Abstract
Coagulation factor IX (FIX) is synthesized by hepatocytes, and the lack of this protein causes hemophilia B. Liver nonparenchymal cells, including liver sinusoidal endothelial cells (LSECs) and extrahepatic cells in the body, are scarcely shown to have an ability to synthesize and secrete FIX. The present study investigated the existence of cells responsible for synthesizing FIX other than hepatocytes in mice using gene expression analyses and FIX-specific clotting assays. Among the several organs investigated, including liver, lung, spleen, kidney, brain, intestine, and tongue, FIX mRNA expressions were observed only in the liver. From the liver, hepatocytes and LSECs were isolated. FIX mRNA expression and FIX protein secretion were observed exclusively in the hepatocytes. Furthermore, the clotting activity of FIX secreted from the cultured hepatocytes was found to be dependent on the concentration of vitamin K2. These findings indicated that the hepatocyte is the only cell type that biochemically produces functional FIX in vivo. This highlights the importance of hepatocytes or cells that are fully differentiated toward the hepatic lineage for possible application for regenerative medicine and for targeting gene delivery to establish new cell-based treatments for hemophilia B.
Keywords: Factor IX, Hemophilia B, Hepatocyte, Nonparenchymal cell
Introduction
Coagulation factor IX (FIX) is one of the vitamin K-dependent serine proteases essential for blood coagulation. The lack of this protein causes hemophilia B, a recessive X-chromosome-linked congenital bleeding disorder (3,13). Patients having this inherited disorder can suffer from unpredictable, recurrent, spontaneous bleeding in various areas, including soft tissues, major joints, and occasionally in internal organs. Standard treatment for hemophilia B is either on-demand or prophylactic therapy with plasma-derived or recombinant human FIX concentrate. However, commercially available FIX concentrates are expensive, and this type of treatment requires lifelong frequent intravenous infusion, which can give a significant impact on economic resources as well as the quality of life for the patient. Under these circumstances, curable therapeutic options that are free from FIX concentrates have been desired and investigated. FIX is mainly produced in the liver, and so liver-directed gene therapy (15) and liver transplantation (14) have been attempted as new types of therapies for hemophilia B. Considering the fact that only 1% elevation in biologically active FIX levels in plasma can attenuate the bleeding diathesis of hemophilia B (3), the symptom of hemophilia B could be attenuated by cell-based therapy using FIX-producing cells.
FIX has been reported to be synthesized by mature hepatocytes in the liver (4). Based on this report, our laboratory transplanted hepatocytes isolated from wildtype mice to hemophilia B mice (20,26). Hepatocyte transplantation to either the liver or under the kidney capsule sites of hemophilia B mice led to 1–3% increase in plasma FIX activity level in the recipient mice (20,26). Apart from FIX, hepactocytes produce many forms of coagulation factors including fibrinogen, factor VII, and factor VIII (4). Our group has previously reported the therapeutic potential of hepatocyte-based therapy for factor VIII deficiency using a mice model (21). At present, however, the hepatocytes used in clinical hepatocyte transplantation are isolated from the discarded liver pieces that are unable to be used for liver transplantation due to their marginal quality. This severely limits the number of viable donor hepatocytes (22).
In this study, to further develop cell-based therapies for hemophilia B, we investigated the existence of cells, which can synthesize FIX, other than hepatocytes in mice using gene expression analyses and FIX-specific clotting assays. More specifically, nonparenchymal cells in the liver, particularly liver sinusoidal endothelial cells (LSECs), and extrahepatic organs were isolated from mice to detect and assess the function of biologically active FIX. Therefore, if alternate cell types or organs could produce functionally active FIX other than hepatocytes, obstacles regarding donor cell shortage are resolved.
Materials and Methods
Animals
Female wild-type FVB/N mice (CLEA Japan, Tokyo, Japan) were used at 8–12 weeks of age. Experimental protocols were developed in accordance with the guidelines outlined by the Institutional Animal Care and Use Committee at Tokyo Women's University.
