Abstract
The blood-brain barrier (BBB) is a major obstacle in drug delivery for diseases of the brain, and today there is no standardized route to surpass it. One technique to locally and transiently disrupt the BBB, is focused ultrasound in combination with gas-filled microbubbles. However, the microbubbles used are typically developed for ultrasound imaging, not BBB disruption. Here we describe efficient opening of the BBB using the promising novel Acoustic Cluster Therapy (ACT), that recently has been used in combination with Abraxane® to successfully treat subcutaneous tumors of human prostate adenocarcinoma in mice. ACT is based on the conjugation of microbubbles to liquid oil microdroplets through electrostatic interactions. Upon activation in an ultrasound field, the microdroplet phase transfers to form a larger bubble that transiently lodges in the microvasculature. Further insonation induces volume oscillations of the activated bubble, which in turn induce biomechanical effects that increase the permeability of the BBB. ACT was able to safely and temporarily permeabilize the BBB, using an acoustic power 5-10 times lower than applied for conventional microbubbles, and successfully deliver small and large molecules into the brain.
Keywords: Blood-brain barrier opening, focused ultrasound, acoustic cluster therapy (ACT), enhanced drug delivery
Introduction
The blood-brain barrier (BBB) maintains the homeostasis of the brain and protects it from unwanted or harmful substances. Unfortunately, the BBB also blocks >98% of small drugs (<600 Da) and all larger therapeutic molecules from entering the brain, unless active transport of the substances is possible 1. Thus, the presence of an intact BBB limits the distribution of a large number of therapeutic agents, including anti-cancer and anti-viral drugs, as well as novel therapeutic approaches that do not translate to clinical practice because of this biological barrier. For this reason, diseases like brain cancer, Alzheimer's disease, amyotrophic lateral sclerosis (ALS) and more, remain untreatable. New ways to pass the BBB have to be explored for every single drug lead, resulting in a severe restriction on the development of medicinal therapies for brain diseases. Since its introduction in 2001 2, focused ultrasound (FUS) in combination with microbubbles has been explored to increase the permeability of the BBB in various pre-clinical settings 3. In brief, insonation of the vascular compartment containing microbubbles leads to a variety of biomechanical effects that increase the permeability of the endothelial barrier both paracellularly and transcellularly 4. The increased permeability leads to enhanced extravasation, distribution and uptake of drug molecules in the target tissue 5-7. This approach is currently being evaluated in two separate clinical trials (ClinicalTrials.gov identifier NCT02343991 8 and NCT02253212) 9, 10. Most studies using FUS for BBB disruption (BBBD) are employing commercial ultrasound contrast agent microbubbles, such as Definity®, Sonovue® and Optison® 2, 11-16. To improve treatment strategies, incorporation of drugs into custom-made microbubbles has also been investigated 17-19. Whereas the concept clearly holds merit, it also has limitations. The microbubbles need to be close to the endothelial wall to maximize biomechanical effects 20, 21. However, regular contrast microbubbles are small (1-3 µm), and their average distance to the vessel wall is often too large to induce a significant biomechanical effect. Furthermore, the circulation lifetime of most microbubbles is typically in the order of 2-3 min, thus limiting the exposure time. Although conventional microbubbles have shown promise for opening the BBB and for drug delivery to the brain, these microbubbles were developed for diagnostic imaging, not for therapy. Microbubble formulations designed for therapy are likely to be needed to enable optimal treatment regimens.
