Abstract
Objective:
To evaluate the use of the 13C-glucose breath test (13C-GBT) for insulin resistance (IR) detection in adolescents through comparison with fasting and post-glucose stimulus surrogates.
Methods:
One hundred thirty-three adolescents aged between 10 and 16 years received an oral glucose load of 1.75 g per kg of body weight dissolved in 150 mL of water followed by an oral dose of 1.5 mg/kg of U-13C-Glucose, without a specific maximum dose. Blood samples were drawn at baseline and 120 minutes, while breath samples were obtained at baseline and at 30, 60, 90, 120, 150, and 180 minutes. The 13C-GBT was compared to homeostasis model assessment (HOMA) IR (≥p95 adjusted by gender and age), fasting plasma insulin (≥p90 adjusted by gender and Tanner stage), results of 2-h oral glucose tolerance test (OGTT), insulin levels (≥65 μU/mL) in order to determine the optimal cut-off point for IR diagnosis.
Results:
13C-GBT data, expressed as adjusted cumulative percentage of oxidized dose (A% OD), correlated inversely with fasting and post-load IR surrogates. Sexual development alters A% OD results, therefore individuals were stratified into pubescent and post-pubescent. The optimal cut-off point for the 13C-GBT in pubescent individuals was 16.3% (sensitivity=82.8% & specificity=60.6%) and 13.0% in post-pubescents (sensitivity=87.5% & specificity=63.6%), when compared to fasting plasma insulin. Similar results were observed against HOMA and 2-h OGTT insulin.
Conclusion:
The 13C-GBT is a practical and non-invasive method to screen for IR in adolescents with reasonable sensitivity and specificity.
Keywords: 13C-glucose breath-test, insulin resistance, oral glucose tolerance test, adolescents
WHAT IS ALREADY KNOWN ON THIS TOPIC?
The 13C-glucose breath test (13C-GBT) is an accurate and reliable method to detect glucose metabolism disorders in adults, however, the use of this technique to assess insulin resistance (IR) in pediatric individuals is still under examination.
WHAT THIS STUDY ADDS?
The 13C-GBT was evaluated in a large population that included individuals with different body mass indexes and stages of pubertal development, furthermore, the A% oxidized dose was compared against IR surrogates in both fasting and post-load scenarios. This protocol suggests cut-off points to identify IR with reasonable sensitivity and specificity.
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a prevalent chronic disease and represents a grievous public health problem (1). This condition is initially asymptomatic, but it is a definite risk factor for cardiovascular disease, nephropathy, and neuropathy. Morbidity- and mortality-related T2DM decreases with adequate metabolic control, therefore, an early diagnosis is imperative (2). Insulin resistance (IR) is defined as a state in which a normal or elevated insulin level produces an attenuated biological response and constitutes a physiopathological basis for development of T2DM (3). IR is associated to a sedentary lifestyle and an unbalanced diet - risk factors commonly observed in adolescence. Thus, adolescence is a critical period of life for diagnosis and initiation of lifestyle intervention in at-risk individuals (4,5,6).
The gold standard to diagnose IR is the hyperinsulinemic euglycemic clamp (HEC) because it provides a direct, dynamic, and accurate assessment (7); nonetheless, this is an expensive and highly invasive method, unfeasible for standard clinical pediatric practice (8). Surrogate IR markers are frequently used to detect IR in ordinary settings, such as the homeostasis model assessment (HOMA-IR) which is based on fasting plasma glucose and insulin concentrations (9) and the Matsuda and DeFronzo (10) insulin sensitivity index (ISI-Composite) derived from plasma glucose and insulin levels throughout an oral glucose tolerant test (OGTT). These measurements have a moderate to good correlation with the HEC technique but remain invasive and poorly reproducible (11). The development of practical and non-invasive screening tests to detect IR is imperative (12).
