Abstract
We sought to determine whether hepatic progenitor cells can be isolated from cirrhotic liver using epithelial cell adhesion molecule (EpCAM) or Thy-1 markers. Liver tissue with cirrhosis secondary to biliary atresia (BA) was collagenase digested, and nonparenchymal cells (NPCs) were cultivated for 24 h. Noncirrhotic NPCs derived from patients with carbamyl phosphate synthetase and ornithine transcarbamylase deficiencies were used as controls. Flow cytometric analysis demonstrated that the percentages of EpCAM- and Thy-1-positive cells were significantly higher in NPC populations derived from BA liver than in those derived from control liver. Reverse transcription polymerase chain reaction analysis revealed that EpCAM-positive sorted cells expressed EpCAM, Thy-1, albumin, and CK-19, whereas Thy-1-positive sorted cells expressed Thy-1, albumin, and CK-19. These findings indicate that EpCAM- or Thy-1-positive hepatic progenitor cells can be more efficiently isolated from BA liver than from control liver and suggest that the properties of EpCAM-positive cells are somewhat different from those of Thy-1-positive cells.
Keywords: Biliary atresia, Cirrhosis, Hepatic progenitor cells, Flow cytometry, EpCAM, Thy-1
Introduction
Hepatic stem cells are an alternative to liver transplantation for repopulation of the liver after various injuries. Hepatic stem cell oval or oval-like cells have been shown to be present in human liver (7,12). Hepatic stem cells also occur in many different chronic liver diseases. In alcoholic liver disease, nonalcoholic fatty liver disease, and cirrhosis, hepatocyte proliferation is inhibited (1,8,10). Under these conditions, the appearance and activity of hepatic stem cells are markers of disease severity. Biliary atresia (BA) is a rare and serious liver disease in newborn infants and is often followed by cirrhosis. This finding suggests that hepatic stem cells may be activated to proliferate in BA liver and that these cells may serve as a potential source for autologous use for cell transplantation.
The existence of bipotential progenitor cells in human liver has been demonstrated using different phenotypic markers (9). Reid's group have reported that epithelial cell adhesion molecule (EpCAM) is expressed by hepatic stem cells, hepatoblasts, and committed progenitors, but not by mature hepatocytes, and that sorting of EpCAMpositive cells from pediatric and adult livers results in a cell population composed mainly of hepatic stem cells (9). It has recently been reported that Thy-1 is a useful marker for the successful isolation of hepatic progenitor cells from human fetal and adult livers (6,13).
In this study, we investigated whether hepatic progenitor cells can be isolated from livers with cirrhosis secondary to BA using EpCAM or Thy-1 as markers of such cells.
Materials and Methods
Liver Tissue
Liver specimens were obtained from patients with cirrhosis secondary to BA (BA liver; n = 5; age range, 5–10 months old) and from patients with carbamyl phosphate synthetase (CPS) deficiency and ornithine transcarbamylase (OTC) deficiency (control liver; n = 3; age range, 5–10 months old) at the National Center for Child Health and Development (Tokyo, Japan). The experimental protocol was approved by the ethics committee for human experimentation at the National Center for Child Health and Development and the Kohno Clinical Medicine Research Institute (Tokyo, Japan), and informed consent was obtained from all patients.
Liver specimens from patients with CPS or OTC deficiency were used as controls in this study because of the absence of cirrhosis in these livers. Histological examination showed that all liver specimens with BA exhibited cirrhosis (Fig. 1). However, no signs of cirrhosis were observed in control liver tissues (data not shown).
Figure 1.
