and Marchant, 2011, Chan et al., 2015). Briefly, a region corresponding to 46-1353bp of the S7.1R coding sequence was subcloned into the pDONR RNAi vector and transformed into HT115(DE3) RNase deficient bacteria, permitting IPTG induced expression of dsRNA against the target gene product. Bacterial pellets mixed in a slurry of chicken liver homogenate were fed to cohorts of approximately 100 worms three times over the course of one week, followed by a cycle of enforced regeneration (amputation of head and tail fragments). This feeding and regeneration protocol was repeated, and one week after amputation regenerated animals were assayed for movement phenotypes.
3. Results
3.1. Functionality of a heterologous expressed planarian 5-HT GPCR resolved using a cAMP biosensor
Free living planarian flatworms can be a tractable model for predicting drug efficacies and identifying novel druggable targets in parasitic helminths (Chan et al., 2014). For example, the ability to combine genetic (in vivo RNAi) and pharmacological manipulations in this system has provided insight into the pathways engaged by the anthelmintic praziquantel (PZQ) in this model (Nogi et al., 2009, Zhang et al., 2011, Chan et al., 2014), as well as the identification of ligands that phenocopy PZQ action which may augur anthelmintic efficacy (Chan et al., 2014, Chan et al., 2015). Serotonergic G protein coupled receptors (GPCRs) are one such class of targets, highlighting the importance of investigating their pharmacological signatures and roles in flatworm biology.
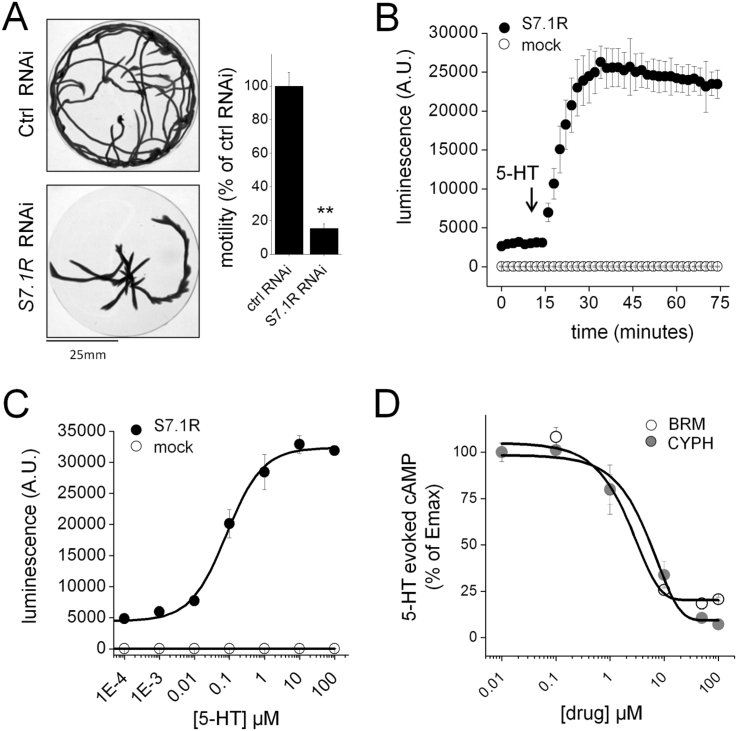
Transcriptomic profiling of the planarian Dugesia japonica has revealed as many as 17 predicted serotonergic GPCRs distributed within three groupings (S1, S4 and S7; (Chan et al., 2015)) defined through homology with Caenorhabditis elegans serotonin receptors (SER1, SER4 & SER7; (Komuniecki et al., 2004, Zamanian et al., 2011)). The most abundantly expressed 5-HT receptor (by FPKM values) was named S7.1R (equivalent to 5HTLpla4 (Saitoh et al., 1997)) and shown to be neuronally expressed (Fraguas et al., 2012). In vivo RNAi experiments revealed S7.1R to regulate planarian mobility and regenerative polarity (Chan et al., 2015). For example, knockdown of S7.1R resulted in a >80% decrease in the distance travelled by individual worms over a 2 min recording period (Fig. 1A). Pharmacological characterization of this receptor and resolution of the effects of S7.1R ligands on neuromuscular function and planarian regeneration is therefore of interest.
Fig. 1.
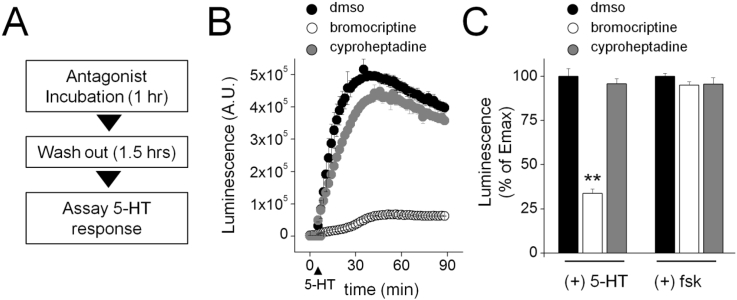
Heterologous expression of a planarian 5-HT receptor. (A) Comparison of mobility of cohorts of D. japonica flatworms with either a control gene or S7.1R targeted by in vivo RNAi. Left – minimal intensity overlay of images from a 2 min recording of respective RNAi cohorts (10 worms) within a watchglass. Right - Quantification of movement for 3 cohorts of 10 worms for each RNAi condition. Data analysed from (Chan et al., 2015). P value, ** = p < 0.01. (B) Kinetics of 5-HT (1 μM) evoked cAMP generation in HEK293 cells transfected with S7.1R and a cAMP dependent luciferase (black circles) or a cAMP-dependent luciferase alone (white circles). (C) Dose response relationship of effects of 5-HT concentration on peak luminescence amplitude in cells transfected with S7.1R and a cAMP dependent luciferase (black circles) or a cAMP-dependent luciferase alone (white circles). (D) 5-HT responsiveness (1 μM) of 7.1R expressing cells co-treated with bromocriptine (BRM, white circles) or cyproheptadine (CYPH, grey circles). Data are representative of n ≥ 3 assays, and represent mean ± s.d (B,C,D),or mean ± s.e.m. (A).
