Abstract
Hepatocyte transplantation is hoped to be an alternative treatment to certain cases of liver transplantation. The most promising indication is for congenital metabolic diseases, especially in infants. To establish a safe protocol for hepatocyte transplantation into infants, we examined physiological changes during treatment using infantile pigs. Recipient pigs (domestic, crossbred with Large-Yorkshire, Landrace, and Duroc; 2.5 kg; 7–14 days old) were anesthetized with isoflurane; a midline incision of minimum length provided access to the superior mesenteric vein. A double lumen catheter was inserted through the vein to within 1 cm of the hepatic portal region. Physiological parameters such as heart rate, systemic blood pressure, and portal pressure were monitored. Cryopreserved porcine hepatocytes isolated from the same strain were suspended in physiological saline and transfused through the catheter. In experiments requiring tracing, cells were stained with fluorescent dye prior to transfusion. Recipient pigs were kept for 1 day and sacrificed for histological liver examination. After preliminary experiments, the optimized number and concentration for hepatocyte transplantation were determined to be 1 × 108 cells/kg and 1 × 107 cells/ml. The cell suspension was transfused at a rate of 0.67 ml/min. No marked anomaly of physiological parameters was observed, whereas light tachycardia occurred in preliminary trials when the transfusion rate was faster than our standard protocol. All five pigs transfused with the established method recovered from anesthesia and survived with good vital signs. Histology revealed that the transfused hepatocytes were integrated in the hepatic tissue.
Keywords: Hepatocyte transplantation, Safe protocol, Infantile pigs
Introduction
Clinical interest for hepatocyte transplantation is becoming stronger with progress in the regenerative medicine research and donor shortage (2,4). Compared with liver transplantation, hepatocyte transplantation is thought to have numerous advantages: (1) it is less invasive; (2) partial or temporary liver support is feasible for congenital metabolic disorders or fulminant hepatic failure; (3) it provides efficient use of marginal donors, because cryopreservation of isolated hepatocytes is easier than that of a whole organ or tissue; (4) it provides a ready-to-use cellular medicine when frozen. On the other hand, the treatment also presents technical and essential difficulties for clinical trial design. For instance, there are many points for consideration, such as purpose, indication, end points, dose, route, hepatocyte characteristics (i.e., auto or allogeneic), fresh or cryopreserved, and so forth. The biggest problem to overcome is the elucidation of therapeutic potential.
To establish an optimal protocol, we need an appropriate animal model to answer each question. Tentatively, we set urea cycle disorders such as ornithine transcarbamylase deficiency as an indication and bridge to liver transplantation. Technically, allogeneic cryopreserved hepatocytes are transfused into the portal vein, as in islet transplantation.
Ornithine transcarbamylase deficiency occurs in 1 in 80,000 live births due to a single gene mutation. The mutation is X-linked recessive and is thus severer in boys. The enzyme ornithine transcarbamylase is responsible for ammonium detoxification. In the fetus, ammonia is detoxified by the maternal urea cycle, but immediately after birth, the ammonia level increases because of a lack of enzyme. Long-lasting hyperammonemia damages the central nervous system and results in irreversible mental retardation. The best and definitive treatment is liver transplantation, although since the treatment involves a size limitation, the infant must wait until his body weight is over 5 kg, preferably 6 kg. Conventional treatments include administration of medicines such as benzoic acid and blood dialysis. However, significant number of cases are nonresponsive to these treatments. Thus, hepatocyte transplantation may provide an effective alternative.
This communication reports the establishment of a safe protocol for hepatocyte transplantation and defines optimal cell number, density, and injection rate in an animal model that closely mimics clinical procedure.
Materials and Methods
Animals
Domestic young pigs (crossbred with Large-Yorkshire, Wrandrace, and Dulroc) for hepatocyte donors (15 kg, 8 weeks old) and infantile recipient pigs (2 kg, 7–10 days old) were purchased from Saitama Experimental Animals (Saitama, Japan).