The 13C-glucose breath test (13C-GBT) has been shown to be an accurate and reliable method to identify glucose metabolism disorders in adults (13,14). The 13C-GBT consists of ingestion of a 13C-glucose dose used as a tracer to label exhaled CO2 together with an oral load of non-labeled glucose to challenge insulin-dependent tissues. Patients with impaired glucose metabolism will have a reduced amount of exhaled 13CO2; this represents an indirect measurement of glucose oxidation via Krebs cycle (13,15). Lewanczuk et al (13) have shown that the 13C-GBT is effective to assess insulin sensitivity in obese individuals with T2DM. Recently, our group demonstrated that the 13C-GBT is a reproducible method to identify glucose metabolism defects also in adults without T2DM (14). We also established that the 13C-GBT is a valid method for screening for metabolic syndrome in adolescents (16).
The 13C-GBT has been extensively studied in adults. However, the use of this method to evaluate IR in pediatric individuals has been insufficiently explored. The purpose of this study was to assess the use of the 13C-GBT for IR detection in adolescents through comparison with fasting and post-glucose stimulus IR surrogates.
METHODS
This cross-sectional study was conducted in the Medical Nutrition Research Unit of the Mexican Social Security Institute in Mexico City, Mexico. The protocol was approved by the Ethics Committee of the said institution (R-2010-3603-35). One hundred thirty-three apparently healthy adolescents aged between 10 and 16 years assented to participate in the study. Informed consent was provided by the parents or by the accompanying adult. Exclusion criteria were: current chronic disease, diagnosed T2DM or presence of a capillary blood glucose level of ≥126 mg/dL, the use of medications that affect glucose metabolism, and fever in the last 48 hours.
Procedures
Voluntary participants arrived to the Medical Nutrition Research Unit with their parents or legal guardian at 8:00 am after an 8-hour fast. Anthropometric measurements (weight, height, and body mass index) were obtained. Subjects received an oral glucose load of 1.75 g per kg of body weight up to a maximum dose of 75 g (ACS reagent; Sigma-Aldrich, St. Louis, MO) dissolved in 150 mL of water, followed by a dose of 1.5 mg/kg of universally labeled 13C-glucose (Cambridge Isotope Laboratories, Inc., Andover, MA, USA) mixed in 50 mL of water, without a specific maximum dose. Blood samples were drawn at baseline and 120 minutes through an antecubital venipuncture. Breath samples were obtained at baseline and at 30, 60, 90, 120, 150, and 180 minutes in 10 mL Exetainer® test tubes (Labco Limited, UK), because in a previous protocol, 13C-GBT results had the highest reproducibility when measured at 180 minutes (14).
Biochemical Determinations
Plasma glucose levels were quantified using an enzymatic method (YSI 2300 Stat Plus™ glucose analyzer; YSI Inc., Yellow Springs, OH), and plasma insulin levels were measured by radioinmmunoassay employing a commercial kit (Millipore, Billerica, MA). The coefficients of variation (CV%) for glucose and insulin were 3.9% and 7.5%, respectively.
As an IR surrogate, the HOMA-IR was calculated with the following formula (9):
HOMA-IR=[fasting glucose (mg/dL) *fasting insulin (µU/mL)]/405.
Breath CO2 Measurements
Carbon 13 in breath samples was determined with an isotope ratio mass spectrometer BreathMat Plus (Finnigan, Bremen, Germany; CV <1%). Breath test data were expressed as cumulative percentage of oxidized dose at 180 minutes (A% OD) as described previously (17).
Statistical Analysis
Data analysis was performed with the SPSS software (version 19; SPSS Inc., Chicago, IL, USA). Kolmogorov-Smirnov test was used to assess data distribution. Data are presented as mean ± standard deviation or median (minimum-maximum) for normal or non-normal distribution, respectively. ANOVA test with Tukey post-hoc analysis or Kruskall-Wallis tests with Mann-Whitney U-test were used for the comparison between groups according to data distribution. Linear multiple regression models were used to summarize associations of insulin resistance surrogates (HOMA-IR, fasting plasma insulin and 2-h OGTT insulin) with sexual development (Tanner stage) and gender. Correlation coefficients were determined with Pearson’s or Spearman’s analyses according to data distribution. To determine the optimal cut-off point for IR diagnosis through the 13C-GBT, several receiver-operating characteristic (ROC) curves were constructed with a 95% confidence interval.
