Abstract
Background: There is accumulating evidence on the negative impacts of childhood poverty on physical and mental health. Previous work has suggested hyperactive neural response to social fear cues, as well as impairment in neural regulatory functions. However, despite differences found between males and females in stress-related and anxiety disorders, possible sex-specific effects of poverty on emotional processing have not been explored. Methods: We analyzed data from three previously reported experiments of childhood poverty effects on emotional processing and regulation, for sex-specific effects. Participants were 52 healthy Caucasian males and females, from a longitudinal cohort of poverty development study, who were recruited for examining the long-term effects of childhood poverty and stress. The three functional MRI studies included emotion regulation task, emotional face assessment task, and shifted attention emotion appraisal task. Brain activations that associated with childhood poverty previously were entered into a regression analysis with interaction of gender by childhood income-to-need ratio as the independent variable, and age and current income-to-need ratio as variables of no interest, separately for males and females. Results: Amygdala reactivity to implicitly processed fearful faces was positively correlated with childhood income-to-need in adult females but not males. On the other hand, activation in dorsolateral and ventrolateral prefrontal regions during emotion regulation by reappraisal was positively correlated with childhood income-to-need in males. Conclusion: Childhood poverty may exert sex-specific effects in adulthood as presented by hypersensitive emotional reactivity of the amygdala in females, and impaired emotion regulatory function of the prefrontal cortex in males. Results suggest further focus on sex-specific effects of childhood poverty.
Keywords: poverty, sex, fMRI, amygdala, sex-specific
1. Introduction
Childhood poverty is linked to increased risk of psychopathology and medical illness in adulthood irrespective of adult socioeconomic status [1,2,3,4,5,6]. One in four children in America are born to poverty [7], and identifying underlying mechanisms leading to long-term effects of poverty on physical and mental health is important in developing measures to prevent these adverse effects. There is accumulating evidence for anatomical and functional brain changes in adults as a result of childhood poverty, suggesting a neurobiological nature to the effects of childhood poverty [8,9,10,11,12,13]. Previous work has found this effect in brain regions involved in emotional response and emotion regulation. Changes in amygdala volume, for example, have been repeatedly reported in adults with history of childhood poverty [8,9,10]. Amygdala hyperactivity in response to threat and fear-related social cues has been replicated in emotion provocation studies of adults with history of childhood poverty [11,12,13]. Reduced cortical thickness in anterior cingulate volume was also liked to history of childhood poverty [14,15]. Our group has previously reported that: adults with a history of childhood poverty had lower ventrolateral prefrontal cortex (VLPFC) and (dorsolateral prefrontal cortex) DLPFC activation during cognitive appraisal of their emotional response to emotional faces [16], and in reappraisal of their emotional response to negative pictures [17].
Although evidence for the sex-specific effects of stress and mental illness is abundant and accumulating, the specific neurobiological mechanisms mediating sex-specific effects are largely unknown. Women have a higher chance of developing major depressive disorder [18] as well as other stress and anxiety-related disorders [19]. While affective and anxiety disorders are more common among women, some disorders of regulatory function such as attention deficit hyperactive disorder, conduct disorder [20], antisocial personality disorder [21], and completed suicide [22] are more common in men. Sex-specific differences in stress response might mediate some of the differences noted above. Cortisol is a key hormonal mediator of acute and chronic stress response in humans, and administration of cortisol during fear conditioning reduces activation in emotion regulatory areas (anterior cingulate, medial prefrontal, and orbitofrontal cortex) in men while increasing activation in these areas in females [23]. Cortisol also reduces psychosocial stress-related amygdala responses in men, while increasing it in women [24]. Females in general have larger startle response to threat-related stimuli [25], and larger left amygdala responses to threat-related pictures [26]. Ohrmann and colleagues [27] reported stronger amygdala and prefrontal cortical response to emotional faces in women with panic disorder compared to men with the same disorder. These data suggest a generally larger sensitivity to social cues in women than men [28,29]. There is also evidence for anatomical differences in relation to emotion regulation strategies between men and women. In a study of frontal cortical maturation, Vijayakumar and colleagues [30] found that greater maturational thinning of the DLPFC and VLPFC cortices during adolescence was correlated with cognitive reappraisal abilities in women, but not men.
With regards to sex-specific effects of poverty, we reported that women with childhood poverty had a larger posterior insula response to infant cry than men with history of poverty [12]. There was no main effect of poverty or gender observed in this area, and the observed effect was an interaction of childhood poverty and sex. The effects of childhood poverty in our cohort, however, were not always mediated by the indices of cumulative stress [13,16] suggesting that additional mechanisms might mediate effects of childhood poverty on sex-specific differences. Clearly, more work examining this question is needed, but the existing literature on such sex-specific effects of childhood poverty is very limited. In the current work, we examined data from three (previously reported) emotion provocation [13] and regulation tasks ([12,16] in a cohort of healthy adults with history of childhood poverty, to explore sex-specific effects of childhood poverty in emotional reactivity and regulation. In the context of the previous reports about the sex-specific effects of stress on emotion reactivity and regulation (more pronounced aberrations of emotion reactivity in females and emotion regulation in males), as well as the effects of poverty in the same areas, we expected to see more pronounced amygdala activation in emotion provocation tasks in women with a history of childhood poverty, and less activation in prefrontal cortical areas of DLPFC and VLPFC in emotion regulation tasks in men with childhood poverty. The brain areas which are chosen to explore in this analysis are based on the previous reports of sex differences in emotion processing, the areas that the tasks used in this work are meant to activate, and our previous findings of effects of childhood poverty on brain activation in emotion processing.
