Abstract
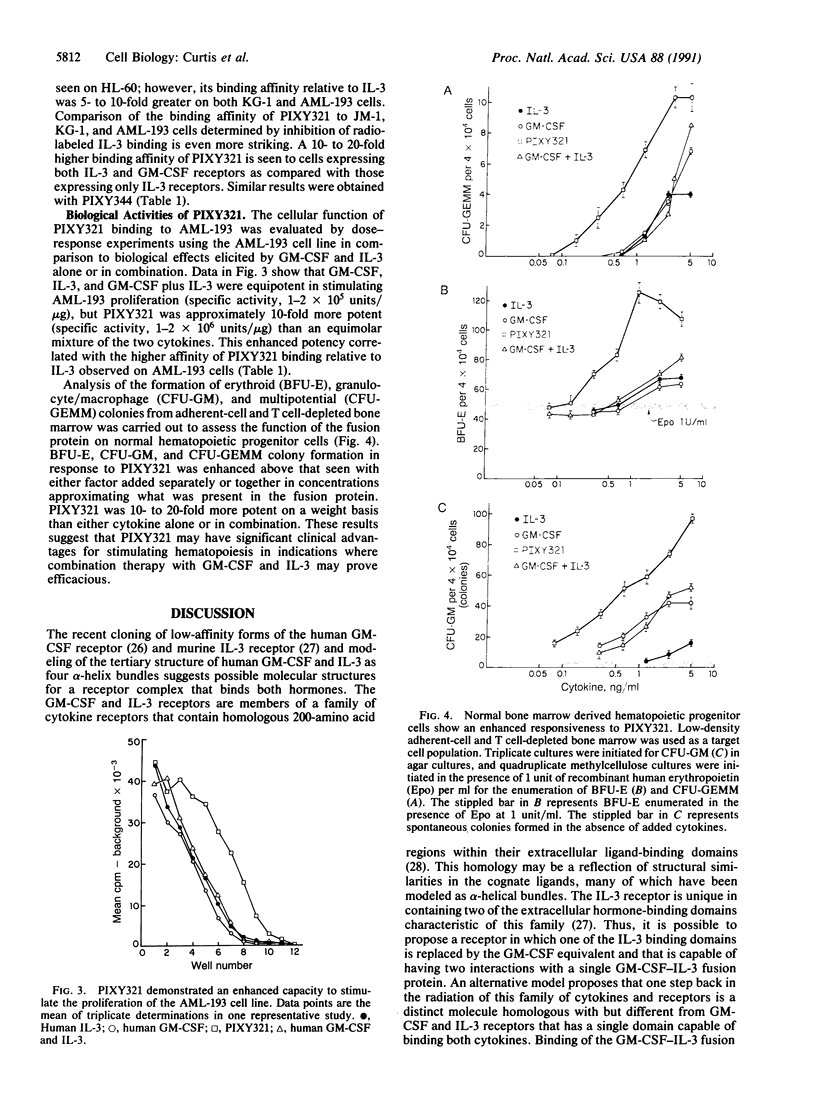
Granulocyte/macrophage colony-stimulating factor-interleukin 3 (GM-CSF-IL-3) fusion proteins were generated by construction of a plasmid in which the coding regions of human GM-CSF and IL-3 cDNAs were connected by a synthetic linker sequence followed by subsequent expression in yeast. Both GM-CSF-IL-3 and IL-3-GM-CSF fusion proteins were purified to homogeneity and shown to bind to cell-surface receptors through either their GM-CSF or IL-3 domains. The fusion proteins exhibited enhanced receptor affinity, proliferative activity, and hematopoietic colony-stimulating activity compared with either IL-3 and/or GM-CSF alone. This suggests that GM-CSF-IL-3 fusion proteins may hold future promise as therapeutic agents.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broxmeyer H. E., Williams D. E., Hangoc G., Cooper S., Gillis S., Shadduck R. K., Bicknell D. C. Synergistic myelopoietic actions in vivo after administration to mice of combinations of purified natural murine colony-stimulating factor 1, recombinant murine interleukin 3, and recombinant murine granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3871–3875. doi: 10.1073/pnas.84.11.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer H. E., Williams D. E. The production of myeloid blood cells and their regulation during health and disease. Crit Rev Oncol Hematol. 1988;8(3):173–226. doi: 10.1016/s1040-8428(88)80016-7. [DOI] [PubMed] [Google Scholar]
- Budel L. M., Elbaz O., Hoogerbrugge H., Delwel R., Mahmoud L. A., Löwenberg B., Touw I. P. Common binding structure for granulocyte macrophage colony-stimulating factor and interleukin-3 on human acute myeloid leukemia cells and monocytes. Blood. 1990 Apr 1;75(7):1439–1445. [PubMed] [Google Scholar]
- Cannistra S. A., Koenigsmann M., DiCarlo J., Groshek P., Griffin J. D. Differentiation-associated expression of two functionally distinct classes of granulocyte-macrophage colony-stimulating factor receptors by human myeloid cells. J Biol Chem. 1990 Jul 25;265(21):12656–12663. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Clark-Lewis I., Hood L. E., Kent S. B. Role of disulfide bridges in determining the biological activity of interleukin 3. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7897–7901. doi: 10.1073/pnas.85.21.7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen F. E., Abarbanel R. M., Kuntz I. D., Fletterick R. J. Turn prediction in proteins using a pattern-matching approach. Biochemistry. 1986 Jan 14;25(1):266–275. doi: 10.1021/bi00349a037. [DOI] [PubMed] [Google Scholar]
- Cohen F. E., Richmond T. J., Richards F. M. Protein folding: evaluation of some simple rules for the assembly of helices into tertiary structures with myoglobin as an example. J Mol Biol. 1979 Aug 15;132(3):275–288. doi: 10.1016/0022-2836(79)90260-2. [DOI] [PubMed] [Google Scholar]
- Cosman D., Lyman S. D., Idzerda R. L., Beckmann M. P., Park L. S., Goodwin R. G., March C. J. A new cytokine receptor superfamily. Trends Biochem Sci. 1990 Jul;15(7):265–270. doi: 10.1016/0968-0004(90)90051-c. [DOI] [PubMed] [Google Scholar]
- Donahue R. E., Seehra J., Metzger M., Lefebvre D., Rock B., Carbone S., Nathan D. G., Garnick M., Sehgal P. K., Laston D. Human IL-3 and GM-CSF act synergistically in stimulating hematopoiesis in primates. Science. 1988 Sep 30;241(4874):1820–1823. doi: 10.1126/science.3051378. [DOI] [PubMed] [Google Scholar]