A recently proposed concept for ultrasound mediated, targeted drug delivery; Acoustic Cluster Therapy (ACT), makes use of similar mechanisms as regular microbubbles, but addresses important shortcomings of the latter. Details of the ACT formulation concept are described previously 22-24. In brief, the ACT formulation is produced by electrostatic complexation between negatively charged microbubbles and positively charged oil microdroplets. The active moiety comprises microbubble/microdroplet clusters engineered to be stable in vivo. After intravenous injection of the clusters, ultrasound is applied to the diseased area and the microbubbles transfer acoustic energy to the attached droplets, which undergo a liquid-to-gas phase shift (the “Activation” Step). The resulting ACT-bubbles undergo a rapid expansion to approximately 25 μm and are temporarily deposited in the local capillary network, transiently blocking blood flow for up to 10 min. Further application of ultrasound (the “Enhancement” step) induces volume oscillations of the ACT-bubbles, which result in non-thermal mechanisms such as cavitation and shear forces that increase the local permeability of the vasculature, increasing transport of co-administered drugs across the capillary wall and through the extracellular matrix 23, 24. Being approx. three orders of magnitude larger in volume than regular contrast microbubbles, ACT bubbles will deliver a significantly larger biomechanical effect. In addition, the ACT bubble is in close contact with the endothelial wall over a significant segment, ensuring optimal coupling between the vessel wall and the oscillating bubble. Finally, the ACT bubble stays for typically 10 minutes, prolonging the treatment time window compared to regular contrast microbubbles. We have recently shown that this concept can be utilized to induce a strong enhancement of the therapeutic efficacy of paclitaxel and nab-paclitaxel (Abraxane®) for treatment of solid tumors such as human prostate adenocarcinoma growing subcutaneously in athymic mice 25. When combining the ACT treatment with a clinically relevant dose of Abraxane®, all of the treated mice were alive at the end of the study (120 days after treatment start, 99 days after treatment end) and 67% were in complete remission. Conversely, the group treated with Abraxane, without ACT treatment, had a median survival time 72 days' post treatment start (0% survival at the end of the study). Furthermore, during treatment, this group showed little tumor regression and more importantly, tumor growth continued immediately after the treatment was stopped. The median survival of the control group time was 28 days'.
In the current paper, we have investigated the ACT concept for opening the BBB for model drugs in rats. With the new ACT approach, it is possible to generate bubbles, in-situ, that more effectively deliver small and large model drugs into the brain, compared to regular contrast microbubbles. In addition, the applied ultrasound pressure in this study is 5 to 10 times lower than typical levels used with regular microbubbles, potentially making the treatment safer.
Materials and Methods
ACT and Sonazoid™
The ACT formulation consisted of a dispersion of microbubble/microdroplet clusters made from reconstituting the ultrasound contrast agent Sonazoid™ with 2 ml of perfluoromethylcyclopentane (PFMCP) microdroplets (3 µl/ml) stabilized with a distearoyl-phosphatidylcholine (DSPC) phospholipid membrane with 3% (mol/mol) stearylamine (SA), dispersed in 5 mM Tris(hydroxymethyl)aminomethane (TRIS) buffer. ACT was diluted 1/8 in (TRIS)-HCl buffer (pH 7.4) before injection 23.
Sonazoid™ (GE Healthcare AS, Norway) is an ultrasound contrast agent comprising perfluorobutane (PFB) microbubbles, stabilized with a hydrogenated egg phosphatidylserine-sodium (HEPS-Na) phospholipid membrane, embedded in a lyophilized sucrose matrix 26. Sonazoid™ was diluted 1/8 in TRIS-HCl buffer (pH 7.4) before injection. Further details on the ACT formulation and the microbubble/microdroplet ratio are provided by Sontum et al 23. ACT and Sonazoid™ were kindly provided by Phoenix Solution AS, Oslo, Norway.
Animals
Healthy female Sprague Dawley rats (NTac:SD; Taconic), 8-12 weeks old with a weight of 240-280 grams, were used. All experimental procedures were conducted in compliance with protocols approved by the Norwegian Animal Research Authorities. Animals were housed in a specific pathogen free environment at a 12h night/day cycle with controlled temperature and humidity. Food and water were provided ad libitum.
Ultrasound Setup and Treatment Regimen
A 1 MHz FUS transducer (Imasonic SAS) with a diameter of 50 mm and a focal length of 125 mm (f-number 2.5) was used. The transducer was positioned in a water bath and a specially designed magnetic resonance imaging (MRI) bed 19, with the animal in a supine position, placed on top of the water tank. The skull of the animal was positioned approximately 120 mm from the transducer.