Hepatocyte Isolation, Preservation, and Thawing
Hepatocytes were isolated from donor pig liver by the collagenase (032-10534 Wako Pure Chemicals, Osaka, Japan) perfusion method (6). Isolated hepatocytes were purified to parenchymal hepatocytes by low-speed centrifugation (50 × g, 1 min, three times). Viability, assessed by trypan blue exclusion test, was approximately 80%. The parenchymal hepatocytes were suspended at a concentration of 1 × 107 live cells/ml in 25 ml of storage solution composed of dimethyl sulfoxide-containing medium (CP-1, Kyokuto Pharmaceutical, Co. Ltd, Tokyo, Japan), packed in a cell storage bag (F-050, NIPRO, Osaka, Japan), and frozen in an ultra low temperature (−80°C) freezer. The frozen hepatocytes were stored in the air phase of a liquid nitrogen reservoir until use. Cryopreserved hepatocytes were thawed in a warm bath (37°C) until the ice crystals disappeared. The cell suspension was diluted with 10 volumes of ice-cold Dulbecco's modified eagle medium and centrifuged at 50 × g for 2 min. The cells were suspended in approximately 10 ml saline and filtered through nylon mesh (pore size, 100 × 100 μm), and cell number and viability were estimated. Prior to injection, cell density was adjusted to 1 × 107 cells/ml by adding saline, and 2 × 108 live cells were drawn into a 30- or 50-ml plastic syringe. Since the thawed cell viability was approximately 60%, the volume of cell suspension ranged from 30 to 35 ml. In some experiments, the hepatocytes were stained with fluorescent dye (CellTracker orange, C-2927; Invitrogen, CA, USA).
Hepatocyte Transplantation
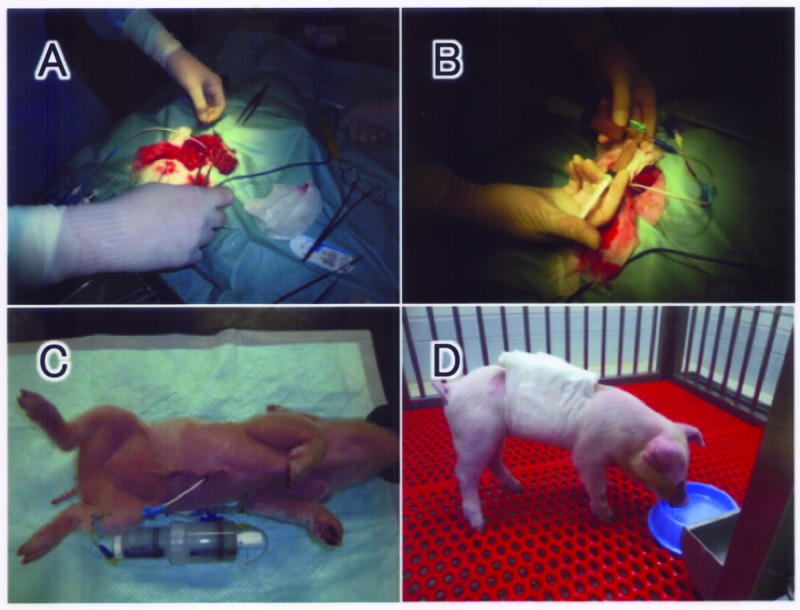
The recipient piglet was anesthetized with inhaled anesthetic (Isoflurane, Abbott Laboratories, IL, USA) and received a minimal midline abdominal incision. A double lumen cannula (18G, Medicut LCV-UK; Tyco Healthcare, NJ, USA) was inserted into the supramesenteric vein to within 1 cm of the hepatic portal region (Fig. 1A). The proximal end was used for portal pressure assessment and the distal end for hepatocyte transplantation. A T-shaped stopcock was connected to the inlet at the distal end of the cannula, and two syringes were set in the stopcock (Fig. 1B). The large syringe was used as a hepatocyte reservoir (30 or 50 ml), and the small one (5 ml) was used as an injection pump. A portion of cell suspension was transferred in a 5-ml syringe and carefully transfused to avoid excessive increase in the portal pressure. The average injection speed was approximately 0.67 ml/min, that is, 20 ml of the cell suspension was transfused in 30 min. After finishing the transfusion, the double lumen catheter was connected to a continuous injector syringe filled with 20% glucose to keep the catheter open (Fig. 1C). The piglets recovered from anesthesia and were kept in the animal room (Fig. 1D). The liver was harvested for histological investigation 1 day after hepatocyte transplantation.