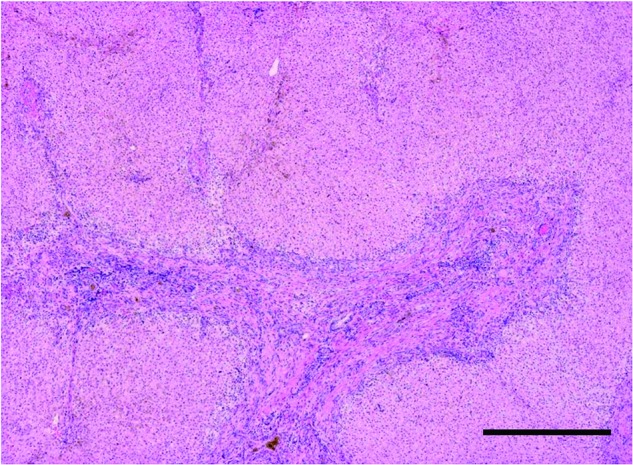
Histology of liver tissue from a patient with cirrhosis secondary to biliary atresia (BA). Hematoxylin and eosin staining. Scale bar: 250 μm.
Isolation and Culture of Nonparenchymal Liver Cells
Liver cell suspensions were prepared using the collagenase perfusion method, as described elsewhere (3). Cells were suspended in Williams' E medium containing 10% fetal bovine serum (FBS), 25 ng/ml epidermal growth factor (EGF) (Sigma Chemical, Louis, MO, USA), 0.1 μM insulin (Wako Chemical, Osaka, Japan), and 1 μM dexamethasone (Sigma) and 1% penicillin–streptomycin (Invitrogen, CA, USA) and were plated on type I collagen-coated dishes in a fully humidified atmosphere containing 5% CO2.
Flow Cytometry
Cells were suspended at a concentration of 1 × 106/100 μl in phosphate-buffered saline (PBS) containing 1% FBS and were then incubated with phycoerythrin (PE)-labeled anti-EpCAM antibody (BioLegend, CA, USA), fluorescein isothiocyanate (FITC)-labeled anti-mouse monoclonal Thy-1 antibody (Beckman Coulter, CA, USA), or with PBS without antibody for 60 min at room temperature. After washing with PBS, the stained cells were analyzed and sorted on an EPICS ARTLA flow cytometer (Beckman Coulter).
RNA Extraction and Reverse Transcription Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted using an RNeasy Mini kit (Qiagen-Japan), treated with DNase (Qiagen), and used as a template for synthesis of cDNA using a first strand cDNA kit (ReverTraAce-α, Toyobo Co., Ltd., Osaka, Japan), as described previously (11). A 5-μl aliquot of cDNA was then amplified by PCR. The PCR reaction was carried out in a total volume of 50 μl in the presence of 0.2 mM dNTP, 0.25 μmol/L primers, and 1.25 U Taq DNA polymerase (MBI Fermentas, MD, USA). The PCR primers used for amplification are as follows: albumin: sense 5′-agcggcacagcacttctctaga-3′, antisense 5′-tccacacggaatg ctgccatgg-3′; EpCAM: sense 5′-ctggccgtaaactgctttgt-3′, antisense 5′-agcccatcattgttctggag-3′; α-fetoprotein (AFP): sense 5′-accaaagttaattttactgaaat-3′, antisense 5′-gtttgtcctca ctgagttggca-3′; cytokeratin-19 (CK-19): sense 5′-tcccgcg actacagccactactacacgacc-3′, antisense 5′-cgcgacttgatgtcca tgagccgctggtac-3′; glyceraldehyde-3-phosphate dehydrogenase (G3PDH): sense 5′-accacagtccatgccatcac-3′, antisense 5′-tccaccaccctgttgctgta-3′; Thy-1: sense 5′-ctagtggaccaga gccttcg-3′, antisense 5′-tggagtgcacacgtgtaggt-3′. A 25- to 40-cycle program was used for all PCR reactions. PCR consisted of denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 2 min. G3PDH was assayed as an internal loading control.
Data Analysis
Student's t test was used to evaluate the statistical significance between experimental groups.
Results
After 24 h of primary culture, the culture media were changed in order to eliminate nonadherent cells including red blood cells. Cells were epithelial-like in morphology, and there was no evidence of mature hepatocytes (data not shown). These nonparenchymal cells (NPCs) were then analyzed using flow cytometry.