To enable such analyses, we heterologously expressed a codon-optimized version of S7.1R in HEK293 cells, together with a genetically encoded biosensor for cAMP. The cAMP biosensor used functions as a luminescence reporter. The probe reports changes in intracellular cAMP through changes in luminescence emission consequent to binding of cAMP to a cAMP-binding domain inserted within the recombinant firefly luciferase (Binkowski et al., 2011a, Binkowski et al., 2011b). Luminescence can therefore be resolved over time in intact cells, enabling kinetic profiling of responses to ligands, an improvement over single (‘endpoint’) measurements from broken cells. The rationale for this approach was based upon the prior demonstration of coupling of the Dugesia tigrina S7.1R homologue (Dt.Ser1, (Zamanian et al., 2012)) and the schistosome S7.1R homologue (Sm.5HTR) to the cAMP signaling pathway (Patocka et al., 2014, Chan et al., 2016b).
Addition of 5-HT to HEK293 cells expressing the biosensor construct failed to change luminescence levels, while addition of 5-HT to HEK293 cells co-expressing S7.1R evoked a robust increase in luminescence (Fig. 1B). Quantification of the amplitude of the 5-HT evoked signal over a range of 5-HT concentrations yielded a dose-response relationship with an EC50 = 82 ± 5 nM, compared with a lack of response from HEK293 cells transfected with the biosensor alone (Fig. 1C). Addition of agonist caused a ∼6–10-fold change in luminescence levels, a smaller range than observed with Sm.5HTR (Chan et al., 2016b), owing to a higher basal level of activity in cells expressing S7.1R. This basal activity likely relates to a low level of constitutive coupling to Gs in cells expressing S7.1R.
The effect of preincubation with serotonergic blockers was then examined. Preincubation with bromocriptine, an ergot alkaloid that inhibits flatworm movement (Chan et al., 2014, Chan et al., 2015) blocked 5-HT evoked signals (Fig. 1D). Similarly cyproheptadine, a known antagonist of 5-HT responses in flatworms (Mellin et al., 1983, Willcockson and Hillman, 1984, Patocka et al., 2014) also caused a dose-dependent inhibition of 5-HT evoked cAMP generation. These experiments demonstrated both these compounds behaved as S7.1 antagonists with similar IC50s (1.8 ± 0.5 μM for bromocriptine, 4.3 ± 0.6 μM for cyproheptadine).
3.2. Comparison of the effects of bromocriptine and cyproheptadine on intact worms
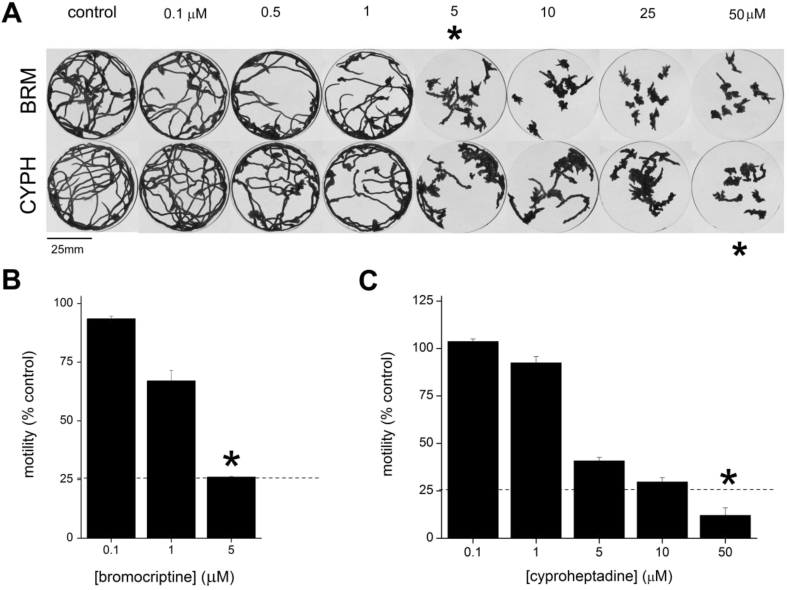
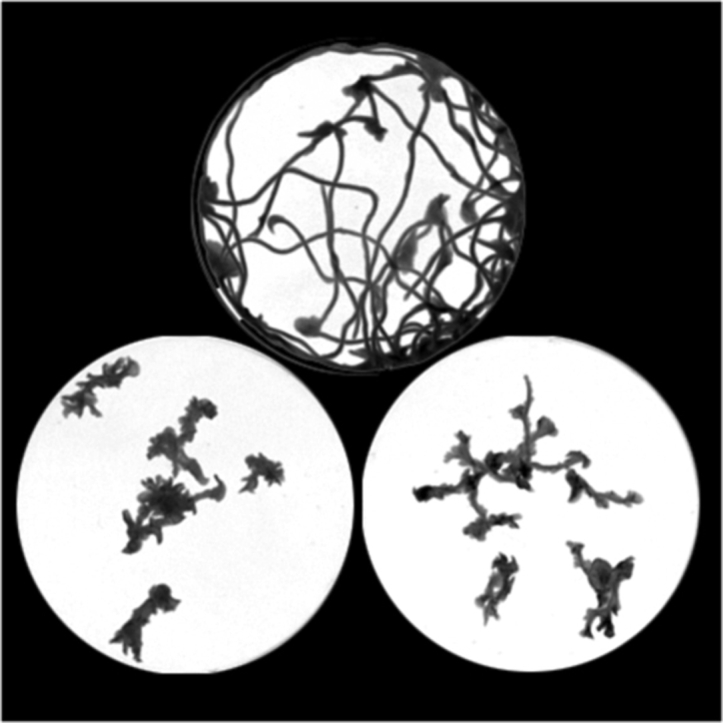
The effects of both S7.1R antagonists were then studied against intact planarians. Exposure of worms to either bromocriptine or cyproheptadine resulted in a dose-dependent inhibition of planarian motility (Fig. 2A). To compare the inhibitory profile of these drugs, movement of individual worms was tracked at various doses of either antagonist. Following image processing, doses were selected at equivalent points on the dose-inhibition curve corresponding to a concentration that caused ≥75% inhibition of worm movement (Fig. 2B&C). This level of inhibition was selected as a dose which caused individual worms to remain localized in the watchglass, such that worm trajectories rarely intersected (asterisks, Fig. 2A). For bromocriptine the selected dose was 5 μM, and for cyproheptadine the selected dose was 50 μM, a higher concentration to effect a similar level of paralysis.
