Abstract
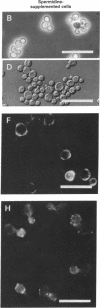
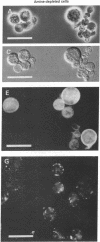
A null mutation in the SPE2 gene of Saccharomyces cerevisiae, encoding S-adenosylmethionine decarboxylase, results in cells with no detectable S-adenosylmethionine decarboxylase, spermidine, and spermine. This mutant has an absolute requirement for spermidine or spermine for growth; this requirement is not satisfied by putrescine. Polyamine-depleted cells show a number of microscopic abnormalities that are similar to those reported for several cell division cycle (cdc) and actin mutants. These include a striking increase in cell size, a marked decrease in budding, accumulation of vesicle-like bodies, absence of specific localization of chitin-like material, and abnormal distribution of actin-like material. The absolute requirement for polyamines for growth and the microscopic abnormalities are not seen if the cultures are grown under anaerobic conditions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A. E., Johnson D. I., Longnecker R. M., Sloat B. F., Pringle J. R. CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J Cell Biol. 1990 Jul;111(1):131–142. doi: 10.1083/jcb.111.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams A. E., Pringle J. R. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J Cell Biol. 1984 Mar;98(3):934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams A. E., Pringle J. R. Staining of actin with fluorochrome-conjugated phalloidin. Methods Enzymol. 1991;194:729–731. doi: 10.1016/0076-6879(91)94054-g. [DOI] [PubMed] [Google Scholar]
- Amatruda J. F., Cannon J. F., Tatchell K., Hug C., Cooper J. A. Disruption of the actin cytoskeleton in yeast capping protein mutants. Nature. 1990 Mar 22;344(6264):352–354. doi: 10.1038/344352a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cabib E., Bowers B. Chitin and yeast budding. Localization of chitin in yeast bud scars. J Biol Chem. 1971 Jan 10;246(1):152–159. [PubMed] [Google Scholar]
- Cabib E., Bowers B. Timing and function of chitin synthesis in yeast. J Bacteriol. 1975 Dec;124(3):1586–1593. doi: 10.1128/jb.124.3.1586-1593.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M. S., Tabor C. W., Tabor H. Identification of a pyruvoyl residue in S-adenosylmethionine decarboxylase from Saccharomyces cerevisiae. J Biol Chem. 1977 Nov 25;252(22):8212–8216. [PubMed] [Google Scholar]
- Cohn M. S., Tabor C. W., Tabor H. Isolation and characterization of Saccharomyces cerevisiae mutants deficient in S-adenosylmethionine decarboxylase, spermidine, and spermine. J Bacteriol. 1978 Apr;134(1):208–213. doi: 10.1128/jb.134.1.208-213.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M. S., Tabor C. W., Tabor H. Regulatory mutations affecting ornithine decarboxylase activity in Saccharomyces cerevisiae. J Bacteriol. 1980 Jun;142(3):791–799. doi: 10.1128/jb.142.3.791-799.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin D. G., Miller K. G., Botstein D. Yeast actin-binding proteins: evidence for a role in morphogenesis. J Cell Biol. 1988 Dec;107(6 Pt 2):2551–2561. doi: 10.1083/jcb.107.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawlitta W., Stockem W. Visualization of actin polymerization and depolymerization cycles during polyamine-induced cytokinesis in living Amoeba proteus. Cell Tissue Res. 1981;215(2):249–261. doi: 10.1007/BF00239112. [DOI] [PubMed] [Google Scholar]