2. Method
2.1. Participants
Healthy unmedicated Caucasian males and females without current or past axis I psychiatric diagnosis confirmed by clinician-conducted Structural Clinical Interview for DSM-IV, enrolled in a 20-year longitudinal cohort of poverty and child development study, were recruited for examining the long-term effects of childhood poverty and stress [13,16,17,31]. The same participants participated in the three tasks reported below. Imaging data for 49 (22 females), 49 (22 females), and 52 (24 females) of these participants were available in the Emotion Regulation Task (ERT) [17], Shifted-Attention Emotion Appraisal Task (SEAT) [16], and Emotional Face Assessment Task (EFAT) [13] respectively for the current analysis. Half of the participants who were recruited at age 9 spent their childhood in low-income households (income-to-need ratio less than 1.5 in New York State) and half grew up in middle-income households (income to need ratio > 1.5). Income-to-need ratio is a per capita index, adjusted annually for costs of living. A ratio equal to or less than 1.0 is defined by the US Census Bureau as “poverty.” All participants were right-handed, and none had a major medical illness or contraindication for MRI (e.g., metallic/ferrous materials in their body). This study was approved by the University of Michigan and Cornell University Institutional Review Boards and all participants provided informed consent. Demographic data are summarized in Table 1. Female and male participants did not significantly differ in age, childhood income-to-need, current income-to-need, or level of education.
Table 1.
Demographic data of the two groups of participants in the three studies. EFAT: Emotional Face Assessment Task; ERT: Emotion Regulation Task; SEAT: Shifting Emotion Attention Task.
| Study | Number of Participants | M/F | Age |
|---|---|---|---|
| EFAT | 52 | 28/24 | 24.4 ± 1.2 |
| ERT | 49 | 27/22 | 23.6 ± 1.3 |
| SEAT | 49 | 27/22 | 23.7 ± 1.3 |
2.2. Experimental Tasks
Detailed descriptions of the tasks are available in original reports; briefly, in the EFAT task [13] designed to examine amygdala reactivity, participants were asked to match one of the two faces on the bottom to the emotion expressed by target face on top. These included angry, fearful, happy, and neutral faces. In participants with history of childhood poverty, we have observed larger amygdala response to Fearful > Happy faces, due to larger response to fearful, and smaller response to happy faces. In other words, the lower the childhood income-to-need, the higher the response in amygdala to fearful faces, and the lower to happy faces. In the ERT task [17], participants were instructed to “Look” at the neutral IAPS pictures presented on the screen, “Maintain” (attend and experience naturally) the emotional state elicited by the aversive pictures (probing explicit emotional response), or “Reappraise” and voluntarily decrease the level of their negative affect in response to aversive pictures through cognitive reappraisal [32]. Here we observed diminished emotion regulatory activation during reappraisal in the DLPFC and VLPFC in relation to poverty. In the SEAT task [16], participants saw a picture of a fearful or neutral face superimposed on a place, and were asked to either identify the gender of the face (Male/Female), whether the place is indoor or outdoor (In/Out), or if they liked or disliked the face (Like/Dislike). Childhood poverty was correlated with impaired DLPFC function during appraisal-related emotion regulation (Like/Dislike > Male/Female) for this task.
In summary, the EFAT examines implicit emotional reactivity of the amygdala to negative and positive emotional faces, SEAT examines implicit regulation of emotional reactivity by appraisal or attention, and ERT examines explicit regulation of emotional response by reappraisal.
2.2.1. Acquisition of MRI Data
All scanning was performed using a Philips 3 Tesla MRI scanner (Philips Medical Systems, Andover, Massachusetts) in the functional MRI laboratory at the Veterans Affair Ann Arbor. A total of 240 T2*-weighted echo planar gradient-recall echo volumes (echo time = 30 ms, repetition time = 2000 ms, 64 × 64 matrix, flip angle = 90 degree, field of view = 22 cm, 42 contiguous 3 mm axial slices per volume), were acquired for each task. Five additional volumes were discarded at the beginning of each run to allow for equilibration of the MRI signal. A high-resolution T1-weighted structural image was also obtained to provide for more precise anatomical localization.
2.2.2. MRI Data Analysis
Details of the preprocessing and first-level analysis can be found in the original reports describing data analysis for each of the tasks [13,16,17]. Data were analyzed using the statistical parametric mapping software package, SPM8 (Welcome Department of Cognitive Neurology, London, UK).