RESULTS
A total of 133 adolescents (62 females and 71 males) living in Mexico City were enrolled during 2011. Mean age was 13 years, weight and abdominal circumference values ranged from 34 to 113 kg and from 63 to 129 cm, respectively. Body mass index (BMI) presented a median of 23 (15.6 to 37.8 kg/m2), and this parameter was used to classify individuals into three groups according to the child growth standards established by the World Health Organization (18), namely, lean (BMI between p3 and p85, 42.1%), overweight (BMI >p85, 14.3%) and obese (BMI >p97, 43.6%). Data describing the study sample and the statistically significant differences between the subgroups are summarized in Table 1.
Table 1. Inter-group comparison of the study group according to body mass index.
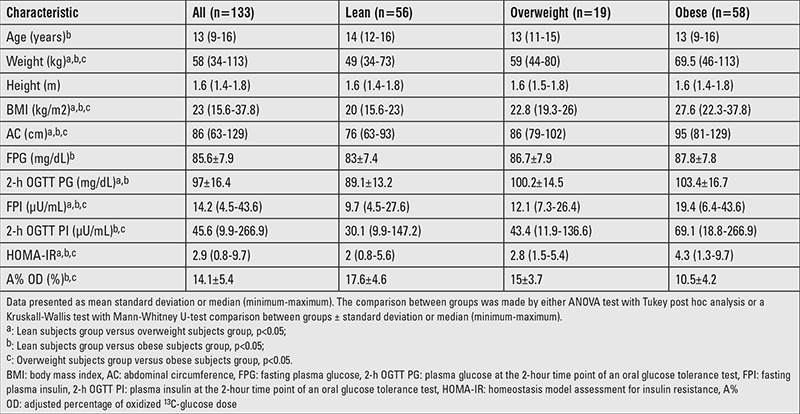
The following parameters had statistically significant differences among the three subgroups: weight, BMI, abdominal circumference, fasting plasma insulin, and HOMA-IR (19). When contrasting lean versus obese and overweight versus obese individuals, 2-h OGTT insulin and A% OD at 180 minutes differed significantly. The comparison of lean versus overweight and lean versus obese subjects revealed that the 2-h OGTT glucose was substantially different. Finally, fasting plasma glucose achieved a statistically relevant difference only between lean and obese individuals.
Three multiple regression models with three different IR surrogates were used to determine the influence of Tanner stage and gender on 13C-GBT; IR was defined as HOMA-IR ≥p95 reference score adjusted by gender and age (19), fasting plasma insulin ≥p90 reference score adjusted by gender and Tanner stage (20), and 2-h OGTT insulin ≥65 μU/mL (21). Gender does not substantially alter 13C-GBT when co-analyzed with HOMA-IR (β=0.8; p=0.361), fasting plasma insulin (β=1.0; p=0.239), and 2-h OGTT insulin (β=1.4; p=0.131). In contrast, it was established that Tanner stage modifies 13C-GBT when co-evaluated with HOMA (β=-2.1; p=0.017), fasting plasma insulin (β=-1.9; p=0.034), and 2-h OGTT insulin (β=-2.1; p=0.017), therefore, in subsequent analyses, the sample was stratified into pubescent (Tanner stages 2 and 3) and post-pubescent (Tanner stages 4 and 5) individuals. Of the total sample, 46.6% were classified as pubescent and 53.4% as post-pubescent.