- Greer C., Schekman R. Actin from Saccharomyces cerevisiae. Mol Cell Biol. 1982 Oct;2(10):1270–1278. doi: 10.1128/mcb.2.10.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarer B. K., Lillie S. H., Adams A. E., Magdolen V., Bandlow W., Brown S. S. Purification of profilin from Saccharomyces cerevisiae and analysis of profilin-deficient cells. J Cell Biol. 1990 Jan;110(1):105–114. doi: 10.1083/jcb.110.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. E., Myers A. M., Koerner T. J., Tzagoloff A. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast. 1986 Sep;2(3):163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- Hosaka K., Yamashita S. Induction of choline transport and its role in the stimulation of the incorporation of choline into phosphatidylcholine by polyamines in a polyamine auxotroph of Saccharomyces cerevisiae. Eur J Biochem. 1981 May;116(1):1–6. doi: 10.1111/j.1432-1033.1981.tb05292.x. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. C., Prendergast J. A., Singer R. A. The Saccharomyces cerevisiae MYO2 gene encodes an essential myosin for vectorial transport of vesicles. J Cell Biol. 1991 May;113(3):539–551. doi: 10.1083/jcb.113.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi K., Taneja S. K., Liu T. Y., Tabor C. W., Tabor H. Spermidine biosynthesis in Saccharomyces cerevisiae. Biosynthesis and processing of a proenzyme form of S-adenosylmethionine decarboxylase. J Biol Chem. 1990 Dec 25;265(36):22321–22328. [PubMed] [Google Scholar]
- Kilmartin J. V., Adams A. E. Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J Cell Biol. 1984 Mar;98(3):922–933. doi: 10.1083/jcb.98.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton L. J., Lee P. L. More sensitive automated detection of polyamines in physiological fluids and tissue extracts with omicron-phthalaldehyde. Clin Chem. 1975 Nov;21(12):1721–1724. [PubMed] [Google Scholar]
- Minton K. W., Tabor H., Tabor C. W. Paraquat toxicity is increased in Escherichia coli defective in the synthesis of polyamines. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2851–2855. doi: 10.1073/pnas.87.7.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Botstein D. Phenotypic analysis of temperature-sensitive yeast actin mutants. Cell. 1985 Feb;40(2):405–416. doi: 10.1016/0092-8674(85)90154-0. [DOI] [PubMed] [Google Scholar]
- Oriol-Audit C. Actin-polyamines interaction : relationship between physicochemical properties and cytokinesis induction. Biochem Biophys Res Commun. 1982 Apr 14;105(3):1096–1101. doi: 10.1016/0006-291x(82)91082-8. [DOI] [PubMed] [Google Scholar]
- Oriol-Audit C. Induction of cytokinesis by interaction between actin and polyamines. Biochimie. 1980;62(10):713–714. doi: 10.1016/s0300-9084(80)80029-0. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohjanpelto P., Virtanen I., Hölttä E. Polyamine starvation causes disappearance of actin filaments and microtubules in polyamine-auxotrophic CHO cells. Nature. 1981 Oct 8;293(5832):475–477. doi: 10.1038/293475a0. [DOI] [PubMed] [Google Scholar]
- Pringle J. R., Preston R. A., Adams A. E., Stearns T., Drubin D. G., Haarer B. K., Jones E. W. Fluorescence microscopy methods for yeast. Methods Cell Biol. 1989;31:357–435. doi: 10.1016/s0091-679x(08)61620-9. [DOI] [PubMed] [Google Scholar]
- Roberts R. L., Bowers B., Slater M. L., Cabib E. Chitin synthesis and localization in cell division cycle mutants of Saccharomyces cerevisiae. Mol Cell Biol. 1983 May;3(5):922–930. doi: 10.1128/mcb.3.5.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Sloat B. F., Pringle J. R. A mutant of yeast defective in cellular morphogenesis. Science. 1978 Jun 9;200(4346):1171–1173. doi: 10.1126/science.349694. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W., Irreverre F. Quantitative determination of aliphatic diamines and polyamines by an automated liquid chromatography procedure. Anal Biochem. 1973 Oct;55(2):457–467. doi: 10.1016/0003-2697(73)90136-x. [DOI] [PubMed] [Google Scholar]
- Whitney P. A., Morris D. R. Polyamine auxotrophs of Saccharomyces cerevisiae. J Bacteriol. 1978 Apr;134(1):214–220. doi: 10.1128/jb.134.1.214-220.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q. W., Tabor C. W., Tabor H. Ornithine decarboxylase in Saccharomyces cerevisiae: chromosomal assignment and genetic mapping of the SPE1 gene. Yeast. 1990 Nov-Dec;6(6):455–460. doi: 10.1002/yea.320060602. [DOI] [PubMed] [Google Scholar]