For the EFAT task, first-level models consisted of regressors for task conditions (angry, fearful, happy, neutral blocks) as well as nuisance regressors consisting of the motion correction parameters from the realignment preprocessing step. We extracted betas for Fearful > Happy contrast using bilateral anatomical (AAL) amygdala masks to examine possible sex-specific implicit emotional response to the faces. We then entered these extracted betas into a regression analysis with childhood income-to-need ratio, gender, and their interaction as the independent variable, and age and current income-to-need ratio as variables of no interest.
For the ERT task, the first-level contrasts included Look (baseline response to neutral images), Maintain > Look (Explicit emotional reactivity), and Reappraise > Maintain (reappraisal-related emotion regulation). A multiple regression was performed with the brain activation as dependent variable, the childhood income-to-needs ratio as an independent variable and the current income-to-needs ratio and age as a covariate of no interest. An initial voxel-wise threshold of p < 0.005 and a minimum cluster size of 265 voxels for the Reappraisal vs. Maintain contrast gave a corrected p < 0.05. The Region of Interest (ROI) for VLPFC was created by placing an 8 mm radius sphere at the left VLPFC peak (x, y, z = −50, 22, 6). To examine reappraisal-related emotion regulation, betas from this ROI, and DLPFC region (x, y, z = −40, 12, 28; 343 voxels; p < 0.05, corrected) were extracted for the contrast Reappraise > Maintain. To ascertain effects of explicit emotional response and explore their consistency with the effects found to the implicit emotional response during EFAT, the “Maintain > baseline” contrast in the ERT task was created to probe explicit emotional reactivity in amygdala. We thus extracted betas for the contrast “Maintain > baseline” for the left and right amygdalas using anatomical AAL bilateral amygdala masks. We then entered these extracted betas into a regression analysis with childhood income-to-need ratio, gender, and their interaction as the independent variable, and age and current income-to-need ratio as variables of no interest.
For the SEAT task, first-level contrast was Like/Dislike > Male/Female (which probes appraisal-related emotion regulation). To ascertain presence of sex-specific effects on implicit emotion regulation, and explore their consistency with the effects found during explicit emotional regulation in the ERT, betas for the DLPFC region were extracted from Like/Dislike > Male/Female contrast using an AAL anatomical mask. We then entered these extracted betas into a regression analysis with childhood income-to-need ratio, gender, and their interaction as the independent variable, and age and current income-to-need ratio as variables of no interest.
Analyses were done using the SPSS software (version 21, IBM, Armonk, NY, USA), with a p value threshold set at <0.05.
3. Results
Results of the regression analysis are summarized in Table 2. For brevity, only areas where there is an effect of gender, or gender by childhood income-to-need ratio are presented in the table.
Table 2.
Results of regression analysis: only results with significant gender, or gender by childhood income-to-need ratio effects are presented. EFAT = Emotional Face Assessment Task, ERT: Emotion Regulation Task, SEAT: Shifted-Attention Emotion Appraisal Task, CITN: childhood income-to-need ratio, AITN: current adult income-to-need ratio.
| Task/Brain Region | Beta Coefficient | T-Ratio | Significance Level |
|---|---|---|---|
| EFAT Right Amygdala Fearful > Happy | |||
| Age | −0.43 | −1.64 | 0.11 |
| CITN | −0.78 | −2.86 | 0.007 |
| AITN | 0.45 | 0.126 | 0.9 |
| Gender | −5.43 | −1.75 | 0.087 |
| Gender by Age | 4.73 | 1.62 | 0.11 |
| Gender by AITN | −0.28 | −0.67 | 0.51 |
| Gender by CITN | 0.80 | 2.25 | 0.03 |
| EFAT Right Amygdala Fearful | |||
| Age | −0.61 | −2.39 | 0.02 |
| CITN | −0.65 | −2.42 | 0.02 |
