Abstract
Repetitive back-and-forth head rotation from vigorous shaking is purported to be a central mechanism responsible for diffuse white matter injury, subdural hemorrhage, and retinal hemorrhage in some cases of abusive head trauma (AHT) in young children. Although animal studies have identified mechanisms of traumatic brain injury (TBI) associated with single rapid head acceleration-decelerations at levels experienced in a motor vehicle crash, few experimental studies have investigated TBI from repetitive head rotations. The objective of this study was to systematically investigate the post-injury pathological time-course after cyclic, low-velocity head rotations in the piglet and compare them with single head rotations. Injury metrics were the occurrence and extent of axonal injury (AI), extra-axial hemorrhage (EAH), red cell neuronal/axonal change (RCNAC), and ocular injury (OI). Hyperflexion/extension of the neck were purposefully avoided in the study, resulting in unscaled angular accelerations at the lower end of reported infant surrogate shaking kinematics. All findings were at the mild end of the injury spectrum, with no significant findings at 6 h post-injury. Cyclic head rotations, however, produced modest AI that significantly increased with time post-injury (p < 0.035) and had significantly greater amounts of RCNAC and EAH than noncyclic head rotations after 24 h post-injury (p < 0.05). No OI was observed. Future studies should investigate the contributions of additional physiological and mechanical features associated with AHT (e.g., hyperflexion/extension, increased intracranial pressure from crying or thoracic compression, and more than two cyclic episodes) to enhance our understanding of the causality between proposed mechanistic factors and AHT in infants.
Keywords: : abusive head trauma, biomechanics, pediatric traumatic brain injury, shaken baby syndrome
Introduction
Traumatic brain injury (TBI) is the most common cause of death and disability in childhood.1 The predominant etiologies of TBI in infants and young children are falls, motor vehicle crashes, firearm incidents, and child abuse.1 Of all traumatic etiologies, young children (<4 years old) die of inflicted injury more than any other single mechanism.2 Few children with inflicted injury present with an accurate history of inflicted trauma, however.3–8
One particularly difficult challenge with identifying causal mechanisms of TBI in infants is that there is often no impartial witness to the traumatic event and no objective means of determining what caused a particular injury. Subsequently, there is much controversy about the mechanisms of TBI related to child abuse. Brain damage with subdural and retinal hemorrhage, with or without evidence of head impact or skeletal injury, and no history of severe trauma is often considered a classic presentation for abusive head trauma (AHT). Each of these injury types can vary from mild to severe, but child abuse is most readily identified when the child's signs and symptoms fall at the more severe end of the injury spectrum. With a significant frequency of elicited or spontaneous confessions of shaking by caregivers among children with inflicted injury, and often no clinically visible indications of head impact (localized scalp swelling, bruising, skull fracture, etc.), it has been hypothesized by many medical providers in the field that subdural hemorrhage, axonal injury (AI), and retinal hemorrhages occur from shaking and that these episodes sometimes occur repeatedly, over time.9–12
In vivo and in vitro animal studies have identified mechanisms of TBIs associated with single rapid head acceleration-decelerations, similar to what might be experienced in a motor vehicle crash,13–20 but only a few experimental studies have investigated TBI from repetitive back-and-forth motions of the head. Smith and associates21 developed a shaking model of brain injury using male Sprague-Dawley rats. Post-natal day (PND) 6 rat pups were equilibrated to hypoxia in a chamber and attached to a pneumatic shaker that shook the rats 20 times over 6 sec (3.3 Hz), followed by a 6 sec rest. This sequence was repeated 60 times for a total of 12 min of shaking in hypoxic conditions per day. The combined hypoxia and shaking exposure was repeated daily for 3 consecutive days, with sacrifice 1 h to 14 days after the last shaking episode.
Cortical hydroxyl radicals, vitamin E levels, extent of cortical hemorrhaging, and rate of cortical wet weight decline were significantly higher in the injured rats compared with shams that were not exposed to hypoxia and shaking. Head kinematics (angular displacement, direction, and velocity) were not measured, nor were the effects of shaking alone or hypoxia alone determined.
In a rodent model of shaking with normoxia,22 Swiss mouse pups (PND8) were shaken for 15 sec on a horizontal rotation shaker at a frequency of 900 cycles per min (15 Hz). White matter hemorrhagic lesions were present at PND13, and cysts developed between PND15 and PND31. Retinal hemorrhages were found in 4 of the 12 eyes examined at PND11 and PND13.
The lack of measured kinematics in all of these rodent studies, the absence of scaling kinematics from the human child to the small rodent brain,23,24 the challenges of interpreting white matter injuries in the lissenphalic rodent brain with minimal white matter, and the differences between rodent and human maturational sequence limit our ability to extrapolate these findings to determine whether cyclic head rotations without impact in children would be associated with intracranial hemorrhage, persistent cerebral damage, and retinal hemorrhage.
More recently, a gyrencephalic lamb model was used to evaluate head injury from unconstrained 30-sec torso shaking sequences at 2 Hz, repeated 10 times over 30 min, resulting in 40 cycles per episode and 400 total cycles per animal.25,26 The shakes caused full head excursion with considerable lateral and rotational head movement and resulted in AI, very mild focal subdural hemorrhages (three of nine lambs), but no retinal hemorrhages at 6 h post-shaking.26
Sheep brains are classified as pre-natal brain developers, and their brains are relatively mature at birth (72% of adult cerebrum weight and 50% myelination as determined by cerebrum lipid content).27 In humans, the brain is approximately 28% of adult brain weight at birth and has only 34% of adult myelination (also determined by cerebrum lipid content).28 The lambs in the study were 5–9 days old, which is the human equivalent of approximately 6–12 months old based on brain weight and lipid content.
All three of the younger subjects (5 days old) died of unknown causes before the 6 h study end-point. No widespread hypoxic-ischemic brain damage was observed in any animal, perhaps because of the lower sensitivity of sheep to hypoxia-ischemia insults compared with humans and pigs.29 In summary, within 6 h of shaking, this large animal model with uncontrolled repeated head movements did not produce widespread hypoxic-ischemic brain injury, subarachnoid or subdural hemorrhage, and retinal hemorrhage, which are characteristics often noted in AHT patterns attributed to shaking.
The pig, with its gyrencephalic brain and well-correlated maturation and brain development sequence with humans,28,30–33 is an established model for TBI and other neurological diseases.34 Using brain growth, myelination, and electrical activity as markers of brain development, the 3–5-day-old piglet brain can be roughly correlated to that of a human infant in the first 2–4 weeks of life.31–33,35
Because of the similarities between the brains of piglets and immature humans, the animal model has been used extensively to investigate mechanisms of pediatric TBI associated with single, nonimpact, rapid head rotations and has successfully identified key attributes of TBI, including the influence of head rotation direction,14,36,37 changes with developmental age,16,19,36 changes with post-injury time,36,38 and behavioral consequences of this injury mechanism.15,39–41 Injuries reported in these studies include hypoxic-ischemic damage, widespread AI, large unilateral/bilateral subdural and subarachnoid hemorrhage, and ocular hemorrhage.42
All of these TBI-associated injuries in the piglet resulted from single head rotations at very high velocities (160–200 rad/sec) and high-magnitude, rapid decelerations, similar to those in high-force accidental trauma. Single head rotations at the high end of this spectrum were repeated once (minutes, days, and weeks later), but white matter injury and functional deficits did not increase significantly above the level of single head rotations.20,38 These studies in the piglet involved velocities and accelerations higher than those documented in carefully instrumented child-like anthropomorphic surrogates subjected to low height falls and shaken vigorously to simulate AHT without impact.24,43,44 These studies did not investigate the effect of cyclic head rotation on TBI. No previous piglet studies have investigated TBI from repetitive head trauma at velocities similar to those measured in surrogate shaking studies.
The objective of this study was to investigate the pathological consequences of cyclic low velocity head rotations in the immature piglet and compare them with single head rotations. Systematically, we investigated: (1) cyclic and noncyclic head rotation; (2) the post-injury time course after cyclic head rotation; (3) the temporal pattern of applied cyclic head rotation; (4) the duration of cyclic head rotation in an episode; and (5) single and repeated cyclic head rotation episodes. Outcome measures for TBI were the occurrence and extent of hypoxic-ischemic damage, AI, extra-axial hemorrhage, and ocular injury (OI) identified through microscopy, gross examination, and indirect funduscopic examination.
Methods
To establish guidelines for animal cyclic loading conditions, four volunteers (two male, two female) were asked to shake an instrumented 1.5-month-old human infant surrogate with at least 10 maximal-effort shakes without impact. This surrogate used for the shakes was our most recently published surrogate45 with average weight for age, and more life-like skull, suture, and neck properties than our earlier published surrogate24 with a unidirectional hinge neck. The surrogate was instrumented with a 9-linear accelerometer array (7264B-2000, Endevco) and an angular velocity transducer (ARS-01, ATA Sensors) to measure three-dimensional head rotations at a sampling rate of 5 kHz. Motion capture (sampled at 100 Hz) was used in conjunction with the surrogate instrumentation to visualize and quantify head and thorax kinematics during shaking.
