Abstract
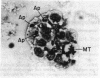
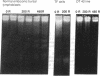
The lymphoid cells of embryonic bursal follicles are engaged in rapid growth and preimmune diversification of immunoglobulin genes. Disruption of follicular architecture by mechanical dispersion of these cells in short-term tissue culture was accompanied by continued cell division and extensive cell death by apoptosis. Apoptosis was suppressed in parallel cultures of intact follicles. gamma Radiation also triggered extensive apoptosis in embryonic bursal follicles within a few hours. Preneoplastic bursal stem cell populations induced by a v-myc oncogene were hypersensitive to induction of apoptosis by follicular dispersion and radiation. In contrast, tumor progression in v-myc- and v-rel-initiated bursal neoplasms was accompanied by development of resistance to induction of apoptosis. A programmed cell death pathway can be activated during normal B-cell development in the bursa, and alterations in the expression of this pathway accompany neoplastic change in this system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baba T. W., Humphries E. H. Formation of a transformed follicle is necessary but not sufficient for development of an avian leukosis virus-induced lymphoma. Proc Natl Acad Sci U S A. 1985 Jan;82(1):213–216. doi: 10.1073/pnas.82.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth C. F., Humphries E. H. Expression of v-rel induces mature B-cell lines that reflect the diversity of avian immunoglobulin heavy- and light-chain rearrangements. Mol Cell Biol. 1988 Dec;8(12):5358–5368. doi: 10.1128/mcb.8.12.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamou L. E., Cazenave P. A., Sarthou P. Anti-immunoglobulins induce death by apoptosis in WEHI-231 B lymphoma cells. Eur J Immunol. 1990 Jun;20(6):1405–1407. doi: 10.1002/eji.1830200630. [DOI] [PubMed] [Google Scholar]
- Carlson L. M., Oettinger M. A., Schatz D. G., Masteller E. L., Hurley E. A., McCormack W. T., Baltimore D., Thompson C. B. Selective expression of RAG-2 in chicken B cells undergoing immunoglobulin gene conversion. Cell. 1991 Jan 11;64(1):201–208. doi: 10.1016/0092-8674(91)90221-j. [DOI] [PubMed] [Google Scholar]
- Collins R. J., Verschuer L. A., Harmon B. V., Prentice R. L., Pope J. H., Kerr J. F. Spontaneous programmed death (apoptosis) of B-chronic lymphocytic leukaemia cells following their culture in vitro. Br J Haematol. 1989 Mar;71(3):343–350. doi: 10.1111/j.1365-2141.1989.tb04290.x. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., Payne L. N., Dent P. B., Burmester B. R., Good R. A. Pathogenesis of avian lymphoid leukosis. I. Histogenesis. J Natl Cancer Inst. 1968 Aug;41(2):373–378. [PubMed] [Google Scholar]
- Eskola J., Toivanen P. Effect of in ovo treatment with cyclophosphamide on lymphoid system in chicken. Cell Immunol. 1974 Sep;13(3):459–471. doi: 10.1016/0008-8749(74)90265-2. [DOI] [PubMed] [Google Scholar]
- Gregory C. D., Dive C., Henderson S., Smith C. A., Williams G. T., Gordon J., Rickinson A. B. Activation of Epstein-Barr virus latent genes protects human B cells from death by apoptosis. Nature. 1991 Feb 14;349(6310):612–614. doi: 10.1038/349612a0. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Weinert T. A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989 Nov 3;246(4930):629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Hasbold J., Klaus G. G. Anti-immunoglobulin antibodies induce apoptosis in immature B cell lymphomas. Eur J Immunol. 1990 Aug;20(8):1685–1690. doi: 10.1002/eji.1830200810. [DOI] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Jalkanen S., Jalkanen M., Granfors K., Toivanen P. Defect in the generation of light-chain diversity in bursectomized chickens. Nature. 1984 Sep 6;311(5981):69–71. doi: 10.1038/311069a0. [DOI] [PubMed] [Google Scholar]
- Linial M., Gunderson N., Groudine M. Enhanced transcription of c-myc in bursal lymphoma cells requires continuous protein synthesis. Science. 1985 Dec 6;230(4730):1126–1132. doi: 10.1126/science.2999973. [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Joshua D. E., Williams G. T., Smith C. A., Gordon J., MacLennan I. C. Mechanism of antigen-driven selection in germinal centres. Nature. 1989 Dec 21;342(6252):929–931. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Deane N., Platt F. M., Nunez G., Jaeger U., McKearn J. P., Korsmeyer S. J. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989 Apr 7;57(1):79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- Neiman P. E., Gehly E. B., Carlson L. M., Cotter R. C., Thompson C. B. Bursal stem cells as targets for myc-induced preneoplastic proliferation and maturation arrest. Curr Top Microbiol Immunol. 1988;141:67–74. doi: 10.1007/978-3-642-74006-0_10. [DOI] [PubMed] [Google Scholar]
- Neiman P., Wolf C., Enrietto P. J., Cooper G. M. A retroviral myc gene induces preneoplastic transformation of lymphocytes in a bursal transplantation assay. Proc Natl Acad Sci U S A. 1985 Jan;82(1):222–226. doi: 10.1073/pnas.82.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud C. A., Anquez V., Grimal H., Weill J. C. A hyperconversion mechanism generates the chicken light chain preimmune repertoire. Cell. 1987 Feb 13;48(3):379–388. doi: 10.1016/0092-8674(87)90189-9. [DOI] [PubMed] [Google Scholar]
- Thompson C. B., Humphries E. H., Carlson L. M., Chen C. L., Neiman P. E. The effect of alterations in myc gene expression on B cell development in the bursa of Fabricius. Cell. 1987 Nov 6;51(3):371–381. doi: 10.1016/0092-8674(87)90633-7. [DOI] [PubMed] [Google Scholar]
- Thompson C. B., Neiman P. E. Somatic diversification of the chicken immunoglobulin light chain gene is limited to the rearranged variable gene segment. Cell. 1987 Feb 13;48(3):369–378. doi: 10.1016/0092-8674(87)90188-7. [DOI] [PubMed] [Google Scholar]
- Trauth B. C., Klas C., Peters A. M., Matzku S., Möller P., Falk W., Debatin K. M., Krammer P. H. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989 Jul 21;245(4915):301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- Vaux D. L., Cory S., Adams J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Rose K. A., Morris R. G., Steel C. M., Foster E., Spandidos D. A. Rodent fibroblast tumours expressing human myc and ras genes: growth, metastasis and endogenous oncogene expression. Br J Cancer. 1987 Sep;56(3):251–259. doi: 10.1038/bjc.1987.186. [DOI] [PMC free article] [PubMed] [Google Scholar]