Abstract
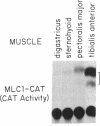
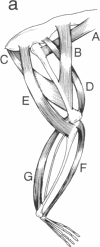
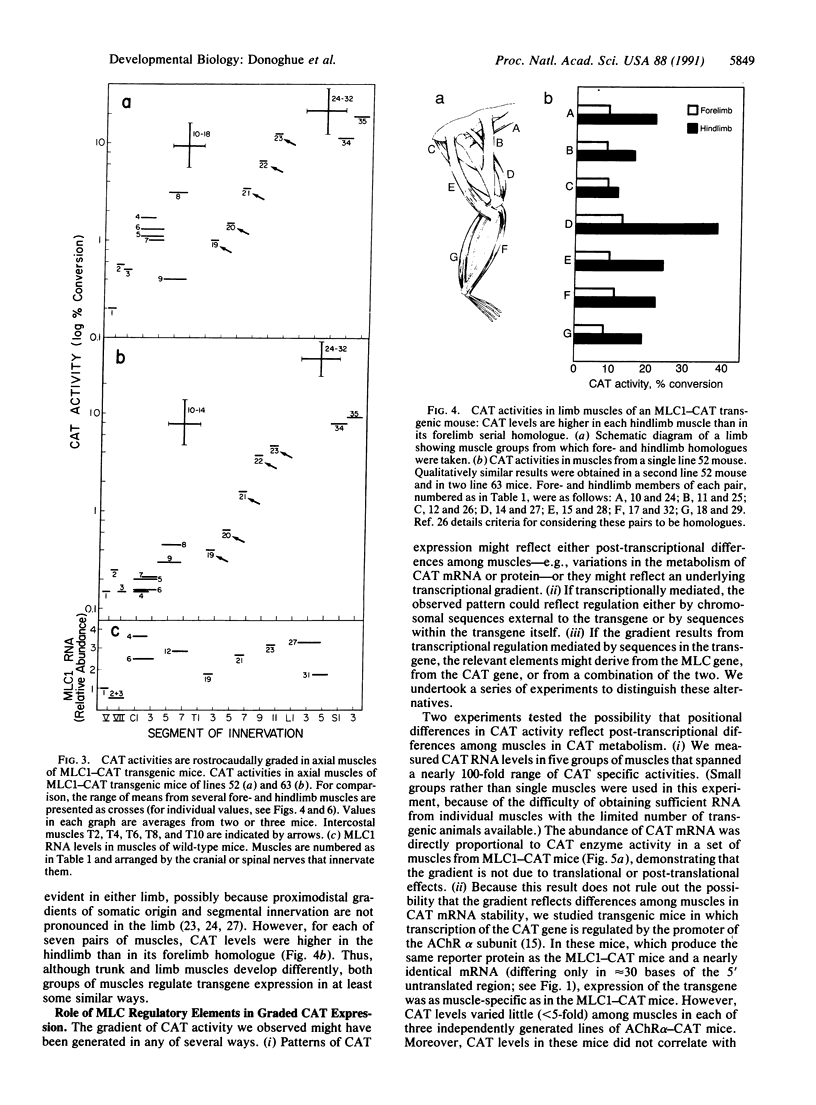
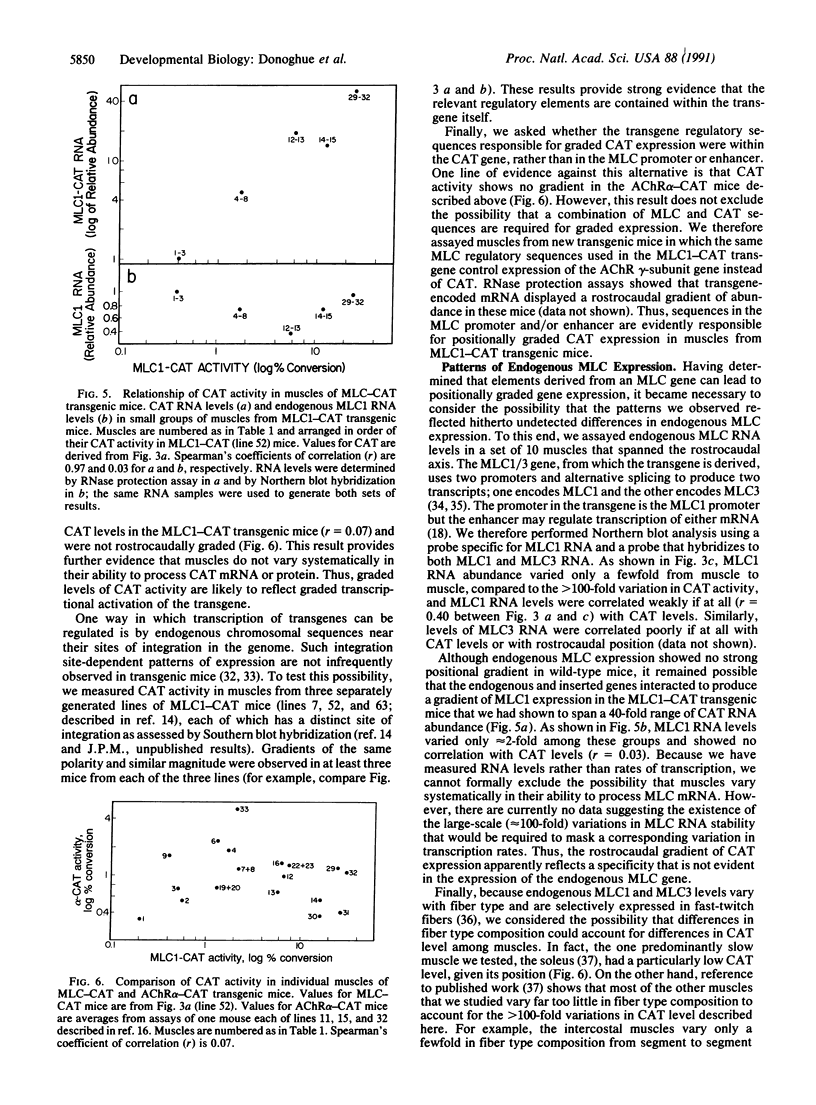
Transgenic mice were produced in which expression of the reporter gene chloramphenicol acetyltransferase (CAT) is controlled by regulatory elements of a rodent myosin light chain gene. CAT activity was readily detectable in muscles of these mice but negligible in a variety of nonmuscle tissues. Unexpectedly, levels of CAT expression varied greater than 100-fold from muscle to muscle, forming a gradient in which a muscle's position in the rostrocaudal axis was correlated with its level of CAT enzyme activity and abundance of CAT mRNA. Thus, rostral muscles (innervated by cranial nerves) had the lowest levels of CAT, thoracic muscles had intermediate levels, and caudal muscles (innervated through lumbar and sacral roots) had the highest levels. We established that myosin light chain sequences are responsible for the gradient of CAT expression but observed no strong gradient of endogenous myosin light chain expression. We argue that elements that are silent or masked by other sequences in their native context are revealed in the transgene and that the rostrocaudal gradient of gene expression they produce reveals the existence of a positionally graded endogenous regulator of gene expression. These transgenic mice provide evidence that cells in adult mammals retain "positional information" of a sort hitherto studied largely in embryos. The transgene they express may provide a means for determining how such positional values are generated and maintained.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akam M. The molecular basis for metameric pattern in the Drosophila embryo. Development. 1987 Sep;101(1):1–22. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brichta A. M., Callister R. J., Peterson E. H. Quantitative analysis of cervical musculature in rats: histochemical composition and motor pool organization. I. Muscles of the spinal accessory complex. J Comp Neurol. 1987 Jan 15;255(3):351–368. doi: 10.1002/cne.902550304. [DOI] [PubMed] [Google Scholar]
- Cunningham J. M., Kaiser K. K., Sanes J. R. Rostrocaudal variation of fiber type composition in rat intercostal muscles. Histochemistry. 1991;95(5):513–517. doi: 10.1007/BF00315748. [DOI] [PubMed] [Google Scholar]
- Dollé P., Izpisúa-Belmonte J. C., Falkenstein H., Renucci A., Duboule D. Coordinate expression of the murine Hox-5 complex homoeobox-containing genes during limb pattern formation. Nature. 1989 Dec 14;342(6251):767–772. doi: 10.1038/342767a0. [DOI] [PubMed] [Google Scholar]
- Donoghue M., Ernst H., Wentworth B., Nadal-Ginard B., Rosenthal N. A muscle-specific enhancer is located at the 3' end of the myosin light-chain 1/3 gene locus. Genes Dev. 1988 Dec;2(12B):1779–1790. doi: 10.1101/gad.2.12b.1779. [DOI] [PubMed] [Google Scholar]