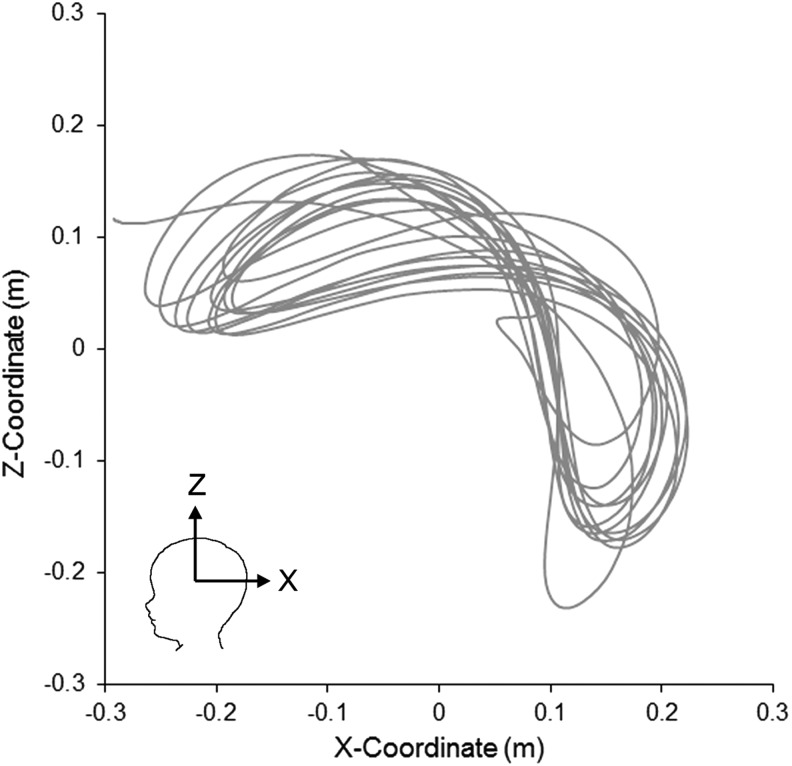
The volunteers moved the torso in a manner that created predominantly sagittal head rotations, but the multidirectional neck allowed unrestricted rotation in all three planes. Only the accelerations associated with sagittal rotations were extracted to compare with our previously published shaking data using surrogates with hinge24 and rubber23 necks. An example of the head motion resulting from one of these shaking episodes is shown in Figure 1.
FIG. 1.
Representative trajectory of the surrogate head center of gravity during anterior-posterior shaking.
As might be expected, there was a large variability between shakes and across subjects. Averaging across the episodes and volunteers, the average sagittal (± standard deviation [SD]) head rotational velocity was 47.12 ± 13.96 rad/sec, angular acceleration was 1512 ± 1295 rad/sec2, and frequency was 2.19 ± 0.49 Hz. Similar to our earliest study showing significantly smaller accelerations for a rubber flexible neck than a hinge neck,23 the velocity, acceleration, and frequency with our new, more biofidelic neck were lower than the 61 ± 9.6 rad/sec, 3480 ± 2467 rad/sec2, and 3.08 ± 1.00 Hz achieved, respectively, with the low-resistance hinge neck of our previous surrogate that restricted movement to purely sagittal head rotations.24
The average infant surrogate kinematics were scaled from the 500 g young infant human brain46 to the 40 g infant piglet brain using conventional mass-scaling approaches.47 This calculation established that equivalent piglet rotation should have a peak-to-peak rotational velocity of 109 rad/sec and an acceleration of 8141 rad/sec2 to produce similar brain tissue deformations during vigorous shaking at approximately 2–3 Hz.
Acceleration and velocity are kinematic byproducts of the rotational excursion of the head and the time course of that motion. The objective of our study was to exclude cervical spine hyperflexion and hyperextension effects, and focus on isolating the pathological effects of repetitive brain motion. Given the narrower physiologic range of the piglet cervical spine and our focus on comparing single head rotations with repetitive head rotation at cycling frequencies of 2–3 Hz, the resulting accelerations and velocities (presented in Results) were similar to the unscaled measurements of the infant surrogate, but lower than average human infant values scaled to the smaller piglet brain.
All animal protocols were approved by the Institute of Animal Care and Use Committee of the University of Pennsylvania. Immature piglets (3–5 days old) were anesthetized with 4% isoflurane via a snout mask. Once fully anesthetized, as determined through absence of a pinch reflex, animals were intubated and maintained under anesthesia with 2–3% isoflurane. Oxygen saturation, end tidal CO2, rectal temperature, heart rate, and respiratory rate were continuously monitored during anesthesia.
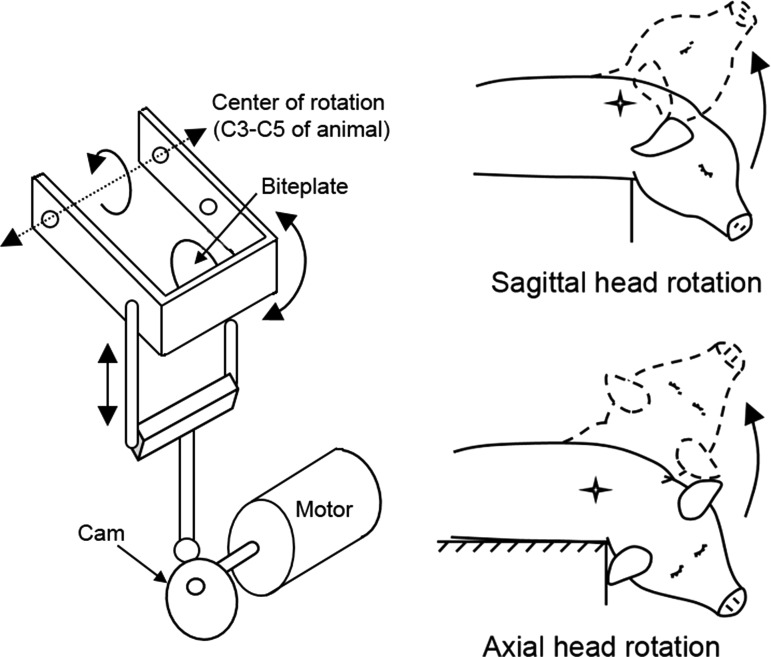
Animals were mounted to the bite plate of a mechanical rotation device (Fig. 2) and underwent either a repetitive back and forth head rotation (cyclic), a single head rotation (noncyclic), or no head rotation (sham). Head rotations were constrained to physiological range of motions with the center of rotation in the cervical spine. Animals experiencing a cyclic head rotation were assigned to three possible cyclic patterns: continuous, double continuous, or episodic. Each of these loading conditions is described in detail below.
FIG. 2.
Schematics of the cyclic device (left) and directions of head rotation in the piglet animal (right). Head rotations were limited to the physiological passive neck range of motions of the piglet (<60 degrees in the sagittal plane, <100 degrees in the axial plane).
Animals were euthanized at 6 h, 24 h, or 6 days after head rotation or sham anesthesia (Table 1). Animals surviving 6 h were anesthetized for the entire 6 hours. Animals surviving 24 h or more were weaned from anesthesia, extubated, and returned to veterinary care after demonstrating capability to ambulate readily to food and water sources.
Table 1.
Breakdown of the Groups and the Number of Animals within Each Group
| Time post-injury | ||||
|---|---|---|---|---|
| Injury group | 6 h | 24 h | 6 days | |
| Cyclic | Episodic |
n = 5 (sagittal) Group A |
- | - |
| Continuous (10 sec) | - |
n = 4 (axial) |
- | |
| Group C | ||||
| Continuous (30 sec) |
n = 8 (6 sagittal, 2 axial) |
n = 8 (axial) |
n = 5 (axial) |
|
| Group B | Group D | Group F | ||
| Double continuous (30 sec) | - |
n = 9 (axial) |
- | |
| Group E | ||||
| Single (noncyclic) |
n = 5 (sagittal) |
n = 6 (sagittal) |
- | |
| Group G | Group H | |||
| Sham | n = 2 | n = 2 | ||
In animals undergoing cyclic head rotation and surviving for ≥24 h, cerebral blood flow (CBF) was measured just before sacrifice. A 2-mm hole was created using a hand drill approximately 2 mm anterior to the coronal suture and 2 mm left of the sagittal suture. A thermal diffusion probe (Bowman Perfusion Monitor, Hemedex, Cambridge, MA) was inserted approximately 5–7 mm into the cortex to access the subcortical white matter. Once stabilized, 30 sec of continuous measurements were recorded. The average measurement over this 30 sec time frame, expressed in units of mL/min per 100 g of tissue, was used for statistical analysis. All animals were sacrificed by a lethal intravenous injection of pentobarbital, and brains were perfusion fixed as described previously.14
Head rotation mechanics
Continuous cyclic rotation (n = 25)
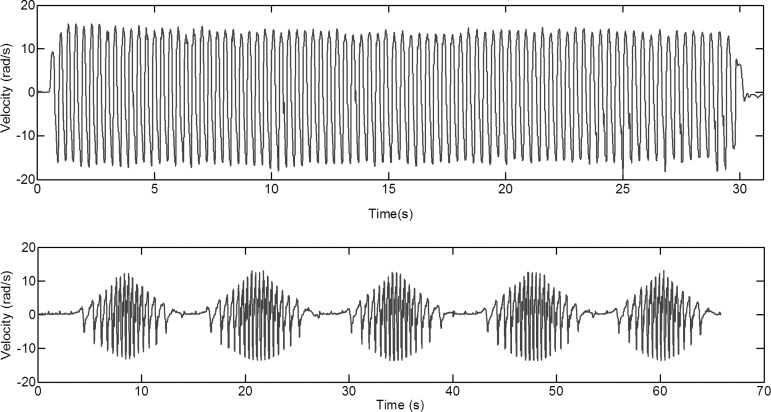
The snout of the animal was attached to a mechanical system designed to apply a back and forth head rotation through a ±30-degree sagittal arc or ±50-degree right-to-left lateral (axial) arc, with 0 degrees defined as the neutral axis position of the neck. This back-and-forth head rotation was applied at a frequency of 2–3 Hz until the total time duration (10 or 30 sec) was reached (Fig. 3A). Subjects thus experienced 30–90 back-and-forth head rotations during this interval. Animals were sacrificed at 6 h (group B), 24 h (groups C, D) or 6 days (group F) after the cyclic head rotation. Details about group sizes are provided in Table 1.