| AITN | 0.16 | 0.05 | 0.96 |
| Gender | −10.80 | −3.58 | 0.001 |
| Gender by Age | 10.17 | 3.60 | 0.001 |
| Gender by AITN | 0.86 | 2.48 | 0.017 |
| Gender by CITN | −0.14 | −0.34 | 0.74 |
| ERT DLPFC Reappraise > Maintain | |||
| Age | −0.63 | −2.12 | 0.04 |
| CITN | −5.41 | −1.20 | 0.05 |
| AITN | 4.16 | 1.03 | 0.3 |
| Gender | −5.01 | −2.24 | 0.03 |
| Gender by Age | 4.98 | 2.23 | 0.03 |
| Gender by AITN | −4.17 | −1.04 | 0.30 |
| Gender by CITN | 5.71 | 2.18 | 0.03 |
| ERT VLPFC Reappraise > Maintain | |||
| Age | −0.53 | −1.58 | 0.12 |
| CITN | −5.67 | −1.84 | 0.07 |
| AITN | 6.73 | 1.47 | 0.15 |
| Gender | −5.20 | −2.05 | 0.046 |
| Gender by Age | 5.21 | 2.07 | 0.05 |
| Gender by AITN | −6.82 | −1.51 | 0.14 |
| Gender by CITN | 5.78 | 1.95 | 0.05 |
3.1. Emotional Face Assessment Task (EFAT)
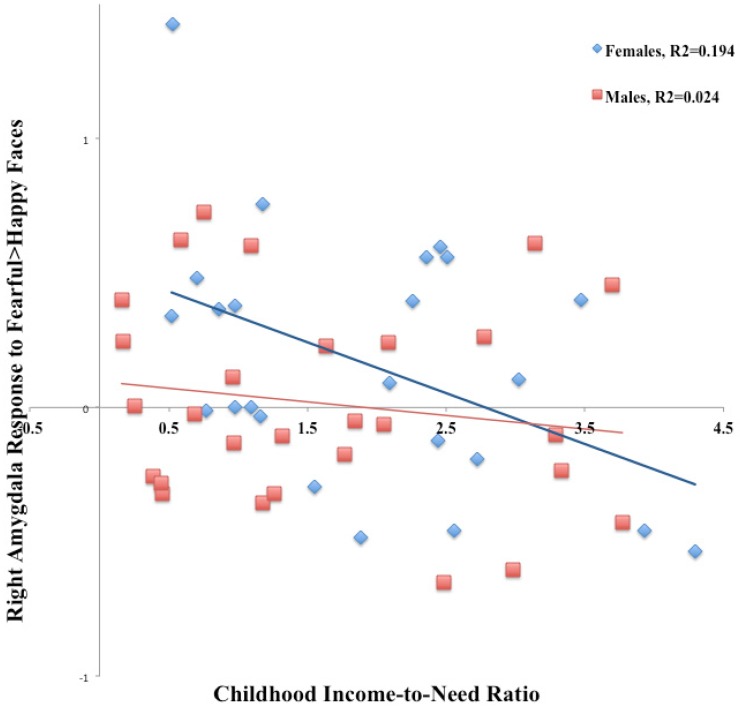
Childhood income-to-need ratio (B = −0.78, t(51) = −2.86, p = 0.007), but not gender (B = −5.43, t(51) = −1.75, p = 0.087) predicted brain activation in right amygdala in the contrast Fearful > Happy. There was a significant interaction of gender and childhood income-to-need ratio predicting brain activation in right amygdala in this contrast (B = 0.80, t(51) = 2.25, p = 0.03). Betas from regression were significantly different between males and females (t(48) = 5.10, p < 0.001), Figure 1. To determine the direction of changes contributing to the observed effect, we did the same regression analysis for Fearful, and Happy faces separately, in both males and females. Right amygdala activation in response to Fearful faces was predicted by childhood income-to-need ratio (B = −0.65, t(51) = −2.42, p = 0.02), gender (B = −10.80, t(51) = −3.58, p = 0.001), and interaction of gender and childhood income-to-need ratio (B = 0.86, t(51) = 2.48, p = 0.017). Betas were significantly different between males and females (t(48) = 10.56, p < 0.001). Right amygdala activation in response to Happy faces was not predicted by interaction of gender and childhood income-to-need ratio (B = −0.36, t(51) = −0.97, p = 0.34).
Figure 1.
Right amygdala activation in response to Fearful > Happy faces in males and females in the Emotional Face Assessment Task (EFAT) tasks.
Left amygdala activation in the contrast Fearful > Happy was predicted by childhood income-to-need ratio (B = −0.70, t(51) = −2.47, p = 0.02), but not by gender (B = −3.75, t(51) = −1.16, p = 0.3). Gender interaction by childhood income-to-need ratio did not predict brain activation in left amygdala in the contrast Fearful > Happy (B = 0.57, t(51) = 1.53, p = 0.13).
3.2. Emotion Regulation Task (ERT)
To examine sex-specific explicit emotional reactivity in the amygdala in response to negative IAPS pictures, we examined the contrast “Maintain > baseline”. In this contrast, childhood income-to-need ratio, gender, or the interaction of gender and childhood income-to-need ratio did not predict activation in left (B = 0.60, t(48) = 1.57, p = 0.13) or right amygdala (B = −0.32, t(48) = −0.72, p = 0.48).
To assess reappraisal-related emotion regulation in the left DLPFC and VLPFC in association with childhood poverty for males and females, separately, we examined the contrast Reappraise > Maintain (reappraisal-related emotion regulation). Left DLPFC activation was predicted by childhood income-to-need ratio (B = −5.41, t(51) = −1.20, p = 0.05), gender (B = −5.01, t(51) = −2.24, p = 0.03), and interaction of gender and childhood income-to-need ratio (B = 5.71, t(48) = 2.18, p = 0.03). Betas were significantly different between males and females t(45) = 3.99, p < 0.001). In the same contrast, left VLPFC activation was marginally predicted by childhood income-to-need ratio (B = −5.67, t(51) = −1.84, p = 0.07), and significantly predicted by gender (B = −5.20, t(51) = −2.05, p = 0.046) and interaction of gender and childhood income-to-need ratio (B = 5.78, t(48) = 1.95, p = 0.05). Betas were significantly different between males and females t(45 = 2.54, p = 0.014).