Fig. 2.
Bromocriptine and cyproheptadine impair D. japonica movement. (A) Dose dependent inhibition of planarian movement following exposure (20min) to the indicated doses of bromocriptine (BRM, top), or cyproheptadine (CYPH, bottom). (B&C) Quantification of worm mobility in (B) bromocriptine or (C) cyproheptadine treated samples relative to controls. Data are representative of n ≥ 3 assays, and represent mean ± s.e.m.
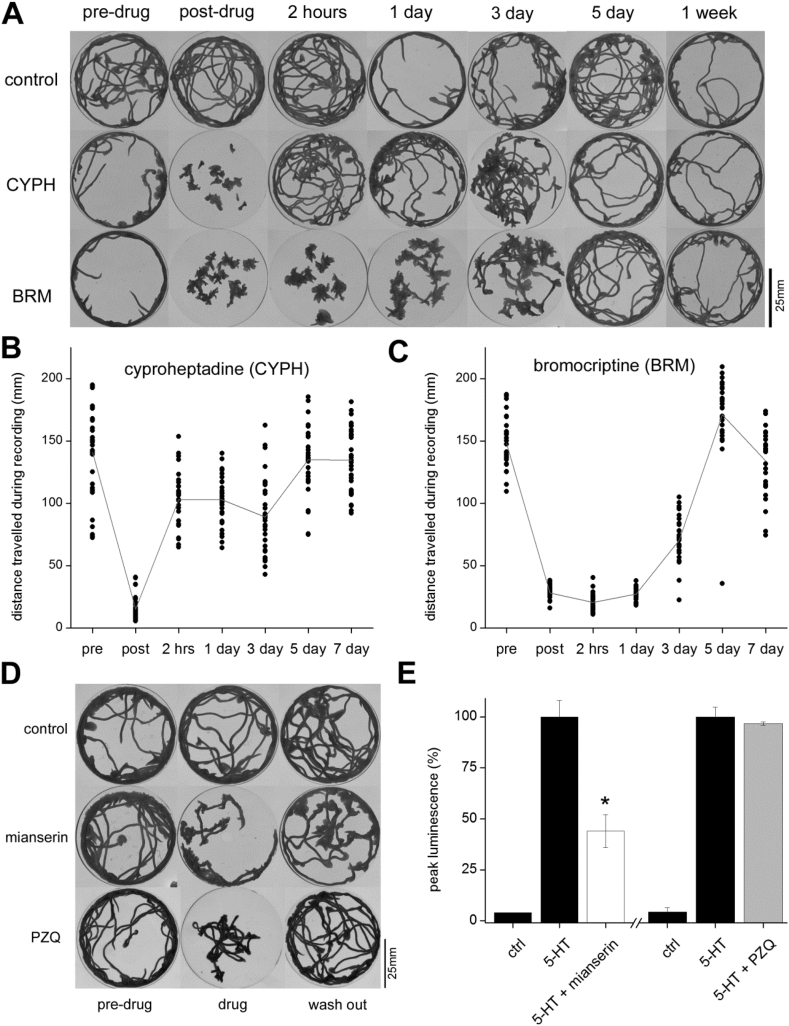
After 20min of exposure to these test doses, the timecourse of recovery of mobility was then examined following a single media exchange to drug-free solution. For cyproheptadine, worms regained normal motility within the course of a few hours (Fig. 3A). In contrast, the duration of inhibition with bromocriptine was considerable: worms remained immobilized >24hrs (Fig. 3A). Quantification of these movement videos underscored the differential kinetics of these drug effects. While worms recovered quickly after cyproheptadine removal (Fig. 3B), worms required several days to regain normal motility following bromocriptine exposure (Fig. 3C). This protracted inhibition of mobility was not due to a deleterious effect of bromocriptine on the animals, as the inhibition was fully reversible within a week under these experiment conditions (Fig. 3A&C). Other drugs that inhibited planarian mobility – mianserin, and the anthelmintic praziquantel – were also examined, and their effects like cyproheptadine were found to be readily reversible (Fig. 3D). Mianserin serves as another example of a S7.1R antagonist, observed to inhibit 5-HT evoked cAMP generation (Fig. 3D). In contrast, the paralysis caused by PZQ was not attributable to S7.1R blockade (Fig. 3D).