- Holland P. W., Hogan B. L. Expression of homeo box genes during mouse development: a review. Genes Dev. 1988 Jul;2(7):773–782. doi: 10.1101/gad.2.7.773. [DOI] [PubMed] [Google Scholar]
- Ingham P. W. The molecular genetics of embryonic pattern formation in Drosophila. Nature. 1988 Sep 1;335(6185):25–34. doi: 10.1038/335025a0. [DOI] [PubMed] [Google Scholar]
- Kessel M., Gruss P. Murine developmental control genes. Science. 1990 Jul 27;249(4967):374–379. doi: 10.1126/science.1974085. [DOI] [PubMed] [Google Scholar]
- Lacy E., Roberts S., Evans E. P., Burtenshaw M. D., Costantini F. D. A foreign beta-globin gene in transgenic mice: integration at abnormal chromosomal positions and expression in inappropriate tissues. Cell. 1983 Sep;34(2):343–358. doi: 10.1016/0092-8674(83)90369-0. [DOI] [PubMed] [Google Scholar]
- Lance-Jones C. The somitic level of origin of embryonic chick hindlimb muscles. Dev Biol. 1988 Apr;126(2):394–407. doi: 10.1016/0012-1606(88)90149-2. [DOI] [PubMed] [Google Scholar]
- Laskowski M. B., Sanes J. R. Topographic mapping of motor pools onto skeletal muscles. J Neurosci. 1987 Jan;7(1):252–260. doi: 10.1523/JNEUROSCI.07-01-00252.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski M. B., Sanes J. R. Topographically selective reinnervation of adult mammalian skeletal muscles. J Neurosci. 1988 Aug;8(8):3094–3099. doi: 10.1523/JNEUROSCI.08-08-03094.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlie J. P., Kornhauser J. M. Neural regulation of gene expression by an acetylcholine receptor promoter in muscle of transgenic mice. Neuron. 1989 Apr;2(4):1295–1300. doi: 10.1016/0896-6273(89)90067-6. [DOI] [PubMed] [Google Scholar]
- Nja A., Purves D. Re-innervation of guinea-pig superior cervical ganglion cells by preganglionic fibres arising from different levels of the spinal cord. J Physiol. 1977 Nov;272(3):633–651. doi: 10.1113/jphysiol.1977.sp012064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980 Oct 30;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Periasamy M., Strehler E. E., Garfinkel L. I., Gubits R. M., Ruiz-Opazo N., Nadal-Ginard B. Fast skeletal muscle myosin light chains 1 and 3 are produced from a single gene by a combined process of differential RNA transcription and splicing. J Biol Chem. 1984 Nov 10;259(21):13595–13604. [PubMed] [Google Scholar]
- Purves D., Thompson W., Yip J. W. Re-innervation of ganglia transplanted to the neck from different levels of the guinea-pig sympathetic chain. J Physiol. 1981;313:49–63. doi: 10.1113/jphysiol.1981.sp013650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert B., Daubas P., Akimenko M. A., Cohen A., Garner I., Guenet J. L., Buckingham M. A single locus in the mouse encodes both myosin light chains 1 and 3, a second locus corresponds to a related pseudogene. Cell. 1984 Nov;39(1):129–140. doi: 10.1016/0092-8674(84)90198-3. [DOI] [PubMed] [Google Scholar]