Flow Cytometric Analysis
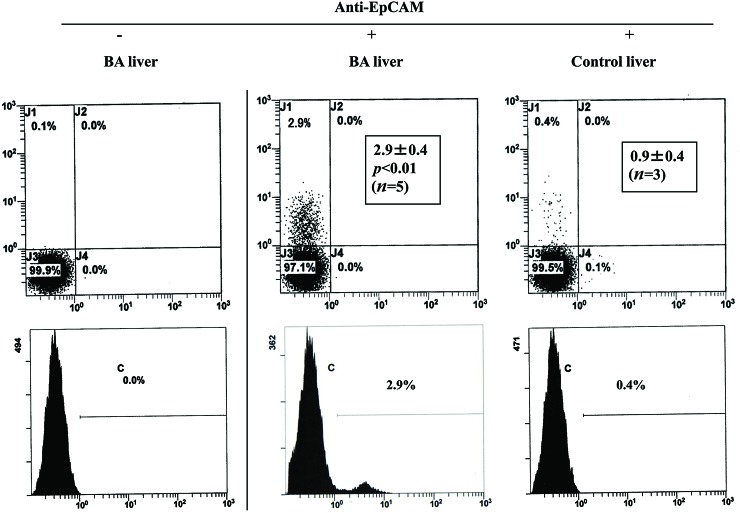
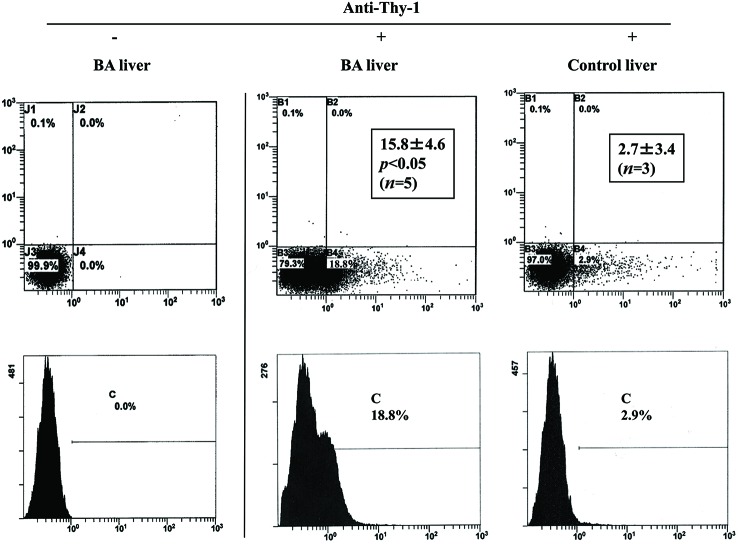
We first investigated the percentage of EpCAM- or Thy-1-positive cells in the NPC populations that were derived from BA and control liver. As shown in Fig. 2, the percentage of EpCAM-positive cells was significantly higher in the NPC populations that were derived from BA liver (2.9 ± 0.4%, n = 5) than in those derived from control liver (0.9 ± 0.4%, n = 3). Similarly, the percentage of Thy-1-positive cells was significantly higher in the NPC populations that were derived from BA liver (15.8 ± 4.6%, n = 5) than in those derived from control liver (2.7 ± 3.4%, n = 3) (Fig. 3).
Figure 2.
Flow cytometric analysis of nonparenchymal cell (NPC) populations derived from biliary atresia (BA) and control liver. Cells were stained with (+) or without (−) phycoerythrin (PE)-labeled anti-epithelial cell adhesion molecule (EpCAM) antibody and were analyzed using flow cytometry. Insets indicate the mean (±SD) percentage of EpCAM-positive cells.
Figure 3.
Flow cytometric analysis of nonparenchymal cell (NPC) populations derived from biliary atresia (BA) and control liver. Cells were stained with (+) or without (−) fluorescein isothiocyanate-labeled anti-mouse monoclonal Thy-1 antibody and were analyzed using flow cytometry. Insets indicate mean (±SD) percentage of Thy-1-positive cells.