Isolation of Primary Hepatocytes and Liver Sinusoidal Endothelial Cells (LSECs)
Mouse primary hepatocytes and liver sinusoidal cells were isolated from the livers of FVB/N wild-type mice using an in situ collagenase perfusion method (1). Isolated hepatocytes were separated from nonparenchymal cells by three rounds of low-speed centrifugation at 50×g, followed by Percoll (Percoll™, American Biosciences, Uppsala, Sweden) isodensity centrifugation. Hepatocytes with viabilities of >90%, as quantified by a trypan blue dye exclusion test, were used in this study. Supernatants after the low-speed centrifugations were used for LSECs isolation. Supernatants containing nonparenchymal cell fractions were filtered through a 40-μm filter and blocked with purified rat antimouse CD16/CD32 antibody (BD Biosciences, San Jose, CA). LSEC fraction was enriched by a magnetic cell sorting instrument (Miltenyi Biotech, Gladbach, Germany) with a magnetic antibody targeted for mouse CD146 (Miltenyi Biotech). Each fraction of isolated cells was partly snap-frozen for subsequent gene expression analyses, and the remaining cells were seeded in type I collagen-coated six-well culture dishes (Iwaki, Tokyo) at a density of 7.5 × 105 cells per well for both hepatocytes and LSECs. After the cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Sigma, St. Louis, MO) containing 10% fetal bovine serum with supplements (L-glutamine, HEPES buffer, penicillin, and streptomycin) for 8 h in humidified culture chamber at 37°C, floating cells were removed, and the culture medium was replaced with hepatocyte culture medium lacking serum. This medium was composed of DMEM supplemented with 20 mmol/L HEPES, 10−7 mol/L dexamethasone (Sigma), 0.5 μg/ml insulin (Wako, Tokyo, Japan), 30 μg/ml L-proline (Wako), 10 mM nicotinamide (Kanto Chemicals, Tokyo, Japan), 10 ng/ml epidermal growth factor (PeproTech, Rocky Hill, NJ), 0.2 mmol/L ascorbic acid-2 phosphate (Wako), 1% di-methyl sulfoxide (DMSO; Sigma), 100 IU/ml penicillin, and 100 μg/ml streptomycin. Vitamin K2 (chemical name: menaquinone; Kaytwo N, Eisai, Tokyo) was also added to the culture medium at final concentrations of 0.1, 1, and 10 μg/ml. After 16-h cell culture, the media were collected for factor IX activity assay.
RNA Isolation and cDNA Synthesis
The liver, lung, spleen, kidney, brain, intestine, and tongue were collected from four FVB/N mice. Total RNA was extracted from each organ or isolated liver cell samples using RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. To eliminate genomic DNA contamination, the extracted total RNA was treated with DNase I (Qiagen). Aliquots of total RNA samples were diluted, and the concentration and purity of each sample was measured at wavelengths of 260 nm (A260) and 280 nm (A280) using a Nanodrop 2000 Spectrophotometer (Thermo Scientific, Wilmington, DE). The A260/A280 ratios for each of the total RNA samples were between 1.9 and 2.1. The total RNA (1 μg) was reverse-transcribed using High Capacity RNA-to-cDNA Master Mix (Applied Biosystems, Foster City, CA), as described by the manufacturer's instructions.
Real-Time PCR for FIX Expression Levels
Quantitative real-time PCR analysis was performed using a PRISM 7300 Sequence Detector with a universal PCR master mix according to the specifications of the manufacturer (Applied Biosystems). TaqMan probes and primers for mouse FIX (Mm01308427_m1) and mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Mm99999915_g1) were chosen from TaqMan Gene Expression Assay (Applied Biosystems). The integrity and purity of the extracted organ samples were evaluated by assessing the expression levels of genes that were known to be specifically expressed in the respective organs: albumin (Alb) (Mm00802090_m1) for the liver, NK2 homeobox 1 (Nkx2-1) (Mm00447558_m1) for the lung, spleen tyrosine kinase (Syk) (Mm01333032_m1) for the spleen, nephrosis 1, congenital, Finnish type (nephrin) (Nphs1) (Mm00497828_m1) for the kidney, sex-determining region Y-box 11 (Sox11) (Mm01281943_s1) for the brain, and defensin-related sequence cryptdin peptide (Defcr-rs1) ( Mm00655850_ m1) for the intestine. All real-time PCR analyses were performed in duplicate using 96-well optical reaction plates (Applied Biosystems), and the following cycling conditions were used: 10 min at 95°C, followed by 40 cycles of 15 s at 95°C, and 1 min at 60°C. For quantifying FIX and GAPDH gene expressions, cDNAs derived from pooled normal mouse livers were used to prepare standard reference curves.