FIG. 3.
(A) Representative velocity-time curve for 30 sec continuous cyclic head rotation. (B) Representative velocity-time curve for episodic cyclic head rotation (group A).
Double continuous cyclic rotation (n = 9)
A 30-sec continuous axial cyclic head rotation was performed as described above. Animals were recovered, returned to the housing facility, re-anesthetized 24 h later, and exposed to a second 30-sec continuous axial cyclic head rotation (group E). Animals in this group were sacrificed 24 h after the second cyclic head rotation.
Episodic cyclic rotation (n = 5)
Episodic head rotations were similar to the continuous cyclic head rotations, but the 30 sec cyclic rotation was interrupted by pauses to simulate repeated episodes of shorter bursts of shaking activity. Specifically, continuous rotations were applied for 6 sec, followed by pauses lasting 30 sec. This pattern repeated five times. The total event duration was 2.5 min, consisting of a total of 30 sec of cyclic head rotation (group A, Fig. 3B).
Single (non-cyclic) rotation (n = 11)
Using our previously published nonimpact head rotation device,19 animals experienced a single head rotation through a 60-degree sagittal arc starting with the neck in full flexion and ending with the neck in full extension. The lower velocity limit of the single rotation device was used to achieve angular velocities comparable to those of the cyclic device. There was no return of the head to the original starting position. Similar to the cyclic rotations, animals surviving 6 h remained anesthetized for the entire 6 h (n = 5, group G). Animals surviving 24 h after a single head rotation (n = 6, group H) were weaned from anesthesia and returned to the animal facility.
Shams (n = 4)
Sham animals were anesthetized and attached to the bite plate of the cyclic device, but their heads were not rotated. Two of the shams survived for 6 h (with continuous anesthesia) to serve as uninjured controls for groups A, B, and G. Two additional shams were weaned from anesthesia and returned to the animal facility. Animals were re-anesthetized 24 h later for CBF measurement, and then euthanized to serve as controls for the animals in the 24 h post-injury survival groups C, D, E, and H.
Tissue processing and injury assessment
Extra-axial hemorrhage (EAH)
To quantify the extent of EAH, digital color images were taken of the brain immediately after brain removal. Care was taken to include any blood in the subdural space at the time of brain removal. These images captured any subdural or subarachnoid hemorrhage present at sacrifice, but did not distinguish between the two hemorrhage types. Images spanned four fields of view (apical, basal, rostral, caudal). After compensating for regions appearing in more than one view, the total image area of brain covered by blood in the cerebrum was summed in all views and divided by the total area of the cerebrum to define the cerebrum EAH score (%). This process was repeated for the cerebellum to identify the cerebellum EAH score. The total brain EAH score was the injured surface area of the cerebrum and cerebellum divided by the total brain surface area.
Axonal injury and red cell neuronal/axonal change (RCNAC)
To quantify the injury within the cerebrum, the perfusion-fixed brains were sectioned into 3 mm-thick coronal blocks through the cerebrum and brainstem, processed and embedded in paraffin, and 6 μm-thick sections from every block were stained with hematoxylin and eosin (H&E), and β-amyloid precursor protein (β-APP) immunohistochemical marker. Immunohistochemical sections were lightly counterstained with Mayer hematoxylin. Regions of β-APP reactivity were marked on digital images of each brain section by the neuropathologist (CS) masked to the injury group, as described previously,36 and used to denote AI.
Ischemic AI identified with β-APP was not included as part of the overall AI. Instead, H&E sections were used to quantify axonal ischemia using RCNAC. Positive RCNAC findings were marked separately on the digital images. The marked regions of AI and RCNAC were digitized to obtain an injured area in each slice, and the boundary of every brain slice was digitized to obtain the total brain area. Volume of AI and RCNAC was defined as the sum of the injured areas across all slices divided by the sum of all the brain slice areas.
Ocular injury
Indirect fundus examinations through pharmacologically dilated pupils were performed by an ophthalmologist (GB) in the anesthetized animals immediately before injury, immediately post-injury, and immediately before sacrifice. Any abnormal findings were recorded. After sacrifice and perfusion, eyes were removed en bloc and placed in a 1.25% gluteraldehyde/1% formaldehyde mixture until analyzed.
Eyes were first examined macroscopically for any signs of hemorrhage or any other ocular abnormalities. Then, the extraocular muscles and surrounding soft tissues were removed and globes were embedded in paraffin wax. Five thin (5 μm) sections of each eye (one section through the pupil-optic nerve-head plane, one section each slightly superior and inferior to that plane, and one section each through the retinal periphery superior and inferior to this plane) were stained with H&E. Slides were examined for the presence or absence of ocular hemorrhage by an ocular pathologist (RLP), masked to the injury group.
Data analysis
Angular velocity for all events was measured with a single axis angular rate transducer (ARS-06, ATA, Albuquerque, NM). Data were sampled at 3000 Hz and filtered using a low-pass Butterworth digital filter with a cutoff frequency determined by the objective function developed by Yu and colleagues48 for kinematic data. The peak-to-peak velocity of each cyclic motion was averaged across the duration of the cyclic event, and injury group averages are reported as mean ± SD of these averages. A one-way analysis of variance was used to determine whether there were significant differences in kinematics across groups.
For every injury group, each outcome (CBF, EAH, AI, RCNAC, and OI) was compared with the appropriate sham group using a Dunnett test or the Dunn method for nonparametric variables. Five different comparisons were performed for each outcome across groups using Pearson chi-square tests to evaluate differences in the occurrence of a positive finding, and Wilcoxon rank sum/Kruskal-Wallis tests to identify significant differences in the continuous injury variables (CBF, EAH, AI, RCNAC). The Wilcoxon test was used because continuous injury variables tended not to be normally distributed. CBF was only measured in a subset of groups (Table 2) and was therefore limited to group comparisons within the subset.
Table 2.
Summary of Kinematic and Injury Data for Each Group
| Frequency (Hz) | Angular velocity (rad/sec) | Axonal injury (%) | Red cell neuronal/axonal change (%) | Total brain EAH (%) | CBF (mL/100 g-min) | |
|---|---|---|---|---|---|---|
| Group A (Episodic 6 h survival) | 2.36 ± 0.32* | 22.96 ± 2.61* | 0 (0%) | 0 (0%) | 0.33 ± 0.75% (20%) | - |
| Group BSAG (30 sec continuous 6 h survival) | 2.65 ± 0.24 | 22.51 ± 4.33* | 0.014 ± 0.034% (17%) | 0 (0%) | 0.20 ± 0.08% (33%) | - |
| Group BAXIAL (30 sec continuous 6 h survival) | 2.74 ± 0.23 | 28.52 ± 4.05 | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Group C (10 sec continuous 24 h survival) | 2.94 ± 0.05 | 30.86 ± 0.77 | 0.13 ± 0.13% (100%) | 0 (0%) | 0.54 ± 0.63% (50%) | 74.93 ± 15.87 |
| Group D (30 sec continuous 24 h survival) | 2.86 ± 0.02 | 28.54 ± 2.67 | 0.067 ± 0.17% (25%) | 0.044 ± 0.052% (50%) | 2.40 ± 5.23% (50%) | 45.48 ± 20.01 |
| Group E (Double continuous 24 h survival) | 2.76 ± 0.17 | 28.75 ± 3.02 | 0.13 ± 0.16% (56%) | 0.082 ± 0.074%# (67%) | 2.70 ± 3.99% (67%) | 48.27 ± 13.80 |
| Group F (30 sec continuous 6 day survival) | 2.91 ± 0.10 | 28.41 ± 3.87 | 0.090 ± 0.085% (80%) | 0.004 ± 0.009% (25%) | 1.24 ± 2.69% (40%) | 64.53 ± 16.11 |
| Group G (noncyclic 6 h survival) | - | 32.19 ± 7.04 | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Group H (noncyclic 24 h survival) | - | 42.86 ± 6.45* | 0.028 ± 0.059% (33%) | 0 (0%) | 0 (0%) | - |
| Shams | - | - | 0 (0%) | 0 (0%) | 0 (0%) | 58.13 ± 3.33 |
EAH, extra-axial hemorrhage; CBF, cerebral blood flow; Sag, saggital.
