Abstract
P-wave dispersion (PWD, Pd or Pdis) is a noninvasive electrocardiographic (ECG) marker for atrial remodeling and predictor for atrial fibrillation (AF). PWD is defined as the difference between the widest and the narrowest P-wave duration recorded from the 12 ECG leads. Increased P-wave duration and PWD reflect prolongation of intraatrial and interatrial conduction time with lack of a well-coordinated conduction system within the atrial muscles, with inhomogeneous, asynchronic, pro-inflammatory and anti-inflammatory effect mediated by interleukin-6 (IL-6) in patients with the CG + GG genotype IL-6 -634C/G polymorphism [1] and discontinuous propagation of sinus impulses mainly between the left and right atria, interstitial/extracellular fibroblast activation and collagen deposition with fibrosis (via TGF-β) in atrial tissue, insufficient blood supply, significant not isotropic myoelectric activity, and thin wall thickness and consequent expansion tendency all well-known electrophysiological characteristics in patients with atrial arrhythmias and especially paroxysmal atrial fibrillation (PAF) [2].
Keywords: P-wave dispersion, P-wave duration, Intraatrial block, Interatrial block, Paroxysmal atrial fibrillation
1. Excess amount of reactive oxygen species (ROS) and P-wave duration and P-wave dispersion relationship
In experimental and even clinical scenarios of AF extracellular fibrosis and inflammation as well as downregulation of several ion channels and gap junctions, nexus or macula communicans have been documented in atrial tissue. Both AF and cardiac heart failure (CHF) are associated with excess amount of reactive oxygen species (ROS). These can cause trigger activity type arrhythmias (early after depolarizations (EADs) and delayed after depolarizations (DADs)), reentry and potential therapeutic targets [3]. Due to triggered activity, arrhythmias are often due to problems in the ion channels in the heart muscle cells. They can also occur as a side effect of certain anti-arrhythmic drugs such as digitalis. Excess amount of ROS alters multiple cardiac ion currents: Activation of CaMKII, c-Src, and PKC may mediate several important effects of ROS on ion currents resulting in arrhythmia. ROS produces Na+ current reduction (via PKC and c-Src, also via abnormal splicing mRNA) and reduction conduction velocity, abnormal splicing, activation of CaMKII, c-Src, and PKC are among emerging new antiarrhythmic therapeutic targets. ROS may also alter intracellular Ca2+ handling in a way that generates arrhythmia.
ROS may stimulate the inward L-type Ca2+ current (direct or via CaMKII activation facilitating after depolarization, which can facilitate EADs) with abnormal depolarization during phase 2 or phase 3. ROS is also responsible by inhibition of K+ channels Ito, IKr, IKs and KATP with consequent abnormal repolarization.
ROS causes adversely affected splicing of mRNA of cardiac Na+ channels resulting in abnormal truncated cardiac Na+ channel proteins and a reduction in normal Na+ channels.
In the extracellular matrix ROS promotes cardiac fibrotic process (via TGF-β) with reduction in conduction velocity and impaired myocyte-myocyte coupling due to collagen deposition.
ROS impairs gap junction affecting assembling of Cx43, resulting in reduced myocyte coupling and velocity facilitation of reentry.
Activation of Ca2+/CaM-dependent kinase II, c-Src tyrosine kinase, protein kinase C, and abnormal splicing of cardiac Na+ channels are among the recently discovered molecular mechanisms of ROS-induced arrhythmia.
ROS produces NCX activation, increasing inward current afterdepolarization and increasing late Na+ current (phase 2 of action potential) facilitating afterdepolarization. A new selective cardiac late Na+ current inhibitor confers concurrent protection against autonomically induced atrial premature beats (APBs), and dual protection against vulnerability to ischemia-induced AF, and reduces atrial and ventricular repolarization abnormalities before and during adrenergic stimulation without negative inotropic effects [4].
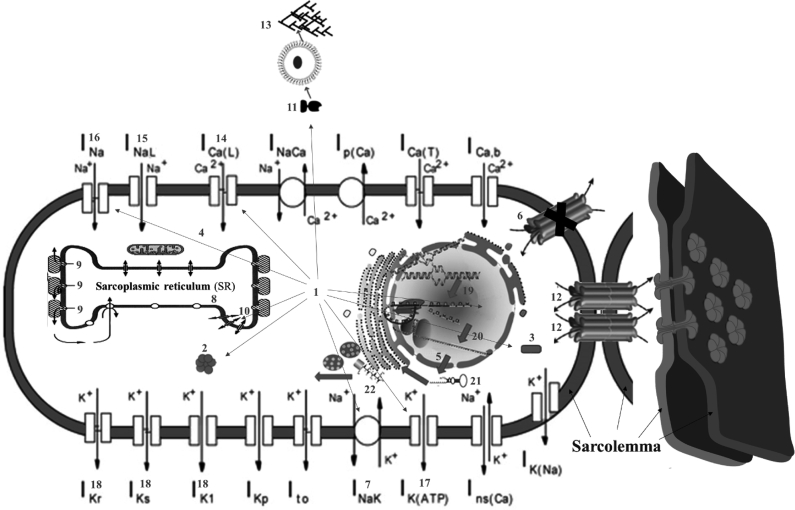
In summary, ROS affects CaMKII: Ca2+/calmodulin-dependent protein kinases II; CX43: connexin 43; NCX: Na+/Ca2+ exchanger; PLB: phospholamban; RYR: ryanodine receptor; SERCA: sarco-/endoplasmic reticulum Ca2+-ATPase; TGF-β: Transforming Growth Factor-β; ZO-1: Zonula Occludens-1. Fig. 1 shows the main action points of ROS.
Fig. 1.
Main action points of ROS.