Measurement of FIX Activity in the Cultured Medium
FIX clotting activities of cultured medium were measured based on one-stage clotting assay with human FIX-deficient plasma and ThromboCheck APTT-SLA (Sysmex, Kobe, Japan) using a KC4 Delta Coagulometer (Trinity Biotech, Co Wicklow, Ireland). Briefly, 50 μl of one tenth of the diluted samples, together with the same amount of FIX-deficient plasma and APTT-SLA, was incubated at 37°C for 4 min, followed by the addition of 50 μl of 0.02 mol/L CaCl2. The activities were calculated from the clotting time based on the standard values of pooled plasma collected from FVB/N mice.
Statistical Analysis
The significant levels of comparisons between two groups were performed by Student's t test. Differences between three or more groups were tested using ANOVA. If ANOVA showed significant differences, the significances were evaluated by the Tukey's HSD test. The level of significance was set at p < 0.05.
Results
Validation of the Extraction of Organ Samples
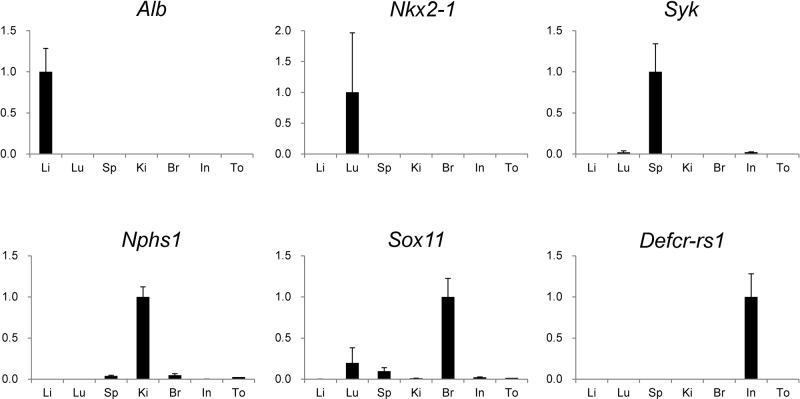
The integrity and purity of the extracted organ samples were validated by assessing their specific gene expressions by real-time PCR (n=4). As demonstrated in Figure 1, Alb, Nkx2-1, Syk, Nphs1, Sox11, and Defcr-rs1 were highly expressed in the liver, lung, spleen, kidney, brain, and intestine, respectively. This indicated that the organ samples were appropriately extracted and processed.
Figure 1.
Validation of the extraction of organ samples. Using extracted organ samples, the gene expression levels of albumin (Alb), NK2 homeobox 1 (Nkx2-1), spleen tyrosine kinase (Syk), nephrosis 1, congenital, Finnish type (nephrin) (Nphs1), sex-determining region Y-box 11 (Sox11), and defensin-related sequence cryptdin peptide (Defcr-rs1) were evaluated by real-time PCR (n=3). Data were normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression levels and analyzed by Δ–ΔCt method. Values were graphed as comparative ratios to the levels in their specific organs and were represented as mean ± standard error of the mean (SEM). Li, liver; Lu, lung; Sp, spleen; Ki, kidney; Br, brain; In, Intestine; To, tongue.
FIX Gene Expression in Liver and Extrahepatic Mouse Organs
Mouse FIX mRNA expression levels in several organs, including liver, lung, spleen, kidney, brain, intestine, and tongue, were evaluated by real-time PCR (n=4). FIX mRNA expression was exclusively detected in the liver, with the expression in other extrahepatic organs being undetectable (Fig. 2).
Figure 2.
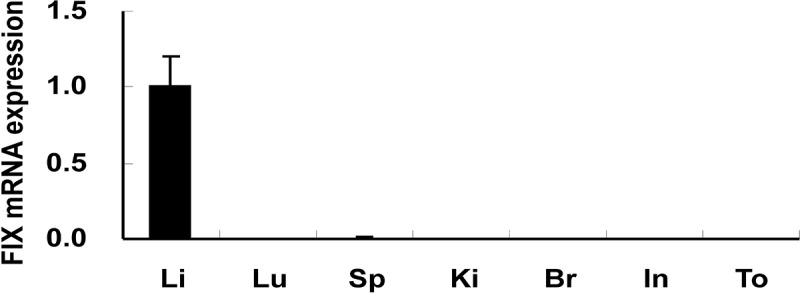
Coagulation factor IX (FIX) gene expression in mouse organs. Mouse FIX mRNA expression levels in several mouse organs were determined by real-time PCR (n=4). Data were normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression levels and graphed as a comparative ratio to the liver. Values were represented as mean±SEM. Li, liver; Lu, lung; Sp, spleen; Ki, kidney; Br, brain; In, Intestine; To, tongue.