Figure 1.
Outline of hepatocyte transplantation. Under inhalation anesthesia, a double lumen cannula was inserted into the supramesenteric vein (A). A T-shaped stopcock was connected to the inlet at the distal end of the cannula, and two syringes were set in the stopcock. The large syringe was used as a hepatocyte reservoir, and the small one for injection (B). After finishing the transfusion, the double lumen catheter was connected with a continuous injector syringe filled with 20% glucose to keep the catheter open (C). The piglets recovered from anesthesia and were kept in the animal room (D).
Ethical Considerations
The institutional animal ethics committee (reference no. 2007-004) approved all experimental procedures.
Results
Calculation of Number of Hepatocytes Required for Treatment
A theoretically necessary number of hepatocytes was calculated in order to design the treatment for hyperammonemia in infants. A prerequisite for treatment is to reduce blood ammonia from 1,000 to 500 μg/dl; the former is regarded as a critical level that may cause irreversible neurological deterioration. Given that the blood volume of an infant weighing 2 kg is 160 ml, the amount of ammonium that should be metabolized is 48 μmol. According to our previous report wherein the ammonia removal activity of isolated rat hepatocytes was 1 × 10−13 mol/h/cell (5), the time (t) required to decrease ammonia is expressed as t = 4.8 × 108/x (x, number of hepatocytes) (Fig. 2). Based on this formula, 1 × 108 hepatocytes will reduce the ammonia level in 4.8 h, and 2 × 108 hepatocytes will reduce it in 2.4 h.
Figure 2.
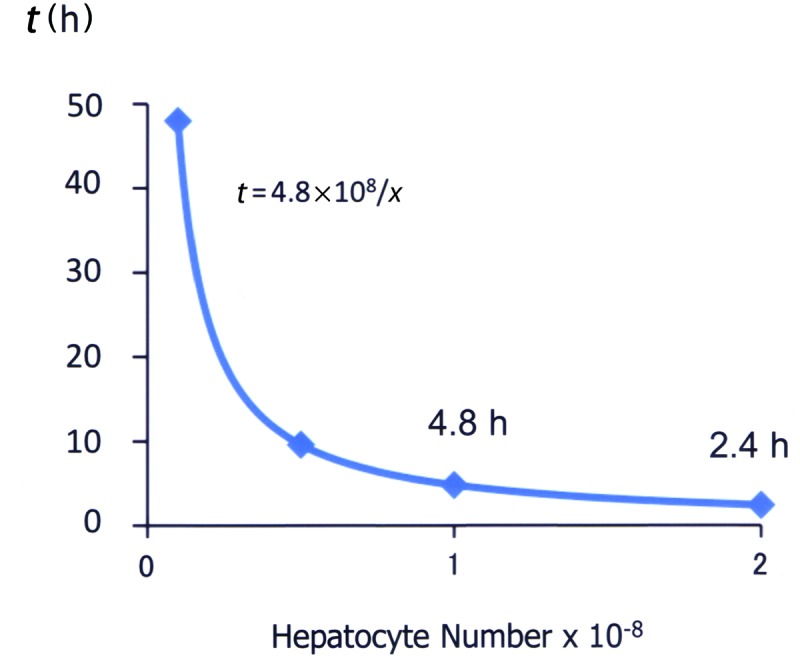
Calculation of number of hepatocytes required for treatment for hyperammonemia in an infant with urea cycle disorder. Given that the blood volume of an infant (2 kg) is 160 ml and that the amount of ammonia that should be metabolized is 48 μmol, the time (t) to decrease ammonia is expressed as t = 4.8 × 108/x, where x is number of hepatocytes. Details were described in the text.
