Abstract
The rat is an excellent cell transplantation model. In accordance with the innovative development of in vivo bioimaging technology, over the last decade we have been developing an engineered rat system based on transgenic technology and have been demonstrating the usefulness of the system with genetically encoded imaging probes such as fluorescent and luminescent proteins. In cooperation with the Japan Society for Organ Preservation and Medical Biology (President: Professor T. Asano), we have also been using luciferase-Tg rats for research into organ preservation and cell transplantation. In this minireview, we introduce the results obtained recently by using these powerful experimental tools during international collaboration in cell transplantation research.
Keywords: Cell transplantation, Living image, Translational research, Transgenic rat
Introduction
For a long time, high priority has been given to the practical application of research results “from bench to clinic.” However, recent advancement of medical sciences and development of high-level techniques are far beyond the reach of one scientist to carry out such a challenging innovative therapy. Then, specialized systems for translational research (TR) play important roles for the clinical application of novel therapeutic strategies developed through experiments in the basic sciences (12). TR is at the forefront of real and relevant improvements in the management of some diseases. This new concept of TR in innovative medical treatment includes an abundance of techniques and knowledge in various fields of science. Researchers who use TR must have high-level experimental skills and knowledge in physiology, immunology, pharmacology, molecular biology, tissue engineering, and ethics; they must also be able to apply their abilities in the clinical setting to continuously improve treatments for patients with severe illnesses.
Efficient experimental animal models are indispensable for the development of innovative medical treatments. The rat is a perfect animal for medical and surgical experiments, because its physiology is well understood, and (unlike mice) rats are large enough for reasonably easy surgical manipulation. Therefore, many rat models of human diseases have been used in research in fields such as physiology, pharmacology, and transplantation immunology. At Jichi Medical University, we have developed transgenic (Tg) technology in rats and have succeeded in establishing a wide variety of Tg rat strains for use in biomedical research. For example, neural stem cells isolated from Tg rats and introduced into the brains of white matter disease models at a specific age of development can generate functional oligodendrocytes capable of contributing to remyelination (3,14,16). Our Tg rat is a valued experimental tool because of its brighter expression than that of any other Tg rat (18). Moreover, we have opened up a new field of bioimaging study using our colorful variations of Tg rats (19), which have great potential for use in the study of cell trafficking after cell transplantation. Tissue organogenesis and transdifferentiation of stem cells are additional targets of TR. In this minireview, we introduce our recent achievements using these Tg rats, focusing on in vivo bioimaging technology.
Fluorescence Technology in Tg Rats
We initially produced green fluorescent proteintransgenic (GFP-Tg) rats as tools for organ transplantation research (6). The GFP gene construct was designed for ubiquitous expression, and the Wistar rat strain was chosen as the Tg carrier. Among the cells obtained from the bone marrow, spleen, and peripheral blood of GFP-Tg Wistar rats, 77%, 91%, and 75%, respectively, were GFP positive. To examine the migration of GFP-positive cells after organ transplantation, we performed pancreas grafts with or without spleen transplantation, heart grafts with or without lung transplantation, and auxiliary liver and small bowel transplantation from GFP-Tg rats to Lewis (LEW) (RT1(1)) rats given tacrolimus at 0.64 mg/kg for 2 weeks. GFP-positive donor cells from these GFP-Tg Wistar rats were detected in the fully allogeneic LEW rats after organ transplantation. These results showed that the GFP-Tg rat is a useful tool for studying cell migration after organ transplantation without the need for donor cell staining.
A Toronto group has studied GFP expression in the tissues of different transgenic rodents—namely, our GFP-Tg Wistar strain, a Sprague–Dawley (SD) rat strain [SD-Tg(GFP)Bal], and an M mouse strain [Tg(GFPU)5Nagy/J]—by direct fluorescence visualization of native GFP expression and by immunohistochemistry (18). Tg strains constitutively expressing GFP showed tissue-specific differences in GFP expression, and GFP immunohistochemistry amplified the fluorescence signals. The Toronto group assessed in vivo the fluorescence of stem/progenitor cells, cultured as neurospheres, from the ependymal region of the spinal cords of adult GFP-SD and -Wistar rat strains. After the cells had been transplanted into the spinal cords of wildtype rats, the trackability of the grafted cells was evaluated in vivo. Cultured stem or progenitor cells from the SD rat strain required GFP immunostaining to be visualized. Likewise, after the transplantation of SD rat-derived cells into the spinal cord, immunohistochemical amplification of the GFP signal was required for detection. In contrast, GFP expression of stem or progenitor cells generated from our Wistar rat strain was readily detected by using direct fluorescence both in vitro and in vivo, without the need for immunohistochemical amplification. The cultured stem or progenitor cells transplanted into the spinal cord survived for at least 49 days after transplantation and continued to express GFP, demonstrating stable expression of the GFP transgene in vivo.