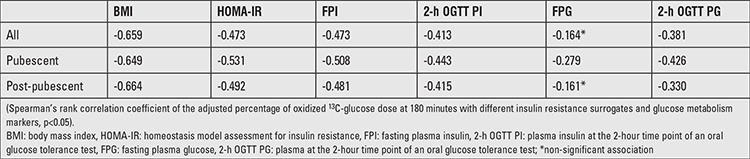
A Spearman’s rank correlation coefficient revealed that BMI, HOMA-IR, fasting plasma insulin, 2-h OGTT insulin, and 2-h OGTT glucose were inversely associated to A% OD at 180 minutes (Table 2). In contrast, fasting plasma glucose did not achieve statistical significance.
Table 2. Correlation of the adjusted percentage of oxidized 13C-glucose dose at 180 minutes with different insulin resistance surrogates.
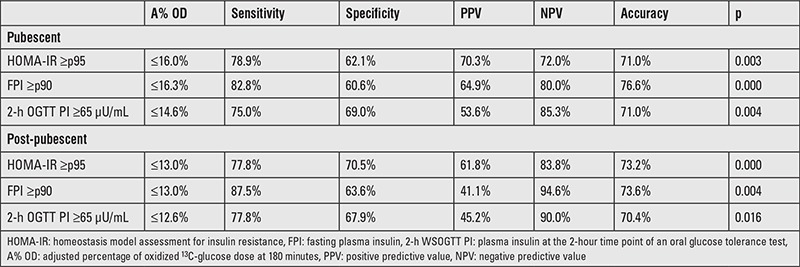
Several ROC curves were plotted to determine the optimal cut-off points for the A% OD at 180 minutes according to different IR surrogates as described previously (Figure 1). Diagnostic attributes of the 13C-GBT for each cut-off point are presented in Table 3. In pubescent and post-pubescent individuals, the 13C-GBT rendered the highest accuracy when compared to fasting plasma insulin. With said parameter, in pubescent individuals, an A% OD at 180 minutes ≤16.3% diagnoses IR with a sensitivity of 82.8%, a specificity of 60.6%, a positive predictive value (PPV) of 64.9%, and a negative predictive value (NPV) of 80.0%. In post-pubescent subjects, an A% OD at 180 minutes ≤13.0% indicates IR with a sensitivity of 87.5%, a specificity of 63.6%, a PPV of 41.1%, and a NPV of 94.6%.
Figure 1. Receiver operating characteristic curves evaluating the sensitivitiy and specificity of the 13C-glucose breath test using different criteria to define insulin resistance. A-C: Performance in pubescent individuals. D-C: Performance in post-pubescent individuals. A, D= HOMA-IR ≥p95 reference score; B, E= fasting plasma insulin ≥p90 reference score; C, F: plasma insulin at the 2-hour time point of an oral glucose tolerance test ≥65 μU/mL.
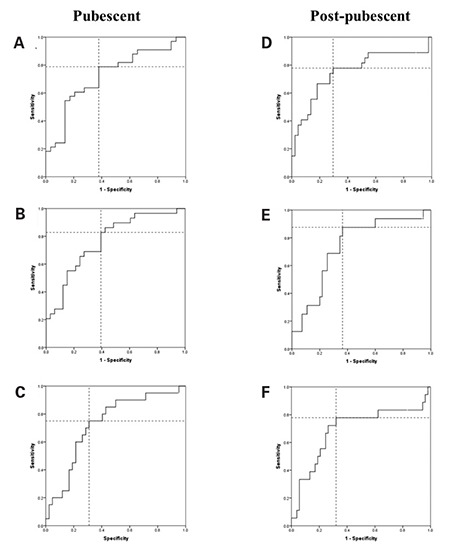
Table 3. Diagnostic attributes of the 13C-glucose breath test (according to different cut-off points for the oxidized 13C-glucose dose at 180 minutes).