In this hypothesis, transplanted hepatocytes are not necessarily engrafted in the liver. As far as the cells metabolize ammonia and do no harm, transplanted hepato cytes can stay in any extrahepatic part of the body. This means the treatment should be fast-acting. In our preliminary experiment, the ammonia removal activity of cryopreserved human hepatocytes was estimated to be 1.12 × 10−13 mol/h/cell (average of two lots; 1.35 and 0.88 × 10−13 mol/h/cell each). Therefore, the above calculation is reasonable and applicable for the clinical study of hepatocyte transplantation.
Physiological Changes During Hepatocyte Transplantation
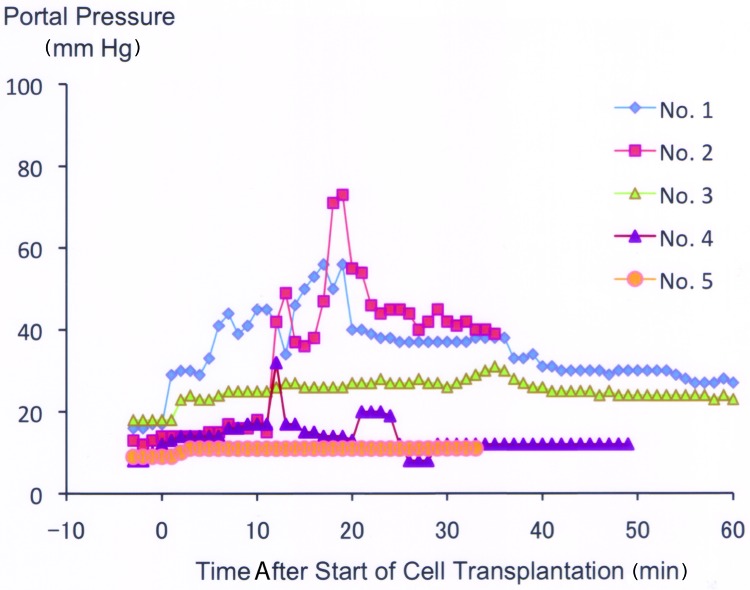
Figure 3 shows time course changes in portal pressure of five trials of hepatocyte transplantation performed by our established procedure. The data are presented in chronological order. Portal pressure increased along with transfusion of hepatocytes in early cases yet, when carefully applied, there was only a minimal increase. A clear learning curve was observed from trial 1 to trial 5. Whereas the pressure values in trials 1 and 2 ranged irregularly and high during transplantation, those of trials 3–5 remained stable. Most notably, the pressure in trial 5 increased only slightly throughout the treatment. Methods to prevent pressure elevation include (1) suspending the cells well using two syringes just before injection and (2) refraining from injection for a while when the portal pressure rises. In a preliminary experiment, light tachycardia occurred when the transfusion rate was faster than our standard protocol (data not shown). All five piglets recovered from anesthesia 2–4 h after the hepatocyte transplantation procedure and were moved back to the animal room.
Figure 3.
Time course changes in portal pressure of five trials of hepatocyte transplantation. The data indicated by serial numbers are aligned in a chronological order.
One day after transplantation, all five piglets showed good vital signs in the animal room (Fig. 1D). The macroscopic observation of harvested liver showed slight irregular shading on the liver surface (Fig. 4A). The histology beneath the patterned area showed the existence of donor cells (Fig. 4B, C).
Figure 4.