FIX Gene Expression in Fractions of Isolated Liver Cells
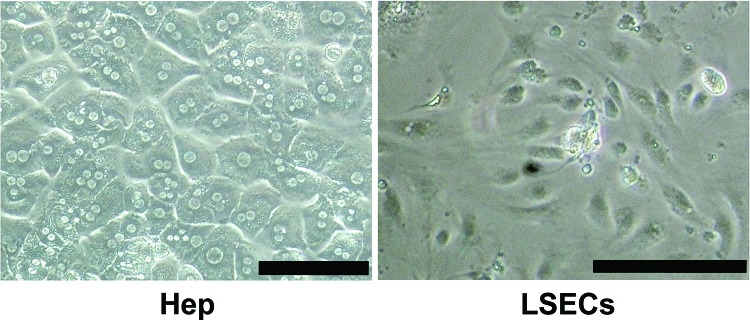
Liver cells were isolated by a collagenase perfusion method from the livers of FVB/N mice. The hepatocyte fraction was purified by Percoll isodensity centrifugation, and LSECs fraction was condensed by magnetic cell sorting. As shown in Figure 3, the former cell fraction showed a cuboidal, organelle-rich, and binucleate cell morphology, which is commonly observed in cultured hepatocytes. On the other hand, the latter cell fraction showed the typical cell morphology of endothelial cells, indicating that these cells were almost corresponding to LSECs. To obtain 1 μg of total RNA to evaluate FIX mRNA expression by real-time RT-PCR, 2.8×104 hepatocytes and 2×106 LSECs were needed. The ratios of FIX to GAPDH were 0.79±0.1 in hepatocytes and 0.11±0.06 in LSECs. Since nearly 70 times more LSECs were necessary to obtain the same amount of total RNA from the hepatocytes, the FIX expression levels per cell were recalculated to be about 0.79 and 0.0016 (0.11/70) in hepatocytes and LSECs, respectively.
Figure 3.
Cell morphology of isolated cell fractions. Liver cells were isolated from the livers of FVB/N mice by a collagenase perfusion method. Hepatocyte (Hep) fraction was purified by Percoll isodensity centrifugation, and liver sinusoidal endothelial cell (LSEC) fraction was condensed by magnetic cell sorting. Isolated cells were seeded on type I collagen-coated six-well culture dishes at a density of 7.5 × 105 cells per well and were cultured for 48 h. Scale bars: 100 μm.
Furthermore, the number of hepatocytes constituting the whole liver is known to be approximately three times more than that of LSECs. Considering this, the contribution of hepatocytes to produce FIX expression in the liver should be far greater. Upon the recalculation of the contribution ratio of both cell types to FIX mRNA expression in the liver, the ratios of hepatocytes and LSECs were 99.93% and 0.07%, respectively (Fig. 4). Therefore, FIX mRNA expression was observed exclusively in the hepatocyte fraction, and FIX expression in the LSEC fraction was below 1% of the hepatocyte fraction. This result clearly indicated that hepatocyte was the sole cell type responsible for FIX production in the liver.
Figure 4.
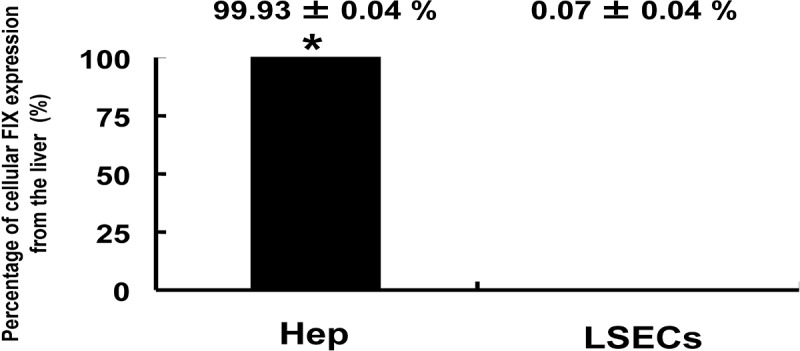
FIX gene expression in fractions of isolated liver cells. Hepatocytes (Hep) and liver sinusoidal endothelial cells (LSECs) were isolated from mouse livers by collagenase perfusion. Mouse FIX mRNA expression levels in each cell fraction were determined by real-time PCR (n=6, respectively). Data were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression levels and graphed as a comparative ratio to the liver. Values were represented as mean±SEM. Asterisks indicate significant differences.