DISCUSSION
The purpose of this study was to explore the use of the 13C-GBT to identify IR in adolescents with different BMIs. Even though the use of 13C-GBT has been extensively described in adults, to our knowledge, definite cut-off points for pediatric populations have not yet been established. In this study, we compare the 13C-GBT with fasting plasma insulin, HOMA-IR, and 2-h OGTT insulin to propose several cut-off points for IR diagnosis. Similar values of sensitivity, specificity, PPV, and NPV were observed among the different IR surrogates contrasted with the 13C-GBT. Our results are similar to those described by Ibarra-Pastrana et al (22) in Mexican adults, where the 13C-GBT rendered a sensitivity of 80% and a specificity of 67.4% when compared to HOMA-IR. Moreover, the cut-off points proposed in this article are comparable with the ones recommended for the detection of metabolic syndrome in adolescents (sensitivity=81.5% and specificity=66.7%) in a recent study published by our group (16).
The 13C-GBT is based on the premise that subjects with impaired insulin sensitivity have a reduced glucose uptake in response to insulin; consequently, the A% OD at 180 minutes is diminished in subjects with IR. Indeed, the 13C-GBT correlates inversely with fasting and post load IR surrogates. Similar associations were described by Jetha et al (15) who compared the 13C-GBT with fasting plasma insulin (r=-0.51, p<0.01), HOMA-IR (r=-0.51, p<0.01) and 2-h OGTT insulin (r=-0.040, p<0.05) in 39 pre-pubescent obese individuals.
Interestingly, even though the A% OD at 180 minutes is inversely associated to BMI, HOMA-IR, fasting plasma insulin, 2-h OGTT insulin and 2-h OGTT, fasting plasma glucose is not statistically correlated, probably because fasting plasma glucose is affected tardily in the pathogenesis of T2DM (23), this supports employing the 13C-GBT for early IR detection.
Undoubtedly, a weakness in this protocol is that the 13C-GBT was not contrasted against the HEC which is considered the gold standard to assess insulin sensitivity, therefore, the suggested cut-off points may undervalue or overestimate the true diagnostic performance of the 13C-GBT. However, the HEC is highly invasive and relatively unsuitable for pediatric subjects, thus, the 13C-GBT was compared against commonly used IR surrogates. Future studies to validate the sensitivity and specificity of the 13C-GBT for IR in adolescents are warranted.
The evaluation of the 13C-GBT in a substantial sample with a wide BMI spectrum is a definite strength of this study. Even though obesity is strongly associated to IR, it is now accepted that physically lean subjects may have defective insulin sensitivity and are candidates for IR screening (24,25). Another valuable asset is that the proposed cut-off points for the 13C-GBT were stratified according to sexual development, since it has been established that sexual hormones influence glucose homeostasis (26,27).
Considering the increasing evidence regarding the existence of noxious metabolic disorders at young ages, the epidemiological relevance of T2DM, the long-term complications associated to impaired glucose homeostasis, and the knowledge that lifestyle interventions in individuals with IR are effective to prevent or delay the onset of T2DM (28), the development of methodologically feasible procedures to screen for IR in large populations is fundamental for the implementation and assessment of public health strategies to face the T2DM epidemic. The use of non-invasive methods to detect IR is particularly appealing for pediatric populations, where pain and emotional stress associated to venipuncture are continuing concerns (29). Moreover, T2DM is recognized as a progressive disease; in fact, a young age at onset of T2DM is associated with a higher incidence of macrovascular complications (30), therefore, early IR detection is distinctly relevant in pediatric subjects. The non-invasive nature of the 13C-GBT and its reasonable diagnostic performance render this method suitable to perform IR screening in large pediatric populations.
Acknowledgments
The authors acknowledge BSc. Filiberto Jasso Saavedra for his technical assistance.
Ethics
Ethics Committee Approval: This protocol was approved by the Ethics Committee of the Mexican Social Security Institute (registry number: R-2010-3603-35) in Mexico City, Mexico, Informed Consent: Informed consent was provided by the parents or by the accompanying adult.
Peer-review: External and Internal peer-reviewed.