The Toronto group also has characterized, in vitro, the native differentiation potential of spinal cord neural progenitor cells (NPCs) isolated from these GFP-Tg Wistar rats (14). Neurospheres were induced to differentiate, immunocytochemistry was performed, and the GFPpositive cells were counted and expressed as percentages of the Hoechst 33342-positive nuclei in 10 random fields. Oligodendrocytes constituted most of the NPC progeny (58.0% per field of differentiated cells; 23.4% of cells in undifferentiated spheres). Astrocytes accounted for 18.0% per field of differentiated cells and neurons for 7.4%, but they accounted for only 2.8% and 0.85%, respectively, of the cells in undifferentiated spheres. The number of differentiated radial glia was 73.0%, and the number of undifferentiated spheres was 80.9%. Unlike ependymal region spheres, the spheres derived from the peripheral white matter of the spinal cord produced glial-restricted precursors. These findings indicate that ependymal NPCs in adult rat spinal cord differentiate preferentially into oligodendrocytes and radial glia; these cells may support axonal regeneration in future trials of transplant therapy for spinal cord injury.
The intensity of GFP expression in Tg rats could not be controlled even when the same ubiquitous promoter was used, because the inserted genes were randomly integrated into the rats' chromosomes; in addition, the copy numbers of the genes expressed upon DNA injection were determined by chance. With our collaboration, PhoenixBio (YS New Technology, Tochigi, Japan) produced several GFP-Tg rat lines. From these lines, we then developed new inbred Tg rat strains with GFP (10). These GFP-Tg rats expressed most of the GFP marker gene ubiquitously, unlike previous Tg LEW rats (Fig. 1). Immunological antigenicity against GFP protein was evaluated by using conventional skin grafting, and the results suggested that GFP-Tg-derived skin was highly immunogenic (data not shown). However, GFP-positive cells from parental Tg rats were still good candidates for the study of cellular fate in sites of immune privilege, such as the brain and spinal cord, without the use of immunosuppressive drugs.
Figure 1.
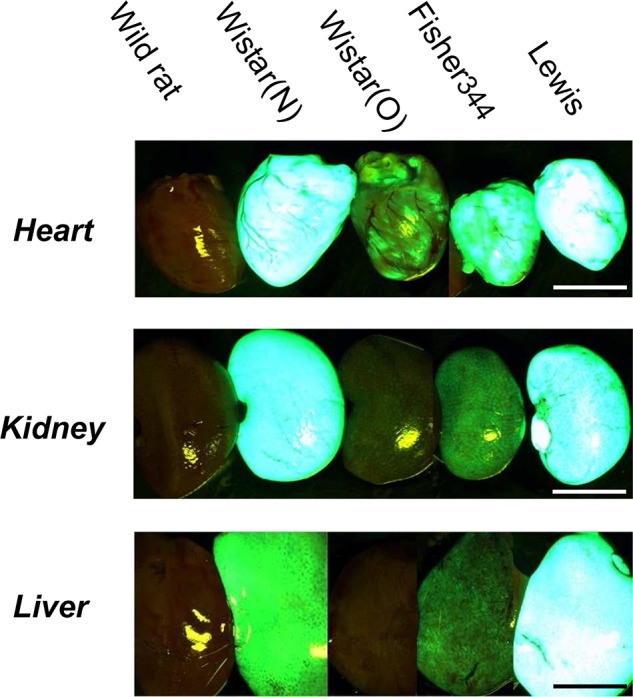
Green fluorescent protein (GFP) expression in various tissues of GFP-transgenic (Tg) rats. Marked expression of GFP was observed under excitation light (wavelength, 489 nm). Wistar (O) [see ref. (6)] and Wistar (N) refer to two different strains of GFP-Tg rat. Scale bar: 1.0 cm.
This new GFP-Tg LEW rat has been the focus of much attention in the in vivo visualization of cell kinetics. Flow cytometric analyses of Tg rat peripheral blood have revealed that this rat has the highest proportion of GFP-positive leucocytes among all Tg rats. Using a heterotopic rat lung transplantation model, we have studied the impact of preservation on the adhesion of circulating leukocytes to the transplanted lung (1). Grafts obtained from wild-type LEW rats were compared with preserved grafts after transplantation into the new GFP-Tg LEW rats. We successfully visualized few adherent GFP-positive leukocytes in the fresh grafts, whereas within the preserved grafts the cells became attached within a few minutes.