Measurement of FIX Activity in the Culture Medium of Cultured Liver Cells
To demonstrate whether cultured hepatocytes could secrete biologically active FIX, FIX clotting activities in the culture medium of hepatocytes and LSECs were measured (Fig. 5). Basal clotting activities were observed in the culture media collected from hepatocytes incubated in the absence of vitamin K2 (0.2±0.03%). In the presence of increasing doses of vitamin K2 at 0.1, 1, and 10 mg/ml, clotting activities were detected to be similarly increased in a dose-dependent manner at 0.28±0.04%, 0.59±0.04%, and 0.96±0.02%, respectively). In marked contrast, there was no measurable FIX activity in the medium harvested from LSECs.
Figure 5.
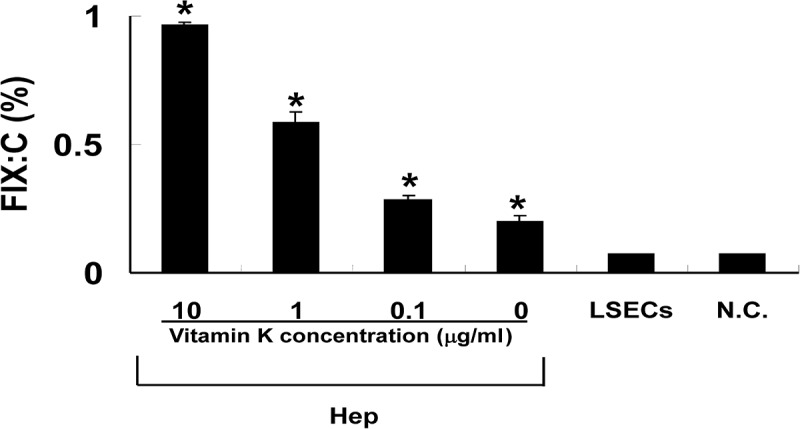
Measurement of FIX activity in the culture medium of cultured liver cells. Isolated hepatocytes and liver sinusoidal endothelial cells were cultured for 16 h in serum-free culture media. Vitamin K2 was added to the media at increasing concentrations from 0.1 to 10 μg/ml. FIX clotting activity in the media was measured by a one-stage clotting assay (n=6, respectively). Data were expressed as the percentage of normal mouse plasma. Values were represented as mean±SEM. Hep, hepatocytes; LSECs, liver sinusoidal endothelial cells; N.C., cell-free control media. Asterisks indicate significant differences versus N.C.
Discussion
The present study was conducted to precisely investigate alternate cell type(s) capable of producing biologically active FIX in vivo. Our results showed that the liver was the only organ that expressed FIX mRNA among the organs and tissues tested in vivo, which was comparable to our previous experimental report using mouse organs from a different strain (C57Bl/6) (10). Furthermore, FIX mRNA expression and FIX production were observed exclusively in isolated hepatocytes, but not in isolated LSECs. This indicated that the hepatocyte was the only cell type that produced FIX in vivo.
The findings obtained in this study elucidated that circulating FIX levels were maintained only by hepatocytes. However, a possible physiological mechanism that maintains a specific concentration of FIX in plasma (approximately 5,000 ng/ml) remains unknown. We have previously elucidated that FIX mRNA expression levels in hepatocytes are significantly suppressed when hepatocytes are in a proliferating state such as liver regeneration induced by partial hepatectomy (27) or primary mitogen (28) in mouse models. FIX mRNA expressions in cultured hepatocytes were also observed to be decreased depending on the number of days in culture (unpublished data). Elucidating the mechanism of FIX production in hepatocytes would allow the mechanism of FIX regulation to be well understood.