Footnotes
Concept: Jorge Maldonado-Hernández, Design: Jorge Maldonado-Hernández, Azucena Martínez-Basila and Alejandra Salas-Fernández, Data Collection or Processing: Azucena Martínez-Basila and Alejandra Salas-Fernández, Analysis or Interpretation: Mónica I. Piña-Aguero, José R. Navarro-Betancourt and Mariela Bernabe-García, Literature Search: Mariela Bernabe-García, Writing: Mónica I. Piña-Aguero and José R. Navarro-Betancourt.
Financial Disclosure: This study was supported with a grant from Consejo Nacional de Ciencia y Tecnología de México (CONACYT), registry number: SALUD-2010-01-139180.
References
- 1.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Cordova-Villalobos JA, Barriguete-Melendez JA, Lara-Esqueda A, Barquera S, Rosas-Peralta M, Hernandez-Avila M, Leon-May ME, de, Aguilar-Salinas CA. [Chronic non-communicable diseases in Mexico: epidemiologic synopsis and integral prevention] Salud Publica Mex. 2008;50:419–427. doi: 10.1590/s0036-36342008000500015. [DOI] [PubMed] [Google Scholar]
- 3.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 4.Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest. 2013;123:2764–2772. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jimenez-Pavon D, Sese MA, Huybrechts I, Cuenca-Garcia M, Palacios G, Ruiz JR, Breidenassel C, Leclercq C, Beghin L, Plada M, Manios Y, Androutsos O, Dallongeville J, Kafatos A, Widhalm K, Molnar D, Moreno LA. Dietary and lifestyle quality indices with/without physical activity and markers of insulin resistance in European adolescents: the HELENA study. Br J Nutr. 2013;110:1919–1925. doi: 10.1017/S0007114513001153. [DOI] [PubMed] [Google Scholar]
- 6.Moran A, Jacobs DR, Steinberger J, Hong CP, Prineas R, Luepker R, Sinaiko AR. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48:2039–2044. doi: 10.2337/diabetes.48.10.2039. [DOI] [PubMed] [Google Scholar]
- 7.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:214–223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 8.Wedin WK, Diaz-Gimenez L, Convit AJ. Prediction of insulin resistance with anthropometric measures: lessons from a large adolescent population. Diabetes Metab Syndr Obes. 2012;5:219–225. doi: 10.2147/DMSO.S33478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 10.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 11.Thompson DS, Boyne MS, Osmond C, Ferguson TS, Tulloch-Reid MK, Wilks RJ, Barnett AT, Forrester TE. Limitations of fasting indices in the measurement of insulin sensitivity in Afro-Caribbean adults. BMC Res Notes. 2014;7:98. doi: 10.1186/1756-0500-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeBoer MD. Obesity, systemic inflammation, and increased risk for cardiovascular disease and diabetes among adolescents: a need for screening tools to target interventions. Nutrition. 2013;29:379–386. doi: 10.1016/j.nut.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewanczuk RZ, Paty BW, Toth EL. Comparison of the [13C]glucose breath test to the hyperinsulinemic-euglycemic clamp when determining insulin resistance. Diabetes Care. 2004;27:441–447. doi: 10.2337/diacare.27.2.441. [DOI] [PubMed] [Google Scholar]
- 14.Maldonado-Hernandez J, Martinez-Basila A, Matute-Gonzalez MG, Lopez-Alarcon M. The [(13)c]glucose breath test is a reliable method to identify insulin resistance in Mexican adults without diabetes: comparison with other insulin resistance surrogates. Diabetes Technol Ther. 2014;16:385–391. doi: 10.1089/dia.2013.0263. [DOI] [PubMed] [Google Scholar]