Recent reports have also raised concerns over the feasibility of differentiating bone marrow (BM) cells into functional hepatocytes. Such augmentation is considered necessary for the potential clinical use of these cells in liver diseases. We studied the kinetics of transplanted BM cells and evaluated the effects of repeated administration of GFP-positive BM cells in rat models of carbon tetrachloride (CCl4)-induced liver damage (4). The early kinetics of transplanted BM cells were evaluated with a charge-coupled device (CCD) camera by using BM cells obtained from GFP-Tg LEW rats. BM cells were transplanted via a peripheral vein or the portal vein once, or repeatedly, by using this system. More BM cells accumulated in the damaged livers than in intact livers. Liver fibrosis was milder in the experimental group receiving repeated BM transplantation than in the group not receiving BM transplantation, and large clusters of albumin-producing cells were detected by albumin immunostaining. The injected BM cells accumulated in the damaged livers. This strategy of repeated BM transplantation has potential clinical use in enhancing the numbers of albumin-producing cells and suppressing liver fibrosis.
A New Jersey group has investigated the gliogenic potential of cells isolated from a new GFP-Tg LEW rat for application to oligodendrocyte replacement in models of white matter insult and disease (3). This Tg rat shows native GFP fluorescence in oligodendrocytes of the central nervous system, with no detectable fluorescence in astrocytes or mature neurons. By targeting a highly gliogenic period of postnatal development, we have shown in vitro that sphere-forming cultures of proliferating cells generated from the GFP-Tg brain give rise to substantial numbers of differentiated oligodendrocytes. Postnatal source tissue was significantly more gliogenic than embryonic source tissue, with more than 50% of postnatally derived cells differentiating into GFP-positive oligodendrocytes. In both isolated culture and coculture with primary neurons, differentiated oligodendrocytes exhibited an increase in the intensity of GFP fluorescence concomitant with the acquisition of mature oligodendrocyte-specific markers. Transplantation of postnatally derived GFP- positive sphere-forming cells into ethidium bromide-lesioned Wistar–Kyoto rats resulted in the engraftment and survival of GFP-positive oligodendrocytes for at least 6 weeks in the host white matter and cerebral cortex. These findings suggest that sphereforming cultures of cells isolated from the early postnatal GFP-Tg LEW rat brain are useful tools for oligodendrocyte replacement studies.
A Freiburg (Germany) group has investigated the potential of this new GFP-Tg LEW rat to supply donor tissue for neural stem cell transplantation (13,14). They compared cells derived from the ventral mesencephalons of GFP-Tg LEW rats at embryonic day (E) 14.5 (i.e., 14.5 days postconception) with those from wild-type rats in vitro and in vivo. In vitro, the GFP-Tg rat-derived cells exhibited 98% GFP expression and did not differ from wild-type rat-derived cells in any of the measured parameters. In vivo, all experimental groups showed significant compensation in rotational behavior after transplantation. There were no differences in rotational behavior or graft morphology and survival pattern, or in GFP expression between immunosuppressed and nonimmunosuppressed animals. The GFP-positive cell population of the graft included 14.7% glial fibrillary acidic protein (GFAP)-positive cells, 56.1% neuronal nuclei (NeuN)-positive cells, and 1.9% tyrosine hydroxylase (TH)-positive cells. Analysis of graft subpopulations revealed that 70.6 ± 7.7% of the GFAP-positive cells, 86.9 ± 8.0% of the NeuN-positive cells, and 80.1 ± 10.3% of the TH-positive cells coexpressed GFP.
Furthermore, the Freiburg group investigated whether fetal NPCs from the GFP-Tg LEW rat retained the ability to differentiate into neuronal cells and to integrate into the hippocampal circuitry after transplantation (16). NPCs were isolated from E14 GFP-Tg LEW and wild-type SD rat embryos. The wild-type and GFP-Tg LEW ratderived cells were expanded and induced to differentiate into neuronal lineages in vitro. GFP-expressing cells were implanted into the hippocampus, and their activity was recorded electrophysiologically 3 months thereafter. Immunohistochemical analysis confirmed neuronal differentiation, and the yield of neuronal cells was determined stereologically.
Luminescent Technology in Tg Rats
Transplantation research involving the use of stem cells requires an appropriate in vivo visualization system to monitor cellular fate over an observation period. The new field of in vivo imaging is being developed with luminescence biotechnology and involves real-time visualization of complex cellular processes in living animals. We created inbred Tg LEW rats with firefly luciferase (5). Immunogenicity against luciferase was evaluated by using a skin grafting test, and the fate of grafts was monitored with an in vivo luminescence technique.
The luciferase-Tg (Luc-Tg) rats ubiquitously expressed the marker gene. Conventional skin grafting suggested long-term acceptance of Luc-Tg rat skin on wild-type rats (>100 days). Strikingly, organ transplants (heart and small bowel) and BM cell transplants showed viability and graft acceptance, demonstrating that cells and organs from Luc-Tg rats are transplantable and that their fate can be tracked for a sufficient amount of time. Taking advantage of less immunogenic luciferase, we also examined the fate of transplanted mature hepatocytes. The transplanted hepatocytes proliferated in damaged livers, but not in healthy livers, and were monitored selectively for more than 60 days. On the basis of these findings, we proposed that the use of the Luc-Tg rat system with modern optical imaging offers a new platform for a better understanding of stem cell biology and transplantation.