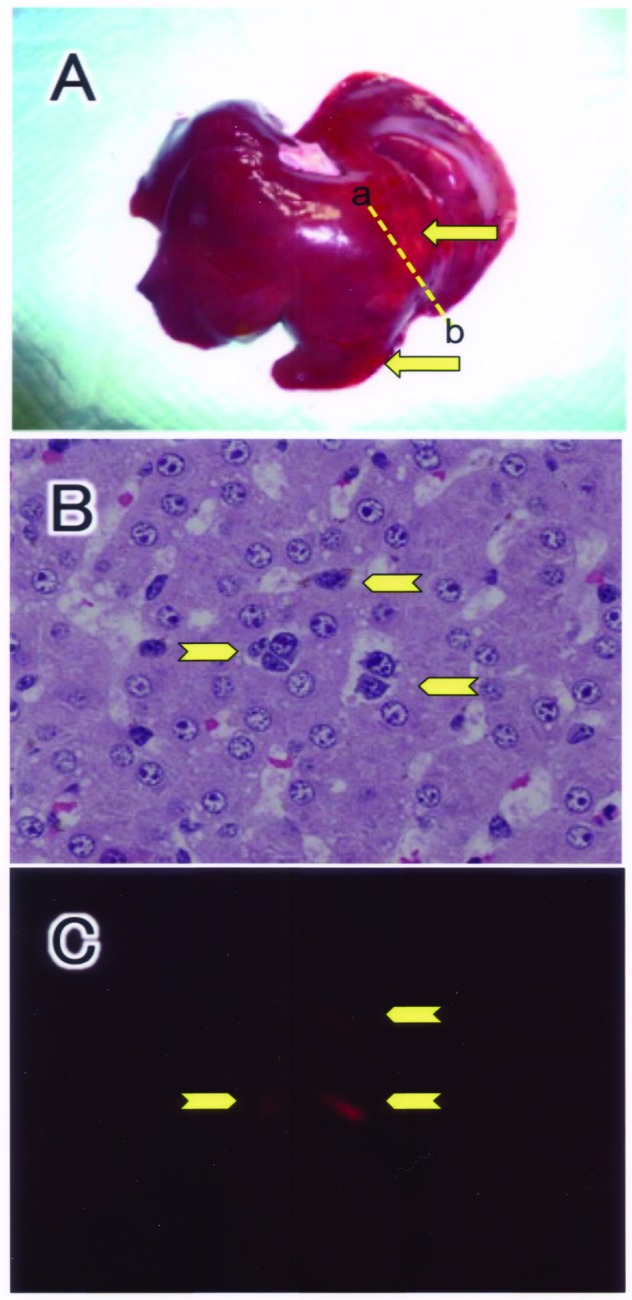
Morphology of the liver 1 day after hepatocyte transplantation. Typical macroscopic view of the liver taken from piglet no. 3 (A). The piglet was transplanted with fluorescencelabeled hepatocytes. The dashed line between a and b indicates the cut plane of the specimen for microscopic examination. Microscopic view of hematoxylin and eosin staining (B) and fluorescence micrograph (C). Transplanted hepatocytes are indicated by arrows.
Discussion
The aim of this communication is the establishment of a safe protocol for hepatocyte transplantation with particular focus on the number and density of cells transfused. Before designing the protocol, we set the indication after a long discussion. Although hepatocyte transplantation is hoped to provide an alternative to liver transplantation, the latter is performed to treat various diseases such as fulminant hepatic failure, cirrhosis, hepatoblastoma, metabolic disorder, etc. Hepatocyte transplantation has been also tried in these diseases (1), although the numbers in each trial were small. Unfortunately, the efficacy of hepatocyte transplantation is still unclear. This lack of clarity may be due to the nature of cell transplantation. While it is mandatory for a transplanted organ to be anastomosed to connect to the recipient circulation, transplanted cells are left passively in the tissue. This feature is prominent when pancreas transplantation and islet transplantation are compared. Even after the Edmonton protocol for islet transplantation was recognized (7), pancreas transplantation is still the first-line choice. The major problem in islet transplantation is disappearance in the transplant site (3), even though islets are much larger than hepatocytes. In addition, it takes time for isolated cells to become integrated and start working in the tissue. Moreover, because the donor cells are substantially damaged at the time of isolation, they may need time for recovery. The latter should be noted as an intrinsic difference from hematopoietic cell transplantation.