1.1. Modified from [3]
1) Excess of reactive oxygen species (ROS); 2) CaMKII activation or Ca2+/calmodulin-dependent protein kinases II; 3) c-Src activation (SRC proto-oncogene, non-receptor tyrosine kinase); 4) PKC (Protein kinase C) enzymes play important roles in several signal transduction cascades. Abnormal splicing, activation of CaMKII, c-Src, and PKC are among emerging new antiarrhythmic; 5) mRNA of Na+ current; 6) Impair gap junction CX43 conduction resulting in reduced myocyte coupling; 7) NCX: Na+/Ca2+ exchanger. The NCX removes a single Ca2+ ion in exchange for the import of three Na+ ions. It is considered one of the most important cellular mechanisms for removing Ca2+; 8) Phospholamban (PLB): It is a 52-amino acid integral membrane protein that regulates the Ca2+ pump in cardiac muscle and skeletal muscle cells; 9) Ryanodine receptor (RyR) participates in different signaling pathways involving Ca2+ release from intracellular organelles. It is the major cellular mediator of Ca2+ induced Ca2+ release (CICR) in animal cells. RyR2 is primarily expressed in myocardium; 10) Sarco-/endoplasmic reticulum Ca2+-ATPase (SERCA) resides in the sarcoplasmic reticulum (SR) within myocytes. It is a Ca2+ ATPase that transfers Ca2+ from the cytosol of the cell to the lumen of the SR at the expense of ATP hydrolysis during muscle relaxation; 11) Transforming Growth Factor-β (TGF-β) leads to the activation of different downstream substrates and regulatory proteins, inducing transcription of different target genes that function in differentiation, chemotaxis, proliferation, and activation of many immune cells; 12) Zonula Occludens-1 (ZO-1) or tight junction protein. It is located on a cytoplasmic membrane surface of intercellular tight junctions. The encoded protein may be involved in signal transduction at cell–cell junctions; 13) Extracellular fibroblasts activation and collagen deposition; 14) Increase in L-type Ca2+ current; 15) Increase in late Na+ current. Selective inhibition of cardiac late INa with eleclazine confers dual protection against vulnerability to ischemia-induced AF and reduces atrial and ventricular repolarization abnormalities before and during adrenergic stimulation without negative inotropic effects. 16) Na+ current reduction; 17) ATP-sensitive K+ channel (KATP channel) inhibition; 18) Ito, IKs and IKr inhibition; 19) Transcription; 20) Splicing; 21) microRNA; 22) Translation.
2. Normal limit values of P-wave dispersion (PWD)
The normal value of PWD is 29 ± 9 ms. Aytemir et al. [5] refer a maximum PWD value of 36 ms. PWD ≥40 ms indicates the presence of heterogeneous electrical activity in different regions of the atrium that might cause atrial tachyarrhythmias (ATAs). Thus, PWD is a strong predictor of ATAs and especially AF.
2.1. Possible scenarios where P-wave dispersion could be present
-
A)Physiological
-
➢Young athletes of high performance: PWD is increased in young athletes of high performance and is positively correlated with training duration and baseline heart rate. The increase in PWD is secondary to a significant decrease in Pmin [6].
-
➢
-
B)Pathological
-
1.Cardiovascular disorders
-
➢Hypertension: hypertension associated with left ventricular hypertrophy, diastolic dysfunction and increased left atrium (LA) diameter/volume are associated with PWD, a marker for AF [7].
-
➢Coronary artery disease Coronary slow flow (CSF): CSF may be related to increased PWD and corrected QT dispersion (QTcd), a prolonged Tp-e interval, and Tp-e/QT ratio, and impaired diastolic filling. Trimetazidine, a cytoprotective anti-ischemic agent may restore these parameters [8].
-
➢Cardiac heart failure (CHF): The systolic and diastolic functions are important prognostic indicators of a number of cardiac conditions, and problems with these functions are significant causes of CHF. It is linked to the development of AF. The LA volume has also been reported to be strongly associated with systolic and diastolic dysfunction and is considered to be an index of atrial remodeling. P-wave abnormalities have been reported to be associated with left atrial enlargement (LAE). The LA diameter was significantly higher, and the left ventricle ejection fraction (LVEF) was significantly lower in patients with dilated cardiomyopathy. A disturbance in interatrial conduction and an inhomogeneous propagation of the sinus impulse in patients with CHF is associated with an increase in PWD, LA volume and a deleterious systolic and diastolic dysfunction [9].
-
➢Valvular heart disease:
-
•Transcatheter aortic valve replacement (TAVR) in severe aortic stenosis (AS): In patients with severe aortic stenosis, P-wave duration and PWD were shown to be increased, indicating atrial electrical remodeling. P-wave duration and PWD were significantly decreased at the 6-months of follow-up on post-TAVR indicating early reverse atrial electrical remodeling [10].
-
•Mitral stenosis (MS) and percutaneous transvenous mitral commissurotomy (PTMC): MS is associated with prolonged inter- and intraatrial electromechanical delays and increased PWD, which are markers of AF risk. Successful PTMC has a favorable early impact on inter and intraatrial electromechanical delays, which are considered as novel parameters of atrial electromechanical remodeling in MS patients [11].
-
•
-
➢Cardiac surgery: PWD predicts AF after cardiac surgery [12].
-
➢Cryoballoon ablation for atrial fibrillation: After cryoablation a significant decrease of PWD was observed compared to preprocedural measurements [13].
-
➢Cardiac resynchronization therapy (CRT): this procedure causes decreased P-wave maximum duration and PWD along with an improvement of LVEF and a reduction of LA diameter with prevention of AF [14].
-
➢Left atrial appendage exclusion in patients with AF to prevent thrombus formation: produces decreased atrial mass and decreased PWD by reverse electrical atrial remodeling [15].
-
➢After pulmonary vein isolation in patients with normal left atrial dimension: P-wave duration ≥120ms and PWD are independently associated with higher recurrence rates of AF after pulmonary vein isolation [16]. There is a significant decrease in P-wave duration and PWD after combined percutaneous left atrial appendage ligation and pulmonary vein isolation (PVI) in patients with persistent AF converted to sinus rhythm [17].
-
➢Children with neurally mediated syncope: PWD is a useful ECG predictor of cardiac autonomic dysfunction in children with vasovagal syncope [18].
-
➢Cardiac tumors (left atrial (LA) myxoma): PWD is a parameter for the prediction of postoperative AF in patients with LA myxoma. Only PWD differed significantly between postoperative AF and non-AF. Logistic regression analysis revealed PWD as independent predictors of postoperative AF [19].
-
➢Congenital heart disease
-
1)Transcatheter closure of an atrial septal defect in children: PWD measurements decrease over time from before the procedure and after one year. There are no PWD differences in transcatheter closures using the Amplatzer septal occluder one year after the procedure [20].
-
2)Transposition of Great Arteries (TGA) after uncomplicated Arterial Switch Operation (ASO): First-degree block and right bundle branch were detected after procedure. In addition, the presence of PWD may increase the risk of atrial arrhythmia [21].
-
1)
-
➢Hypertrophic Cardiomyopathy: PWD together with LA phasic functions, and N-Terminal Pro-Brain Natriuretic Peptide (NT-proBNP) levels, predict development of AF [22].