Several functions of hepatocytes have been reported to have a dependency on their located zone in the liver (7). However, FIX production in hepatocytes was found to be uniformly observed in the liver regardless of their location (25), indicating that no cell sorting was required in preparing hepatocytes for cell-based therapy toward hemophilia B. To develop hepatocyte-based therapy for hemophilia B, preparing enough numbers of hepatocytes, preserving their ability to produce functional FIX, is essential. At present, however, the number of donor livers remains severely restricted, and even if they are available, these livers are frequently of marginal quality (22). Furthermore, conventional procedures for the culture of primary hepatocytes are found to be unable to support extensive cell proliferation (18). To solve this problem, several approaches have been investigated such as (1) hepatocyte propagation systems in mice (25), (2) the technical refinement of hepatocyte cryopreservation for the establishment of hepatocyte bank (24), (3) cell therapy using fetal hepatocytes or hepatocyte progenitor cells (6), and (4) hepatic differentiation from several types of stem cells such as embryonic stem (ES) cells (9), induced pluripotent stem (iPS) cells (10), and mesenchymal stem cells (5).
Considering that cell-based therapy using allogenic cells requires long-term immunosuppression to achieve therapeutic cell engraftment, the safest and most feasible way is applying hemophilia B patients' own cells that will be genetically modified to produce FIX. Ex vivo gene modification of hepatocyte using viral vector has been investigated (12,19). In fact, cell therapies using ex vivo genetically modified hepatocytes have been applied for several types of liver diseases (2,16). This type of therapy is unable to be theoretically limited to hepatocytes, and other cell types that are easily harvested from the patients, such as mesenchymal stem cells derived from bone marrow (5,17) or adipose tissue, and iPS cells (30) could be viable candidate cells. However, FIX protein is known to gain its full functionality after several steps of posttranslational modification such as γ-carboxylation by γ-glutamyl-carboxylase and the propeptide cleavage by furin/paired amino acid-cleaving enzyme (PACE) (11), which are physiologically functioned in hepatocytes. Especially, γ-carboxylation is critical for FIX to obtain a binding ability to calcium ions and phospholipid surface, and this reaction requires the presence of vitamin K and its related internal enzymes including γ- glutamyl-carboxylase and vitamin K epoxide reductase. Actually, this study demonstrated that higher FIX activity was obtained in culture media in the presence of vitamin K compared with the absence of vitamin K (Fig. 5). Therefore, upon the collection of cells in preparing cell-based therapy, the cells should be carefully verified to retain an ability to perform FIX posttranslational modification and vitamin K-related enzymes for efficient FIX protein production. Recently, the generation of modified FIX gene, named triple FIX, that can bear the significant amount of FIX protein in plasma has been reported (8,29), and naturally occurring FIX mutation that demonstrates extraordinary hyper-FIX activity in plasma in spite of its normal antigen levels, leading to X-linked thrombophilia, has been identified (FIX Padua) (23). By using these mutated hyperactive FIX genes as a transduction gene to hemophilic cells, higher therapeutic efficacy could be achieved with a fewer amount of cells.
In conclusion, coagulation FIX is physiologically synthesized only by hepatocytes. Therefore, mature hepatocytes or cells that are fully differentiated to hepatic lineage could be the most appropriate cell source for cell-based therapy in hemophilia B.
Acknowledgments
The authors would like to thank Dr. Norio Ueno (Tokyo Women's Medical University) and Dr. Frank Park (Medical College of Wisconsin) for their critical reading of the manuscript, Dr. Hideki Ohdan (Hiroshima University) for his kind advice in LSEC isolation and culture, and Ms. Mamiko Yoshimura (Department of Surgery, Nara Medical University) and Ms. Yuka Bessyo (Department of Pediatrics, Nara Medical University) for their technical assistance. The present study was supported in part by Special Coordination Funds for Promoting Science and Technology (K.O. and T.O.), Grant-in-Aid (K.O. no. 21300180), and Global Center of Excellence Program (K.O) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) Japan. The authors declare no conflict of interest.