Bone transplantation is important and is often used for bone defect reconstruction after trauma or in malignancy. However, the kinetics of free bone graft-derived cells remain unelucidated. We recently examined the kinetics of graft-derived cells by using transgenic rats systemically expressing firefly luciferase (22). Free iliac bone grafts derived from the Luc-Tg rats were transplanted into the subcutaneous space on the backs of wild-type LEW rats, and the kinetics of the graft-derived cells were evaluated over time by assessing the degree of luminescence. Although the luminescence emitted in the luciferase reaction decreased after transplantation, substantial luminescence was recorded from donor-derived cells, even at 180 days after transplantation, suggesting the longterm survival of the graft-derived cells. In a computed tomographic image analysis of bone grafts retrieved 180 days after transplantation, highly luminescent grafts with a sufficient number of viable graft-derived cells showed significantly greater bone graft volume and polar moment of inertia of area than did poorly luminescent grafts, indicating that the highly luminescent grafts maintained better conditions.Bone graft-derived cells might survive for a long time, and the presence of sufficient numbers of viable graftderived cells is essential for engraftment and remodeling.
The development of organ preservation solutions and the associated technology has been a major effort in tissue transplantation. However, this research takes a great deal of time and resources. We have established a novel method that uses Luc-Tg rat tissue to evaluate preservation solutions, and we have applied this technology to islet preservation (20). Primary islets were hand-picked from the Luc-Tg rat pancreas (2,5,15). The viability and condition of islets preserved with several solutions were then evaluated by using relative photon intensity. Preserved islets were transplanted into the renal capsules of nonobese diabetic–severe combined immunodeficient (NOD-SCID) mice with streptozotocin-induced type 1 diabetes (21); an intraperitoneal glucose tolerance test and a histological analysis were then performed. Luc-Tg rat islet viability was increased in a relative photon intensity-dependent manner. In the recipients of Luc-Tg rat islets preserved in extracellular-type trehalose-Kyoto (ET-K) or University of Wisconsin solution for 1 day, the abnormal hyperglycemic response to glucose injection disappeared. This study demonstrated that the use of the ET-K preservation method allowed tissue ATP synthesis to continue and ameliorated cold ischemic tissue damage during extended (24-h) isolated islet preservation.
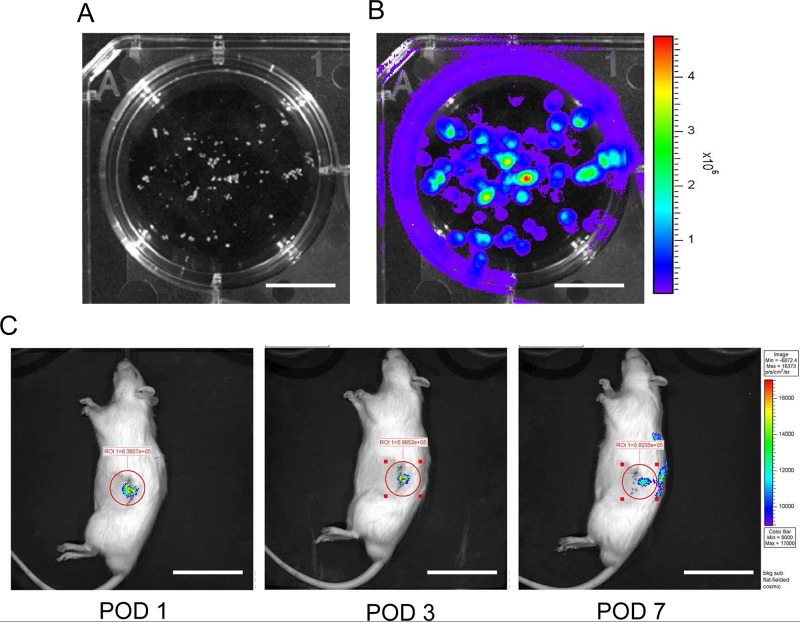
Furthermore, we transplanted Luc-Tg rat islets into the kidneys of wild-type LEW rats with streptozotocin- induced diabetes. Isolated islets were observed in culture with luminescence using the in vivo imaging system (Fig. 2A, B). Transplanted islets (1,500 IEQ per diabetic rat) adhered to the injected kidney for a long time (Fig. 2C). Transplantation of Luc-Tg rat islets into rats with streptozotocin-induced diabetes led to a decline in blood glucose level within 2 days. Thus, the Luc-Tg rat-derived islet system was useful in evaluating the transplant locus, condition, and viability. This simple method will be easily adaptable to the clinical setting and can be used to maximize the usefulness of islet transplantation and for pancreas transplant sharing with remote centers.