RT-PCR Analysis
EpCAM- and Thy-1-positive cells were sorted using flow cytometry and were assayed for expression of phenotypic markers using RT-PCR as shown in Fig. 4. EpCAM-positive sorted cells expressed EpCAM, Thy-1, albumin, and CK-19 (Fig. 4A), while Thy-1-positive sorted cells expressed Thy-1, albumin, and CK-19 (Fig. 4B). These results were identical for either type of liver (data not shown). AFP was not expressed in any of the cell preparations.
Figure 4.
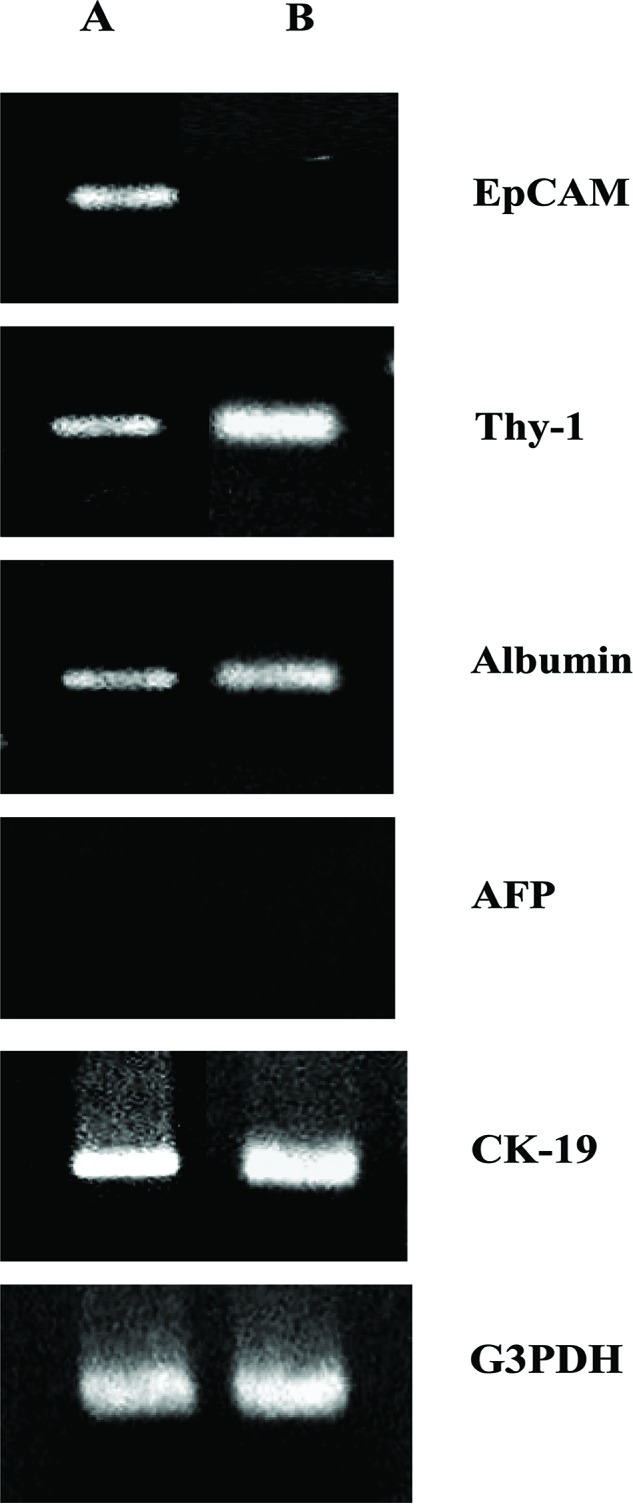
Reverse transcription and polymerase chain reaction (RT-PCR) analysis of epithelial cell adhesion molecule (EpCAM)- and Thy-1-positive sorted cells derived from biliary atresia (BA) liver. The indicated mRNA expression was assessed using RT-PCR and (A) EpCAM-positive cells (A) or Thy-1- positive cells (B). G3PDH was included as an internal loading control. The results are representative of two independent experiments. AFP, α-fetoprotein; G3PDH, glyceraldehyde-3-phosphate dehydrogenase.