- Elliott M. J., Vadas M. A., Eglinton J. M., Park L. S., To L. B., Cleland L. G., Clark S. C., Lopez A. F. Recombinant human interleukin-3 and granulocyte-macrophage colony-stimulating factor show common biological effects and binding characteristics on human monocytes. Blood. 1989 Nov 15;74(7):2349–2359. [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gearing D. P., King J. A., Gough N. M., Nicola N. A. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 1989 Dec 1;8(12):3667–3676. doi: 10.1002/j.1460-2075.1989.tb08541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesner T. G., Mufson R. A., Norton C. R., Turner K. J., Yang Y. C., Clark S. C. Specific binding, internalization, and degradation of human recombinant interleukin-3 by cells of the acute myelogenous, leukemia line, KG-1. J Cell Physiol. 1988 Sep;136(3):493–499. doi: 10.1002/jcp.1041360314. [DOI] [PubMed] [Google Scholar]
- Gillis S., Urdal D. L., Clevenger W., Klinke R., Sassenfeld H., Price V., Cosman D. Production of recombinant human colony stimulating factors in yeast. Behring Inst Mitt. 1988 Aug;(83):1–7. [PubMed] [Google Scholar]
- Huston J. S., Levinson D., Mudgett-Hunter M., Tai M. S., Novotný J., Margolies M. N., Ridge R. J., Bruccoleri R. E., Haber E., Crea R. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5879–5883. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Schreurs J., Gorman D. M., Maruyama K., Ishii A., Yahara I., Arai K., Miyajima A. Cloning of an interleukin-3 receptor gene: a member of a distinct receptor gene family. Science. 1990 Jan 19;247(4940):324–327. doi: 10.1126/science.2404337. [DOI] [PubMed] [Google Scholar]
- Krumwieh D., Weinmann E., Seiler F. R. Human recombinant derived IL-3 and GM-CSF in hematopoiesis of normal cynomolgus monkeys. Behring Inst Mitt. 1988 Aug;(83):250–257. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lopez A. F., Eglinton J. M., Gillis D., Park L. S., Clark S., Vadas M. A. Reciprocal inhibition of binding between interleukin 3 and granulocyte-macrophage colony-stimulating factor to human eosinophils. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7022–7026. doi: 10.1073/pnas.86.18.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Broxmeyer H. E. Comparative influences of phytohemagglutinin-stimulated leukocyte conditioned medium, hemin, prostaglandin E, and low oxygen tension on colony formation by erythroid progenitor cells in normal human bone marrow. Exp Hematol. 1985 Nov;13(10):989–993. [PubMed] [Google Scholar]
- Park L. S., Friend D., Price V., Anderson D., Singer J., Prickett K. S., Urdal D. L. Heterogeneity in human interleukin-3 receptors. A subclass that binds human granulocyte/macrophage colony stimulating factor. J Biol Chem. 1989 Apr 5;264(10):5420–5427. [PubMed] [Google Scholar]
- Park L. S., Waldron P. E., Friend D., Sassenfeld H. M., Price V., Anderson D., Cosman D., Andrews R. G., Bernstein I. D., Urdal D. L. Interleukin-3, GM-CSF, and G-CSF receptor expression on cell lines and primary leukemia cells: receptor heterogeneity and relationship to growth factor responsiveness. Blood. 1989 Jul;74(1):56–65. [PubMed] [Google Scholar]
- Price V., Mochizuki D., March C. J., Cosman D., Deeley M. C., Klinke R., Clevenger W., Gillis S., Baker P., Urdal D. Expression, purification and characterization of recombinant murine granulocyte-macrophage colony-stimulating factor and bovine interleukin-2 from yeast. Gene. 1987;55(2-3):287–293. doi: 10.1016/0378-1119(87)90288-5. [DOI] [PubMed] [Google Scholar]
- Schrimsher J. L., Rose K., Simona M. G., Wingfield P. Characterization of human and mouse granulocyte-macrophage-colony-stimulating factors derived from Escherichia coli. Biochem J. 1987 Oct 1;247(1):195–199. doi: 10.1042/bj2470195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder R. Y., Walder J. A. Oligodeoxynucleotide-directed mutagenesis using the yeast transformation system. Gene. 1986;42(2):133–139. doi: 10.1016/0378-1119(86)90289-1. [DOI] [PubMed] [Google Scholar]
- Williams D. E., Straneva J. E., Shen R. N., Broxmeyer H. E. Purification of murine bone-marrow-derived granulocyte-macrophage colony-forming cells. Exp Hematol. 1987 Mar;15(3):243–250. [PubMed] [Google Scholar]