Figure 2.
Isolated islets from Luc-Tg rat-derived pancreases. In an in vivo imaging system (IVIS), isolated Luc-Tg rat islets emitted luminescent light in the presence of d-luciferin. (A) Phase contrast of Luc-Tg rat islets. (B) Luminescence imaging of Luc-Tg rat islets. (C) Transplanted islets in LEW rats with streptozotocin-induced diabetes were monitored using the IVIS system. Scale bars: 1.0 cm (A and B) and 5.0 cm (C). POD, postoperative day.
Dual Marker Technology by Crossbreeding Between Each Tg Rats
In light of the merits of using inbred Tg rat strains in cell or tissue transplantation, we have been creating Tg rats by using the universally used LEW rat colony.Double transgenic rats expressing GFP × red fluorescent protein (RFP) or GFP × luciferase or luciferase × LacZ were created by crossbreeding established Tg rats. In vivo bioimaging uses fluorescent proteins (e.g., GFP and RFP) as internal biological light sources and offers important opportunities for investigating a wide variety of biological processes in living cells and animals (8,17). The use of d-luciferin–luciferase technology enables the fate of transplanted cells within living animals to be detected. Use of the LacZ strain enables the presence of the translocated cells to be confirmed by using b-galactosidase (X-gal; 5-bromo-4-chloro-indolyl-b-d-galactopyranoside) staining.
Dual Fluorescent (GFP × RFP) Tg Rat
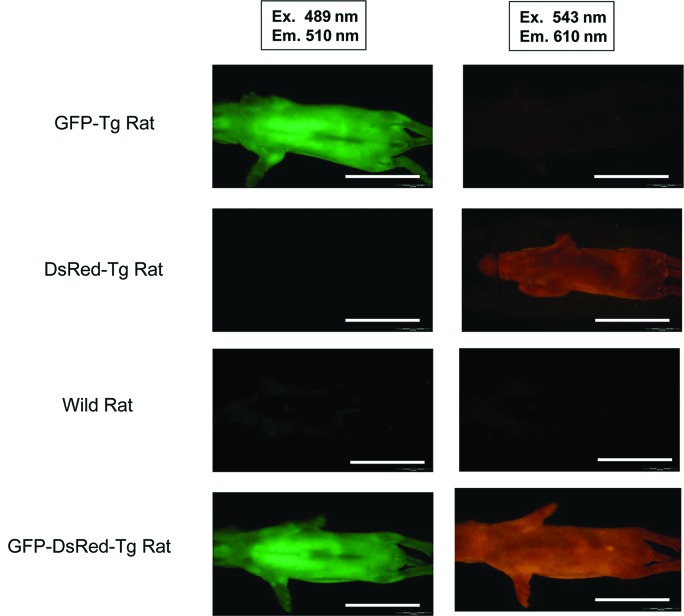
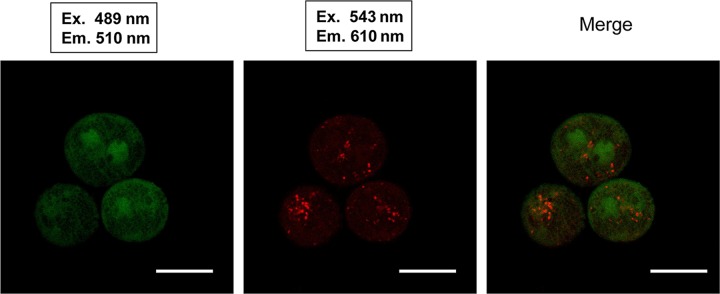
To examine the distribution of fluorescent proteins in the cells or tissues of Tg rats, we mated GFP-Tg rats and RFP-Tg rats to yield dual fluorescent marker Tg rats. We examined the fluorescence of Tg rat islets by using a CCD camera under excitation light with a wavelength of 489 nm (GFP) or 543 nm (RFP) (Olympus OV110 imaging system). Mating of a GFP-Tg rat and an RFP-Tg rat yielded one GFP–RFP dual-Tg rat (Table 1). The whole body of the transgenic rat could be detected when the rat was placed under excitation lights (Fig. 3). From the GFP–RFP dual-Tg rat, we isolated fresh hepatocytes and observed their GFP and RFP fluorescence under confocal microscopy (Fig. 4). These fluorescent proteins of Tg rats were driven by a ROSA promoter sequence. However, there was differentially localized expression of GFP and RFP in the hepatocytes from the dual-Tg rat. There were areas of GFP positivity within the cytoplasm, but RFP protein was aggregated within these areas of GFP expression. This result suggested that the differences in expression of the fluorescent proteins reflected differences in the expression regulated by the promoter sequence.