Significantly (p < 0.05) different than other groups in column.
Significantly (p < 0.05) different than shams.
-Measurement not applicable or not collected.
Animals without injury were given a value of zero and included in the averages ± standard deviation. Parentheses indicate the percentage of animals in the group with the injury. CBF units are mL/min/100 g tissue.
Episodic cyclic head rotation was first compared with continuous cyclic head rotation (group A vs. group BSAG). Then, the duration of the cyclic head rotation was evaluated by comparing a single 10 sec continuous cycling event with a single 30 sec continuous cycling event (group C vs. group D). Next, a single cyclic event was compared with two cyclic loading events 24 h apart (group D vs. group E). Then, the influence of post-injury survival time on outcome was compared for 6 h, 24 h, and 6 day survivals after a single 30 sec continuous cycling event. (groups B, D, and F).
Finally, a 30 sec cyclic rotation event was compared with a single noncyclic head rotation at 6 h (groups B and G) and 24 h (groups D and H) post-injury survival time. Some groups have a greater number of subjects because of a desire to add statistical power to groups with positive findings and large variability. Significance was defined as p < 0.05 for all statistical analyses. All results are reported as means ± standard deviations.
Results
Head kinematics
All cyclic head rotations achieved the targeted shaking frequency (complete back-and-forth cycles per second) of 2–3 Hz (Table 2). All of group A and six subjects in group B (BSAG) experienced sagittal plane head rotations, with an average peak-to-peak angular velocity of 22.71 ± 3.49 rad/sec and average peak angular acceleration of 606.21 ± 160.30 rad/sec2. To maximize the angular velocity within the physiological cervical spine range of motion, frequency was increased to the higher end of the target range, and the primary direction of head rotation was changed to the axial plane for the remaining cyclic studies (two animals in group B, and all animals in groups C, D, E, and F), because axial head rotation has a larger range of motion in the pig than sagittal head rotation (Fig. 2).
Making this change significantly (p < 0.001) increased the average frequency from 2.52 ± 0.31 Hz to 2.84 ± 0.13, and significantly increased peak-to-peak angular velocity and peak angular acceleration (p < 0.0001) to 28.92 ± 2.85 rad/sec and 780.08 ± 118.03 rad/sec2, respectively. These kinematics are similar to the low end of the unscaled sagittal rotational velocity and acceleration spectrum measured in vigorous shaking in our new surrogate (47.12 ± 13.96 rad/sec; 1512 ± 1295 rad/sec2, mean ± SD). The velocities and accelerations of the single, noncyclic head rotations were significantly higher (p < 0.002) than the cyclic head rotations. Specifically, group G had a significantly higher acceleration (32.19 ± 7.04 rad/sec and 2857.40 ± 1682.91 rad/sec2), and group H had a significantly higher velocity (42.86 ± 6.45 rad/sec and 866.33 ± 213.92 rad/sec2).
Comparison of injury groups with shams
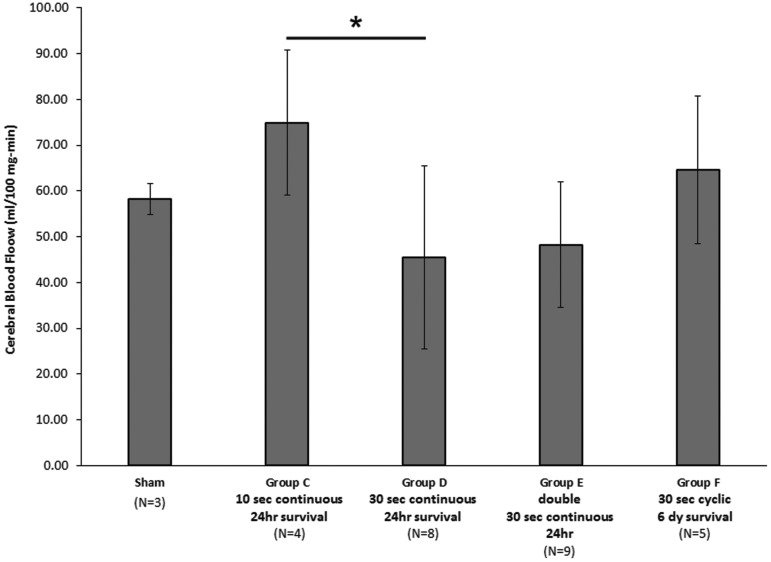
Unintended mortality and morbidity was zero. For survivals >6 h, all animals returned to the animal husbandry unit, were able to ambulate to food, and did not display signs of distress. No AI, RCNAC, or EAH was observed in the sham animals, and average CBF (58.13 ± 3.33 mL/min-100 g tissue) was within the range we have published previously for infant piglets anesthetized with isoflurane.49 While there were notable differences between the average CBF in injured animals (particularly groups C, D, and E) and shams, they did not reach statistical significance, likely because of large variability in many of the groups and small sample sizes (Fig. 4).
FIG. 4.
Cerebral blood flow measurements for sham and injured animals. There were no significant differences between injured groups and sham animals, but a 30 sec continuous cyclic head rotation had a significantly lower cerebral blood flow than a 10 sec continuous cyclic head rotation at 24 h post-injury (*p = 0.047).
Very modest or no AI (Fig. 5, Table 2), RCNAC, and EAH (Fig. 6, Table 2) were noted in animals surviving 6 h post-injury (groups A, B, G), and a Dunn method for joint ranking (the nonparametric equivalent to the Dunnett test) revealed no significant differences between these acute-survival animals and shams. The six animals experiencing sagittal head rotations in group B had very little to no injury and were not significantly different from zero. The two axial rotation animals in group B also had no injury and were not different from zero. Therefore, we concluded that the two axial rotation animals in group B were not different from the sagittal animals in group B and combined them for all injury analysis.
FIG. 5.
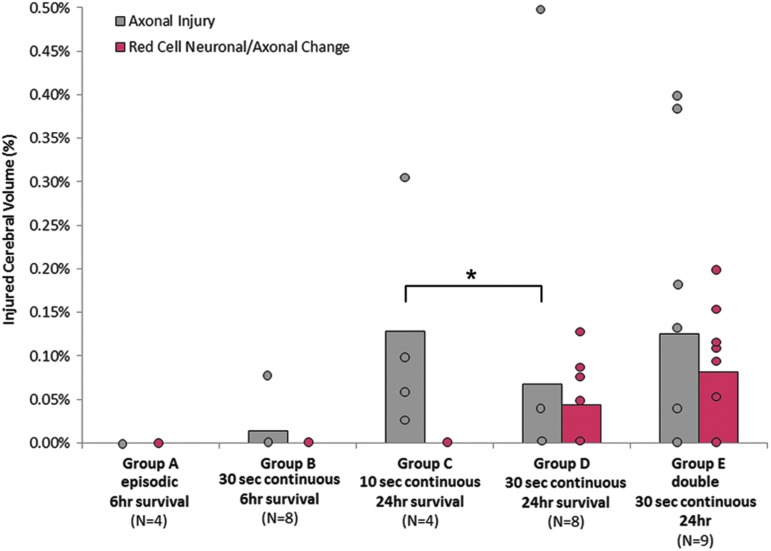
Axonal injury after episodic, 10 sec continuous, 30 sec continuous, or two 30 sec continuous cyclic events held 24 h apart. *Axonal injury occurred significantly more often in the 10 sec continuous group compared with the 30 sec continuous group after 24 h post-injury (Pearson chi-square, p = 0.014). Color image is available online at www.liebertpub.com/neu
FIG. 6.