-
➢Channelopathies: Intraatrial conduction delay with significantly longer P-wave duration, PWD and more percentage of late potentials than the matched controls. In literature, a special attention is seen devoted to the use of techniques to average the signals in studying P-wave, with the aim of detecting patients susceptible to AF. Several studies proved that individuals with a clinical history of PAF [2] have intraatrial and interatrial conduction significantly longer in sinus rhythm. The presence of atrial LPs indicates risk of AF, since P-wave-triggered signal-averaged ECG (SAECG) enables to detect slow conduction, even in small areas of the atrium [23]. Atrial heterogeneity may exist in Brugada syndrome (BrS) patients, especially those showing type 1 ECG patterns. These atrial electrical abnormalities could be a substrate for atrial reentrant tachycardia such as AF [24]. Among the studied 12-lead ECG parameters, BrS subjects with ATAs exhibit increased values of P-wave duration, PWD, PR interval in lead II, QRS duration, Tpeak-end interval in lead II and V2, and Tpeak-end dispersion of the 12 leads in relation to those without ATAs. Among the assessed electrophysiological parameters, atrial-His (AH) and His-ventricular (HV) intervals are significantly prolonged in subjects with ATAs. All these parameters are independent predictors of ATAs in subjects with BrS [25].
-
➢
-
2.Metabolic-Endocrine diseases
-
➢Diabetes mellitus: Diabetes mellitus is an independent and strong risk factor for development of AF. There is a significant atrial electromechanical delay and PWD in patients with type 2 diabetes mellitus when compared with healthy volunteers [26].
-
➢Insulin resistance in obese adolescents: It is a significant, independent predictor of PWD [27].
-
➢Hypothyroidism: In the pediatric population hypothyroidism causes atrial electromechanical conduction delay, abnormal P-wave dispersion, and ventricle diastolic dysfunction [28].
-
➢Diabetes insipidus: Diabetes insipidus patients without any cardiovascular disease or risk factors displayed significantly shorter QRS duration and increased PWD compared with controls. It was significantly correlated with serum osmolality and hyponatremia observed in severe cases [29].
-
➢Non-alcoholic fatty liver disease (NAFLD) without cardiac disease, diabetes, or hypertension: in a population of NAFLD without any clinical diagnosis of cardiac disease, diabetes, hypertension, increased left atrial volume, PWD was significantly higher when compared with controls [30].
-
➢Weight loss and P-wave dispersion in obese patients: P-wave duration and PWD are significantly reduced in patients who lost more than 5% of weight and this decrease is highly related to the extent of weight loss [31].
-
➢
-
3.Renal diseases
-
➢Renal failure: PWD is positively associated with overall and cardiovascular mortality in hemodialysis patients [32].
-
➢Hemodialysis (HD) versus hemodiafiltration (HDF): P-wave duration and PWD are significantly increased during HD. On the other hand, HDF has a more beneficial effect on P-wave duration and PWD than HD, because the LA diameter decreased significantly only during HDF. The decrease in LA diameter during HDF is negatively correlated with the incidence of APBs. HDF has a more beneficial effect on P-wave duration and PWD than HD [33].
-
➢
-
4.Respiratory diseases
-
➢Chronic obstructive pulmonary disease (COPD): secondary pulmonary hypertension attributable to COPD can affect right atrial (RA) function without causing gross enlargement in the early stages leading to secondary atrial dysfunction and atrial arrhythmias. Secondary pulmonary hypertension is included in the comorbidity criteria of CHF, a condition associated with PWD by heterogeneous atrial depolarization that predisposes to AF in COPD independent of lung function, blood gas and electrolyte levels, and atrial function [34].
-
➢Moderate/severe obstructive sleep apnea-hypopnea index ≥15 treated with nasal continuous positive airway pressure: Three-month therapy significantly decreased PWD [35].
-
➢Respiratory maneuvers simulating Obstructive Sleep Apnea in healthy subjects and in patients with PAF: intrathoracic pressure swings PWD and therefore may represent an independent trigger factor for the development of PAF [36].
-
➢Nasal Septum Deviation: Preoperative PWD and QTcd values were significantly higher in nasal septum deviation patients than in the control group (Pd: 57.40 ± 14.21 vs 34.11 ± 7.12 milliseconds, P < 0.001 [37].
-
➢
-
5.Neurological/psychiatric disorders
-
➢Myotonic dystrophy type 1 (MD1): a significantly increased P-wave duration and PWD in these patients compared with age and sex-matched healthy controls. Additionally, a statistically significant increase in PDW and Pmax in MD1 patient's subgroup with AF compared to MD1 patients with no arrhythmias [38].
-
➢Multiple sclerosis: In the group of patients, mean P-wave duration, and PWD were significantly longer than normal controls [39].
-
➢Anorexia nervosa: Increased PWD is observed as a risk for AF in adolescents with anorexia nervosa when compared with the group of controls [40].
-
➢Anxiety disorders [5].
-
➢Hypochondriac patients: they have significantly higher PWD than those of healthy matched controls.
-
➢
- 6.
-
7.Gastrointestinal diseases
-
➢Celiac disease: Patients with celiac disease have significantly increased PWD and significantly higher interatrial, intra-left atrial, and intra-RA time than matched healthy controls [43].
-
➢
-
8.Gynecologic diseases
-
➢Polycystic ovary syndrome (PCOS): Patients with PCOS had increased inter and intraatrial conduction delays, and decreased LA passive emptying volumes and fractions [44].
- ➢
-
➢Women with intrahepatic cholestasis of pregnancy: In this entity P-wave duration and PWD are significantly prolonged when compared to the normal controls [47].
-
➢
-
9.Drugs and PWD
-
➢Combined sedation with midazolam and propofol: PWD values increased after endoscopy with a combination of midazolam and propofol sedation [48].
-
➢The effect of trimetazidine: It is an anti-ischemic (antianginal) metabolic agent, which improves myocardial glucose utilization through inhibition of fatty acid metabolism, also known as fatty acid oxidation inhibitor. Additionally, the drug improves cardiac function in CHF patients and may improve Pmax and PWD [49].
-
➢
-
10.Hematological entities
-
➢The association of red cell distribution width (RDW) and PWD: RDW is a measure of the variation in sizes of peripheral blood erythrocytes. The parameter is an independent risk factor for new-onset AF such as PWD. Elevated RDW values are caused by ferropenic anemia, deficit of vitamin B12, or folic acid; increased erythrocyte damage; and chronic inflammation. RDW is an independent predictor of CHA2RDS2-VASc scores [50]. Xiao et al. [51] observed association between red cell distribution width and PWD in patients with ATAs).