Cell Culture
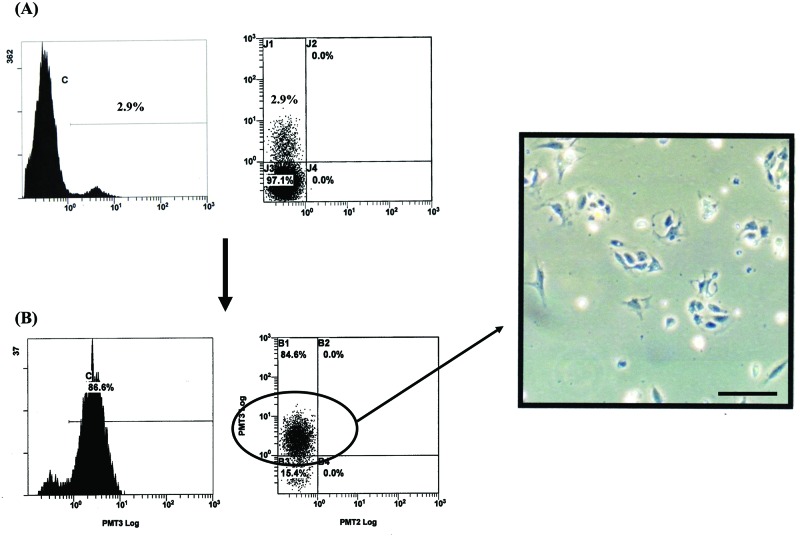
The EpCAM-positive sorted cells that were derived from BA liver were inoculated on collagen-coated plates and were cultured in growth medium. Various epitheliallike cells were observed on day 2 of culture (Fig. 5).
Figure 5.
Culture of epithelial cell adhesion molecule (EpCAM)-positive sorted cells derived from biliary atresia (BA) liver. Flow cytometric analysis of cells from BA liver before (A) and after (B) cell sorting for EpCAM-positive cells. The cells in (B) were epithelial-like on day 2 of culture as determined by phase contrast microscopy. Scale bar: 250 μm.
Discussion
Availability of human hepatic progenitor cells would be an invaluable therapeutic tool for induction of liver generation or for the support of liver function by cell transplantation (13). To obtain such progenitor cells, human hepatic stem/progenitor-like cells have been isolated and characterized from normal and diseased livers (1,4,5,10). However, there have been few attempts to isolate hepatic progenitor cells from BA liver. BA is a rare and serious liver disease in newborn infants and is often followed by cirrhosis. We investigated whether hepatic progenitor cells can be isolated from BA liver using EpCAM or Thy-1 as markers of such cells.
Many studies suggest that EpCAM-positive cells are hepatic progenitors (9). The present study shows that the percentage of EpCAM-positive cells was significantly higher in NPC populations that were derived from BA liver, suggesting that hepatic progenitor-like cells are activated to proliferate in BA liver.
RT-PCR analysis showed that these cells express both hepatic (albumin) and biliary (CK19) markers, suggesting that they express the bipotential nature expected of hepatic progenitor cells. Recently, it has been reported that Thy-1 is a useful marker for successful isolation of hepatic progenitor cells from human fetal and adult livers (6,13). It has also been reported that Thy-1 is not a marker of oval cells but is present on a subpopulation of myofibroblasts/stellate cells (2). In this study, we found a higher percentage of Thy-1-positive cells than EpCAMpositive cells, regardless of whether the cells were derived from BA or from control livers. Furthermore, RT-PCR analysis showed that EpCAM-positive cells expressed both EpCAM and Thy-1, whereas Thy-1-positive cells expressed only Thy-1. The combined results suggest that the properties of the isolated EpCAM-positive cells are somewhat different from those of the isolated Thy-1-positive cells.
Currently, studies are underway in order to culture the EpCAM- and Thy-1-positive cells, to characterize their phenotypes, and to compare their differentiation potentials in vitro and in vivo.