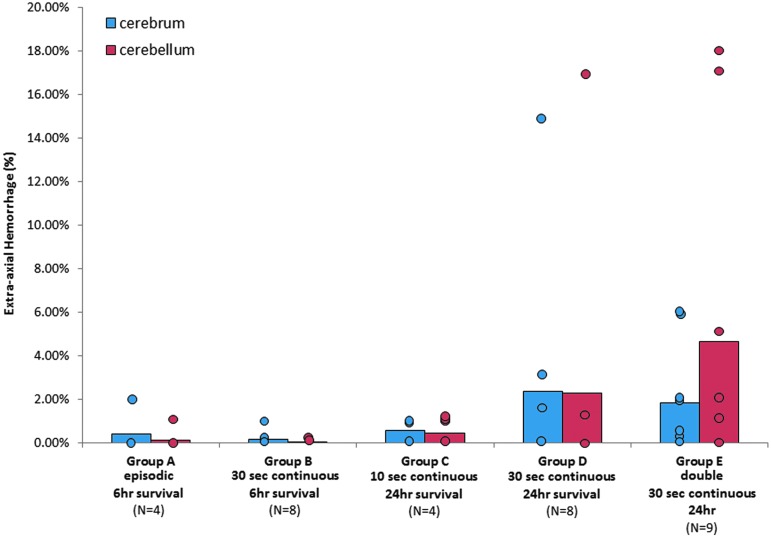
Extra-axial hemorrhage after episodic, 10 sec continuous, 30 sec continuous, or two 30 sec continuous cyclic events held 24 h apart. Color image is available online at www.liebertpub.com/neu
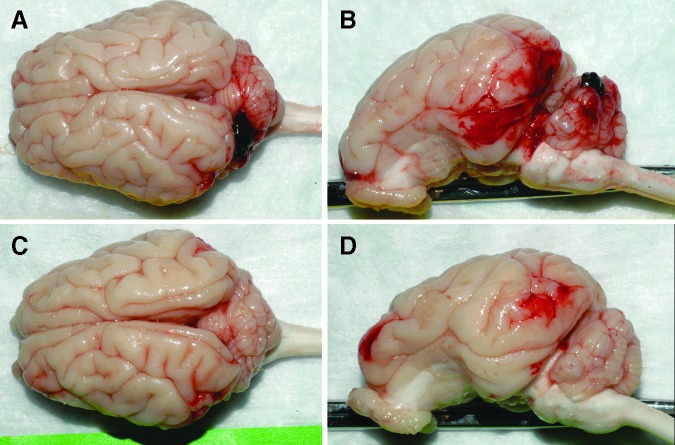
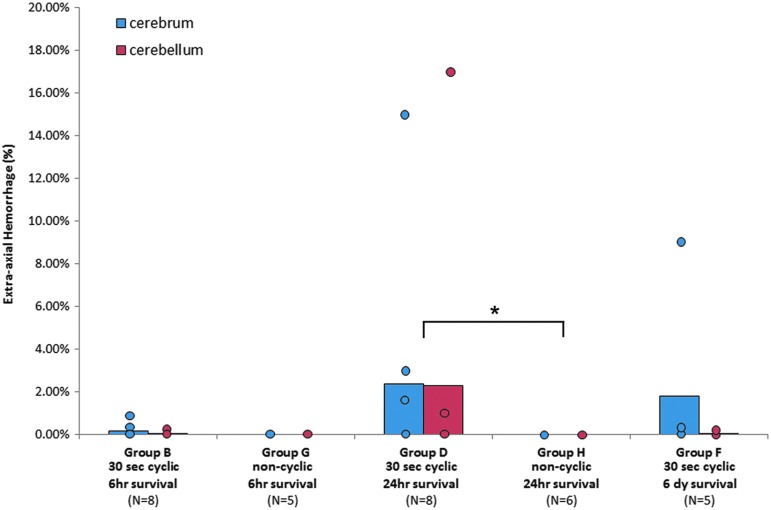
Injury (i.e., AI, RENAC, or EAH) was seen in 88.5% of the cyclic animals surviving 24 h and 6 days (groups C, D, E, F), but the presence of several animals without injury resulted in low overall averages for these groups, and only the percent of RCNAC for group E (two cyclic head rotation exposures 24 h apart) was significantly greater than shams (p < 0.042). Representative images displaying the worst and average EAH scores in group E are provided in Figure 7. No ocular injury was found in any of the animal studies.
FIG. 7.
Superior and lateral views of piglet brains representing 9.75% (A, B) and 2.1% (C, D) total extra-axial hemorrhage. Cerebrum and cerebellum hemorrhage was 6.3% and 17.7%, respectively, in A, B and 2.3% and 1.6%, respectively, in C, D. Both images are from group E (double, 30 sec continuous cyclic head rotation). Color image is available online at www.liebertpub.com/neu
Episodic versus continuous cyclic head rotation
We observed no significant differences in the occurrence (number of animals) or extent (brain volume) of AI (Fig. 5) or EAH (Fig. 6) at 6 h post-injury when comparing animals that experienced five 6-sec episodes (group A) of cyclic head rotation with those exposed to 30 sec of continuous (group B) head rotation. No animals in the episodic group had axonal injury, and only one (20%) of those animals showed cerebral or cerebellum EAH (Table 2, Fig. 5). Only one animal in the 30-sec continuous group had AI (group B, Table 2). No animals had RCNAC. In summary, episodic and continuous cyclic head rotations for a total of 30 sec were similar at 6 h after the head rotation in this animal model.
Duration of cyclic head rotation
When survival after a single episode was increased to 24 h, we observed significantly more animals with AI after continuous cyclic head rotations for 10 sec (100%, group C) than those after 30 sec of continuous head rotation (25%, group D, Table 2, Pearson chi-square p = 0.014). There was no significant difference in the extent of AI or RCNAC. The occurrence and extent of EAH did not differ between groups (Table 2, Fig. 6). CBF was significantly lower (p = 0.047) in the 30 sec group D (45.48 ± 20.01 mL/100 g-min) than the 10 sec group C (74.93 ± 15.87 mL/100 g-min, Table 2, Fig. 4).
In summary, while the decreased duration had a higher occurrence of AI, increasing the duration by 20 sec resulted in a significant decrease in CBF. Increasing the duration did not have an effect on the occurrence or extent of EAH or the extent of AI.
Repeated cyclic head rotation
Animals that experienced a second cyclic head rotation 24 h after the initial cyclic head rotation (group E) showed a slightly higher occurrence and extent of AI, RCNAC, and EAH when compared with the animals with only one continuous cyclic head rotation (group D), but these observations did not reach statistical significance (Table 2, Fig. 5 and 6). CBF was not altered by a second injury compared with the single cyclic head rotation group (Table 2). In summary, in our small cohort, repeating the cyclic head rotations 24 h after the first cyclic head rotation did not significantly increase injuries.
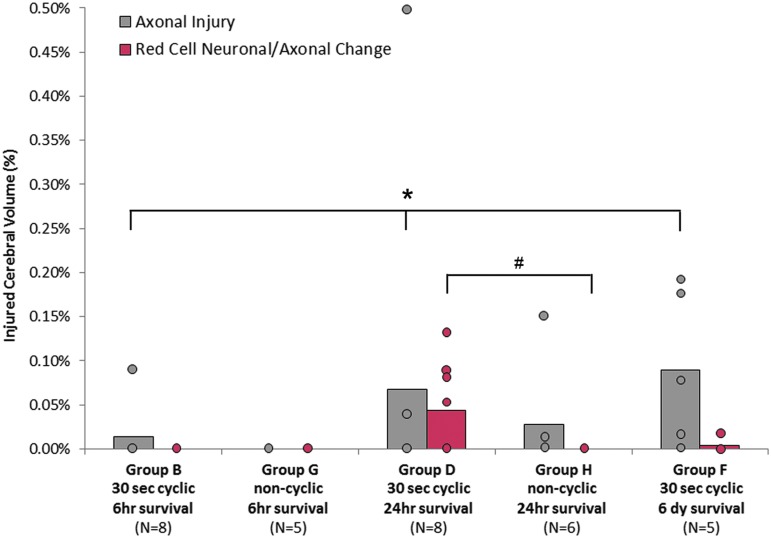
Temporal response of cyclic head rotation
To evaluate the injury temporal response, animals from the 30 sec continuous cyclic groups with post-injury survival times of 6 h, 24 h, and 6 days (groups B, D, and F) were compared. The number of animals with AI increased significantly with increasing post-injury survival time (p = 0.035). The average extent of AI increased with post-injury survival time, but this increase did not quite reach statistical significance (p = 0.059, Table 2, and Fig. 8). RCNAC was not detected at 6 h post-injury, increased to 0.0435 ± 0.0523% at 24 h post-injury (n = 4), and then decreased to 0.004 ± 0.009% by 6 days (n = 1). These changes did not reach significance (p = 0.054).
FIG. 8.
Axonal injury dependent on time course or cyclic/noncyclic. Significance indicator is a significant difference in the occurrence. *The number of animals with axonal injury increased significantly with time (Pearson chi-square, p = 0.035). #The number of animals with red cell neuronal/axonal changes was significantly greater in the cyclic group compared to the noncyclic group at 24 h (Pearson chi-square, p = 0.04). Color image is available online at www.liebertpub.com/neu
The number of animals with EAH (occurrence) increased between 6 h survival times (20% for the combined group B) and the 24 h (50%, group D) and 6 day survival times (40%, group F), but this increased occurrence did not reach statistical significance (Fig. 9). The average extent of EAH between the groups was not significantly different. No statistically significant changes in CBF were found between group D (24 h post-injury) and group F (6 days post-injury) as shown in Figure 4.
FIG. 9.
Temporal and cyclic/noncyclic for extra-axial hemorrhage. *The number of animals with hemorrhage was significantly greater in the cyclic group after a survival time of 24 h (p < 0.05). Color image is available online at www.liebertpub.com/neu
Cyclic versus noncyclic head rotation
We compared 30 sec cyclic head rotations with single, noncyclic head rotations in extension at post-rotation survival intervals of 6 h (groups B and G) and 24 h (groups D and H). Both types of head rotations consisted of similar head excursions; however, group H (single rotation, 24 h survival) had a significantly higher angular velocity than all other groups (p < 0.0001, Table 2). The physical lower velocity limits of the single head rotation device were 15 rad/sec greater than the physical upper velocity limits of the cyclic device.
At 6 h after head rotation, little to no AI, RCNAC, or EAH was found in either the cyclic or noncyclic head rotation groups. Animals surviving 24 h after a cyclic or single head rotation (groups D and H) showed no significant difference in the occurrence or extent of AI; however, cyclic injury had a slightly higher average AI extent (Fig. 8). Fifty percent of the animals in the cyclic group (n = 4, group D) had RCNAC after 24 h post-injury, which was significantly higher than the single head rotation group (group H, p = 0.04), where no RCNAC were observed. Despite this significant increase in the number of animals with hypoxic-ischemic injury, the amount of RCNAC in the cyclic group (0.0435 ± 0.0523%) was not significantly different from zero (p = 0.052).