-
➢
-
1.
3. Main items to be analyzed in P-wave
P-wave has six components essential for P-wave analysis. They are:
P-wave duration, P-wave amplitude or voltage, P-wave polarity, P-wave axis in the frontal plane, P-wave shape or morphology, and PWD (main object of our revision).
-
I.
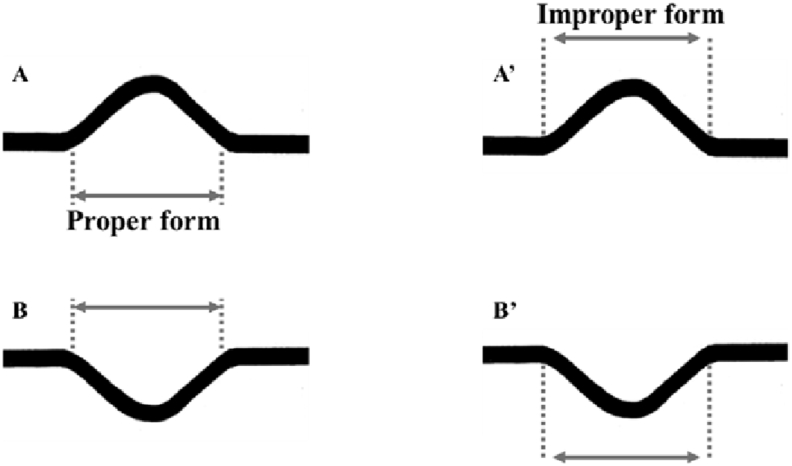
P-wave duration: normal value in adults is 60–110 ms (1.5–2.75 small boxes) < 120 ms or 0.12 s (three small boxes). Over the years, the P-wave duration progressively increases. Table 1 shows normal maximal values of the P-wave duration with the advancement of age. Fig. 2 shows the proper mode of measurement of P-wave duration.
Table 1.
Normal maximal values of the P-wave duration with the advancement of age [52].
| Age | P-wave duration (ms) |
|---|---|
| From the 18th to the 22nd week of pregnancy | 44 |
| ≥37 weeks | 52.9 |
| Premature newborn baby | 80 (60 ± 20) |
| Full-term newborn baby at 12 months | 80 (2 small boxes) |
| From 1 to 12 years old | 90 |
| From 12 years old until the adult life | The normal maximal value is 110 ms (2.5 small squares). In adults, P-wave ≥ 110 ms indicates the presence of LAE, anomaly in LA activation by Bachman's fascicle. |
| Elderly people | ≤120 ms |
LA: Left atrial; LAE: Left atrial enlargement.
Fig. 2.
Appropriate measurement of P-wave duration.
P-wave duration measurement onset of the P-wave is defined as the point of first visible upward slope from baseline for positive waveforms (A), and as the point of first downward slope from baseline for negative waveforms (B). The return to baseline was considered as the end of the P-wave.
A′ and B′ represent the improper form of P-wave duration measurement.
-
II.
P-wave amplitude/voltage: maximal normal value < 2.5 mm or 0.25 mV. P-wave amplitude is between 5 mm or 0.05 mV to 2.5 mm 0.25 mV (0.5–2.5 small boxes).
In the precordial leads normal P-wave amplitude is always <1.5 mm.
-
III.
P-wave polarity: always positive in II, I, aVF and from V3 to V6, always negative in aVR and variable in III, aVL and V1—V2. The normal P-wave is typically biphasic in V1, with similar sizes of the positive and negative deflections. Left atrial enlargement causes widening (>40 ms wide) and deepening (>1 mm deep) in V1 of the terminal negative portion of the P-wave.
-
IV.
P-wave axis in the frontal plane: Normal P-axis was considered between 0 and + 75° by manually constructing the mean frontal plane electrical P-axis from standard limb leads [53].
-
V.P-wave shape or morphology: The shape of a P-wave is usually smooth and rounded. We may see notched P-waves in the frontal plane in partial interatrial block (P-IAB) and LAE produces a broad (≥120 ms), bifid P-wave in lead II (P mitrale). P-IAB does not have a terminal delayed negative portion of the P-wave in V1. On the other hand, LAE has a positive Morris index [54] (P terminal force in V1 (PTF-V1) = 0.04 mm/s). P-terminal force >0.04 mm/s is indicative of LAE. This is the terminal, negative part of the P-wave in lead V1 expressed as the multiplication of its depth in millimeters and width in seconds (mm/s). The normal PTF-V1 and P-IAB do not exceed 0.04 s wide and 1 mm deep, i.e., 0.04 mm/s. Platonov divided the P-wave morphology using orthogonal leads in three types:
-
•Type 1: Upright P-waves in all orthogonal leads — common in healthy subjects below 50 years of age;
-
•Type 2: Upright P-waves in leads X and Y and biphasic in lead Z — common in patients with PAF, LAE but may also be seen in healthy patients older than 50;
-
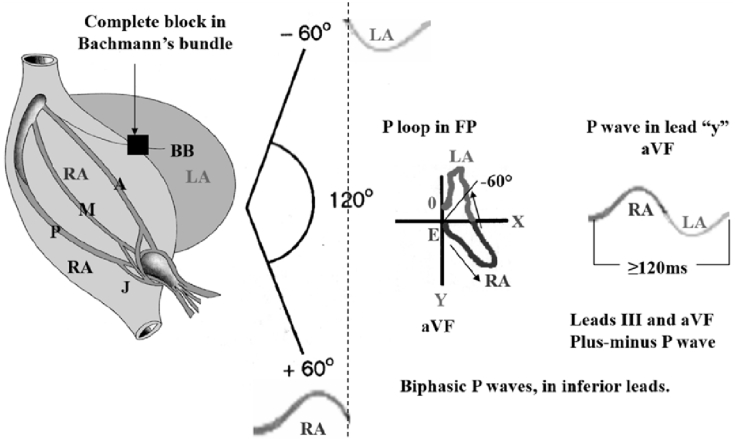
•Type 3: Upright in X but biphasic in leads Y and Z — advanced, complete or third degree interatrial block (A-IAB), often associated with prolongation of P-wave >120 ms. This P-wave morphology is uncommon in healthy subjects. Electrical impulse is blocked/delayed in Bachmann's muscular interatrial bundle (BB), but retrograde left atrial activation usually occurs [55]. Note the existence of an open angle between the vector of the first portion of the P-wave (RA) and the last portion (LA). The electrophysiological study demonstrates retrograde activation of the LA. Consequently, P loop/wave in orthogonal lead Y, aVF and III is biphasic (+/-) or “plus-minus”. LA activation occurs by an alternate route rather than proceeding from right to left via the BB [56] (Fig. 3).
