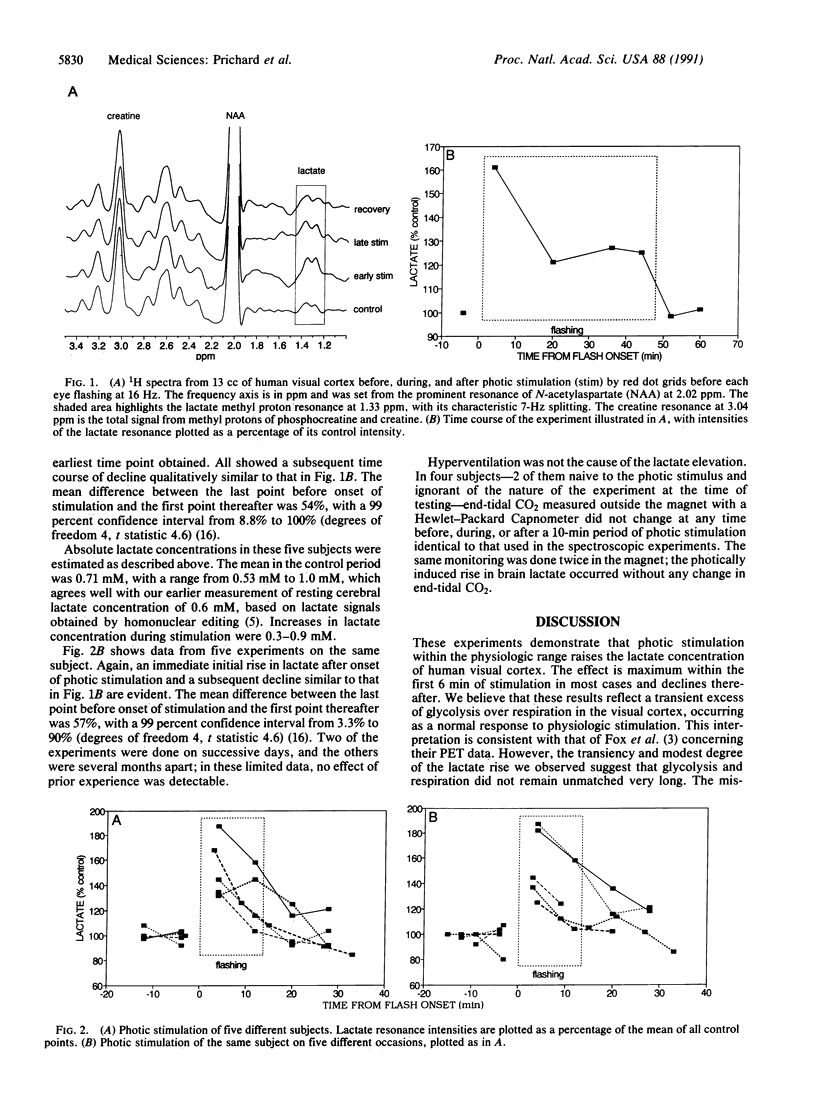
Abstract
Brain lactate concentration is usually assumed to be stable except when pathologic conditions cause a mismatch between glycolysis and respiration. Using newly developed 1H NMR spectroscopic techniques that allow measurement of lactate in vivo, we detected lactate elevations of 0.3-0.9 mM in human visual cortex during physiologic photic stimulation. The maximum rise appeared in the first few minutes; thereafter lactate concentration declined while stimulation continued. The results are consistent with a transient excess of glycolysis over respiration in the visual cortex, occurring as a normal response to stimulation in the physiologic range.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behar K. L., den Hollander J. A., Stromski M. E., Ogino T., Shulman R. G., Petroff O. A., Prichard J. W. High-resolution 1H nuclear magnetic resonance study of cerebral hypoxia in vivo. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4945–4948. doi: 10.1073/pnas.80.16.4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall M. R., Pegg D. T. Theoretical description of depth pulse sequences, on and off resonance, including improvements and extensions thereof. Magn Reson Med. 1985 Apr;2(2):91–113. doi: 10.1002/mrm.1910020202. [DOI] [PubMed] [Google Scholar]
- Borowsky I. W., Collins R. C. Metabolic anatomy of brain: a comparison of regional capillary density, glucose metabolism, and enzyme activities. J Comp Neurol. 1989 Oct 15;288(3):401–413. doi: 10.1002/cne.902880304. [DOI] [PubMed] [Google Scholar]
- Chen W., Ackerman J. J. Surface coil single-pulse localization in vivo via inhomogeneous surface spoiling magnetic gradient. NMR Biomed. 1989 Apr;1(4):205–207. doi: 10.1002/nbm.1940010409. [DOI] [PubMed] [Google Scholar]
- Dager S. R., Rainey J. M., Kenny M. A., Artru A. A., Metzger G. D., Bowden D. M. Central nervous system effects of lactate infusion in primates. Biol Psychiatry. 1990 Jan 15;27(2):193–204. doi: 10.1016/0006-3223(90)90649-m. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E., Mintun M. A., Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988 Jul 22;241(4864):462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Frahm J., Bruhn H., Gyngell M. L., Merboldt K. D., Hänicke W., Sauter R. Localized high-resolution proton NMR spectroscopy using stimulated echoes: initial applications to human brain in vivo. Magn Reson Med. 1989 Jan;9(1):79–93. doi: 10.1002/mrm.1910090110. [DOI] [PubMed] [Google Scholar]