Fig. 3.
Bromocriptine effects a long lasting impairment of planarian movement. (A) D. japonica mobility before (pre) or at various time periods after a 20 min exposure (post drug, spanning time = ‘0’ to 1 week) to the S7.1R antagonists bromocriptine (BRM, 5 μM) and cyproheptadine (CYPH, 50 μM). (B&C) Quantification of images shown in (A) before and after drug washout. Each datapoint represents the mobility of a single worm (3 petri dishes, 10 worms in each dish). Plotted line connects the population mean for each condition. (D) Minimum intensity projections show movement profiles for worms treated with no drug (top), mianserin (middle; 10 μM, 20 min) and PZQ (bottom; 75 μM, 15 min), before drug exposure (left), during drug exposure (middle) and 2hrs after drug removal (right). (E) Effects of mianserin (10 μM) and praziquantel (100 μM) on 5-HT (1 μM) evoked cAMP signaling through the S7.1R. PZQ, unlike mianserin, did not block 5-HT action at the planarian S7.1R. Data are representative of n ≥ 3 assays, and represent mean ± s.e.m. P value, * = p < 0.05.
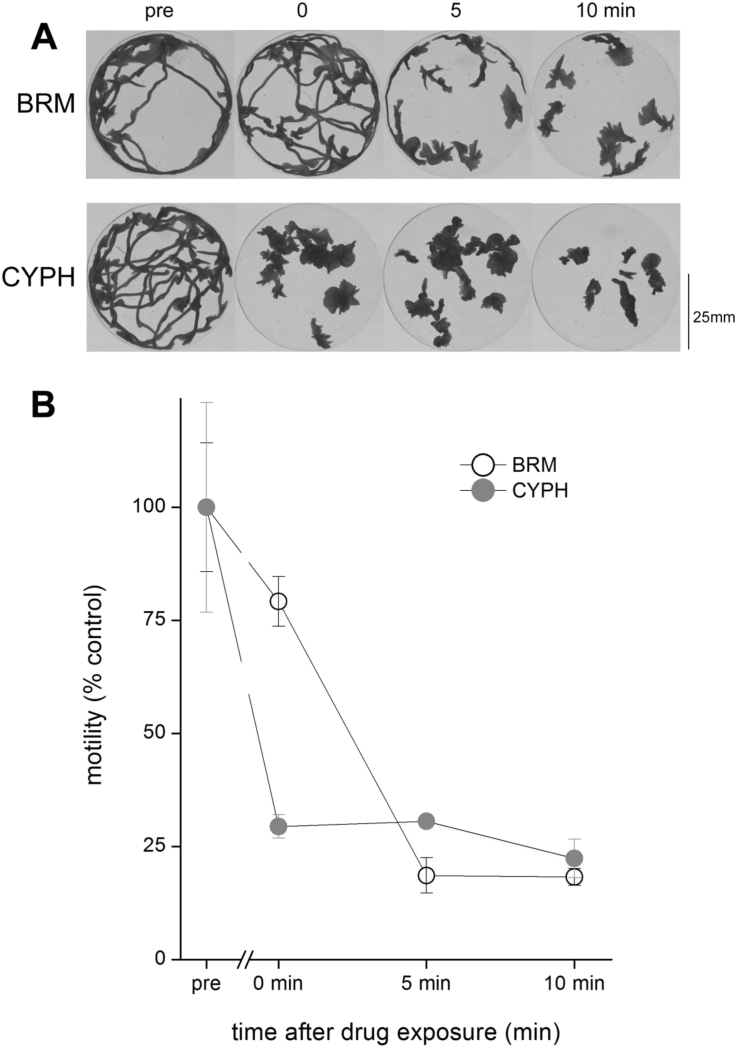
To examine the possibility that slow exchange of bromocriptine with worm tissues contributed to the protracted paralysis, the kinetics of onset of paralysis was examined. Continual recording of worm motility after drug exposure confirmed a slower onset of paralysis for bromocriptine compared with cyproheptadine (Fig. 4). Reversal of bromocriptine evoked paralysis was also accelerated by repeated solution exchanges (data not shown), further implicating a slower equilibration of bromocriptine in the intact organism. From these experiments, we conclude that the S7.1R antagonists bromocriptine and cyproheptadine (Fig. 1) both inhibit planarian mobility (Fig. 2). However the compounds differ in their timecourse of inhibition of planarian movement (Fig. 3, Fig. 4), with bromocriptine causing a potent, protracted inhibition of movement which reverses slowly in vivo.
Fig. 4.
Kinetics of movement inhibition evoked by S7.1R blockers. (A) Minimum intensity projections showing worm tracks prior to bromocriptine (5 μM, top) or cyproheptadine (50 μM, bottom) exposure (‘pre’), immediately after drug addition (‘0’) and at 5 min intervals thereafter. (B) Mean worm motility (3 petri dishes, 10 worms in each dish) from experiments as shown in (A) prior to, and after exposure to indicated drugs. Data are representative of n ≥ 3 assays, and represent mean ± s.e.m.