-
•
Fig. 3.
Outline of atrial activation in advanced interatrial block (A-IAB), third degree or complete IAB.
Electrical impulse is blocked/delayed in Bachmann's muscular interatrial bundle (BB), but retrograde LA activation usually occurs [55]. Note the existence of an open angle between the vector of the first portion of P-wave (RA) and the last portion (LA). Electrophysiological study demonstrates retrograde activation of the LA. Consequently, P-loop/wave in orthogonal lead Y, aVF and III is biphasic (+/-) or “plus-minus”. LA activation occurs by an alternate route rather than proceeding from right to left via the BB [56].
A: Anterior internodal bundle; BB: Bachmann's bundle; FP: frontal plane; J: bypass James fascicle; LA: left atrium; M: Middle internodal or Wenckebach bundle; P: Posterior internodal or Thorel Bundle; RA: right atrium.
-
VI.
P-wave dispersion: is defined as the difference between the maximum and the minimum P-wave duration recorded from multiple different-surface ECG leads. It has been known that increased P-wave duration and PWD reflect prolongation of intraatrial and interatrial conduction time and the inhomogeneous atrial propagation of sinus impulses [1], which are well-known electrophysiological characteristics in patients with atrial arrhythmias and especially PAF. Maximum and minimum P-wave durations are calculated from the standard ECG during sinus rhythm. PWD is derived by subtracting the minimum P-wave duration from the maximum in any of the 12 ECG leads. PWD can be calculated by manual measurements (hand-held caliper measurements) or computerized methods. Manual measurement with hand-held calipers is performed by increasing the ECG rate to 50 mm/s and the voltage to 1 mV/cm, accompanied by use of magnification. However, hand-held caliper measurements have less accuracy compared with digital measurements.
4. Measurement of P-Wave dispersion
P maximum duration and PWD are calculated on a standard surface ECG. They are simple ECG markers that could be used to identify the patients with idiopathic PAF. A P maximum value of 106 ms separated patients with PAF from control subjects with a sensitivity of 83%, a specificity of 72%, and a positive predictive accuracy of 79%. A PWD value ≥ 36 ms separated patients from control subjects with a sensitivity of 77%, a specificity of 82%, and a positive predictive accuracy of 85% [5]. In elderly people in sinus rhythm three dimensional LA volume index is correlated with PWD. This parameter index is measured by both 2-dimensional and 3-dimensional echocardiography and categorized as normal when ≤ 34 mL/m2 or mild increased (35–41 mL/m2; moderate, 42–48 mL/m2; severe, ≥ 49 mL/m2. Consequently, PWD might be helpful in discriminating patients prone to PAF [57].
Measurement of PWD has not been standardized yet, however, PWD is calculated by subtracting minimum P-wave duration (P min) from maximum P-wave duration (Pmax), measured from the standard 12-lead surface ECG during sinus rhythm obtained from each patient in supine position following 15 min of rest and room temperature and lighting kept constant (PWD and Pmax are lower in the right lateral decubitus lying position than in other positions) [58]. The paper speed must be of 50 mm/s and amplitude at 20 mm/mV for the recording calibration. PWD can be calculated by manual measurements on paper or computerize methods. Manual measurement has less accuracy compared with a computerized digital system. The onset of the P-wave is defined as the point of first detectable upward or downward slope from the isoelectric line for positive or negative waveforms, respectively. Return to the isoelectric line is considered as the end of the P-wave.
Table 2 shows the ECG and electrophysiological markers prone to AF (predictors).
Table 2.
Main electrocardiographic and electrophysiological markers for atrial fibrillation.
| A prolonged P-wave duration (≥120ms). the global conduction slowing is not an obligatory requirement for development of AF [59] |
| A prolonged PWD (≥36ms between the widest and the narrowest P-wave) |
| Biphasic configuration of P-waves: Increased PTF-V1: Biphasic “plus-minus” P-wave with terminal negative portion > 40 ms duration and > 1 mm deep in lead V1. This parameter is specific but less sensitive ECG marker of LAE [60]. |
| The P-wave amplitude in leads II and V1 is a significant independent factors for the prediction of new-onset AF [61]. |
| A PR interval prolongation: Between 160 to 190 ms (minimally increase risk), ≥200 ms (highest risk) [62]. It is an independent predictor of AF [63]. |
| Incomplete Right Bundle Branch Block: It is a novel electrocardiographic strongly and independent marker for early lone AF [63]. |
| Congenital Short QT syndrome [64]: these patients have extremely short atrial effective refractory period (between 120 and 180 ms) |
| Ion channel gene variants in families segregating AF in SCN5A Gene [65] |
| A prolonged atrial SAECGs or prolonged signal-averaged P-wave duration: mean unfiltered P-wave duration of 132 ± 22 ms in X orthogonal lead, 133 ± 23ms in Y orthogonal lead and 154 ± 23 ms in Z orthogonal lead. The positive predictive value of atrial SAECGs in predicting the risk of AF is considerably lower than the negative predictive value [66]. |
| Values of effective atrial refractory periods < 220 ms (shortened refractoriness) were significantly more frequent in patients with post-pacing AF than in patients without [67]. |
AF: Atrial fibrillation; LAE: Left atrial enlargement; PTF-V1: P-terminal force in V1; PWD: P-wave dispersion; SAECGs: Signal-averaged ECGs.
5. Summary
PWD is revealed as an important parameter of easy measurement that indicates a greater tendency to the appearance of supraventricular arrhythmias, particularly PAF. A PWD value close to 40ms (one small box with a paper speed of 50ms) is considered increased having been observed both in physiological and pathological scenarios. Increased PWD in elite of athletes explains the higher incidence of AF in this population. A task force to determine definitively the PWD normal limits is mandatory. The molecular substrate involves driving changes affecting the atrial tissue with affectation of numerous sarcolemmal ion channels, gap junctions (Cx43 and Cx40) leading to degrees of fibrosis in the extracellular tissue of the atria, insufficient blood supply, significant anysoisotropic myoelectric activity, thin wall thickness and consequent expansion tendency all well-known electrophysiological alterations.