- Gaudry-Talarmain Y. M. The effect of lactate on acetylcholine release evoked by various stimuli from Torpedo synaptosomes. Eur J Pharmacol. 1986 Oct 7;129(3):235–243. doi: 10.1016/0014-2999(86)90433-4. [DOI] [PubMed] [Google Scholar]
- Hanstock C. C., Rothman D. L., Prichard J. W., Jue T., Shulman R. G. Spatially localized 1H NMR spectra of metabolites in the human brain. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1821–1825. doi: 10.1073/pnas.85.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten P. R., Anderson C. M., den Hollander J. A. 1H NMR relaxation measurements of human tissues in situ by spatially resolved spectroscopy. Magn Reson Med. 1987 May;4(5):431–440. doi: 10.1002/mrm.1910040504. [DOI] [PubMed] [Google Scholar]
- Ordidge R. J. Random noise selective excitation pulses. Magn Reson Med. 1987 Jul;5(1):93–98. doi: 10.1002/mrm.1910050113. [DOI] [PubMed] [Google Scholar]
- Petroff O. A., Spencer D. D., Alger J. R., Prichard J. W. High-field proton magnetic resonance spectroscopy of human cerebrum obtained during surgery for epilepsy. Neurology. 1989 Sep;39(9):1197–1202. doi: 10.1212/wnl.39.9.1197. [DOI] [PubMed] [Google Scholar]
- Prichard J. W., Petroff O. A., Ogino T., Shulman R. G. Cerebral lactate elevation by electroshock: a 1H magnetic resonance study. Ann N Y Acad Sci. 1987;508:54–63. doi: 10.1111/j.1749-6632.1987.tb32894.x. [DOI] [PubMed] [Google Scholar]
- Reiman E. M., Raichle M. E., Robins E., Mintun M. A., Fusselman M. J., Fox P. T., Price J. L., Hackman K. A. Neuroanatomical correlates of a lactate-induced anxiety attack. Arch Gen Psychiatry. 1989 Jun;46(6):493–500. doi: 10.1001/archpsyc.1989.01810060013003. [DOI] [PubMed] [Google Scholar]
- Segebarth C. M., Balériaux D. F., Luyten P. R., den Hollander J. A. Detection of metabolic heterogeneity of human intracranial tumors in vivo by 1H NMR spectroscopic imaging. Magn Reson Med. 1990 Jan;13(1):62–76. doi: 10.1002/mrm.1910130108. [DOI] [PubMed] [Google Scholar]
- Silver M. S., Joseph R. I., Chen C. N., Sank V. J., Hoult D. I. Selective population inversion in NMR. Nature. 1984 Aug 23;310(5979):681–683. doi: 10.1038/310681a0. [DOI] [PubMed] [Google Scholar]
- Ueki M., Linn F., Hossmann K. A. Functional activation of cerebral blood flow and metabolism before and after global ischemia of rat brain. J Cereb Blood Flow Metab. 1988 Aug;8(4):486–494. doi: 10.1038/jcbfm.1988.89. [DOI] [PubMed] [Google Scholar]