The presence of EAH occurred significantly more often in the cyclic group (50%) compared with the noncyclic group (0%) for animals surviving 24 h, despite lower velocities used in the cyclic head rotations compared with the single head rotation (p < 0.05, Table 2, Fig. 8). This increased occurrence of EAH in the cyclic group at 24 h resulted in an increased average extent of EAH that nearly reached statistical significance (p = 0.052).
In summary, low levels of continuous cyclic head rotations for 30 sec resulted in more EAH and hypoxic-ischemic insult than a single head rotation, but was detectable only after a 24 h delayed presentation. It should be noted that the single head rotations (groups D and H) were performed in the sagittal plane, while the cyclic head rotations (groups B and G) were performed in the axial plane. Previous research in our laboratory has shown that sagittal head rotation in piglets results in more AI and EAH than axial head rotation50; therefore, the little to no injury found in groups D and H would be even lower with axial head rotations, and our conclusions would remain the same.
Discussion
The purpose of this study was to systematically investigate the effect of cyclic, low-velocity head rotations on TBI in a large pediatric animal model. This objective was accomplished by comparing brain injury from different cyclic head rotation patterns and also from single, noncyclic head rotations. Overall injury was lowest in the 6-h survival groups, indicating that mild injury may develop or only be faintly visualized around this time period. Considering all experimental groups, which consisted of low-velocity head rotations within the physiological range of motion, we found that repeated cyclic events could produce modest AI and EAH. Specifically, these injury findings were not evident 6 h after head rotation, were detectable by 24 h after head rotation, and were still present 6 days later. The percentage of AI increased between 24 h and 6 days, whereas the amount of EAH did not.
In groups with the highest level of injuries (groups D, E, and F), the injuries were mild in magnitude in comparison with those seen in our previous piglet studies, which resulted in clinical signs or symptoms associated with mild to moderate TBI.36,41 The magnitude of AI averaged less than 0.15% of the cerebral volume, the RCNAC indicative of hypoxic/ischemic injury averaged less than 0.08%, and the extent of EAH averaged less than 3% of the total brain surface (Fig. 7). There was no ocular injury.
In our previous piglet studies of the same age, we observed a 10-fold higher extent of AI and EAH at the same time point after the injury (24 h).36 Specifically, AI was present in 1.3% of the cerebrum volume; EAH covered 25–30% of the brain surface. The previous studies consisted of single, sagittal head rotations with five times higher velocity than the present study (150 rad/sec). Even with higher levels of velocity producing several-fold greater extents of AI, the animals in these previous studies returned to the animal facility and were able to eat and ambulate hours later, without life-support or interventions. However, these animals, as well as those experiencing axial head rotations at even higher velocities (190 rad/sec), demonstrated persistent cognitive and behavioral deficits when tested 1–2 weeks after injury.15,39,41 Retinal hemorrhage at the vitreous base occurred in 8% of the animals experiencing a sagittal or axial high-velocity head rotation.42
These injury descriptions from our previous piglet studies, associated with high rotational velocities, may be described as mild to moderate compared with the characteristic widespread hypoxic-ischemic brain injury,51 subdural hemorrhage,52–54 and retinal hemorrhage55–58 often described in human AHT (which may include confessions of shaking). Therefore, the injuries in the present animal study, associated with single or repeated low-velocity cyclic head rotations, might be classified as very mild.
Our objective was to examine the injury response of the young brain to single and repeated episodes of cyclic head rotations using a large animal model with similarities to the human brain, not to prove or disprove whether shaking alone can cause serious injury in human infants. To this extent, there are some important distinctions between this study and typical descriptions of nonaccidental trauma that might account for differences in the injuries observed.
First, head impact injuries were excluded from the design of our animal model study. Instead, we focused on the pathological findings produced by rapid head rotations exclusive of other modes of brain injury. Second, although recent clinical data suggest that cervical spine pathology is common in victims of AHT,59 potentially injurious cervical hyperextension and hyperflexion were purposefully avoided in this study to ensure an isolated and controlled measurement of the effect of cyclic head rotation on the brain itself.
Head rotations were limited to the physiological passive neck range of motions of the piglet (60 degrees in the sagittal plane, 100 degrees in the axial plane). Motion capture of the instrumented infant surrogate head during shaking illustrated a figure eight-like pattern with a maximum head rotation of 160 degrees (Fig. 1). In 2-month-old human infants, the passive range of motion for axial rotation and lateral bending of the neck is 105.2 degrees and 68.1 degrees, respectively.60 The passive sagittal range of neck motion is not published for the infant. Active axial rotation, lateral bending, and sagittal range of motion of an adolescent human neck are 143 degrees, 103 degrees, and 137 degrees, respectively.61
Given that the passive measurements of the infant are lower than the active measurements in the adolescent, one might conservatively estimate that a sagittal rotation in the infant beyond 140 degrees is representative of some degree of hyperextension or hyperflexion. Therefore, we may add in a deliberate hyperflexion or hyperextension in future studies to evaluate the specific contribution of these motions to brain injury.
A third important distinction between this study and no-accidental trauma is that all animals in this study were anesthetized during the shaking events. A common trigger for abusive shaking is prolonged and intense crying of an infant.9,62 During intense crying, infants typically contract their abdominal muscles, creating a Valsalva maneuver, thus impeding cerebral venous return.63 This increases intracranial pressure by 15 to 20 mm Hg64 and increases cerebral blood volume 17.4 times baseline measurements.63 When the infant pauses crying for deep inspiration, the cerebral blood volume rapidly decreases, but not to cerebral blood volume levels before the crying. This process repeats and results in a gradual increase in overall cerebral blood volume.63
The increased intracranial pressure and cerebral blood volume may result in a preliminary intravascular load applied to the brain. In addition, there may be further increased intracranial pressure from increased intrathoracic pressure if a child is held tightly. When these pre-loads are coupled with a shaking mechanical load, it is possible that injury could be exacerbated. The contribution of this physiological pre-loading to brain injury will be investigated in future studies.
Because of technical and physiologic constraints described earlier, the levels of velocity and acceleration achieved in our study were similar in magnitude to those generated during vigorous shaking of an instrumented infant surrogate, but were not at the higher levels necessary to replicate human infant brain deformation in the much smaller piglet brain. Therefore, the results of this study should not be considered as a demonstration of the upper limits of injuries that might occur from shaking a human infant. Instead, one should conclude that cyclic loading increases AI and EAH compared with just a single, noncyclic head rotation, but only by marginal amounts.
Further, we have documented that AI persists longer41 and CBF is significantly reduced13,14 after sagittal head rotations compared with axial head rotations. Therefore, our findings at 24 h post-injury might have been more substantial if we were able to achieve the same velocities with a sagittal head rotation instead of the axial head rotations. We recommend that future studies include sagittal head rotations for comparison.
We found slight increases in AI and EAH in animals that received a second cyclic head rotation 24 h after the first, but these increases were not statistically significant. This agrees with our previously published data for two single (noncyclic) head rotations after an acute period. Two repeated episodes of shaking, however, may not be enough to capture the spectrum of abusive shaking exposure. In a retrospective study of 29 AHT confessions in which shaking was an admitted part of the injury mechanism, perpetrators admitted to 2–30 episodes of infant shaking.9 The average number of episodes among all of the confessions was 10 times.
Our previous piglet studies have shown that multiple head rotations increase axonal damage.20,38 Therefore, the number of exposures to shaking, the physiological state of the child (including intracranial changes from crying), the mechanical loading parameters of the abuse (velocity, head excursion, head rotation direction, chest grip force, and impact force), the developmental state of the brain, and previous damage could all contribute to eventual mechanical damage in the brain. Future studies should systematically explore each of these factors to determine whether and how they contribute to injury presentation and progression.
To our knowledge, there is only one other large animal model of cyclic head rotation. Finnie and coworkers26,65 investigated brain injury in nine immature (5–9 day old) lambs shaken by gripping the thorax of the body. At 6 h post-injury, they report small (1 cm ×1 cm) focal subdural hemorrhage in three of the nine lambs (33%) and no retinal hemorrhage. The occurrence of EAH in our piglet model at 6 h post-injury was similar (33% in group BSAG), and we also found no retinal hemorrhage. At 24 h post-injury, however, we found a significantly higher occurrence of EAH and slightly larger extent.
AI was present in all nine of the lambs in Finnie and associates26,65 at 6 h post-injury, but only one of the six piglets in our study had axonal injury at 6 h post-injury after a sagittal head rotation. The older lambs (7–9 days old) had an average of 12% of white matter with AI and the young lambs (5 days old) averaged 29% AI. The percentages of AI reported in our study were based on total brain volume, and when we express them as a fraction of the white matter (approximately 19% of total cerebrum), we estimate 0.07% of the white matter of our 6 h sagittal cyclic head rotations had injury, which is three orders of magnitude lower than the Finnie studies.26,65
It is challenging to interpret these similarities and differences, however, because no angular kinematic measurements of the lamb head rotations were reported,25 and it is unclear how the mechanical forces applied to the lambs compared with that of the pigs in this study. The lamb's neck is also longer than that of the pig, and the head movement was not constrained, so we speculate that hyperflexion and hyperextension of the lamb's neck may have occurred.