References
- 1. Berry M. N.; Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: A biochemical and fine structural study. J. Cell Biol. 43:506–520;1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Birraux J.; Menzel O.; Wildhaber B.; Jond C.; Nguyen T. H.; Chardot C. A step toward liver gene therapy: Efficient correction of the genetic defect of hepatocytes isolated from a patient with Crigler-Najjar syndrome type 1 with lentiviral vectors. Transplantation 87:1006–1012;2009. [DOI] [PubMed] [Google Scholar]
- 3. Bolton-Maggs P. H.; Pasi K. J. Haemophilias A and B. Lancet 361:1801– 1809; 2003. [DOI] [PubMed] [Google Scholar]
- 4. Boost K. A.; Auth M. K.; Woitaschek D.; Kim H. S.; Hilgard P.; Nadalin S.; Blaheta R. A. Long-term production of major coagulation factors and inhibitors by primary human hepatocytes in vitro: Perspectives for clinical application. Liver Int. 27:832–844;2007. [DOI] [PubMed] [Google Scholar]
- 5. Coutu D. L.; Cuerquis J.; El Ayoubi R.; Forner K. A.; Roy R.; Francois M.; Griffith M.; Lillicrap D.; Yousefi A. M.; Blostein M. D.; Galipeau J. Hierarchical scaffold design for mesenchymal stem cell-based gene therapy of hemophilia B. Biomaterials 32:295–305;2011. [DOI] [PubMed] [Google Scholar]
- 6. Fiegel H. C.; Lange C.; Kneser U.; Lambrecht W.; Zander A. R.; Rogiers X.; Kluth D. Fetal and adult liver stem cells for liver regeneration and tissue engineering. J. Cell. Mol. Med. 10:577–587;2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goto Y.; Ohashi K.; Utoh R.; Yamamoto M.; Okano T. Hepatocyte transplantation through the hepatic vein: A new route of cell transplantation to the liver. Cell Transplant. 20:1259–1270;2011. [DOI] [PubMed] [Google Scholar]
- 8. Kao C. Y.; Lin C. N.; Yu I. S.; Tao M. H.; Wu H. L.; Shi G. Y.; Yang Y. L.; Kao J. T.; Lin S. W. FIX-Triple, a gain-of-function factor IX variant, improves haemostasis in mouse models without increased risk of thrombosis. Thromb. Haemost. 104:355–365;2010. [DOI] [PubMed] [Google Scholar]
- 9. Kasuda S.; Kubo A.; Sakurai Y.; Irion S.; Ohashi K.; Tatsumi K.; Nakajima Y.; Saito Y.; Hatake K.; Pipe S. W.; Shima M.; Yoshioka A. Establishment of embryonic stem cells secreting human factor VIII for cell-based treatment of hemophilia A. J. Thromb. Haemost. 6:1352–1359;2008. [DOI] [PubMed] [Google Scholar]
- 10. Kasuda S.; Tatsumi K.; Sakurai Y.; Kato J.; Taminishi S.; Takeda T.; Ohashi K.; Okano T.; Hatake K.; Shima M. Expression of coagulation factors from murine iPS-derived liver cells. Blood Coagul. Fibrinolysis 22:271–279;2011. [DOI] [PubMed] [Google Scholar]
- 11. Kaufman R. J. Post-translational modifications required for coagulation factor secretion and function. Thromb. Haemost. 79:1068–1079;1998. [PubMed] [Google Scholar]
- 12. Kuge H.; Ohashi K.; Yokoyama T.; Kanehiro H.; Hisanaga M.; Koyama F.; Bumgardner G. L.; Kosai K.; Nakajima Y. Genetic modification of hepatocytes towards hepatocyte transplantation and liver tissue engineering. Cell Transplant. 15:1–12;2006. [PubMed] [Google Scholar]
- 13. Kurachi K.; Davie E. W. Isolation and characterization of a cDNA coding for human factor IX. Proc. Natl. Acad. Sci. USA 79:6461–6464;1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Merion R. M.; Delius R. E.; Campbell D. A. Jr.; Turcotte J. G. Orthotopic liver transplantation totally corrects factor IX deficiency in hemophilia B. Surgery 104:929–931;1988. [PubMed] [Google Scholar]
- 15. Nathwani A. C.; Davidoff A. M.; Tuddenham E. G. Prospects for gene therapy of haemophilia. Haemophilia 10:309–318;2004. [DOI] [PubMed] [Google Scholar]