3.3. Protracted blockade of S7.1R by bromocriptine in vitro
Is the long lasting action of bromocriptine solely attributable to differential pharmacokinetics of drug accumulation and efflux in vivo? To address this issue, the inhibitory profile of these two antagonists were rescreened against the heterologously expressed S7.1R in vitro. To characterize the kinetics of S7.1R inhibition in HEK293 cells by bromocriptine and cyproheptadine, ligands were incubated with experimental samples (60mins) then removed from cells followed by a drug-free incubation period (90mins), prior to reassessment of 5-HT responsiveness (Fig. 5A). For cyproheptadine, 5-HT responsiveness was fully regained following removal of cyproheptadine, with the kinetics of 5-HT evoked cAMP elevation resembling control cells (Fig. 5B). In contrast, 5-HT signaling remained depressed in cells previously exposed to bromocriptine (Fig. 5B), despite drug removal. These data indicate a transient exposure to bromocriptine caused a prolonged inhibition of S7.1R signaling activity when the receptor was heterologously expressed in mammalian cells. This effect was not associated with a prolonged inhibition of adenylate cyclase, as forskolin evoked cAMP generation remained robust after incubation with either antagonist (Fig. 5C). Therefore, the kinetics of S7.1R blockade by bromocriptine and cyproheptadine were also distinct ex vivo.
Fig. 5.
Bromocriptine results in a long lasting impairment of S7.1R activity. (A) Outline of experimental workflow. HEK293 cells were co-transfected with S7.1R and a cAMP reporter and pre-incubated (60 min) with vehicle (DMSO), or S7.1R antagonist (bromocriptine, cyproheptadine; 10 μM), which was washed out prior to assaying for 5-HT responsiveness (90 min later). (B) Kinetics of 5-HT (1 μM) evoked cAMP generation in cells pre-treated with either vehicle control (DMSO, black circles), bromocriptine (10 μM, white circles) or cyproheptadine (10 μM, grey circles). (C) Quantification of 5-HT (1 μM) response (left) and forskolin (20 μM) response (right) for individual drug treatments. Data are representative of n ≥ 3 assays, and represent mean ± s.d (B) or mean ± s.e.m (C). P value, ** = p < 0.01.
4. Discussion
Here, we have demonstrated the application of a genetically encoded cAMP biosensor to profile the properties of a planarian serotonin receptor (S7.1R). The S7.1 receptor is of interest as it is the most abundant planarian 5-HT receptor and has a demonstrated role in regulating planarian neuromuscular biology (Fig. 1A) and regenerative polarity (Chan et al., 2015). The application of this methodology is important, as it will be enabling for efforts to characterize the behavior and pharmacological properties of flatworm GPCRs. The technique is sensitive, compatible with real time measurements in live cells (Fig. 1) and is amenable to miniaturization to facilitate higher throughput drug screening campaigns against flatworm GPCRs (Chan et al., 2016b). Obviously, this approach method is useful only for GPCRs that modulate cellular cAMP levels (Gs, Gi), but this reflects a coupling specificity of the majority of flatworm GPCRs examined in heterologous systems to date (Omar et al., 2007, Taman and Ribeiro, 2009, Zamanian et al., 2012, Patocka et al., 2014, MacDonald et al., 2015). Probes for other second messenger pathways (for example genetically encoded Ca2+ indicators) are available and have also been shown to be useful for deorphanizing lophotrochozoan GPCR activity (Bauknecht and Jekely, 2015).
Here, we demonstrate the utility of this cAMP biosensor for resolving aspects of planarian GPCR function. These experiments yielded the interesting observation that the kinetics of S7.1 receptor inhibition varied considerably with different S7.1R ligands. While both bromocriptine and cyproheptadine inhibit 5-HT evoked cAMP generation in a heterologous expression assay (Fig. 1), the duration of receptor inhibition persisted considerably longer for bromocriptine (Fig. 5). Cyproheptadine (and mianserin) inhibition reversed more rapidly, consistent with these drugs behaving as competitive, reversible S7.1R antagonists. A similar kinetic divergence was also observed for these two drugs at the schistosome Sm.5HTR receptor (Chan et al., 2016b). In intact planarians, divergent kinetic profiles were also resolved, with bromocriptine-evoked inhibition of worm motility persisting for days, whereas normal motility was regained within 2 h after removal of an equivalent dose of cyproheptadine (Fig. 3). Transient exposure to bromocriptine also caused a long lasting inhibition of adult schistosome movement (Chan et al., 2016b). This observation that bromocriptine has temporally penetrant effects beyond the period of exposure to a bolus dosage has potential significance for treating parasitic flatworm infections, if a single dose regimen in the field effects a persistent paralysis of the parasite. For praziquantel, the standard anthelmintic therapy, the duration for which plasma PZQ concentrations exceed the minimal effective concentration is brief, necessitating multiple dosing on a single day, ideally reinforced by subsequent treatments, for maximal clinical efficacy (King et al., 2011, Olliaro et al., 2014). Such a multiple dosing regimen can rarely be executed in disadvantaged health care environments for practical reasons. Therefore, the observed kinetics properties of bromocriptine interaction with flatworm serotonin receptors could represent a highly attractive piece of ligand-receptor phenomenology to exploit for treating parasitic disease.
What is the mechanism underpinning this behavior? The short answer is that we do not currently know, but several options merit discussion. A first consideration would be whether bromocriptine causes a protracted inhibition because the drug exhibits a lengthy elimination timecourse in vivo. This could occur if the drug was converted into an active metabolite, or the physiochemical properties cause the drug to be sequestered within flatworm tissues. Further experiments would be needed to investigate these possibilities, but our observations that this behavior is manifest in vitro on the S7.1 receptor heterologously expressed in mammalian cells lessens the likelihood that whole organism pharmacokinetics provide the complete explanation.