The animal was sedated by gas anesthesia (isoflurane ~ 4% and 2% induction and maintenance, respectively, in 78% medical air/20% O2). The head of the animal was shaved using a trimmer, and depilatory cream was applied to the shaved area for 1 min to remove remaining hair. The tail vein was cannulated (BD Neoflon 24G, Becton Dickinson & Co), and the animal placed on the MRI bed. Omniscan (1 ml/kg, 0.5 mmol gadododiamide/kg) was injected and MR images were recorded. Sonazoid™ (1 ml/kg) or ACT (1 ml/kg) was injected and the cannula was flushed with 0.1 ml of 1 U/ml heparin. Immediately after injection, the ultrasound treatment was initiated. The treatment was divided into an activation step followed by an enhancement step using the same transducer and the same sonication sequence with two different mechanical indexes (MIs). The MI was calculated from the estimated in situ pressure, assuming 40% attenuation of the ultrasound power through the skull of the animal 19. For the activation step, the parameters were: MI 0.28, 4 µs pulse length, pulse repetition frequency (PRF) 1 kHz, sonication time 30 s. For the enhancement step, MI was 0.09, 4 µs pulse length, PRF 1 kHz, sonication time 10 min. Each animal was treated two times in two different positions, posterior and anterior of the cerebrum, and the two treatments were sometimes from different treatment groups. The contralateral side of the treated position was used as an internal negative control. After treatment, MR images were recorded. The animals were intravenously injected with pentobarbital and perfused with phosphate saline buffer 1x (PBS) and 4% paraformaldehyde (PFA) in PBS and the brain was submerged in formalin (10%) for at least 24 h, before being sectioned for histology. The experimental timeline is shown in Fig. 1.
Figure 1.
Treatment timeline. Grey bar indicates activation step period and black bold bar indicates enhancement step period.
Five treatment groups were investigated.
1. ACT + Activation ultrasound + Enhancement ultrasound (ACT+A+E) (8 animals for BBBD, 4 animals for BBB recovery, 2 animals for bioluminescence of IRDye 800CW-PEG).
2. ACT + Activation ultrasound (ACT+A). (4 animals for BBBD, 2 animals for bioluminescence of IRDye 800CW-PEG).
3. ACT + Enhancement (ACT+E) (5 animals).
4. Sonazoid™ + Activation ultrasound + Enhancement ultrasound (Sonazoid™+A+E) (3 animals).
5. Saline + Activation ultrasound + Enhancement ultrasound (Saline+A+E) (3 animals).
MRI
MR images were acquired using a 7.05 T horizontal bore magnet (Biospec 70/20 Avance III, Bruker Biospin) with an 86 mm volume resonator for RF transmission and a phased array rat brain surface coil for reception. A pressure sensitive- and a temperature probe recorded respiration and temperature, respectively, of the rat (SA Instruments). Gas anesthesia (isoflurane ~2% in 78% medical air/20% O2) was adjusted appropriately and the body temperature was maintained at 37 °C by water circulation in the MRI bed. The animal was placed in the scanner, coils were tuned and matched and a tri-pilot with navigator scan (30 s) was acquired. MRI was acquired pre- and post-treatment. To verify BBBD and to detect hemorrhage in vivo, a Fast-Low Angle Shot (FLASH) sequence was used (Flip angle of 60°, TE/TR 5/350 ms, zero fill acceleration of 1.3, 10 averages, lasting 6 min and 8 s). The geometry of the MR sequence had a field of view of 40x27 mm, matrix size of 200x135 and 12 slices á 1 mm.
Analysis of BBBD
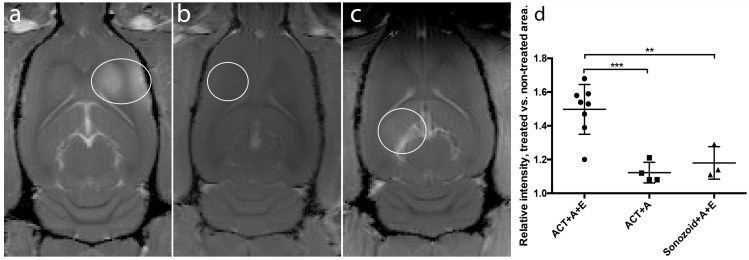
To evaluate the effect of BBBD, the intensity of the contrast agent in the MR images from each treatment region was estimated. When BBBD was visibly detectable, an ROI was drawn around the opened area and the same ROI was used on the non-treated contralateral side of the brain. The ratio of the average intensities of the ROIs was calculated using ImageJ 27. Positioning of the ultrasound was manual, thus the exact coordinates for the BBBD was not available. To quantify the BBBD, it was therefore required to be able to observe the contrast agent in the image. Hence, quantitative data is only available for 3 of the 5 groups in Fig. 2.