The three younger (5-day-old) lambs in the Finnie studies26.65 died prematurely at 2, 3, and 5 h. It is unclear whether this premature death was because of increased sensitivity to anesthesia, increased damage from hyperflexion/hyperextension, or differences in applied mechanical forces between the age groups. It is of interest that none of the lambs had significant subdural hemorrhage or widespread coalescent parenchymal damage of the type seen in highly morbid or fatal cases of inflicted head injury, either unilaterally or bilaterally.
AHT is rarely witnessed by an unbiased party, and there is variability in the manner in which children are abused. Confessions and observations dating back to the mid-1950s repeatedly invoke shaking as an important component of the physical activities associated with an inflicted mechanism of injury, which often include a head impact at some point in the event,9,11,12,66 but there is still controversy regarding the precise contribution of shaking alone to TBI.
Biomechanical studies report that the angular acceleration during shaking is significantly lower than the head angular accelerations required to produce subdural hemorrhage and/or AI in adult nonhuman primates undergoing a single head rotation.23,24 With this evidence, some claim that these low-level accelerations in children are not enough to cause TBI. To date, however, laboratory studies have not fully recreated the clinical scenarios that characterize infant abuse, including the physiologic changes from crying.
Our present study suggests that under controlled circumstances, cyclic, low-velocity head rotations can produce more injury after 24 h than is seen after a single head rotation at the same load magnitude. These injuries are pathologically and clinically at the mild end of the injury spectrum and still do not replicate the constellation of pathoanatomic injury types seen in infants who sustain severe AHT. Future efforts that more closely replicate the complex physiological and mechanical environments associated with these abusive events will improve our understanding of the pathophysiology of infant abuse.
Acknowledgments
Research funding was provided by NIH NINDS T32 NS043126, K12 EY015398, R01 NS39679 and its associated ARRA administrative supplemental award. The authors are grateful to Jill Ralston for assistance with animal handling, tissue processing, and scoring of axonal injury and red cell neuronal/axonal change; Sarah Sullivan for assistance with extra-axial hemorrhage scoring and statistical analysis; Justin Jones for assistance with data analysis; and Dr. Andrew Merryweather for using data motion capture to evaluate head motion during surrogate shaking.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Langlois J., Rutland-Brown W. and Thomas K. (2004). Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA [Google Scholar]
- 2.CDC (2015). Percent distributions of TBI-related deaths by age group and injury mechanism—United States, 2006–2010. Available at: http://www.cdc.gov/traumaticbraininjury/data/dist_hosp.html Accessed: April19, 2016
- 3.Bechtel K., Stoessel K., Leventhal J., Ogle E., Teague B., Lavietes S., Banyas B., Allen K., Dziura J., and Duncan C. (2004). Characteristics that distinguish accidental from abusive injury in hospitalized young children with head trauma. Pediatrics 114, 165–168 [DOI] [PubMed] [Google Scholar]
- 4.Duhaime A., Alario A., Lewander W., Schut L., Sutton L.N., Seidl T.S., Nudelman S., Budenz D., Hertle R., Tsarias W., and Loporchio S. (1992). Head injury in very young children: mechanisms, injury types, and ophthalmologic findings in 100 hospitalized patients younger than 2 years of age. Pediatrics 90, 179–185 [PubMed] [Google Scholar]
- 5.Hettler J., and Greenes D.S. (2003). Can the initial history predict whether a child with a head injury has been abused? Pediatrics 111, 602–607 [DOI] [PubMed] [Google Scholar]
- 6.Reece R.M., and Sege R. (2000). Childhood head injuries: accidental or inflicted? Arch. Pediatr. Adolesc. Med. 154, 11–15 [PubMed] [Google Scholar]
- 7.Vinchon M., de Foort-Dhellemmes S., Desurmont M., and Delestret I. (2010). Confessed abuse versus witnessed accidents in infants: comparison of clinical, radiological, and ophthalmological data in corroborated cases. Childs Nerv. Syst. 26, 637–645 [DOI] [PubMed] [Google Scholar]
- 8.Christian C.W.; Committee on Child Abuse and Neglect, American Academy of Pediatrics. (2015). The evaluation of suspected child physical abuse. Pediatrics 135, e1337–1354 [DOI] [PubMed] [Google Scholar]
- 9.Adamsbaum C., Grabar S., Mejean N., and Rey-Salmon C. (2010). Abusive head trauma: judicial admissions highlight violent and repetitive shaking. Pediatrics 126, 546–555 [DOI] [PubMed] [Google Scholar]
- 10.Christian C.W., andBlock R.; Committee on Abuse and Neglect; American Academy of Pediatrics. (2009). Abusive head trauma in infants and children. Pediatrics 123, 1409–1411 [DOI] [PubMed] [Google Scholar]
- 11.Bell E., Shouldice M., and Levin A.V. (2011). Abusive head trauma: a perpetrator confesses. Child Abuse Negl. 35, 74–77 [DOI] [PubMed] [Google Scholar]
- 12.Starling S.P., Patel S., Burke B.L., Sirotnak A.P., Stronks S., and Rosquist P. (2004). Analysis of perpetrator admissions to inflicted traumatic brain injury in children. Arch. Pediatr. Adolesc. Med. 158, 454–458 [DOI] [PubMed] [Google Scholar]
- 13.Eucker S., Friess S., Ralston J., and Margulies S. (2008). Regional Cerebral Blood Flow Response Following Brain Injury Depends on Direction of Head Motion. National Neurotrauma Society: Orlando, FL, pps.854–935 [Google Scholar]
- 14.Eucker S.A., Smith C., Ralston J., Friess S.H., and Margulies S.S. (2011). Physiological and histopathological responses following closed rotational head injury depend on direction of head motion. Exp. Neurol. 227, 79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friess S.H., Ichord R.N., Owens K., Ralston J., Rizol R., Overall K.L., Smith C., Helfaer M.A., and Margulies S.S. (2007). Neurobehavioral functional deficits following closed head injury in the neonatal pig. Exp. Neurol. 204, 234–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibrahim N.G., Ralston J., Smith C., and Margulies S.S. (2010). Physiological and pathological responses to head rotations in toddler piglets. J. Neurotrauma 27, 1021–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margulies S.S., Meaney D.F., Smith D.H., Chen X.H., Miller R. and Raghupathi R. (1999). A Comparison of Diffuse Brain Injury in the Newborn and Adult Pig. International Research Committee on the Biomechanics of Impact: Barcelona, Spain [Google Scholar]
- 18.Prange M., and Margulies S. (2001). Tissue strain thresholds for axonal injury in the infant brain. Kamm R. (ed). The American Society of Mechanical Engineers, Bioengineering Conference.: Snowbird, UT, pps. 833–834 [Google Scholar]
- 19.Raghupathi R., and Margulies S.S. (2002). Traumatic axonal injury after closed head injury in the neonatal pig. J. Neurotrauma 19, 843–853 [DOI] [PubMed] [Google Scholar]
- 20.Raghupathi R., Mehr M., Helfaer M., and Margulies S. (2004). Traumatic axonal injury is exacerbated following repetitive closed head injury in the neonatal pig. J. Neurotrauma 21, 307–316 [DOI] [PubMed] [Google Scholar]
- 21.Smith S., Andrus P., Gleason D., and Hall E. (1998). Infant rat model of the shaken baby syndrome: preliminary characterization and evidence for the role of free radicals in cortical hemorrhaging and progressive neuronal degeneraiton. J. Neurotrauma 15, 693–705 [DOI] [PubMed] [Google Scholar]
- 22.Bonnier C., Mesples B., Carpentier S., Henin D., and Gressens P. (2002). Delayed white matter injury in a murine model of shaken baby syndrome. Brain Pathol. 12, 320–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duhaime A.C., Gennarelli T.A., Thibault L.E., Bruce D.A., Margulies S.S., and Wiser R. (1987). The shaken baby syndrome: a clinical, pathological, and biomechanical study. J. Neurosurg. 66, 409–415 [DOI] [PubMed] [Google Scholar]
- 24.Prange M.T., Coats B., Duhaime A.C., and Margulies S.S. (2003). Anthropomorphic simulations of falls, shakes, and inflicted impacts in infants. J. Neurosurg. 99, 143–150 [DOI] [PubMed] [Google Scholar]
- 25.Anderson R., Sandoz B., Dutschke J., Finnie J., Turner R., Blumbergs J., Manavis J., and Vink R. (2014). Biomechanical studies in an ovine model of non-accidental head injury. J. Biomech. 47, 2578–2583 [DOI] [PubMed] [Google Scholar]
- 26.Finnie J.W., Blumbergs P.C., Manavis J., Turner R.J., Helps S., Vink R., Byard R.W., Chidlow G., Sandoz B., Dutschke J., and Anderson R.W. (2012). Neuropathological changes in a lamb model of non-accidental head injury (the shaken baby syndrome). J. Clin. Neurosci. 19, 1159–1164 [DOI] [PubMed] [Google Scholar]