Second, do the properties of the S7.1 receptor provide any insight into this phenomenon? The S7.1 receptor is the most predominantly expressed serotonin receptor in Dugesia japonica (FPKM values, (Chan et al., 2015)). The S7 clade, named on account of homology to the C. elegans SER7 receptor, most closely resembles the human 5-HT7 receptor (Hs.5HTR7). Intriguingly, Hs.5HTR7 displays an unusual phenomenon of persistent inactivation caused by a subset of ligands (Smith et al., 2006). Bromocriptine has been identified as one such ‘inactivating antagonist’ (Knight et al., 2009). Other ‘inactivating antagonists’ derive from diverse chemical classes, and include risperidone, 9-OH risperidone (a metabolite, see above), methiothepin and several ergot alkaloids (lisuride, metergoline and bromocriptine). The persistent inactivation of receptor signaling has been ascribed to a pseudo-irreversible binding of these ‘inactivator’-like compounds to Hs.5HTR7, and potentially other GPCRs (Teitler and Klein, 2012), stabilizing a receptor conformation that inactivates associated G proteins and effectors (Toohey et al., 2009). The same class of compounds identified as inactivating antagonists at Hs.5HTR7 cause a protracted inactivation at the predominant schistosome 5.HTR (Sm.5HTR, (Chan et al., 2016b)) and on the basis of the data reported here (Fig. 4), this property appears conserved at a free living flatworm serotonergic GPCR.
Third, does the compound class provide any insight? Bromocriptine is a synthetic brominated ergopeptide, belonging to the ergot alkaloid class of compounds which we have recently shown are potent regulators of planarian neuromuscular function (Chan et al., 2015). The action of ergot alkaloids against smooth muscle has long been appreciated, as has the efficacy of ergot alkaloids against flatworms (Hillman et al., 1974, Tomosky et al., 1974). Despite the historical importance of this compound grouping, much remains to be resolved about the interaction of ergots with bioaminergic GPCRs and their ability to bias signaling outcomes (Wacker et al., 2013, Wang et al., 2013), their action in vivo (Carhart-Harris et al., 2016), and the therapeutic malleability that derives from their notorious polypharmacology (Besnard et al., 2012). Of particular interest to our observations is the observation that ergot alkaloids display slow receptor association and dissociation kinetics at a heterologously expressed human 5-HT2B GPCR (Unett et al., 2013). For example, the half time for dissociation of cabergoline was ∼17-fold longer than for 5-HT (100mins versus 6mins). These data evidencing slow on and off rates of ergot alkaloids at the receptor level provides another explanation for the lengthy duration of the bromocriptine-evoked paralysis.
In conclusion, use of a real-time cAMP biosensor to study the properties of an abundant planarian 5-HT receptor has revealed a protracted inhibition of receptor signaling caused by bromocriptine. These data advance bromocriptine as a pharmacological tool for effecting protracted inhibition of 5-HT signaling in platyhelminths (akin to a pharmacological ‘knockout’). Whatever the mechanistic explanation for this effect, the key point is that this receptor phenomenology occurs at a prevalent and widely conserved 5-HT GPCR in flatworms and affords an encouraging vulnerability for directed drug design. Application of this screening method to other GPCRs should aid discovery of new ligands to help dissect the basic biology of GPCR signaling in flatworms.
Acknowledgements
This work was supported by the National Science Foundation (MCB1615538).
References
- Bauknecht P., Jekely G. Large-scale combinatorial deorphanization of platynereis neuropeptide GPCRs. Cell Rep. 2015;12:684–693. doi: 10.1016/j.celrep.2015.06.052. [DOI] [PubMed] [Google Scholar]
- Besnard J., Ruda G.F., Setola V., Abecassis K., Rodriguiz R.M., Huang X.P., Norval S., Sassano M.F., Shin A.I., Webster L.A., Simeons F.R., Stojanovski L., Prat A., Seidah N.G., Constam D.B., Bickerton G.R., Read K.D., Wetsel W.C., Gilbert I.H., Roth B.L., Hopkins A.L. Automated design of ligands to polypharmacological profiles. Nature. 2012;492:215–220. doi: 10.1038/nature11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkowski B.F., Butler B.L., Stecha P.F., Eggers C.T., Otto P., Zimmerman K., Vidugiris G., Wood M.G., Encell L.P., Fan F., Wood K.V. A luminescent biosensor with increased dynamic range for intracellular cAMP. ACS Chem. Biol. 2011;6:1193–1197. doi: 10.1021/cb200248h. [DOI] [PubMed] [Google Scholar]
- Binkowski B.F., Fan F., Wood K.V. Luminescent biosensors for real-time monitoring of intracellular cAMP. Methods Mol. Biol. 2011;756:263–271. doi: 10.1007/978-1-61779-160-4_14. [DOI] [PubMed] [Google Scholar]