Figure 2.
Comparison of gadodiamide signal intensities from MR-images. White rings indicate BBBD, (a) ACT+A+E, (b) ACT+A, (c) Sonazoid™ +A+E. (d) Scatter plot with mean and standard deviation. ** (p<0.01), *** (p<0.001).
Bioluminescence optical imaging
Successful delivery of a near infrared pegylated macromolecule (IRDye 800CW-PEG, LI-COR Biosciences Ltd) was verified using bioluminescence imaging (Pearl Impulse Imager, LI-COR Biosciences Ltd.). The excitation and emission settings were 785 and 820 nm, respectively. Animals were treated according to treatment group 1 and 2 with IRDye 800CW-PEG (10 nmol/kg) injected immediately after gadodiamide. The pegylated dye of approximately 45 kDa was used for assessing macromolecule vascular permeability 28. 1 h after FUS treatment, the animals were injected with pentobarbital and perfused with PBS, before the brain was removed and imaged.
BBB recovery
To evaluate recovery of the BBB, treated animals were taken off anesthesia and decannulated. The animals were imaged again approximately 24 h and 72 h post treatment. For each MRI session, the animals were sedated and cannulated as described and Omniscan (1 ml/kg) was injected to evaluate the BBB recovery.
Histology
Paraformaldehyde fixated brains were cast into paraffin and cut into 4 µm thick sections. The sections were stained by haematoxylin-eosin-saffron (HES). Sections were imaged on an EVOS FL Auto (objective, 20x/ 0.25, air, Invitrogen/ThermoFisher). Images were stitched using the built-in software.
Statistical analysis
All statistical analysis was performed using Prism 6 (GraphPad Software, Inc.). Mean, standard deviation (with N-1 degree's freedom), analysis of variance (ANOVA) and Bonferroni's multiple comparison test were calculated for the BBBD groups (Fig. 2). Mean, standard deviation and monoexponential decay (f(t)=a*e(-K*t) +b) were calculated for the BBB recovery group (Fig. 4).
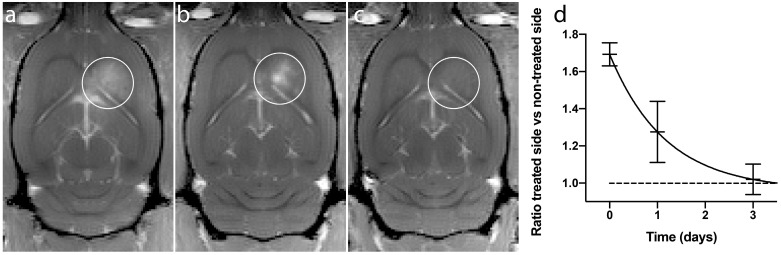
Figure 4.
BBBD from a representative animal. (a) Immediately after treatment, (b) 1 day after treatment and (c) 3 days after treatment. (d) Ratio of treated vs non-treated side of the brain, mean and standard deviation from 4 animals.
Results and discussion
Gadodiamide extravasation to the brain
ACT in combination with the activation and enhancement step clearly opened the BBB allowing MRI contrast agent to enter into the brain tissue (Fig. 2). Fig. 2 shows strong gadodiamide signal in the brain treated with ACT and activation and enhancement FUS, whereas only giving ACT and the activation FUS exposure showed hardly any signal. ACT was also much more efficient than the commercial Sonazoid in opening the BBB in combination with ultrasound. To obtain more quantitative data, the ratio of gadodiamide signal in the treated part vs. the non-treated contralateral part of the brain was calculated. In average, the ratio increased 50% for ACT+A+E treatment, compared to 18% for the Sonazoid™+A+E treatment (Fig. 2d). The extravasation of gadodiamide into the brain caused by ACT+A+E was significantly higher (p<0.01, Figure 2d) than with Sonazoid™+A+E (groups 1 and 4, respectively). ACT+A (group 2) caused similar gadodimamide extravasation as for Sonazoid™+A+E. ACT+E only (group 3) and Saline+A+E (group 5) did not induce BBBD and are thus not included in Fig. 2.