3.3. Shifted-Attention Emotion Appraisal Task (SEAT)
To examine sex-specific differences in implicit emotion regulation using appraisal, we examined the contrast Like/Dislike > Male/Female. In the contrast Like/Dislike > Male/Female (appraisal-related emotion regulation), left DLPFC activation was marginally predicted by childhood income-to-need ratio (B = 0.49, t(51) = 1.81, p = 0.07), but not predicted by gender (B = 1.34, t(51) = 0.43, p = 0.7) or interaction of gender and childhood income-to-need ratio (B = −0.45, t(48) = −0.13, p = 0.9).
4. Discussion
In this work, we aimed to explore sex-specific effects in a sample of healthy adults with history of childhood poverty. We examined sex-specific effects in implicit and explicit emotional reactivity, using EFAT and ERT accordingly. We also examined sex-specific effects of childhood poverty on implicit and explicit emotional regulation, using appraisal condition in SEAT and reappraisal condition in ERT, accordingly. Our findings suggest that the effects of poverty on implicit emotional reactivity in amygdala are mainly seen in females. The previously observed negative bias toward social fear cues (fearful faces) and away from positive cues (happy faces) in the EFAT task was derived by the female group. This effect was specifically more pronounced on the right side. Interestingly, this effect was not present during explicit emotional reactivity (ERT Maintain > baseline contrast). On the other hand, deficits in brain activation in explicit reappraisal-related emotion regulation were an effect of male participants.
Although human studies of sex-specific effects of early life stress on brain anatomy and function are scarce, animal research provides intriguing evidence for sex-specific effects. Hypothalamus Pituitary Axis (HPA) reactivity is more susceptible to prenatal stress in female rats such that adult female rats show larger corticosterone level at baseline and in response to stress [33,34]. Female rats with early life stress exhibit greater nociceptive responses than males with early life stress [35,36]. Even central administration of female hormones can induce visceral hypersensitivity in female rats [37]. In humans, the social stress test, which triggers a robust HPA response and increases cortisol level, shows sex-specific effects on fear conditioning. This effect is very much in concert with our findings and suggests reduced prefrontal activation in males, and increased amygdala activation in females in response to the fear-conditioned stimulus [24]. Also, administration of cortisol in a fear-conditioning study led to reduced activation in prefrontal regulatory areas in men, and increased activation in these areas in women in response to conditioned stimulus [23]. Our findings suggest sensitized implicit amygdala reactivity to emotional faces as an effect of childhood poverty in adult females. This is in line with previous reports of larger amygdala response to threat pictures in healthy females [26], and emotional faces in female patients with anxiety disorders ([27]. Indeed, prevalence of anxiety disorders is higher in adolescents with history of childhood poverty (for a review see [5]). It is thus possible that a more sensitive salience detection in female amygdala in response to social cues [28] may lead to sensitization to negative social signals as an effect of early life stress, as well as to subsequent development of anxiety disorders with heightened threat detection. Explicit awareness of the emotional response to aversive stimuli (ERT Maintain task) did not show the sex-specific effects of poverty in amygdala activity, which were observed during implicit emotional reactivity (EFAT task). Emotional faces processed implicitly might be a more sensitive probe of amygdala reactivity than explicit processing of emotional pictures that might involve some measure of cognitive processing. Interaction effects in amygdala were lateralized, and seen on the right side and in females. Multiple studies have previously shown left amygdala response to emotional pictures, or recall of emotional memories in females, and right amygdala response in males [26,38,39]. Absence of similar findings in the left amygdala in our study (B = 0.565, t(48) = 1.53, p = 0.13) could be due to the small sample size and low power. On the other hand, right amygdala response could be more vulnerable to the effects of poverty.
Our findings of impaired function in the emotion regulatory areas as an effect of poverty in males is consistent with some earlier findings reported in the animal and human literature. Several animal studies have shown that adult male, but not female, rats with prenatal or infancy exposure to stress had decreased dendritic complexity in medial prefrontal cortex (mPFC) and prelimbic cortex [40]. Blaze and colleagues [41] showed sex-specific changes in methylation of BDNF and reelin genes in the mPFC in male mice maltreated during infancy. Their findings suggest sex-specific epigenetic changes in expression of genes with a role in brain development and synaptic plasticity in emotion regulatory areas in male mice. Similarly in human studies, [27] cortisol reduced mPFC activation in fear-conditioning paradigm in males, but not females. This indirect evidence is consistent with our findings, and together suggest that structure and function of the prefrontal emotion regulatory areas in males might be more sensitive to developmental effects in general and to stress effects in particular.