- 27.Patterson D., Sweasey D., and Hebert C. (1971). Changes occurring in the chemical composistion of the central nervous system during foetal and post-natal development of the sheep. J. Neurochem. 18, 2027–2040 [DOI] [PubMed] [Google Scholar]
- 28.Dobbing J., and Sands J. (1973). Quantitative growth and development of human brain. Arch. Dis. Child 48, 757– 767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duhaime A.C. (2006). Large animal models of traumatic injury to the immature brain. Dev. Neurosci. 28, 380–387 [DOI] [PubMed] [Google Scholar]
- 30.Buckley N. (1986). Maturation of circulatory system in three mamalian models of human development. Comp. Biochem. Physiol. A Comp. Physiol 83, 1–7 [DOI] [PubMed] [Google Scholar]
- 31.Dickerson J., and Dobbing J. (1966). Prenatal and postnatal growth and development of the central nervous system of the pig. Proc. R Soc. London, Series B; 166, 384–395 [DOI] [PubMed] [Google Scholar]
- 32.Pampiglione G. (1971). Some aspects of development of cerebral function in mammals. Proc. R Soc. Med. 64, 429–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dobbing J., and Sands J. (1979). Comparative aspects of the brain growth spurt. Early Hum. Dev. 3, 79–83 [DOI] [PubMed] [Google Scholar]
- 34.Lind N.M., Moustgaard A., Jelsing J., Vajta G., Cumming P., and Hansen A.K. (2007). The use of pigs in neuroscience: modeling brain disorders. Neurosci. Biobehav. Rev. 31, 728–751 [DOI] [PubMed] [Google Scholar]
- 35.Flynn T.J. (1984). Developmental changes of myelin-related lipids in brain of miniature swine. Neurochem. Res. 9, 935–945 [DOI] [PubMed] [Google Scholar]
- 36.Weeks D., Sullivan S., Kilbaugh T., Smith C., and Margulies S.S. (2014). Influences of developmental age on the resolution of diffuse traumatic intracranial hemorrhage and axonal injury. J. Neurotrauma 31, 206–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sullivan S., Eucker S.A., Gabrieli D., Bradfield C., Coats B., Maltese M.R., Lee J., Smith C., and Margulies S.S. (2015). White matter tract-oriented deformation predicts traumatic axonal brain injury and reveals rotational direction-specific vulnerabilities. Biomech. Model Mechanobiol. 14, 877–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friess S.H., Ichord R.N., Ralston J., Ryall K., Halfaer M.A., Smith C., and Margulies SS. (2009). Repeated traumatic brain injury affects composit cognitive function in piglets. J. Neurotrauma 26, 1111–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naim M., Friess S., Smith C., Ralston J., Ryall K., Helfaer M., and Margulies S.S. (2010). Folic acid enhances early functional recovery in a piglet model of pediatric head injury. Dev. Neurosci. 32, 466–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sullivan S., Friess S.H., Ralston J., Smith C., Propert K.J., Rapp P.E., and Margulies S.S. (2013). Improved behavior, motor, and cognition assessments in neonatal piglets. J. Neurotrauma 30, 1770–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sullivan S., Friess S.H., Ralston J., Smith C., Propert K.J., Rapp P.E., and Margulies S.S. (2013). Behavioral deficits and axonal injury persistence after rotational head injury are direction dependent. J. Neurotrauma 30, 538–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coats B., Binenbaum G., Peiffer R.L., Forbes B.J., and Margulies S.S. (2010). Ocular hemorrhages in neonatal porcine eyes from single, rapid rotational events. Invest. Ophthalmol. Vis. Sci. 51, 4792–4797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cory C.Z., and Jones B.M. (2003). Can shaking alone casue fatal brain injury? A biomechanical assessment of the Duhaime shaken baby syndrome model. Med. Sci. Law 43, 317–333 [DOI] [PubMed] [Google Scholar]
- 44.Sullivan S., Coats B., and Margulies S. (2015). Biofidelic neck influences head kinematics of parietal and occipital impacts following short falls in infants. Accid. Anal. Prev. 82, 143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coats B., and Margulies S.S. (2008). Potential for head injuries in infants from low-height falls. J. Neurosurg. Pediatr. 2, 321–330 [DOI] [PubMed] [Google Scholar]
- 46.Margulies S.S., and Thibault L.E. (1992). A proposed tolerance criterion for diffuse axonal injury in man. J. Biomech. 25, 917–923 [DOI] [PubMed] [Google Scholar]
- 47.Ommaya A.K., and Hirsch A.E. (1971). Tolerances for cerebral concussion from head impact and whiplash in primates. J. Biomech. 4, 13–21 [DOI] [PubMed] [Google Scholar]
- 48.Yu B., Gabriel D., Noble L., and An K. (1999). Estimate of the optimum cutoff frequency for the Butterworth low-pass digital filter. J. Appl. Biomech. 15, 318–329 [Google Scholar]
- 49.Eucker S.A., Hoffman B.D., Natesh R., Ralston J., Armstead W.M,. and Margulies S.S. (2010). Development of a fluorescent microsphere technique for rapid histological determination of cerebral blood flow. Brain Res. 1326, 128–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eucker S. (2009). Effect of Head Rotation Direction on Closed Head Injury in Neonatal Piglets. Bioengineering, University of Pennsylvania; Philadelphia [Google Scholar]
- 51.Ichord R., Naim M., Pollock A.N., Nance M.L., Margulies S.S., and Christian C.W. (2007). Hypoxic-ischemic injury complicates inflicted and accidental traumatic brain injury in young children: the role of diffusion weighted imaging. J. Neurotrauma 24, 106–118 [DOI] [PubMed] [Google Scholar]
- 52.Jayawant S., Rawlinson A., Gibbon F., Price J., Schulte J., Sharples P., Sibert J.R., and Kemp A.M. (1998). Subdural haemorrhages in infants: population based study. BMJ 317, 1558–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matschke J., Voss J., Obi N., Gorndt J., Sperhake J.P., Puschel K., and Glatzel M. (2009). Nonaccidental head injury is the most common cause of subdural bleeding in infants <1 year of age. Pediatrics 124, 1587–1594 [DOI] [PubMed] [Google Scholar]
- 54.Trenchs V., Curcoy A., Morales M., Serra A., Navarro R., and Pou J. (2008). Retinal hemorrhages in head trauma resulting from falls: differential diagnosis with non-accidental trauma in patients younger than 2 years of age. Childs Nerv. Syst. 24, 815–820 [DOI] [PubMed] [Google Scholar]
- 55.Bhardwaj G., Chowdhury V., Jacobs M.B., Moran K.T., Martin F.J.,and Coroneo M.T. (2010). A systematic review of the diagnostic accuracy of ocular signs in pediatric abusive head trauma. Ophthalmology 117, 983–992 [DOI] [PubMed] [Google Scholar]
- 56.Binenbaum G., Mirza-George N., Christian C., and Forbes B. (2009). Odds of abuse associated with retinal hemorrhages in children suspected of child abuse. J AAPOS 13, 268–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maguire S., Pickerd N., Farewell D., Mann M., Tempest V., and Kemp A. (2009). Which clinical features distinguish inflicted from non-inflicted brain injury? A systematic review. Arch. Dis. Child. 94, 860–867 [DOI] [PubMed] [Google Scholar]
- 58.Vinchon M., DeFoort-Dhellemmes S., Desurmont M., and Dhellemmes P. (2005). Accidental and nonaccidental head injuries in infants: a prospective study. J. Neurosurg. 102, Suppl 4, 380–384 [DOI] [PubMed] [Google Scholar]
- 59.Choudhary A., Ishak R., Zacharia T., and Dias M. (2014). Imaging of spinal injury in abusive head trauma: a retrospective study. Pediatr. Radiol. 44, 1130–1140 [DOI] [PubMed] [Google Scholar]
- 60.Ohman A., and Beckung E. (2008). Reference values for range of motion and muscle function of the neck in infants. Pediatr. Phys. Ther. 20, 53–58 [DOI] [PubMed] [Google Scholar]
- 61.Tommasi D.G., Foppiani A.C., Galante D., Lovecchio N., and Sforza C. (2009). Active head and cervical range of motion: effect of age in healthy females. Spine 34, 1910–1916 [DOI] [PubMed] [Google Scholar]
- 62.Lee C., Barr R., Catherine N., and Wicks A. (2007). Age-related incidence of publicly reported shaken baby syndrome cases: is crying a trigger for shaking? J. Dev. Behav. Pediatr. 28, 288–293 [DOI] [PubMed] [Google Scholar]
- 63.Brazy J. (1988). Effects of crying on cerebral blood volume and cytochrome aa3. J. Pediatr. 112, 457–461 [DOI] [PubMed] [Google Scholar]
- 64.Stow P., McLeod M., Burrows F., and Creighton R. (1988). Anterior fontanelle pressure responses to tracheal intubation in the awake and anaesthetized infant. Br. J. Anaesthesiol. 60, 167–170 [DOI] [PubMed] [Google Scholar]
- 65.Finnie J., Manavis J., and Blumbergs P. (2010). Diffuse neuronal perikaryal amyloid precursor protein immunoreactivity in an ovine model of non-accidental head injury (the shaken baby syndrome). J. Clin. Neurosci. 17, 237–240 [DOI] [PubMed] [Google Scholar]
- 66.(1956). The boys jeered her. In: Newsweek, pp. 90