With the ultrasound activation and enhancement regimes applied, the ACT treatment clearly induced a significantly higher extravasation of gadodiamide to the brain than regular, small contrast microbubbles. Several important distinctions may contribute to this difference in effect level. The ACT-bubbles are in close contact with the endothelium over a significant segment of the vessel wall, ensuring a large interfacing area between the oscillating bubble and the endothelial cells. Sonazoid™, on the other hand, is free flowing and the average distance between the microbubble surface and the endothelial cells may limit the biomechanical effect level. ACT-bubbles are approximately 1000 times larger (by volume) than Sonazoid™ microbubbles and the biomechanical effects these large bubbles induce, even at low ultrasound pressures, should be orders of magnitude larger than with regular contrast microbubbles. Moreover, the lodged ACT-bubble will cause an increased microvascular pressure on the arterial side of the bubble and the induced transcapillary pressure gradient will enhance extravasation.
Comparing ACT+A with ACT+A+E, the 10 min, low MI enhancement step in the later treatment scheme is clearly increasing extravasation. Whether this is due to generation of larger openings with prolonged treatment, greater influx from longer treatment time, or a combination, cannot be determined from these experiments. Moreover, the enhancement step alone fails to produce BBBD with ACT, indicating that the phase shift event is a prerequisite for an effective BBBD.
In this study, Sonazoid™ has been used to represent commercial microbubbles, as it is a constituent of ACT. The commercial microbubbles have different properties with respect to size, size-distribution and shell properties. Nonetheless, the acoustic parameters used for effective BBBD are often similar for these MBs. This has been investigated in the case of Optison vs. Definity by McDannold et al 16 and for microbubbles of different sizes by Samiotaki et al. 14. Another phase shift microbubble, based on acoustically active oil nanodroplets, has previously been used to open the BBB 29. The nanodroplets had a size of 100-200 nm and ultrasound activation of the volatile oil created a microbubble of similar size to those in conventional ultrasound contrast agents. The study found that the nanodroplets were less effective in opening the BBB than commercial contrast agents at similar ultrasound settings. The main advantages of phase shift nanodroplets are that their size makes it possible for the nanodroplet to enter into the interstitium and cells, enabling abluminal activation, and their long circulation time, hours compared to minutes for conventional microbubbles, enabling prolonged treatment time. Except for the phase shift, nanodroplets and ACT are not easily compared; for instance, the nanodroplet is similar in size and blood circulation half-life to standard contrast microbubbles after activation, while the size and circulation half-life of ACT are more similar before activation.
IRDye 800CW-PEG extravasation into the brain
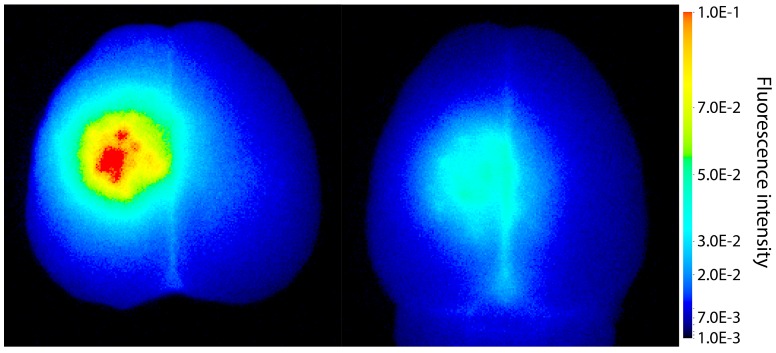
To explore if ACT facilitate extravasation of larger molecules, IRDye 800CW-PEG was injected before the treatment. IRDye 800CW-PEG extravasated into brain tissue both after ACT+A+E and ACT+A treatment (Fig. 3), the former clearly at a much higher level. IRDye 800CW-PEG is a macromolecule with a molecular weight of approximately 45 kDa, which is a relevant size for many applications within medicinal therapy where macromolecules are used. In a number of therapeutic approaches, including immunotherapy, larger molecules such as proteins or other nanosized agents are applied, hence, enabling delivery of such large molecules to the brain is an imperative for the success of these therapeutic agents. Even though the literature states that the BBB can be open for several days post treatment, this is only true for smaller molecules, such as gadodiamide 19, 30, 31. Larger openings generated, allowing larger molecules to enter the brain, are only present for a short time 32. According to Marty et al. 32, any molecule larger than 15-20 nm would in principle only benefit from FUS mediated BBBD for the duration of the FUS treatment. Hence, the large and deposited ACT bubbles, which are present for a longer time than regular microbubbles and in closer contact with the endothelial wall, are likely to be more effective for delivery of large therapeutic molecules. Furthermore, the efficient delivery observed shows that larger molecules can be delivered to a specific volume of the brain.