- 16. Nguyen T. H.; Mainot S.; Lainas P.; Groyer-Picard M. T.; Franco D.; Dagher I.; Weber A. Ex vivo liver-directed gene therapy for the treatment of metabolic diseases: Advances in hepatocyte transplantation and retroviral vectors. Curr. Gene Ther. 9:136–149;2009. [DOI] [PubMed] [Google Scholar]
- 17. Oh T.; Peister A.; Ohashi K.; Park F. Transplantation of murine bone marrow stromal cells under the kidney capsule to secrete coagulation factor VIII. Cell Transplant. 15:637–645;2006. [DOI] [PubMed] [Google Scholar]
- 18. Ohashi K.; Park F.; Kay M. A. Hepatocyte transplantation: Clinical and experimental application. J. Mol. Med. 79:617–630;2001. [DOI] [PubMed] [Google Scholar]
- 19. Ohashi K.; Park F.; Schwall R.; Kay M. Efficient gene transduction to cultured hepatocytes by HIV-1 derived lentiviral vector. Transplant. Proc. 34:1431–1433;2002. [DOI] [PubMed] [Google Scholar]
- 20. Ohashi K.; Tatsumi K.; Utoh R.; Takagi S.; Shima M.; Okano T. Engineering liver tissues under the kidney capsule site provides therapeutic effects to hemophilia B mice. Cell Transplant. 19:807–813;2010. [DOI] [PubMed] [Google Scholar]
- 21. Ohashi K.; Waugh J. M.; Dake M. D.; Yokoyama T.; Kuge H.; Nakajima Y.; Yamanouchi M.; Naka H.; Yoshioka A.; Kay M. A. Liver tissue engineering at extra-hepatic sites in mice as a potential new therapy for genetic liver diseases. Hepatology 41:132–140;2005. [DOI] [PubMed] [Google Scholar]
- 22. Ostrowska A.; Bode D. C.; Pruss J.; Bilir B.; Smith G. D.; Zeisloft S. Investigation of functional and morphological integrity of freshly isolated and cryopreserved human hepatocytes. Cell Tissue Bank. 1:55–68;2000. [DOI] [PubMed] [Google Scholar]
- 23. Simioni P.; Tormene D.; Tognin G.; Gavasso S.; Bulato C.; Iacobelli N. P.; Finn J. D.; Spiezia L.; Radu C.; Arruda V. R. X-linked thrombophilia with a mutant factor IX ( factor IX Padua). N. Engl. J. Med. 361:1671–1675;2009. [DOI] [PubMed] [Google Scholar]
- 24. Stephenne X.; Najimi M.; Sokal E. M. Hepatocyte cryopreservation: Is it time to change the strategy? World J. Gastroenterol. 16:1–14;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tatsumi K.; Ohashi K.; Kataoka M.; Tateno C.; Shibata M.; Naka H.; Shima M.; Hisanaga M.; Kanehiro H.; Okano T.; Yoshizato K.; Nakajima Y.; Yoshioka A. Successful in vivo propagation of factor IX-producing hepatocytes in mice: Potential for cell-based therapy in haemophilia B. Thromb. Haemost. 99:883–891;2008. [DOI] [PubMed] [Google Scholar]
- 26. Tatsumi K.; Ohashi K.; Shima M.; Nakajima Y.; Okano T.; Yoshioka A. Therapeutic effects of hepatocyte transplantation on hemophilia B. Transplantation 86:167–170;2008. [DOI] [PubMed] [Google Scholar]
- 27. Tatsumi K.; Ohashi K.; Taminishi S.; Sakurai Y.; Ogiwara K.; Yoshioka A.; Okano T.; Shima M. Regulation of coagulation factors during liver regeneration in mice: Mechanism of factor VIII elevation in plasma. Thromb. Res. 128:54–61;2011. [DOI] [PubMed] [Google Scholar]
- 28. Tatsumi K.; Ohashi K.; Taminishi S.; Takagi S.; Utoh R.; Yoshioka A.; Shima M.; Okano T. Effects on coagulation factor production following primary hepatomitogen- induced direct hyperplasia. World J. Gastroenterol. 15: 5307–5315;2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu Y. M.; Kao C. Y.; Huang Y. J.; Yu I. S.; Lee H. S.; Lai H. S.; Lee P. H.; Lin C. N.; Lin S. W. Genetic modification of donor hepatocytes improves therapeutic efficacy for hemophilia B in mice. Cell Transplant. 19:1169–1180;2010. [DOI] [PubMed] [Google Scholar]
- 30. Xu D.; Alipio Z.; Fink L. M.; Adcock D. M.; Yang J.; Ward D. C.; Ma Y. Phenotypic correction of murine hemophilia A using an iPS cell-based therapy. Proc. Natl. Acad. Sci. USA 106:808–813;2009. [DOI] [PMC free article] [PubMed] [Google Scholar]