Table 1.
Results of Crossbreeding Between Green Fluorescent Protein (GFP) - and Red Fluorescent Protein (RFP) -Tg Rats
| Male | Female | Total Newborn | Type | No. |
|---|---|---|---|---|
| RFP-Tg rat | GFP-Tg rat | 12 | RFP-Tg rat | 7 |
| GFP-Tg rat | 3 | |||
| Wild-type rat | 2 | |||
| RFP-GFP-Tg rat | 0 | |||
| GFP-Tg rat | RFP-Tg rat | 7 | RFP-Tg rat | 1 |
| GFP-Tg rat | 3 | |||
| Wild-type rat | 2 | |||
| RFP-GFP-Tg rat | 1 |
Figure 3.
Differential fluorescence expression patterns in single-Tg rats (GFP or DsRed) and the dual-Tg rat (GFP-DsRed) under an Olympus-OV110 imaging system (Olympus Corp., Tokyo, Japan). Scale bar: 5.0 cm.
Figure 4.
Results of confocal microscopy in [GFP × red fluorescent protein (RFP)] dual-Tg rat-derived hepatocytes. Scale bar: 20 µm.
F1 (GFP × Luc) Tg Rat
Mesenchymal stem cells (MSCs) derived from BM have the capacity for self-renewal and differentiation and can give rise to cells of muscle, bone, adipose, or cartilage lineages. In light of this potential, MSCs are expected to be useful in cell therapy for human diseases. Intriguingly, MSCs migrate to various in vivo locations, including sites of injury or tumors. However, their fate and distribution remain unclear. To examine the characteristics of migration of MSCs to injury sites, we have examined the potential of using a photogenic Tg rat that expresses GFP and a luminescent protein (7). When synergized with modern advances in optical imaging, the photogenic rat system will provide innovative preclinical tools and a new platform on which to further our understanding of stem cell biology.
F1(Luc × LacZ) Tg Rat
The synovium plays a pivotal role during the natural course of meniscal healing and contains MSCs with high chondrogenic potential. We also have investigated whether synovium-derived MSCs injected intra-articularly enhance meniscal regeneration in massive meniscal defects in the rat. To track the injected cells, we developed transgenic rats expressing Luc and LacZ genes (7,9). Cells derived from the synovia of the rats demonstrated colonyforming ability and multipotentiality, both of which are characteristics of MSCs. Hierarchical clustering analysis revealed that the pattern of gene expression in the meniscal cells was closer to that of synovium-derived MSCs than to that of BM-MSCs. Two to eight weeks after 5 million Luc/LacZ-positive synovium-derived MSCs were injected into the massively meniscectomized stifle joints of wild-type rats, macroscopic examination revealed that the menisci had regenerated much better than in the control group. After 12 weeks, transmission electron microscopy revealed that the regenerated menisci were LacZ positive, produced type 2 collagen, and had meniscal features. Luminescence analysis revealed an increase in the abundance of photons in the meniscus-resected stifles over a 3-day period, then a decrease, without detection in any other organs. The LacZ gene derived from MSCs could not be detected in organs other than the synovium by real-time PCR. Synovium-derived MSCs injected into the massively meniscectomized stifles adhered to the lesions, differentiated directly into meniscal cells, and promoted meniscal regeneration without mobilization to distant organs.
Recently, using Luc/LacZ-Tg rats, we found that MSC transplantation provides trophic support to the ischemia-reperfusion-injured liver by inhibiting hepatocellular apoptosis and stimulating regeneration (11). We examined the fate of transplanted Luc/LacZpositive MSCs in luciferase-expressing rats by using in vivo luminescence imaging. The MSC-transplanted group showed peak luciferase activity of transplanted MSCs in the remnant liver 24 h after reperfusion, after which luciferase activity gradually declined. Elevation of serum alanine transaminase levels was significantly reduced by MSC injection. Analysis of X-gal staining revealed that almost all of the LacZ-positive MSCs were distributed around the portal triad and interlobular connective tissue 6 h after reperfusion (Fig. 5). Histopathological examination showed that there was less vacuolar change in the MSC-transplanted group than in the control group. In addition, there was a significantly smaller percentage of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells in the MSC-transplanted group than in the controls. The rate of regeneration of the remnant liver was accelerated in the MSC-transplanted group.
Figure 5.