Acknowledgements
The authors do not report any conflict of interest regarding this work.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Geng H.H., Li R., Su Y.M., Pan H.Y., Pan M., Ji X.P. A functional single-nucleotide polymorphism in interleukin-6 promoter is associated with p wave dispersion in hypertensive subjects with atrial fibrillation. Int J Clin Exp Med. 2014;7(11):4434–4440. [PMC free article] [PubMed] [Google Scholar]
- 2.Okutucu S., Aytemir K., Oto A. P-wave dispersion: what we know till now? JRSM Cardiovasc Dis. 2016;5 doi: 10.1177/2048004016639443. 2048004016639443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sovari A.A. Cellular and molecular mechanisms of arrhythmia by oxidative stress. Cardiol Res Pract. 2016;2016:9656078. doi: 10.1155/2016/9656078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Justo F., Fuller H., Nearing B.D., Rajamani S., Belardinelli L., Verrier R.L. Inhibition of the cardiac late Na+ current with eleclazine protects against ischemia-induced vulnerability to atrial fibrillation and reduces atrial and ventricular repolarization abnormalities in the absence and presence of concurrent adrenergic stimulation. Heart Rhythm. 2016 doi: 10.1016/j.hrthm.2016.06.020. S1547–5271(16)30438-6. (in press) [DOI] [PubMed] [Google Scholar]
- 5.Aytemir K., Ozer N., Atalar E. P wave dispersion on 12-lead electrocardiography in patients with paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2000;23(7):1109–1112. doi: 10.1111/j.1540-8159.2000.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 6.Puerta R.C., Aliz E.L., Lopez-Calleja M.A., Ramirez R.R., Pena G.P. Increased p wave dispersion in elite athletes. Indian Pacing Electrophysiol J. 2011;11(3):73–80. [PMC free article] [PubMed] [Google Scholar]
- 7.Ertem A.G., Erdoğan M., Keleş T., Durmaz T., Bozkurt E. P-wave dispersion and left ventricular diastolic dysfunction in hypertension. Anatol J Cardiol. 2015;15(1):78–79. doi: 10.5152/akd.2014.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suner A., Cetin M. The effect of trimetazidine on ventricular repolarization indexes and left ventricular diastolic function in patients with coronary slow flow. Coron Artery Dis. 2016;27(5):398–404. doi: 10.1097/MCA.0000000000000373. [DOI] [PubMed] [Google Scholar]
- 9.Kim D.H., Kim G.C., Kim S.H. The relationship between the left atrial volume and the maximum P-wave and P-wave dispersion in patients with congestive heart failure. Yonsei Med J. 2007;48(5):810–817. doi: 10.3349/ymj.2007.48.5.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dursun H., Tanriverdi Z., Colluoglu T., Kaya D. Effect of transcatheter aortic valve replacement on P-wave duration, P-wave dispersion and left atrial size. J Geriatr Cardiol. 2015;12(6):613–617. doi: 10.11909/j.issn.1671-5411.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beig J.R., Tramboo N.A., Rather H.A. Immediate effect of percutaneous transvenous mitral commissurotomy on atrial electromechanical delay and P-wave dispersion in patients with severe mitral stenosis. Indian Heart J. 2015;67(Suppl 2):S46–S54. doi: 10.1016/j.ihj.2015.10.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazzeroni D., Parati G., Bini M. P-wave dispersion predicts atrial fibrillation following cardiac surgery. Int J Cardiol. 2016;203:131–133. doi: 10.1016/j.ijcard.2015.10.143. [DOI] [PubMed] [Google Scholar]
- 13.Kizilirmak F., Demir G.G., Gokdeniz T. Changes in electrocardiographic P wave parameters after Cryoballoon ablation and their association with atrial fibrillation recurrence. Ann Noninvasive Electrocardiol. 2016 doi: 10.1111/anec.12364. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding L., Hua W., Zhang S. Improvement of P wave dispersion after cardiac resynchronization therapy for heart failure. J Electrocardiol. 2009;42(4):334–338. doi: 10.1016/j.jelectrocard.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Kawamura M., Scheinman M.M., Lee R.J., Badhwar N. Left atrial appendage ligation in patients with atrial fibrillation leads to a decrease in atrial dispersion. J Am Heart Assoc. 2015;4(5):e001581. doi: 10.1161/JAHA.114.001581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mugnai G., Chierchia G.B., de Asmundis C. P-wave indices as predictors of atrial fibrillation recurrence after pulmonary vein isolation in normal left atrial size. J Cardiovasc Med Hagerst. 2016;17(3):194–200. doi: 10.2459/JCM.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 17.Badhwar N., Lakkireddy D., Kawamura M. Sequential percutaneous LAA ligation and pulmonary vein isolation in patients with persistent AF: initial results of a feasibility study. J Cardiovasc Electrophysiol. 2015;26(6):608–614. doi: 10.1111/jce.12655. [DOI] [PubMed] [Google Scholar]
- 18.Köse M.D., Bağ Ö Güven B., Meşe T., Öztürk A., Tavlı V. P-wave dispersion: an indicator of cardiac autonomic dysfunction in children with neurocardiogenic syncope. Pediatr Cardiol. 2014;35(4):596–600. doi: 10.1007/s00246-013-0825-y. [DOI] [PubMed] [Google Scholar]
- 19.Sahin M., Tigen K., Dundar C. Postoperative atrial fibrillation in patients with left atrial myxoma. Cardiovasc J Afr. 2015;26(3):120–124. doi: 10.5830/CVJA-2014-069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baspinar O., Kervancioglu M., Koruk S., Kilinc M., Irdem A. Follow-up of P dispersion after transcatheter closure of an atrial septal defect in children. J Pak Med Assoc. 2014 May;64(5):546–548. [PubMed] [Google Scholar]
- 21.Amoozgar H., Amirghofran A.A., Salaminia S. Evaluation of electrocardiographic changes after arterial switch operation. Int Cardiovasc Res J. 2014;8(3):99–104. [PMC free article] [PubMed] [Google Scholar]