Figure 3.
Extravasation of the fluorescent macromolecule IRDye 800CW-PEG to the brain after treatment with (left) ACT+A+E and (right) ACT+A.
Recovery of BBBD
The recovery after the BBBD following ACT+A+E procedure was not complete 1 day after treatment (Fig. 4a-c). At this point, the extravasation of gadodiamide revealed a spot like pattern, rather than a diffuse extravasation pattern, which was observed immediately after ultrasound exposure. This pattern was seen in all recovery animals, and can also be seen in animals injected with IRDye 800CW-PEG (Fig. 3). The spots might be a result of the in situ ultrasound beam profile being heterogenous in the sonication focus. Although the beam profile measured in a water thank is homogenous 19, penetrating the skull might generate a more heterogeneous beam profile. This resulted in some areas where the opening was more substantial and consequently prolonged the recovery time. At 3 days' post treatment, a little gadodiamide extravasation into the brain was detectable for some animals, indicating that the BBB was close to or fully recovered. A monoexponential fit (f(t)=0.73·e -0.85t +0.96, R2=0.89) gave a half-life of BBBD recovery of 0.82 days. Previous studies have shown that the BBB closes after 1 to several days 19, 30, 31. The present recovery kinetics of BBBD are thus similar to those for regular FUS microbubble treatment.
Histology
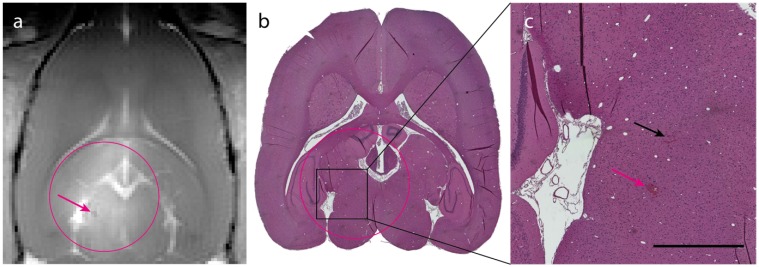
The in situ MI applied in this study was well below the threshold for what has previously been published as risk for damage to the brain/vasculature 33. This is corroborated by the T1-FLASH and HES-staining, which revealed no signs of hemorrhage. Brains from group 1, 2 and 4 were stained with HES and analyzed for any signs of damage from the different treatments. Group 2 (ACT+A) and 4 (Sonazoid™+A+E), that showed the weakest BBBD, had no signs of adverse effects. Group 1 (ACT+A+E) showed only a few occurrences of focal microhemorrhages (exemplified in Fig. 4a-c), but the damage scored mild and was of no clinical relevance. No signs of eosinophilic degeneration of neurons, gliosis, inflammation or microglial/macrophage reaction were detected.
Conclusions
The present study demonstrates that ACT can be utilized to open the BBB in a safe manner, using a lower pressure FUS regimen than regular contrast microbubbles. ACT is clearly more efficient than regular microbubbles in opening the BBB in combination with ultrasound. The ACT treatment induces a significantly enhanced extravasation of gadodiamide as well as delivery of macromolecules.
This study demonstrates a new application for ACT which previously has shown great promise in enhancing the delivery of co-injected drugs to solid tumors growing in mice 24 and in treating tumors growing in mice 25.
Figure 5.
Histological analysis of ACT+A+E treatment. (a) T1-FLASH image showing BBB opening after treatment (magenta circle indicates treated area; magenta arrow indicates microhemorrhage). (b) HES stained image of the brain (magenta circle indicates treated area). (c) Magnified area of b showing two sites of extravasated erythrocytes (magenta arrow corresponds to the microhemorrhage in panel a, black arrow indicates small amount of extravasated erythrocytes; scale bar: 1 mm).
Acknowledgments
Histological samples were prepared by the Cellular and Molecular Imaging Core Facility (CMIC), MRI was performed at the MR core facility, and housing and care of animals was provided by the Comparative Medicine Core Facility (CoMed), all at the Norwegian University of Science and Technology (NTNU) and funded by the Faculty of Medicine and Central Norway Regional Health Authority.