Several limitations of the reported findings have to be acknowledged. Our low sample size might have led to limited power in detection of gender by childhood income-to-need interaction. The imaging tasks were not designed to probe differences in emotional processing between males and females, thus more gender-specific paradigms and analyses (e.g., differential response to female or male threat faces, or gender-specific negative images) could provide more specific data for possible threat-specific differences in neural responses of men and women. Secondly, while the absence of sex-specific effects in amygdala reactivity in the ERT task and the presence of these effects in the EFAT could be, as we suggested, due to difference between explicit and implicit emotional reactivity, other between-task differences could have contributed to these “inconsistencies.” ERT tasks involved presentation of aversive IAPS images, and not faces, and some previous studies reported higher amygdala activation in response to negative emotional faces than to negative IAPS images [42,43]. Weaker responses to pictures compared to faces may have reduced our power of detecting possible effects in amygdala in response to IAPS pictures. Furthermore, as is inherent to the majority of laboratory studies, complexity and intensity of the emotional stimuli are lower than in real-life experiences. Hence, expanding the results into in vivo emotion processing should be done cautiously. Finally, our work does not address the question of which mechanisms accompanying early life experience of poverty can lead to differential effects in neurocircuitry of emotion processing between males and females.
5. Conclusions
In summary, this is the first report on sex-specific effects of childhood poverty on social emotional processing and regulation. Our findings suggest differential effects of childhood poverty, such that in females it is associated with higher threat detection response in amygdala (and smaller response to positive social cues), while in males with impaired function of emotion regulatory regions. Our findings underline the importance of exploration of possible differential effects of early life stress, as well as poverty in larger studies, as these differences have been rarely explored. These results also suggest exploring possible sex-specific behavioral consequences of childhood stress (e.g., possibility of higher avoidance behavior due to higher threat detection in females versus impulsivity in males). However, at this stage, more studies exploring these differential effects are needed to map the potential differences in underlying neurocircuitry.
Acknowledgments
The authors highly appreciate the selfless enormous help and support of Michael Angstadt in analysis of the data.
Abbreviations
| AAL | Automated Anatomical Labeling |
| DLPFC | Dorsolateral Prefrontal Cortex |
| DSM | Diagnostic and Statistical Manual |
| EFAT | Emotional Face Assessment Task |
| ERT | Emotion Regulation Task |
| MRI | Magnetic Resonance Imaging |
| SEAT | Shifting Emotion Attention Task |
| VLPFC | ventrolateral Prefrontal Cortex |
Author Contributions
Arash Javanbakht was involved in study design, data analysis, interpretation, and writing the manuscript; Pilyoung Kim was involved in study design, data analysis, and writing the manuscript; James E. Swain was involved in study design, data collection, interpretation of the results, and writing the manuscript; Gary W. Evans was involved in study design, data analysis, interpretation of the results, and writing the manuscript; K. Luan Phan was involved in the study design and writing the manuscript; Israel Liberzon was involved in the study design, interpretation of the results, and writing the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. Declaration: Ethics approval and consent to participate: This study was approved by the internal review board of the University of Michigan. All participants signed informed consent before participating in this study. Consent for publication: This study does not contain any individual person’s data. Availability of data and materials: Please contact corresponding author for data requests. Funding: This research was partially funded by the National Institute on Minority Health and Health Disparities, 5RC2MD00467, the W.T. Grant Foundation, the John D. and Catherine T. Mac Arthur Foundation Network on Socioeconomic Status and Health, the Robert Wood Johnson Foundation and the University of Michigan’s Injury Center (Centers for Disease Control & Prevention Award Number U49/CE002099) and Center for Human Growth and Development. Authors’ information: Please see authors’ information on page 1.