- 22.Tuluce K., Yakar Tuluce S., Kahya Eren N. Predictors of future atrial fibrillation development in patients with hypertrophic cardiomyopathy: a prospective follow-up study. Echocardiography. 2016;33(3):379–385. doi: 10.1111/echo.13093. [DOI] [PubMed] [Google Scholar]
- 23.Fukunami M., Yamada T., Ohmori M. Detection of patients at risk for paroxysmal atrial fibrillation during sinus rhythm by P wave-triggered signal-averaged electrocardiogram. Circulation. 1991;83(1):162–169. doi: 10.1161/01.cir.83.1.162. [DOI] [PubMed] [Google Scholar]
- 24.Furukawa Y., Yamada T., Okuyama Y. Increased intraatrial conduction abnormality assessed by P-wave signal-averaged electrocardiogram in patients with Brugada syndrome. Pacing Clin Electrophysiol. 2011;34(9):1138–1146. doi: 10.1111/j.1540-8159.2011.03122.x. [DOI] [PubMed] [Google Scholar]
- 25.Letsas K.P., Weber R., Astheimer K. Predictors of atrial tachyarrhythmias in subjects with type 1 ECG pattern of Brugada syndrome. Pacing Clin Electrophysiol. 2009;32(4):500–505. doi: 10.1111/j.1540-8159.2009.02311.x. [DOI] [PubMed] [Google Scholar]
- 26.Demir K., Avci A., Kaya Z. Assessment of atrial electromechanical delay and P-wave dispersion in patients with type 2 diabetes mellitus. J Cardiol. 2016 Apr;67(4):378–383. doi: 10.1016/j.jjcc.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Sert A., Aslan E., Buyukınan M., Pirgon O. Correlation of P-wave dispersion with insulin sensitivity in obese adolescents. Cardiol Young. 2016:1–7. doi: 10.1017/S1047951116000366. in press. [DOI] [PubMed] [Google Scholar]
- 28.Irdem A., Aydın Sahin D., Kervancioglu M. Evaluation of P-Wave dispersion, diastolic function, and atrial electromechanical conduction in pediatric patients with Subclinical hypothyroidism. Echocardiography. 2016;33(9):1397–1401. doi: 10.1111/echo.13255. [DOI] [PubMed] [Google Scholar]
- 29.Deniz F., Kepez A., Ay S.A. Evaluation of electrocardiographic parameters in patients with diabetes insipidus. Wien Klin Wochenschr. 2015;127(21–22):871–876. doi: 10.1007/s00508-015-0874-8. [DOI] [PubMed] [Google Scholar]
- 30.Ozveren O., Izgi C., Eroglu E. Doppler tissue evaluation of atrial conduction properties in patients with non-alcoholic fatty-liver disease. Ultrason Imaging. 2016;38(3):225–235. doi: 10.1177/0161734615595015. [DOI] [PubMed] [Google Scholar]
- 31.Falchi A.G., Grecchi I., Muggia C., Tinelli C. Weight loss and P wave dispersion: a preliminary study. Obes Res Clin Pract. 2014;8(6) doi: 10.1016/j.orcp.2014.08.005. e614–7. [DOI] [PubMed] [Google Scholar]
- 32.Chen S.C., Su H.M., Huang J.C. Association of P-Wave dispersion with overall and cardiovascular mortality in hemodialysis patients. Am J Nephrol. 2015;42(3):198–205. doi: 10.1159/000440776. [DOI] [PubMed] [Google Scholar]
- 33.Páll A., Czifra Á., Sebestyén V. Hemodiafiltration and hemodialysis differently affect P wave duration and dispersion on the surface electrocardiogram. Int Urol Nephrol. 2016;48(2):271–277. doi: 10.1007/s11255-015-1144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tükek T., Yildiz P., Akkaya V. Factors associated with the development of atrial fibrillation in COPD patients: the role of P-wave dispersion. Ann Noninvasive Electrocardiol. 2002;7(3):222–227. doi: 10.1111/j.1542-474X.2002.tb00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cicek D., Balcioğlu A.S., Lakadamyali H., Müderrisoğlu H. Effects of three month nasal continuous positive airway pressure treatment on electrocardiographic, echocardiographic and overnight polysomnographic parameters in newly diagnosed moderate/severe obstructive sleep apnea patients. Int Heart J. 2015;56(1):94–99. doi: 10.1536/ihj.14-085. [DOI] [PubMed] [Google Scholar]
- 36.Gaisl T., Wons A.M., Rossi V. Simulated obstructive sleep apnea increases P-Wave duration and P-Wave dispersion. PLoS One. 2016;11(4):e0152994. doi: 10.1371/journal.pone.0152994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uluyol S., Kilicaslan S., Gur M.H. Effects of nasal septum deviation and Septoplasty on cardiac arrhythmia risk. Otolaryngol Head Neck Surg. 2016;155(2):347–352. doi: 10.1177/0194599816642432. [DOI] [PubMed] [Google Scholar]
- 38.Russo V., Di Meo F., Rago A. Paroxysmal atrial fibrillation in myotonic dystrophy type 1 patients: P wave duration and dispersion analysis. Eur Rev Med Pharmacol Sci. 2015;19(7):1241–1248. [PubMed] [Google Scholar]
- 39.Razazian N., Hedayati N., Moradian N., Bostani A., Afshari D., Asgari N. P wave duration and dispersion and QT interval in multiple sclerosis. Mult Scler Relat Disord. 2014;3(5):662–665. doi: 10.1016/j.msard.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Ertuğrul İ., Akgül S., Derman O., Karagöz T., Kanbur N. Increased P-wave dispersion a risk for atrial fibrillation in adolescents with anorexia nervosa. Eat Disord. 2016;24(3):289–296. doi: 10.1080/10640266.2015.1042310. [DOI] [PubMed] [Google Scholar]
- 41.Aksoy H., Okutucu S., Sayin B.Y. Assessment of cardiac arrhythmias in patients with ankylosing spondylitis by signal-averaged P wave duration and P wave dispersion. Eur Rev Med Pharmacol Sci. 2016;20(6):1123–1129. [PubMed] [Google Scholar]
- 42.Guler H., Seyfeli E., Sahin G. P wave dispersion in patients with rheumatoid arthritis: its relation with clinical and echocardiographic parameters. Rheumatol Int. 2007;27(9):813–818. doi: 10.1007/s00296-007-0307-8. [DOI] [PubMed] [Google Scholar]
- 43.Bayar N., Çekin A.H., Arslan Ş. Assessment of left atrial function in patients with celiac disease. Echocardiography. 2015;32(12):1802–1808. doi: 10.1111/echo.12963. [DOI] [PubMed] [Google Scholar]