Acknowledgments
This work was supported by grants from the National Center for Child Health and Development (22A6 and 22A8). The authors declare no conflict of interest.
References
- 1. Crosby H. A.; Kelly D. A.; Strain A. J. Human hepatic stemlike cells isolated using c-kit or CD34 can differentiate into biliary epithelium. Gastroenterology 120:534–544;2001. [DOI] [PubMed] [Google Scholar]
- 2. Dezso K.; Jelnes P.; Laszlo V.; Baghy K.; Bodor C.; Paku S.; Tygstrup N.; Bisgaard H. C.; Nagy P. Thy-1 is expressed in hepatic myofibroblasts and not oval cells in stem cell-mediated liver generation. Am. J. Pathol. 171:1529–1537;2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Enosawa S. Hepatocyte isolation from human liver tissue. Organ Biol. 16:361–370;2009. [Google Scholar]
- 4. Herrera M. B.; Bruno S.; Buttiglieri S.; Tetta C.; Gatti S.; Deregibus M. C.; Bussolati B.; Camussi G. Isolation and characterization of a stem cell population from adult human liver. Stem Cells 24:2840–2850;2006. [DOI] [PubMed] [Google Scholar]
- 5. Hussain S. Z.; Strom S. C.; Kirby M. R.; Burns S.; Langemeijer S.; Ueda T.; Hsieh M.; Tisdale J. F. Side population cells derived from adult human liver generate hepatocytelike cells in vitro. Dig. Dis. Sci. 50:1755–1763;2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Masson N. M.; Currie I. S.; Terrance J. D.; Garden O. J.; Parks R. W.; Ross J. A. Hepatic progenitor cells in human fetal liver express the oval cell marker Thy-1. Am. J. Gastrointest. Liver Physiol. 291:45–54;2006. [DOI] [PubMed] [Google Scholar]
- 7. Parent R.; Marion M. J.; Furio L.; Trepo C.; Petit M. A. Origin and characterization of a human bipotent liver progenitor cell line. Gastroenterology 126:1147–1156;2004. [DOI] [PubMed] [Google Scholar]
- 8. Roskams T. Progenitor cell involvement in cirrhotic human liver diseases: From controversy to consensus. J. Hepatol. 39:431–434;2003. [DOI] [PubMed] [Google Scholar]
- 9. Schmelzer E.; Wauthier E.; Reid L. M. The phenotypes of pluripotent human hepatic progenitors. Stem Cells 24: 1852–1858;2006. [DOI] [PubMed] [Google Scholar]
- 10. Selden C.; Chalmers S-A.; Jones C.; Standish R.; Quaglia A.; Roland N.; Burroughs A. K.; Rolles K.; Dhillon A.; Hodgson H. J. F. Epithelial colonies cultured from human explanted liver in subacute liver failure exhibit hepatocyte, biliary epithelial, and stem cell phenotypic markers. Stem Cells 21:624–631;2003. [DOI] [PubMed] [Google Scholar]
- 11. Tokiwa T.; Yamazaki T.; Ono M.; Enosawa S.; Tsukiyama T. Cloning and Characterization of liver progenitor cells from the scattered cell clusters in primary culture of porcine livers. Cell Transplant. 17:179–186;2008. [DOI] [PubMed] [Google Scholar]
- 12. Tokiwa T.; Yamazaki T.; Xin W.; Sugae N.; Noguchi M.; Enosawa S.; Tsukiyama T. Differentiation potential of an immortalized non-tumorigenic human liver epithelial cell line as liver progenitor cells. Cell Biol. Int. 30:992–998;2006. [DOI] [PubMed] [Google Scholar]
- 13. Weiss T. S.; Lichtenauer M.; Kirchner S.; Stock P.; Aurich H.; Christ B.; Brockhoff G.; Kunz-Schughart L. A.; Jauch K. W.; Schlitt H. J.; Thasler W. E. Hepatic progenitor cells from adult human livers for cell transplantation. Gut 57:1129–1138;2008. [DOI] [PubMed] [Google Scholar]