References
- 1.Adler N.E., Rehkopf D.H. U.S. disparities in health: Descriptions, causes, and mechanisms. Annu. Rev. Public Health. 2008;29:235–252. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- 2.Birnie K., Cooper R., Martin R.M., Kuh D., Sayer A.A., Alvarado B.E., Bayer A., Christensen K., Cho S.I., Cooper C., et al. Childhood socioeconomic position and objectively measured physical capability levels in adulthood: A systematic review and meta-analysis. PLoS ONE. 2011;6:e15564. doi: 10.1371/journal.pone.0015564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen J., Cohen P., West S.G., West L.S. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Erlbaum Press; New York, NY, USA: 2003. pp. 375–388. [Google Scholar]
- 4.Shonkoff J.P., Boyce W.T., McEwen B.S. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 5.Wadsworth M.E., Evans G.W., Grant K., Carter J.S., Duffy S. Poverty and the development of psychopathology. In: Cicchetti D., editor. Developmental Psychopathology. Wiley; New York, NY, USA: 2016. [Google Scholar]
- 6.Poulton R., Caspi A., Milne B.J., Thomson W.M., Taylor A., Sears M.R., Moffitt T.E. Association between children’s ex-perience of socioeconomic disadvantage and adult health: A life-course study. Lancet. 2002;360:1640–1645. doi: 10.1016/S0140-6736(02)11602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattingly M.J., Carson J.A., Schaefer A. 2012 National Child Poverty Rate Stagnates at 22.6 Percent: New Hampshire Child Poverty Jumps 30 Percent Since 2011. Carsey Institute, University of New Hampshire; Durham, NH, USA: 2013. National Issue Brief #65. [Google Scholar]
- 8.Hanson J.L., Nacewicz B.M., Sutterer M.J., Cayo A.A., Schaefer S.M., Rudolph K.D., Shirtcliff E.A., Pollak S.D., Davidson R.J. Behavioral Problems After Early Life Stress: Contributions of the Hippocampus and Amygdala. Biol. Psychiatry. 2015;77:314–323. doi: 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luby J., Belden A., Botteron K., Marrus N., Harms M.P., Babb C., Nishino T., Barch D. The effects of poverty on childhood brain development: The mediating effect of caregiving and stressful life events. JAMA Pediatr. 2013;167:1135–1142. doi: 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noble K.G., Houston S.M., Kan E., Sowell E.R. Neural correlates of socioeconomic status in the developing human brain. Dev. Sci. 2012;15:516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gianaros P.J., Horenstein J.A., Hariri A.R., Sheu L.K., Manuck S.B., Matthews K.A., Cohen S. Potential neural embedding of parental social standing. Soc. Cogn. Affect. Neurosci. 2008;3:91–96. doi: 10.1093/scan/nsn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim P., Ho S.S., Evans G.W., Liberzon I., Swain J.E. Childhood social inequalities influences neural processes in young adult caregiving. Dev. Psychobiol. 2015;57:948–960. doi: 10.1002/dev.21325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Javanbakht A., King A.P., Evans G.W., Swain J.E., Angstadt M., Phan K.L., Liberzon I. Childhood poverty predicts adult amygdala and frontal activity and connectivity in response to emotional faces. Front. Behav. Neurosci. 2015;9:154. doi: 10.3389/fnbeh.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gianaros P.J., Horenstein J.A., Cohen S., Matthews K.A., Brown S.M., Flory J.D., Critchley H.D., Manuck S.B., Hariri A.R. Perigenual anterior cingulate morphology covaries with perceived social standing. Soc. Cogn. Affect. Neurosci. 2007;2:161–173. doi: 10.1093/scan/nsm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silverman M.E., Muennig P., Liu X., Rosen Z., Goldstein M.A. The impact of socioeconomic status on the neural substrates associated with pleasure. Open Neuroimage J. 2009;3:58–63. doi: 10.2174/1874440000903010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberzon I., Ma S.T., Okada G., Shaun Ho S., Swain J.E., Evans G.W. Childhood poverty and recruitment of adult emotion regulatory neurocircuitry. Soc. Cogn. Affect. Neurosci. 2015;10:1596–1606. doi: 10.1093/scan/nsv045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim P., Evans G.W., Angstadt M., Ho S.S., Sripada C.S., Swain J.E., Liberzon I., Phan K.L. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc. Natl. Acad. Sci. USA. 2013;110:18442–18447. doi: 10.1073/pnas.1308240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kessler R.C., McGonagle K.A., Nelson C.B., Hughes M., Swartz M., Blazer D.G. Sex and depression in the National Comorbidity Survey. II: Cohort effects. J. Affect. Disord. 1994;1:15–26. doi: 10.1016/0165-0327(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 19.Bangasser D.A., Valentino R.J. Sex differences in stress-related psychiatric disorders: Neurobiological perspectives. Front. Neuroendocrinol. 2014;35:303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merikangas K.R., He J.P., Burstein M., Swanson S.A., Avenevoli S., Cui L., Benjet C., Georgiades K., Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kessler R.C., McGonagle K.A., Zhao S., Nelson C.B., Hughes M., Eshleman S., Wittchen H.U., Kendler K.S. Lifetime and 12 month prevalence of, D.S.M-111-R psychiatric disorders in the United States. Arch. Gen. Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 22.Thomas K., Gunnell D. Suicide in England and Wales 1861–2007: A time-trends analysis. Int. J. Epidemiol. 2010;39:1464–1475. doi: 10.1093/ije/dyq094. [DOI] [PubMed] [Google Scholar]