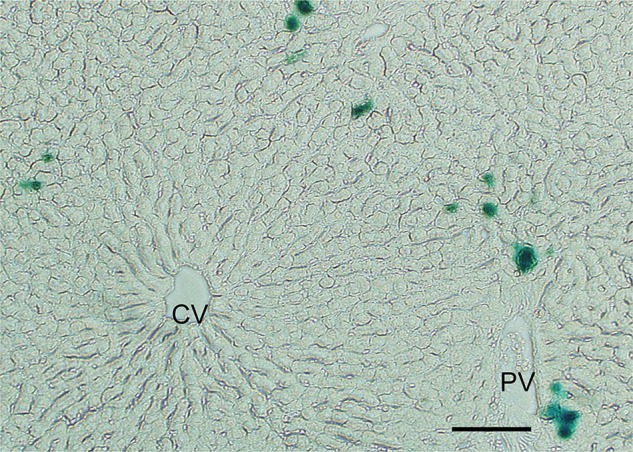
Photomicrograph of LacZ-Tg rat-derived mesenchymal stem cells (MSCs) (blue stain) distributed around the periportal area. The liver section was stained with X-gal from a rat 6 h after reflow. CV, central vein; PV, portal vein. Scale bar: 100 µm.
Conclusion
Cell transplantation strategies have emerged as potential approaches to restoring form and function to damaged tissues and to thwarting the progression of cancer. Transplanted stem cells that produce various differentiated types of cells are capable of generating tissues, and the capacity to unequivocally identify and track the transplanted cells in the host is very important. In addition, a variety of rat experimental systems, including disease models, has been developed over the past century. Coupled with the use of recent advances in genetic engineering in the rat, the use of the transgenic rats described in this minireview will likely provide innovative animal tools and help broaden our understanding of the new fields of biomedical research and cell transplantation.
Acknowledgments
Professor G. Nikkhah (Freiburg, Germany) and Professor P. Leone (New Jersey, USA) have greatly advanced the use of our GFP rats in the field of neuroscience. Professor J. Becker (Hanover, Germany) has demonstrated cell trafficking in renal disease using these rats. Professor R. H. Tolba (Aachen, Germany) has initiated the use of our imaging rats in the animal center at his university. Professor A. Keating (Toronto, Canada) has used several Tg rats. We have also collaborated with Professor Q. F. Li (Shanghai, PR China) and Professor F. C. Wei (Taipei, Taiwan) in the use of the firefly Tg rat.
Our Tg rats are available from the National Bio Resource Project for the Rat (Professor T. Serikawa; nbrprat@anim.med. kyoto-u.ac.jp) in Japan and from the Rat Resource and Research Center (Dr. Elizabeth Bryda.; RRRC, http://www.rrrc.us/) in the US. PheonixBio Co., Ltd. (Tochigi, Japan) produced our Tg rats, and many of the staff of Jichi Medical University have helped to characterize the usefulness of these rats as research tools.
Eiji Kobayashi is a Chief Scientific Advisor to Otsuka Pharmaceutical Co., Ltd. There are no patents, products in development, or marketed products to declare. The position held by E. Kobayashi does not alter the authors' adherence to all of the Cell Medicine policies on the sharing of data and materials, as described in detail online in the guide for authors. The other author declares no competing financial interests.
References
- 1. Enomoto A.; Kikuchi T.; Seo N.; Matsuno K.; Kobayashi E. Impact of cold preservation on leukocyte adhesion to the transplanted rat lung. Microsurgery 27:228–233; 2007. [DOI] [PubMed] [Google Scholar]
- 2. Fraga D. W.; Sabek O.; Hathaway D. K.; Gaber A. O. A comparison of media supplement methods for the extended culture of human islet tissue. Transplantation 65:1060–1066; 1998. [DOI] [PubMed] [Google Scholar]
- 3. Francis J. S.; Olariu A.; Kobayashi E.; Leone P. GFP-transgenic Lewis rats as a cell source for oligodendrocyte replacement. Exp. Neurol. 205:177–189; 2007. [DOI] [PubMed] [Google Scholar]
- 4. Haga J.; Wakabayashi G.; Shimazu M.; Tanabe M.; Takahara T.; Azuma T.; Sato Y.; Hakamata Y.; Kobayashi E.; Kitajima M. In vivo visualization and portally repeated transplantation of bone marrow cells in rats with liver damage. Stem Cells Dev. 16:319–328; 2007. [DOI] [PubMed] [Google Scholar]
- 5. Hakamata Y.; Murakami T.; Kobayashi E. “Firefly rats” as an organ/cellular source for long-term in vivo bioluminescent imaging. Transplantation 27:1179–1184; 2006. [DOI] [PubMed] [Google Scholar]
- 6. Hakamata Y.; Tahara K.; Uchida H.; Sakuma Y.; Nakamura M.; Kume A.; Murakami T.; Takahashi M.; Takahashi R.; Hirabayashi M.; Ueda M.; Miyoshi I.; Kasai N.; Kobayashi E. Green fluorescent protein-transgenic rat: A tool for organ transplantation research. Biochem. Biophys. Res. Commun. 286:779–785; 2001. [DOI] [PubMed] [Google Scholar]