However, live cells have unknown biological effects (1). For instance, our previous work showed that an extracorporeal bioartificial liver containing a relatively small number of hepatic cells prolonged the survival of hepatic failure pigs.
Taking all circumstances into consideration, our purpose was set on keeping an infantile patient of hyperammonemia to bridge to liver transplantation, avoiding irreversible mental disorder by ammonemia. In summary, we established a basic protocol for an experimental hepatocyte transplantation model. Using this protocol, we will study hepatocyte survival, side effects, feasibility of repeated transfusion, and ammonia removal efficacy.
Acknowledgments
The authors thank Mr. Taichi Kawaguchi for his skillful technical assistance. This work was supported by grants from the National Center for Child Health and Development (22A6 and 22A8). The authors declare no conflict of interest.
References
- 1. Enosawa S.; Miyashita T.; Saito T.; Omasa T.; Matsumura T. The significant improvement of survival times and pathological parameters by bioartificial liver with recombinant HepG2 in porcine liver failure model. Cell Transplant. 15:873–880; 2006. [DOI] [PubMed] [Google Scholar]
- 2. Fisher R. A.; Strom S. C. Human hepatocyte transplantation: Worldwide results. Transplantation 82:441–449; 2006. [DOI] [PubMed] [Google Scholar]
- 3. Johansson H.; Lukinius A.; Moberg L.; Lundgren T.; Berne C.; Foss A.; Felldin M.; Källen R.; Salmela K.; Tibell A.; Tufveson G.; Ekdahl K. N.; Elgue G.; Korsgren O.; Nilsson B. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes 54:1755–1762; 2005. [DOI] [PubMed] [Google Scholar]
- 4. Lee K. W.; Lee J. H.; Shin S. W.; Kim S. J.; Joh J. W.; Lee D. H.; Kim J. W.; Park H. Y.; Lee S. Y.; Lee H. H.; Park J. W.; Kim S. Y.; Yoon H. H.; Jung D. H.; Choe Y. H.; Lee S. K. Hepatocyte transplantation for glycogen storage disease type Ib. Cell Transplant. 16:629–637; 2007. [DOI] [PubMed] [Google Scholar]
- 5. Omasa T.; Yamanaka M.; Tanimura N.; Katakura Y.; Kishimoto M.; Suga K.; Enosawa S. Expression and amplification of glutamine synthetase gene endows HepG2 cells with ammonia-metabolizing activity for bioartificial liver support system. Enzyme Microb. Technol. 35:519–524; 2004. [Google Scholar]
- 6. Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 13:29–83; 1976. [DOI] [PubMed] [Google Scholar]
- 7. Shapiro A. M. J.; Ricordi C.; Hering B. J.; Auchincloss H.; Robert Lindblad R.; Robertson R. P.; Secchi A.; Brendel M. D.; Berney T.; Brennan D. C.; Cagliero E.; Alejandro R.; Ryan E. A.; DiMercurio B.; Morel P.; Polonsky K. S.; Reems J. A.; Bretzel R. G.; Bertuzzi F.; Froud T.; Kandaswamy R.; Sutherland D. E.; Eisenbarth G.; Segal M.; Preiksaitis J.; Korbutt G. S.; Barton F. B.; Viviano L.; Seyfert-Margolis V.; Bluestone J.; Lakey J. R. International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med. 355:1318–1330; 2006. [DOI] [PubMed] [Google Scholar]