- Branson K., Robie A.A., Bender J., Perona P., Dickinson M.H. High-throughput ethomics in large groups of Drosophila. Nat. Methods. 2009;6:451–457. doi: 10.1038/nmeth.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris R.L., Muthukumaraswamy S., Roseman L., Kaelen M., Droog W., Murphy K., Tagliazucchi E., Schenberg E.E., Nest T., Orban C., Leech R., Williams L.T., Williams T.M., Bolstridge M., Sessa B., McGonigle J., Sereno M.I., Nichols D., Hellyer P.J., Hobden P., Evans J., Singh K.D., Wise R.G., Curran H.V., Feilding A., Nutt D.J. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc. Natl. Acad. Sci. U. S. A. 2016;113:4853–4858. doi: 10.1073/pnas.1518377113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.D., Marchant J.S. Pharmacological and functional genetic assays to manipulate regeneration of the planarian Dugesia japonica. J. Vis. Exp. 2011;54 doi: 10.3791/3058. pii: 3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.D., Agbedanu P.N., Zamanian M., Gruba S.M., Haynes C.L., Day T.A., Marchant J.S. ’Death and axes’; unexpected Ca2+ entry phenologs predict new anti-schistosomal agents. PLoS Pathog. 2014;10:e1003942. doi: 10.1371/journal.ppat.1003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.D., Agbedanu P.N., Grab T., Zamanian M., Dosa P.I., Day T.A., Marchant J.S. Ergot alkaloids (Re)generate new leads as antiparasitics. PLoS Negl. Trop. Dis. 2015;9:e0004063. doi: 10.1371/journal.pntd.0004063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.D., Acharya S., Day T.A., Marchant J.S. Pharmacological profiling an abundantly expressed schistosome serotonergic GPCR identifies nuciferine as a potent antagonist. Int. J. Parasitol. Drugs Drug Resist. 2016;6:364–370. doi: 10.1016/j.ijpddr.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.D., McCorvy J.D., Acharya S., Johns M.E., Day T.A., Roth B.L., Marchant J.S. A miniaturized screen of a schistosoma mansoni serotonergic G protein-coupled receptor identifies novel classes of parasite-selective inhibitors. PLoS Pathog. 2016;12:e1005651. doi: 10.1371/journal.ppat.1005651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clister T., Mehta S., Zhang J. Single-cell analysis of G-protein signal transduction. J. Biol. Chem. 2015;290:6681–6688. doi: 10.1074/jbc.R114.616391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley D.G., Bustinduy A.L., Secor W.E., King C.H. Human schistosomiasis. Lancet. 2014;383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F., Binkowski B.F., Butler B.L., Stecha P.F., Lewis M.K., Wood K.V. Novel genetically encoded biosensors using firefly luciferase. ACS Chem. Biol. 2008;3:346–351. doi: 10.1021/cb8000414. [DOI] [PubMed] [Google Scholar]
- Fraguas S., Barberan S., Ibarra B., Stoger L., Cebria F. Regeneration of neuronal cell types in Schmidtea mediterranea: an immunohistochemical and expression study. Int. J. Dev. Biol. 2012;56:143–153. doi: 10.1387/ijdb.113428sf. [DOI] [PubMed] [Google Scholar]
- Hillman G.R., Olsen N.J., Senft A.W. Effect of methysergide and dihydroergotamine on Schistosoma mansoni. J. Pharmacol. Exp. Ther. 1974;188:529–535. [PubMed] [Google Scholar]
- King C.H., Olbrych S.K., Soon M., Singer M.E., Carter J., Colley D.G. Utility of repeated praziquantel dosing in the treatment of schistosomiasis in high-risk communities in Africa: a systematic review. PLoS Negl. Trop. Dis. 2011;5:e1321. doi: 10.1371/journal.pntd.0001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight J.A., Smith C., Toohey N., Klein M.T., Teitler M. Pharmacological analysis of the novel, rapid, and potent inactivation of the human 5-Hydroxytryptamine7 receptor by risperidone, 9-OH-Risperidone, and other inactivating antagonists. Mol. Pharmacol. 2009;75:374–380. doi: 10.1124/mol.108.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuniecki R.W., Hobson R.J., Rex E.B., Hapiak V.M., Komuniecki P.R. Biogenic amine receptors in parasitic nematodes: what can be learned from Caenorhabditis elegans? Mol. Biochem. Parasitol. 2004;137:1–11. doi: 10.1016/j.molbiopara.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Kotlikoff M.I. Genetically encoded Ca2+ indicators: using genetics and molecular design to understand complex physiology. J. Physiol. 2007;578:55–67. doi: 10.1113/jphysiol.2006.120212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald K., Kimber M.J., Day T.A., Ribeiro P. A constitutively active G protein-coupled acetylcholine receptor regulates motility of larval Schistosoma mansoni. Mol. Biochem. Parasitol. 2015;202:29–37. doi: 10.1016/j.molbiopara.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellin T.N., Busch R.D., Wang C.C., Kath G. Neuropharmacology of the parasitic trematode, Schistosoma mansoni. Am. J. Trop. Med. Hyg. 1983;32:83–93. doi: 10.4269/ajtmh.1983.32.83. [DOI] [PubMed] [Google Scholar]
- Nogi T., Zhang D., Chan J.D., Marchant J.S. A novel biological activity of praziquantel requiring voltage-operated Ca2+ channel β subunits: subversion of flatworm regenerative polarity. PLoS Negl. Trop. Dis. 2009;3:e464. doi: 10.1371/journal.pntd.0000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olliaro P., Delgado-Romero P., Keiser J. The little we know about the pharmacokinetics and pharmacodynamics of praziquantel (racemate and R-enantiomer) J. Antimicrob. Chemother. 2014;69:863–870. doi: 10.1093/jac/dkt491. [DOI] [PubMed] [Google Scholar]