- 23.Stark R., Wolf O.T., Tabbert K., Kagerer S., Zimmermann M., Kirsch P., Schienle A., Vaitl D. Influence of the stress hormone cortisol on fear conditioning in humans: Evidence for sex differences in the response of the prefrontal cortex. Neuroimage. 2006;32:1290–1298. doi: 10.1016/j.neuroimage.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 24.Merz C.J., Wolf O.T., Schweckendiek J., Klucken T., Vaitl D., Stark R. Stress differentially affects fear conditioning in men and women. Psychoneuroendocrinology. 2013;38:2529–2541. doi: 10.1016/j.psyneuen.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Bradley M.M., Cuthbert B.N., Lang P.N. Affect and the startle reflex. In: Dawson M.E., Schell A.M., Bohmelt A.H., editors. Startle Modification: Implications for Neuroscience, Cognitive Science, and Clinical Science. Cambridge University Press; New York, NY, USA: 1999. pp. 157–183. [Google Scholar]
- 26.Canli T., Desmond J.E., Zho Z., Gabrieli J.D. Sex differences in the neural basis of emotional memories. Proc. Natl. Acad. Sci. USA. 2002;99:10789–10794. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohrmann P., Pedersen A., Braun M., Bauer J., Kugel H., Kersting A., Domschke K., Deckert J., Suslow T. Effect of gender on processing threat-related stimuli in patients with panic disorder: Sex does matter. Depress. Anxiety. 2010;27:1034–1043. doi: 10.1002/da.20721. [DOI] [PubMed] [Google Scholar]
- 28.McClure E.B., Monk C.S., Nelson E.E., Zarahn E., Leibenluft E., Bilder R.M., Charney D.S., Ernst M., Pine D.S. A developmental examination of gender differences in brain engagement during evaluation of threat. Biol. Psychiatry. 2004;55:1047–1055. doi: 10.1016/j.biopsych.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Brody L.R., Hall J.A. Gender and Emotion. In: Lewis M., Haviland J.M., editors. Handbook of Emotions. Guilford; New York, NY, USA: 1993. pp. 447–460. [Google Scholar]
- 30.Vijayakumar N., Whittle S., Yücel M., Dennison M., Simmons J., Allen N.B. Thinning of the lateral prefrontal cortex during adolescence predicts emotion regulation in females. Soc. Cogn. Affect. Neurosci. 2014;9:1845–1854. doi: 10.1093/scan/nst183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans G.W. A multimethodological analysis of cumulative risk and allostatic load among rural children. Dev. Psychol. 2003;39:924–933. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- 32.Phan K.L., Fitzgerald D.A., Nathan P.J., Moore G.J., Uhde T.W., Tancer M.E. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biol. Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 33.Weinstock M., Matlina E., Maor G.I., Rosen H., McEwen B.S. Prenatal stress selectively alters the reactivity of the hypothalamic-pituitary adrenal system in the female rat. Brain Res. 1992;595:195–200. doi: 10.1016/0006-8993(92)91049-K. [DOI] [PubMed] [Google Scholar]
- 34.McCormick C.M., Smythe J.W., Sharma S., Meaney M.J. Sex-specific effects of prenatal stress on hypothalamic–pituitary–adrenal responses to stress and brain glucocorticoid receptor density in adults rats. Dev. Brain Res. 1995;84:55–61. doi: 10.1016/0165-3806(94)00153-Q. [DOI] [PubMed] [Google Scholar]
- 35.Kayser V., Berkley K.J., Keita H., Gautron M., Guilbaud G. Estrous and sex variations in vocalization thresholds to hind paw and tail pressure stimulation in the rat. Brain Res. 1996;742:352–354. doi: 10.1016/S0006-8993(96)01108-0. [DOI] [PubMed] [Google Scholar]
- 36.Chaloner A., Greenwood-Van Meerveld B. Sexually dimorphic effects of unpredictable early life adversity on visceral pain behavior in a rodent model. J. Pain. 2013;14:270–280. doi: 10.1016/j.jpain.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Myers B., Schulkin J., Greenwood-Van Meerveld B. Sex steroids localized to the amygdala increase pain responses to visceral stimulation in rats. J. Pain. 2011;12:486–494. doi: 10.1016/j.jpain.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Cahill L., Haier R.J., White N.S., Fallon J., Kilpatrick L., Lawrence C., Potkin S.G., Alkire M.T. Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiol. Learn. Mem. 2001;75:1–9. doi: 10.1006/nlme.2000.3999. [DOI] [PubMed] [Google Scholar]
- 39.Cahill L., Uncapher M., Kilpatrick L., Alkire M.T., Turner J. Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: An fMRI investigation. Learn. Mem. 2004;11:261–266. doi: 10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suenaga T., Yukie M., Gao S., Nakahara D. Sex-specific effects of prenatal stress on neuronal development in the medial prefrontal cortex and the hippocampus. Neuroreport. 2012;23:430–435. doi: 10.1097/WNR.0b013e3283529805. [DOI] [PubMed] [Google Scholar]
- 41.Blaze J., Scheuing L., Roth T.L. Differential methylation of genes in the medial prefrontal cortex of developing and adult rats following exposure to maltreatment or nurturing care during infancy. Dev. Neurosci. 2014;35:306–316. doi: 10.1159/000350716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hariri A.R., Tessitore A., Mattay V.S., Fera F., Weinberger D.R. The amygdala response to emotional stimuli: A comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- 43.Britton J.C., Taylor S.F., Sudheimer K.D., Liberzon I. Facial expressions and complex IAPS pictures: Common and differential networks. Neuroimage. 2006;31:906–919. doi: 10.1016/j.neuroimage.2005.12.050. [DOI] [PubMed] [Google Scholar]