- 44.E1 Gazi, Gencer M., Hanci V. Atrial conduction time, and left atrial mechanical and electromechanical functions in patients with polycystic ovary syndrome: interatrial conduction delay. Cardiovasc J Afr. 2015;26(6):217–221. doi: 10.5830/CVJA-2015-046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kirbas O., Biberoglu E.H., Kirbas A. P-wave duration changes and dispersion in preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2014;183:141–145. doi: 10.1016/j.ejogrb.2014.10.042. [DOI] [PubMed] [Google Scholar]
- 46.İnci S., Nar G., Aksan G., Sipahioğlu H., Soylu K., Dogan A. P-wave dispersion and atrial electromechanical delay in patients with preeclampsia. Med Princ Pract. 2015;24(6):515–521. doi: 10.1159/000435857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Biberoglu E.H., Kirbas A., Kirbas O. Prediction of cardiovascular risk by electrocardiographic changes in women with intrahepatic cholestasis of pregnancy. J Matern Fetal Neonatal Med. 2015;28(18):2239–2243. doi: 10.3109/14767058.2014.983895. [DOI] [PubMed] [Google Scholar]
- 48.Bolat E., Çelikbilek M., Sarıkaya S. Effects of balanced propofol sedation on QT, corrected QT, and P-wave dispersion on upper endoscopy. Anatol J Cardiol. 2016;16(5):328–332. doi: 10.5152/AnatolJCardiol.2015.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gunes Y., Tuncer M., Guntekin U., Akdag S., Gumrukcuoglu H.A. The effects of trimetazidine on p-wave duration and dispersion in heart failure patients. Pacing Clin Electrophysiol. 2009;32(2):239–244. doi: 10.1111/j.1540-8159.2008.02208.x. [DOI] [PubMed] [Google Scholar]
- 50.Kurt M., Tanboga I.H., Buyukkaya E., Karakas M.F., Akcay A.B., Sen N. Relation of red cell distribution width with CHA2DS2-VASc score in patients with nonvalvular atrial fibrillation. Clin Appl Thromb Hemost. 2014;20(7):687–692. doi: 10.1177/1076029613478157. [DOI] [PubMed] [Google Scholar]
- 51.Xiao Q., Li Y., Guo H., Zhang G. Association between red cell distribution width and P-wave dispersion in patients with atrial tachyarrhythmia. Int J Clin Exp Med. 2015;8(5):8141–8146. [PMC free article] [PubMed] [Google Scholar]
- 52.Macfarlane P.W., Coleman E.N., Pomphrey E.O., McLaughlin S., Houston A., Aitchison T. Normal limits of the high-fidelity pediatric ECG. Preliminary observations. J Electrocardiol. 1989;22(Suppl):162–168. doi: 10.1016/s0022-0736(07)80118-x. [DOI] [PubMed] [Google Scholar]
- 53.Prajapat L., Ariyarajah V., Frisella M.E., Apiyasawat S., Spodick D.H. Association of P-wave duration, dispersion, and terminal force in relation to P-wave axis among outpatients. Ann Noninvasive Electrocardiol. 2007;12(3):210–215. doi: 10.1111/j.1542-474X.2007.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morris J.J., Jr., Estes E.H., Jr., Whalen R.E., Thompson H.K., Jr., Mcintosh H.D. P-wave analysis in valvular heart disease. Circulation. 1964;29:242–252. doi: 10.1161/01.cir.29.2.242. [DOI] [PubMed] [Google Scholar]
- 55.Ariyarajah V., Spodick D.H. Advanced interatrial block: a classic electrocardiogram. Cardiology. 2005;104(1):33–34. doi: 10.1159/000086052. [DOI] [PubMed] [Google Scholar]
- 56.Spodick D.H., Ariyarajah V., Apiyasawat S. Higher prevalence of cardiovascular events among patients with abnormal atrial depolarization and coronary artery disease at 18 months' post-exercise tolerance testing. Am Heart Hosp J. 2007;5(4):236–240. doi: 10.1111/j.1541-9215.2007.07361.x. [DOI] [PubMed] [Google Scholar]
- 57.Ozyigit T., Kocas O., Karadag B., Ozben B. Three dimensional left atrial volume index is correlated with P wave dispersion in elderly patients with sinus rhythm. Wien Klin Wochenschr. 2016;128(5–6):182–186. doi: 10.1007/s00508-016-0973-1. [DOI] [PubMed] [Google Scholar]
- 58.Oylumlu M., Dogan A., Ozer O., Yuce M., Ercan S., Davutoglu V. Effects of lying position on P-wave dispersion in patients with heart failure. Med Princ Pract. 2014;23(6):556–560. doi: 10.1159/000365510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Platonov P.G. Atrial conduction and atrial fibrillation: what can we learn from surface ECG? Cardiol J. 2008;15(5):402–407. [PubMed] [Google Scholar]
- 60.Tsao C.W., Josephson M.E., Hauser T.H. Accuracy of electrocardiographic criteria for atrial enlargement: validation with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2008;10:7. doi: 10.1186/1532-429X-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshizawa T., Niwano S., Niwano H. Prediction of new onset atrial fibrillation through P wave analysis in 12 lead ECG. Int Heart J. 2014;55(5):422–427. doi: 10.1536/ihj.14-052. [DOI] [PubMed] [Google Scholar]
- 62.Schnabel R.B., Sullivan L.M., Levy D., Pencina M.J., Massaro J.M., D'Agostino R.B., Sr. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373(9665):739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nielsen J.B., Olesen M.S., Tangø M., Haunsø S., Holst A.G., Svendsen J.H. Incomplete right bundle branch block: a novel electrocardiographic marker for lone atrial fibrillation. Europace. 2011;13(2):182–187. doi: 10.1093/europace/euq436. [DOI] [PubMed] [Google Scholar]
- 64.Pérez Riera A.R., Paixão-Almeida A., Barbosa-Barros R. Congenital short QT syndrome: landmarks of the newest arrhythmogenic cardiac channelopathy. Cardiol J. 2013;20(5):464–471. doi: 10.5603/CJ.a2013.0052. [DOI] [PubMed] [Google Scholar]
- 65.John R.M., Kumar S. Sinus node and atrial arrhythmias. Circulation. 2016;133(19):1892–1900. doi: 10.1161/CIRCULATIONAHA.116.018011. [DOI] [PubMed] [Google Scholar]
- 66.Rosenheck S. Signal-averaged P wave in patients with paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 1997;20(10 Pt 2):2577–2586. doi: 10.1111/j.1540-8159.1997.tb06107.x. [DOI] [PubMed] [Google Scholar]
- 67.De Sisti A., Attuel P., Manot S. Electrophysiological determinants of atrial fibrillation in sinus node dysfunction despite atrial pacing. Europace. 2000;2(4):304–311. doi: 10.1053/eupc.2000.0118. [DOI] [PubMed] [Google Scholar]