- 7. Hara M.; Murakami T.; Kobayashi E. In vivo bioimaging using photogenic rats: Fate of injected bone marrow-derived mesenchymal stromal cells. J. Autoimmun. 30:163–171; 2008. [DOI] [PubMed] [Google Scholar]
- 8. Hoffman R. M. The multiple uses of fluorescent proteins to visualize cancer in vivo. Nat. Rev. Cancer 10:796–806; 2005. [DOI] [PubMed] [Google Scholar]
- 9. Horie M.; Sekiya I.; Muneta T.; Ichinose S.; Matsumoto K.; Saito H.; Murakami T.; Kobayashi E. Intra-articular Injected synovial stem cells differentiate into meniscal cells directly and promote meniscal regeneration without mobilization to distant organs in rat massive meniscal defect. Stem Cells 27:878–887; 2009. [DOI] [PubMed] [Google Scholar]
- 10. Inoue H.; Ohsawa I.; Murakami T.; Kimura A.; Hakamata Y.; Sato Y.; Kaneko T.; Takahashi M.; Okada T.; Ozawa K.; Francis J.; Leone P.; Kobayashi E. Development of New inbred transgenic strains of rats with LacZ or GFP. Biochem. Biophys. Res. Commun. 329:288–295; 2005. [DOI] [PubMed] [Google Scholar]
- 11. Kanazawa H.; Fujimoto Y.; Teratani T.; Iwasaki J.; Kasahara N.; Negishi K.; Tsuruyama S.; Uemoto S.; Kobayashi E. Bone marrow-derived mesenchymal stem cells ameliorate hepatic ischemia reperfusion injury in a rat model. PLoS ONE 6:e19195; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kobayashi E.; Montero E. F. A new concept of translational research in surgery. Act. Cir. Bras. 20:194; 2005. [DOI] [PubMed] [Google Scholar]
- 13. Krause M.; Ganser C.; Kobayashi E.; Papazoglou A.; Nikkhah G. The Lewis GFP transgenic rat strain is a useful cell donor for neural transplantation. Cell Transplant. Epub ahead of print; 2012. [DOI] [PubMed] [Google Scholar]
- 14. Kulbatski I.; Mothe A. J.; Keating A.; Hakamata Y.; Kobayashi E.; Tator C. H. Oligodendrocytes and radial glia derived from adult rat spinal cord progenitors: Morphological and immunocytochemical characterization. J. Histochem. Cytochem. 55:209–222; 2007. [DOI] [PubMed] [Google Scholar]
- 15. Lacy P. E.; Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes 16:35–39; 1967. [DOI] [PubMed] [Google Scholar]
- 16. Lepski G.; Jannes C. E.; Wessolleck J.; Kobayashi E.; Nikkhah G. Equivalent neurogenic potential of wild-type and GFP-labeled fetal-derived neural progenitor cells before and after transplantation into the rodent hippocampus. Transplantation 91:390–397; 2011. [DOI] [PubMed] [Google Scholar]
- 17. Lippincott-Schwartz J.; Patterson G. H. Development and use of fluorescent protein markers in living cells. Science 300:87–91; 2003. [DOI] [PubMed] [Google Scholar]
- 18. Mothe A. J.; Kulbatski I.; van Bendegem R. L.; Lee L.; Kobayashi E.; Keating A.; Tator C. H. Analysis of green fluorescent protein expression in transgenic rats for tracking transplanted neural stem/progenitor cells. J. Histochem. Cytochem. 53:1215–1226; 2005. [DOI] [PubMed] [Google Scholar]
- 19. Murakami T.; Kobayashi E. Color-engineered rats and luminescent LacZ imaging: A new platform to visualize biological processes. J. Biomed. Optics 10:41204; 2005. [DOI] [PubMed] [Google Scholar]
- 20. Negishi K.; Teratani T.; Iwasaki J.; Kanazawa H.; Kasahara N.; Lefor A. T.; Uemoto S.; Fujimoto Y.; Kobayashi E. Luminescence technology in preservation and transplantation for rat islet. Islets 3:111–117; 2011. [DOI] [PubMed] [Google Scholar]
- 21. Soria B.; Roche E.; Berná G.; León-Quinto T.; Reig J. A.; Martín F. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes 49:157–162; 2000. [DOI] [PubMed] [Google Scholar]
- 22. Yamaguchi A.; Murakami T.; Takahashi M.; Kobayashi E.; Sugawara Y. Luminescence imaging of regenerating free bone graft in rats. Plast. Reconstr. Surg. 127:78–87; 2011. [DOI] [PubMed] [Google Scholar]