The project is supported by The Research Council of Norway, NANO2021 grant number 220005 and grant number 228604, Central Norway Regional Health Authority and Center for Innovative Ultrasound Solutions (CIUS).
References
- 1.Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32:1959–72. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220:640–6. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- 3.Etame AB, Diaz RJ, Smith CA, Mainprize TG, Hynynen K, Rutka JT. Focused ultrasound disruption of the blood-brain barrier: a new frontier for therapeutic delivery in molecular neurooncology. Neurosurg Focus. 2012;32:E3. doi: 10.3171/2011.10.FOCUS11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, Hynynen K. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med Biol. 2004;30:979–89. doi: 10.1016/j.ultrasmedbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Khokhlova TD, Haider Y, Hwang JH. Therapeutic potential of ultrasound microbubbles in gastrointestinal oncology: recent advances and future prospects. Therap Adv Gastroenterol. 2015;8:384–94. doi: 10.1177/1756283X15592584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin KH, Dayton PA. Current status and prospects for microbubbles in ultrasound theranostics. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5:329–45. doi: 10.1002/wnan.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unga J, Hashida M. Ultrasound induced cancer immunotherapy. Adv Drug Deliv Rev. 2014;72:144–53. doi: 10.1016/j.addr.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Blood-Brain Barrier Disruption Using Transcranial MRI-Guided Focused Ultrasound. U.S. National Institute of Health; 2016. [Google Scholar]
- 9.Safety of BBB Opening With the SonoCloud. U.S. National Institute of Health; 2016. [Google Scholar]
- 10.Carpentier A, Canney M, Vignot A, Reina V, Beccaria K, Horodyckid C, Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Science translational medicine; 2016. p. 8. [DOI] [PubMed] [Google Scholar]
- 11.McDannold N, Vykhodtseva N, Raymond S, Jolesz FA, Hynynen K. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: histological findings in rabbits. Ultrasound Med Biol. 2005;31:1527–37. doi: 10.1016/j.ultrasmedbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Chopra R, Vykhodtseva N, Hynynen K. Influence of exposure time and pressure amplitude on blood-brain-barrier opening using transcranial ultrasound exposures. ACS Chem Neurosci. 2010;1:391–8. doi: 10.1021/cn9000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu HL, Hua MY, Chen PY, Chu PC, Pan CH, Yang HW. et al. Blood-Brain Barrier Disruption with Focused Ultrasound Enhances Delivery of Chemotherapeutic Drugs for Glioblastoma Treatment. Radiology. 2010;255:415–25. doi: 10.1148/radiol.10090699. [DOI] [PubMed] [Google Scholar]
- 14.Samiotaki G, Vlachos F, Tung YS, Konofagou EE. A quantitative pressure and microbubble-size dependence study of focused ultrasound-induced blood-brain barrier opening reversibility in vivo using MRI. Magn Reson Med. 2012;67:769–77. doi: 10.1002/mrm.23063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan CH, Liu HL, Ting CY, Lee YH, Huang CY, Ma YJ. et al. Submicron-Bubble-Enhanced Focused Ultrasound for Blood-Brain Barrier Disruption and Improved CNS Drug Delivery. PloS one. 2014;9:e96327. doi: 10.1371/journal.pone.0096327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDannold N, Vykhodtseva N, Hynynen K. Use of ultrasound pulses combined with Definity for targeted blood-brain barrier disruption: a feasibility study. Ultrasound in medicine & biology. 2007;33:584–90. doi: 10.1016/j.ultrasmedbio.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan CH, Ting CY, Lin HJ, Wang CH, Liu HL, Yen TC. et al. SPIO-conjugated, doxorubicin-loaded microbubbles for concurrent MRI and focused-ultrasound enhanced brain-tumor drug delivery. Biomaterials. 2013;34:3706–15. doi: 10.1016/j.biomaterials.2013.01.099. [DOI] [PubMed] [Google Scholar]