- Omar H.H., Humphries J.E., Larsen M.J., Kubiak T.M., Geary T.G., Maule A.G., Kimber M.J., Day T.A. Identification of a platyhelminth neuropeptide receptor. Int. J. Parasitol. 2007;37:725–733. doi: 10.1016/j.ijpara.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Patocka N., Sharma N., Rashid M., Ribeiro P. Serotonin signaling in Schistosoma mansoni: a serotonin-activated G protein-coupled receptor controls parasite movement. PLoS Pathog. 2014;10:e1003878. doi: 10.1371/journal.ppat.1003878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth B.L., Kroeze W.K. Integrated approaches for genome-wide interrogation of the druggable non-olfactory G protein-coupled receptor superfamily. J. Biol. Chem. 2015;290:19471–19477. doi: 10.1074/jbc.R115.654764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saberi A., Jamal A., Beets I., Schoofs L., Newmark P.A. GPCRs direct germline development and somatic gonad function in planarians. PLoS Biol. 2016;14:e1002457. doi: 10.1371/journal.pbio.1002457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh O., Yuruzume E., Watanabe K., Nakata H. Molecular identification of a G protein-coupled receptor family which is expressed in planarians. Gene. 1997;195:55–61. doi: 10.1016/s0378-1119(97)00152-2. [DOI] [PubMed] [Google Scholar]
- Smith C., Rahman T., Toohey N., Mazurkiewicz J., Herrick-Davis K., Teitler M. Risperidone irreversibly binds to and inactivates the h5-HT7 serotonin receptor. Mol. Pharmacol. 2006;70:1264–1270. doi: 10.1124/mol.106.024612. [DOI] [PubMed] [Google Scholar]
- Taman A., Ribeiro P. Investigation of a dopamine receptor in Schistosoma mansoni: functional studies and immunolocalization. Mol. Biochem. Parasitol. 2009;168:24–33. doi: 10.1016/j.molbiopara.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Teitler M., Klein M.T. A new approach for studying GPCR dimers: drug-induced inactivation and reactivation to reveal GPCR dimer function in vitro, in primary culture, and in vivo. Pharmacol. Ther. 2012;133:205–217. doi: 10.1016/j.pharmthera.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomosky T.K., Bennett J.L., Bueding E. Tryptaminergic and dopaminergic responses of Schistosoma mansoni. J. Pharmacol. Exp. Ther. 1974;190:260–271. [PubMed] [Google Scholar]
- Toohey N., Klein M.T., Knight J., Smith C., Teitler M. Human 5-HT7 receptor-induced inactivation of forskolin-stimulated adenylate cyclase by risperidone, 9-OH-risperidone and other “inactivating antagonists”. Mol. Pharmacol. 2009;76:552–559. doi: 10.1124/mol.109.056283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai I.J., Zarowiecki M., Holroyd N., Garciarrubio A., Sanchez-Flores A., Brooks K.L., Tracey A., Bobes R.J., Fragoso G., Sciutto E., Aslett M., Beasley H., Bennett H.M., Cai J., Camicia F., Clark R., Cucher M., De Silva N., Day T.A., Deplazes P., Estrada K., Fernandez C., Holland P.W., Hou J., Hu S., Huckvale T., Hung S.S., Kamenetzky L., Keane J.A., Kiss F., Koziol U., Lambert O., Liu K., Luo X., Luo Y., Macchiaroli N., Nichol S., Paps J., Parkinson J., Pouchkina-Stantcheva N., Riddiford N., Rosenzvit M., Salinas G., Wasmuth J.D., Zamanian M., Zheng Y., Taenia solium Genome, C., Cai X., Soberon X., Olson P.D., Laclette J.P., Brehm K., Berriman M. The genomes of four tapeworm species reveal adaptations to parasitism. Nature. 2013;496:57–63. doi: 10.1038/nature12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unett D.J., Gatlin J., Anthony T.L., Buzard D.J., Chang S., Chen C., Chen X., Dang H.T., Frazer J., Le M.K., Sadeque A.J., Xing C., Gaidarov I. Kinetics of 5-HT2B receptor signaling: profound agonist-dependent effects on signaling onset and duration. J. Pharmacol. Exp. Ther. 2013;347:645–659. doi: 10.1124/jpet.113.207670. [DOI] [PubMed] [Google Scholar]
- Wacker D., Wang C., Katritch V., Han G.W., Huang X.P., Vardy E., McCorvy J.D., Jiang Y., Chu M., Siu F.Y., Liu W., Xu H.E., Cherezov V., Roth B.L., Stevens R.C. Structural features for functional selectivity at serotonin receptors. Science. 2013;340:615–619. doi: 10.1126/science.1232808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Jiang Y., Ma J., Wu H., Wacker D., Katritch V., Han G.W., Liu W., Huang X.P., Vardy E., McCorvy J.D., Gao X., Zhou X.E., Melcher K., Zhang C., Bai F., Yang H., Yang L., Jiang H., Roth B.L., Cherezov V., Stevens R.C., Xu H.E. Structural basis for molecular recognition at serotonin receptors. Science. 2013;340:610–614. doi: 10.1126/science.1232807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcockson W.S., Hillman G.R. Drug effects on the 5-HT response of Schistosoma mansoni. Comp. Biochem. Physiol. C. 1984;77:199–203. doi: 10.1016/0742-8413(84)90151-8. [DOI] [PubMed] [Google Scholar]
- Zamanian M., Kimber M.J., McVeigh P., Carlson S.A., Maule A.G., Day T.A. The repertoire of G protein-coupled receptors in the human parasite Schistosoma mansoni and the model organism Schmidtea mediterranea. BMC Genomics. 2011;12:596. doi: 10.1186/1471-2164-12-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian M., Agbedanu P.N., Wheeler N.J., McVeigh P., Kimber M.J., Day T.A. Novel RNAi-mediated approach to G protein-coupled receptor deorphanization: proof of principle and characterization of a planarian 5-HT receptor. PLoS ONE. 2012;7:e40787. doi: 10.1371/journal.pone.0040787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Chan J.D., Nogi T., Marchant J.S. Opposing roles of voltage-gated Ca2+ channels in neuronal control of stem cell differentiation in vivo. J. Neurosci. 2011;31:15983–15995. doi: 10.1523/JNEUROSCI.3029-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]