- Rosenthal N., Kornhauser J. M., Donoghue M., Rosen K. M., Merlie J. P. Myosin light chain enhancer activates muscle-specific, developmentally regulated gene expression in transgenic mice. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7780–7784. doi: 10.1073/pnas.86.20.7780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoon D., Lyons G., Wright W. E., Lin V., Lassar A., Weintraub H., Buckingham M. Expression of two myogenic regulatory factors myogenin and MyoD1 during mouse embryogenesis. Nature. 1989 Sep 28;341(6240):303–307. doi: 10.1038/341303a0. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Tamkun J. W., Hartzell G. W., 3rd The structure and function of the homeodomain. Biochim Biophys Acta. 1989 Jul 28;989(1):25–48. doi: 10.1016/0304-419x(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Hollyday M. The development and postnatal organization of motor nuclei in the rat thoracic spinal cord. J Comp Neurol. 1983 Oct 10;220(1):16–28. doi: 10.1002/cne.902200104. [DOI] [PubMed] [Google Scholar]
- Staron R. S., Pette D. Correlation between myofibrillar ATPase activity and myosin heavy chain composition in rabbit muscle fibers. Histochemistry. 1986;86(1):19–23. doi: 10.1007/BF00492341. [DOI] [PubMed] [Google Scholar]
- Strehler E. E., Periasamy M., Strehler-Page M. A., Nadal-Ginard B. Myosin light-chain 1 and 3 gene has two structurally distinct and differentially regulated promoters evolving at different rates. Mol Cell Biol. 1985 Nov;5(11):3168–3182. doi: 10.1128/mcb.5.11.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzue T., Kaprielian Z., Patterson P. H. A monoclonal antibody that defines rostrocaudal gradients in the mammalian nervous system. Neuron. 1990 Oct;5(4):421–431. doi: 10.1016/0896-6273(90)90081-p. [DOI] [PubMed] [Google Scholar]
- Wigston D. J., Sanes J. R. Selective reinnervation of adult mammalian muscle by axons from different segmental levels. Nature. 1982 Sep 30;299(5882):464–467. doi: 10.1038/299464a0. [DOI] [PubMed] [Google Scholar]
- Wigston D. J., Sanes J. R. Selective reinnervation of intercostal muscles transplanted from different segmental levels to a common site. J Neurosci. 1985 May;5(5):1208–1221. doi: 10.1523/JNEUROSCI.05-05-01208.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert L. Positional information revisited. Development. 1989;107 (Suppl):3–12. doi: 10.1242/dev.107.Supplement.3. [DOI] [PubMed] [Google Scholar]
- Yu L., LaPolla R. J., Davidson N. Mouse muscle nicotinic acetylcholine receptor gamma subunit: cDNA sequence and gene expression. Nucleic Acids Res. 1986 Apr 25;14(8):3539–3555. doi: 10.1093/nar/14.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Shawi R., Kinnaird J., Burke J., Bishop J. O. Expression of a foreign gene in a line of transgenic mice is modulated by a chromosomal position effect. Mol Cell Biol. 1990 Mar;10(3):1192–1198. doi: 10.1128/mcb.10.3.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]