- 18.Lammers T, Koczera P, Fokong S, Gremse F, Vogt M, Storm G. et al. Theranostic USPIO-loaded Microbubbles for MR-controlled Blood-Brain Barrier Permeation. Adv Funct Mater. 2015;7:36–43. doi: 10.1002/adfm.201401199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Åslund AKO, Berg S, Hak S, Morch Y, Torp SH, Sandvig A. et al. Nanoparticle delivery to the brain - By focused ultrasound and self-assembled nanoparticle-stabilized microbubbles. J Control Release. 2015;220:287–94. doi: 10.1016/j.jconrel.2015.10.047. [DOI] [PubMed] [Google Scholar]
- 20.Liu HL, Fan CH, Ting CY, Yeh CK. Combining microbubbles and ultrasound for drug delivery to brain tumors: current progress and overview. Theranostics. 2014;4:432–44. doi: 10.7150/thno.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kooiman K, Vos HJ, Versluis M, de Jong N. Acoustic behavior of microbubbles and implications for drug delivery. Adv Drug Deliv Rev. 2014;72:28–48. doi: 10.1016/j.addr.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Healey AJ, Sontum PC, Kvåle S, Eriksen M, Bendiksen R, Tornes A. et al. Acoustic Cluster Therapy: In Vitro and Ex Vivo Measurement of Activated Bubble Size Distribution and Temporal Dynamics. Ultrasound Med Biol. 2016;42:1145–66. doi: 10.1016/j.ultrasmedbio.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Sontum P, Kvåle S, Healey AJ, Skurtveit R, Watanabe R, Matsumura M. et al. Acoustic Cluster Therapy (ACT)-A novel concept for ultrasound mediated, targeted drug delivery. Int J Pharm. 2015;495:1019–27. doi: 10.1016/j.ijpharm.2015.09.047. [DOI] [PubMed] [Google Scholar]
- 24.van Wamel A, Healey A, Sontum PC, Kvåle S, Bush N, Bamber J. et al. Acoustic Cluster Therapy (ACT) - pre-clinical proof of principle for local drug delivery and enhanced uptake. J Control Release. 2016;224:158–64. doi: 10.1016/j.jconrel.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 25.van Wamel A, Sontum PC, Healey A, Kvåle S, Bush N, Bamber J. et al. Acoustic Cluster Therapy (ACT) enhances the therapeutic efficacy of paclitaxel and Abraxane(R) for treatment of human prostate adenocarcinoma in mice. J Control Release. 2016 doi: 10.1016/j.jconrel.2016.06.018. DOI: 10.1016/j.jconrel.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 26.Sontum PC. Physicochemical characteristics of Sonazoid, a new contrast agent for ultrasound imaging. Ultrasound Med Biol. 2008;34:824–33. doi: 10.1016/j.ultrasmedbio.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Billaud M, Ross JA, Greyson MA, Bruce AC, Seaman SA, Heberlein KR. et al. A new method for in vivo visualization of vessel remodeling using a near-infrared dye. Microcirculation. 2011;18:163–71. doi: 10.1111/j.1549-8719.2011.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen CC, Sheeran PS, Wu SY, Olumolade OO, Dayton PA, Konofagou EE. Targeted drug delivery with focused ultrasound-induced blood-brain barrier opening using acoustically-activated nanodroplets. Journal of controlled release: official journal of the Controlled Release Society. 2013;172:795–804. doi: 10.1016/j.jconrel.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang S, Samiotaki G, Olumolade O, Feshitan JA, Konofagou EE. Microbubble type and distribution dependence of focused ultrasound-induced blood-brain barrier opening. Ultrasound Med Biol. 2014;40:130–7. doi: 10.1016/j.ultrasmedbio.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samiotaki G, Konofagou EE. Dependence of the reversibility of focused- ultrasound-induced blood-brain barrier opening on pressure and pulse length in vivo. IEEE transactions on ultrasonics, ferroelectrics, and frequency control. 2013;60:2257–65. doi: 10.1109/TUFFC.2013.6644731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marty B, Larrat B, Van Landeghem M, Robic C, Robert P, Port M. et al. Dynamic study of blood-brain barrier closure after its disruption using ultrasound: a quantitative analysis. J Cereb Blood Flow Metab. 2012;32:1948–58. doi: 10.1038/jcbfm.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDannold N, Vykhodtseva N, Hynynen K. Blood-brain barrier disruption induced by focused ultrasound and circulating preformed microbubbles appears to be characterized by the mechanical index. Ultrasound Med Biol. 2008;34:834–40. doi: 10.1016/j.ultrasmedbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]