- 15.Jetha MM, Nzekwu U, Lewanczuk RZ, Ball GD. A novel, non-invasive 13C-glucose breath test to estimate insulin resistance in obese prepubertal children. J Pediatr Endocrinol Metab. 2009;22:1051–1059. doi: 10.1515/jpem.2009.22.11.1051. [DOI] [PubMed] [Google Scholar]
- 16.Salas-Fernandez A, Maldonado-Hernandez J, Martinez-Basila A, Martinez-Razo G, Jasso-Saavedra F. The 13C-glucose breath test is a valid non-invasive screening tool to identify metabolic syndrome in adolescents. Clin Chem Lab Med. 2015;53:133–138. doi: 10.1515/cclm-2014-0412. [DOI] [PubMed] [Google Scholar]
- 17.Braden B, Lembcke B, Kuker W, Caspary WF. 13C-breath tests: current state of the art and future directions. Dig Liver Dis. 2007;39:795–805. doi: 10.1016/j.dld.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Onis M. 4.1 The WHO Child Growth Standards. World Rev Nutr Diet. 2015;113:278–294. doi: 10.1159/000360352. [DOI] [PubMed] [Google Scholar]
- 19.Aradillas-Garcia C, Rodriguez-Moran M, Garay-Sevilla ME, Malacara JM, Rascon-Pacheco RA, Guerrero-Romero F. Distribution of the homeostasis model assessment of insulin resistance in Mexican children and adolescents. Eur J Endocrinol. 2012;166:301–306. doi: 10.1530/EJE-11-0844. [DOI] [PubMed] [Google Scholar]
- 20.Garcia Cuartero B, Garcia Lacalle C, Jimenez Lobo C, Gonzalez Vergaz A, Calvo Rey C, Alcazar Villar MJ, Diaz Martinez E. [The HOMA and QUICKI indexes, and insulin and C-peptide levels in healthy children. Cut off points to identify metabolic syndrome in healthy children] An Pediatr (Barc) 2007;66:481–490. doi: 10.1157/13102513. [DOI] [PubMed] [Google Scholar]
- 21.Sebekova K, Stefikova K, Polakovicova D, Spustova V, Dzurik R. Does magnesium dysbalance participate in the development of insulin resistance in early stages of renal disease? Physiol Res. 2002;51:605–612. [PubMed] [Google Scholar]
- 22.Ibarra-Pastrana E, Alvarez G, Valencia ME. Estimation of Insulin Resistance in Mexican Adults by the [(13)C]Glucose Breath Test Corrected for Endogenous Total CO(2) Production. Int J Endocrinol. 2012;2012:907818. doi: 10.1155/2012/907818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayans L. Metabolic Syndrome: Insulin Resistance and Prediabetes. FP Essent. 2015;435:11–16. [PubMed] [Google Scholar]
- 24.Parker VE, Semple RK. Genetics in endocrinology: genetic forms of severe insulin resistance: what endocrinologists should know. Eur J Endocrinol. 2013;169:71–80. doi: 10.1530/EJE-13-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goyal A, Nimmakayala KR, Zonszein J. Is there a paradox in obesity? Cardiol Rev. 2014;22:163–170. doi: 10.1097/CRD.0000000000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cunningham GR. Testosterone and metabolic syndrome. Asian J Androl. 2015;17:192–196. doi: 10.4103/1008-682X.148068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho YH, Craig ME, Donaghue KC. Puberty as an accelerator for diabetes complications. Pediatr Diabetes. 2014;15:18–26. doi: 10.1111/pedi.12112. [DOI] [PubMed] [Google Scholar]
- 28.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM Diabetes Prevention Program Research G. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baxter AL, Cohen LL, McElvery HL, Lawson ML, von Baeyer CL. An integration of vibration and cold relieves venipuncture pain in a pediatric emergency department. Pediatr Emerg Care. 2011;27:1151–1156. doi: 10.1097/PEC.0b013e318237ace4. [DOI] [PubMed] [Google Scholar]
- 30.Hillier TA, Pedula KL. Complications in young adults with early-onset type 2 diabetes: losing the relative protection of youth. Diabetes Care. 2003;26:2999–3005. doi: 10.2337/diacare.26.11.2999. [DOI] [PubMed] [Google Scholar]


